Abstract
Vinylation of aryl N-(2-pyridylsulfonyl) aldimines with versatile 1-alkenyl-1,1-borozinc heterobimetallic reagents is disclosed. In situ hydroboration of air-stable B(pin)-alkynes followed by chemoselective transmetallation with dimethylzinc and addition to aldimines provides B(pin)-substituted allylic amines in 60–93% yield in a one-pot procedure. The addition step can be followed by either B–C bond oxidation to provide α-amino ketones (71–98% yield) or Suzuki cross-coupling to furnish trisubstituted 2-arylated (E)-allylic amines (51–73% yield).
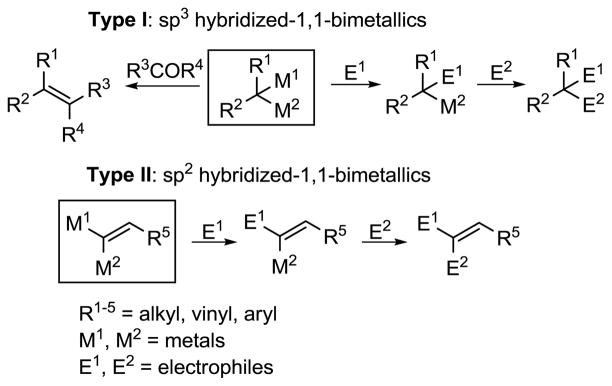
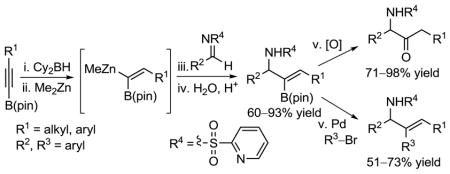
Highly stereoselective construction of C–C double bonds remains a challenge in organic synthesis.1 In this regard, sp3 and sp2 hybridized heterobimetallic reagents of type I and II (Scheme 1) are potentially useful intermediates, because each metal-carbon bond can be chemoselectively exploited in C–C bond forming reactions.2,3,4,6 Furthermore, these versatile heterobimetallic reagents can be employed in tandem reactions, minimizing isolation and purification of intermediates.5 These attributes allow for rapid development of molecular complexity from simple building blocks.
Scheme 1.
1,1-Heterobimetallics in Organic Synthesis
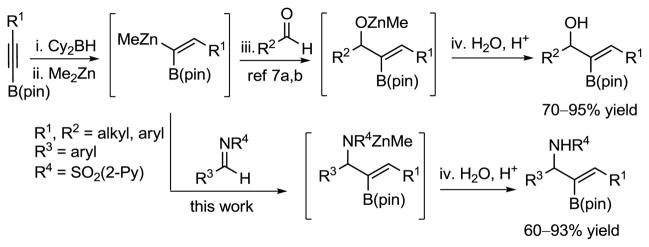
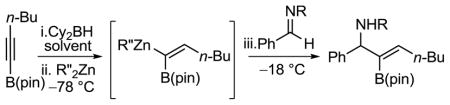
As part of our program in developing stereoselective C–C bond forming reactions,6 we have reported the generation of 1-alkenyl-1,1-heterobimetallic reagents based on boron and zinc from readily available, air-stable B(pin)-substituted alkynes (Scheme 2).7a Thus, regioselective hydroboration of B(pin)-alkynes generates the 1,1-bis (boro) intermediates.7a,8 Chemoselective transmetallation of the more reactive vinyl-BCy2 bond generates 1-alkenyl-1,1-heterobimetallic reagents. The difference in reactivity between Zn–C vs. B–C bonds allows for selective reaction at the Zn–C bond with aldehydes to yield B(pin)-substituted allylic zinc alkoxide intermediates. The alkoxide intermediates were then employed in various tandem reactions to form an array of compounds such as B(pin)-substituted allylic alcohols,7a,b,c α-hydroxy ketones,7a trisubstituted (E)-allylic alcohols,7a B(pin)-substituted cyclopropyl alcohols7b and B(pin)-substituted allylic acetates.7d
Scheme 2.
Generation of 1-Alkenyl-1,1-heterobimetallics of Boron/Zinc and Additions to Electrophiles
Herein, we report the addition of alkenyl-1,1-heterobimetallic reagents to N-(2-pyridylsulfonyl) aldimines to furnish B(pin)-substituted allylic amines (Scheme 2, lower part). The addition can be followed by oxidation of the B–C bond to provide α-aminoketones or by Suzuki cross-coupling to provide densely functionalized trisubstituted (E)-allylic amines.
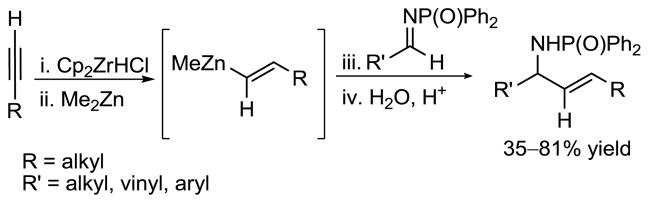
Allylic amines9 are important pharmacophores that can exhibit significant biological properties. Examples include Acrivastine (Semprex),10 Flunarizine,11 and several GABA uptake inhibitors.12 As a result, additions to imines have attracted considerable attention. For example, Wipf and coworkers reported the addition of vinylzinc reagents to aldimines activated with a diphenylphosphonoyl moiety (Scheme 3).13 Carretero14a,b and co-workers demonstrated that the reactivity of N-sulfonyl imines could be increased in the presence of an appropriately positioned heteroaryl group. Using this strategy, they developed the alkylation of aryl N-(2-pyridylsulfonyl) aldimines with organozinc halides.14b The Carretero and Toru groups both have utilized the N-pyridylsulfonyl as a novel stereocontrol element in enantioselective Mannich-type reactions with silyl enol ethers in the presence of chiral copper catalysts.15 Various related nucleophilic reagents, such as dialkyl zinc,5,16,17 alkynylzinc,5,18 diethylaluminium cyanide19 and Danishefsky’s diene20 have also been investigated in imine addition reactions to yield the desired amines.
Scheme 3.
Wipf’s Vinylation of Aryl Diphenylphosphonoyl Imines via Vinylzinc Reagents
Our first task in the addition of bimetallics to imines was to find a suitable imine activating group. The bimetallic reagent was generated and allowed to react with activated imines at −18 °C (Table 1). N-Tosylimines gave trace addition product with our alkenyl heterobimetallic reagents (entry 1). Rather, a significant amount of reduction product was isolated. The N-Boc imine behaved similarly, failing to furnish the desired amine (entry 2). When the activating group was changed to diphenylphosphinoyl, less than 30% of the allylic amine was isolated. Gratifyingly, the bimetallic addition to N-pyridyl sulfonyl imine occurred smoothly in 73% yield in toluene at −18 °C to furnish the desired product (entry 4). The addition was then optimized with the N-pyridyl sulfonyl imines. Switching the solvent from toluene to dicholoromethane improved the yields slightly (entry 4 vs. 7), while in THF, almost no product was formed (entry 5). Dimethylzinc performed better than diethylzinc (entry 7 vs. 9). Increasing the reaction temperature from −18 °C to −10 °C led to diminished yield (entry 6 vs. 7). With the optimized conditions in entry 7, the scope of the reaction was examined.
Table 1.
Optimization of the Addition of Alkenyl-1,1-heterobimetallics to N-Pyridyl Sulfonyl Imines
 | ||||
|---|---|---|---|---|
| entry | R″2Zn | solvent | R | yield (%)a |
| 1 | Me2Zn | toluene | SO2Tol | trace |
| 2 | Me2Zn | toluene | Boc | trace |
| 3 | Me2Zn | toluene | P(O)Ph2 | <30 |
| 4 | Me2Zn | toluene | SO2(2-Py) | 73 |
| 5 | Me2Zn | THF | SO2(2-Py) | trace |
| 6 | Me2Zn | CH2Cl2 | SO2(2-Py) | 68b |
| 7 | Me2Zn | CH2Cl2 | SO2(2-Py) | 80 |
| 8 | Et2Zn | toluene | SO2(2-Py) | 64 |
| 9 | Et2Zn | CH2Cl2 | SO2(2-Py) | 65 |
Isolated yields,
Reaction performed at −10°C
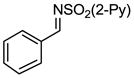
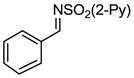
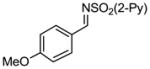
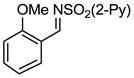
Aryl aldimines with electron donating or electron withdrawing groups were good substrates, providing the B(pin) substituted allylic amines in 60–93% yield (Table 2). The air-stable B(pin)-substituted alkynes can contain aromatic or aliphatic substituents (R = aryl, alkyl). Even the bulky tert-butyl-substituted B(pin) alkyne underwent addition to generate the corresponding allylic amine in 60% yield (entry 5). Substitution at the ortho position of the aldimine resulted in slightly lower yield (entry 7 vs 3–5).
Table 2.
Addition of Alkenyl-1,1-hetrobimetallics to N-Pyridyl Sulfonyl Imines
| entry | borane | imine | allylic amines | yield (%)a |
|---|---|---|---|---|
| 1 |
|
|
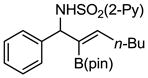 1a 1a
|
80 |
| 2 |
|
|
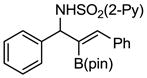 1b 1b
|
68 |
| 3 |
|
|
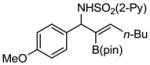 1c 1c
|
87 |
| 4 |
|
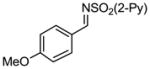
|
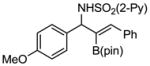 1d 1d
|
93 |
| 5 |
|
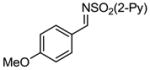
|
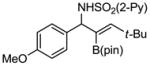 1e 1e
|
60 |
| 6 |
|
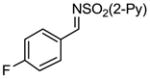
|
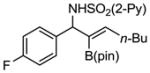 1f 1f
|
70 |
| 7 |
|
|
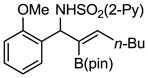 1g 1g
|
53 |
Isolated yields
Having established vinylation of aldimines with our heterobimetallics, we sought to examine tandem reactions involving the B–C bond. Two such reactions are B–C bond oxidation and Suzuki cross-coupling.
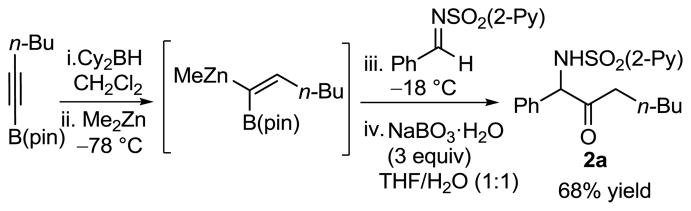
We envisioned that oxidation of the 2-B(pin)-substituted allylic amines would provide access to valuable α-amino ketones, which have important biological activity.21 In the presence of NaBO3·H2O22 in THF/H2O (1:1) at rt, B(pin)-substituted allylic amines were smoothly oxidized to the corresponding α-amino ketones in 71–98% yield (Table 3). The addition/oxidation reaction can also be executed in a tandem fashion. Thus, after the completion of the bimetallic addition to the aldimine, the reaction mixture was subjected to NaBO3·H2O to provide the α-amino ketone in 68% yield in one pot (Scheme 4).
Table 3.
Oxidation of Allylic Amines to α-Amino Ketones
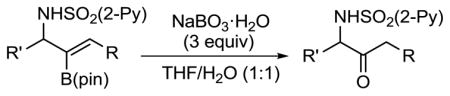 | |||
|---|---|---|---|
| entry | allylic amines | amino ketones | yield %a |
| 1 | 1a |
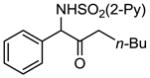 2a 2a
|
80 |
| 2 | 1b |
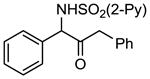 2b 2b
|
75 |
| 3 | 1c |
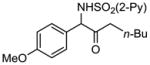 2c 2c
|
96 |
| 4 | 1d |
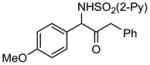 2d 2d
|
98 |
| 5 | 1e |
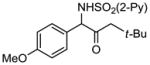 2e 2e
|
87 |
| 6 | 1f |
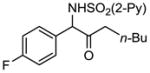 2f 2f
|
71 |
| 7 | 1g |
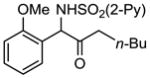 2g 2g
|
87 |
Isolated yields
Scheme 4.
Tandem Addition/B–C Bond Oxidation to Yield α-Amino Ketone 2a
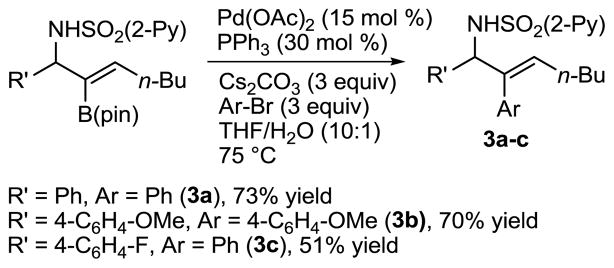
We next utilized the B–C bond in Suzuki cross-coupling reactions. In the presence of Pd(OAc)2 (15 mol %), PPh3 (30 mol %), Cs2CO3 (3 equiv) and aryl bromide (3 equiv) in THF/H2O (10:1) at 75 °C, the B(pin)-substituted allylic amines smoothly underwent cross-coupling to furnish the 2-arylated trisubstituted (E)-allylic amines in 51–73% yield (Scheme 5). No (Z)-double bond isomers were observed in these reactions.
Scheme 5.
Suzuki Cross-Coupling of Allylic Amines
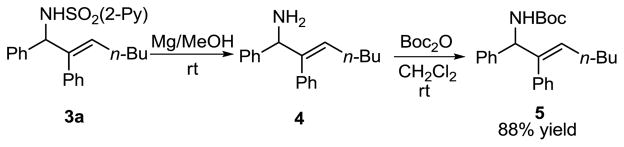
Although the 2-pyridyl sulfonyl group is essential for the addition step, its removal is important for applications of the products. The 2-pyridyl sulfonyl group was readily cleaved on treatment of 1a with magnesium in MeOH to liberate the free amine 4 (Scheme 6).23,24 The free amine 4 was then transformed into its Boc-derivative 5 on treatment with Boc2O at rt in 88% overall yield (Scheme 6).
Scheme 6.
Removal of the 2-Pyridyl Sulfonyl Group followed by Boc-protection
In summary, the nucleophilic addition of 1-alkenyl-1,1-borozinc heterobimetallic reagents to aryl N-(2-pyridylsulfonyl) aldimines has been developed. This protocol provides a variety of B(pin)-substituted allylic amines in good yields. The addition step can be followed by a tandem oxidative cleavage of the B–C bond to furnish valuable α-amino ketones or by Suzuki cross-coupling to form 2-arylated trisubstituted (E)-allylic amines. It is noteworthy that 2-arylated trisubstituted (E)-allylic amines are not currently accessible via the Tsuji-Trost reaction, because 2-arylated allylic acetates are not good substrates for the allylic substitution reaction.7d Given that amino ketones and allylic amines are important pharmacophores, 10–12,21 we anticipate that the methods described herein will be useful to the synthetic community.
Supplementary Material
Acknowledgments
We acknowledge the NIH (NIGMS GM58101) and the NSF (CHE–0848467). Funds for instrumentation were provided by the NIH for a MS (1S10RR023444). MMH thanks the University of Pennsylvania SAS for a Dissertation Completion Fellowship.
Footnotes
Supporting Information Available: Procedures and full characterization of new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Corey EJ, Cheng X-M. The Logic of Chemical Synthesis. John Wiley & Sons; New York: 1988. [Google Scholar]
- 2.Marek I, Normant J-F. Chem Rev. 1996;96:3241. doi: 10.1021/cr9600161. [DOI] [PubMed] [Google Scholar]
- 3.Marek I. Chem Rev. 2000;100:2887. doi: 10.1021/cr990288e. [DOI] [PubMed] [Google Scholar]; (b) Marek I. Actual Chim. 2003:15. [Google Scholar]
- 4.Waas JR, Sidduri A, Knochel P. Tetrahedron Lett. 1992;33:3717. [Google Scholar]
- 5.Ho TL. Tandem Organic Reactions. Wiley and Sons; New York: 1992. p. 502. [Google Scholar]; (b) Wender PA, Miller BL. In: Organic Synthesis: Theory and Applications. Hudlicky T, editor. Vol. 2. JAI Press; Greenwich, CT: 1993. p. 27. [Google Scholar]; (c) Parsons PJ, Penkett CS, Shell AJ. Chem Rev. 1996;96:195. doi: 10.1021/cr950023+. [DOI] [PubMed] [Google Scholar]; (d) Schmalz H-G, Geis O. In: Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi E, editor. Vol. 2. Wiley; New York: 2002. p. 2377. [Google Scholar]; (e) Nicolaou KC, Montagnon T, Snyder SA. Chem Commun. 2003:551. doi: 10.1039/b209440c. [DOI] [PubMed] [Google Scholar]; (f) Tietze LF, Beifuss U. Angew Chem, Int Ed. 1993;32:131. [Google Scholar]; (g) Walsh PJ, Kozlwoski MC. Fundamentals of Asymmetric Catalysis. University Science Books; Sausalito, CA: 2008. [Google Scholar]
- 6.Hussain MM, Walsh PJ. Acc Chem Res. 2008;41:883. doi: 10.1021/ar800006h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Li H, Carroll PJ, Walsh PJ. J Am Chem Soc. 2008;130:3521. doi: 10.1021/ja077664u. [DOI] [PubMed] [Google Scholar]; (b) Hussain MM, Li H, Hussain N, Ureńa M, Carroll PJ, Walsh PJ. J Am Chem Soc. 2009;131:6516. doi: 10.1021/ja900147s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hussain MM, Hernández-Toribio J, Carroll PJ, Walsh PJ. Angew Chem Int Ed. 2011;50:6337. doi: 10.1002/anie.201005742. [DOI] [PubMed] [Google Scholar]; (d) Hussain MM, Walsh PJ. Angew Chem Int Ed. 2010;49:1834. doi: 10.1002/anie.200905399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srebnik M. Tetrahedron Lett. 1991;32:2449. [Google Scholar]
- 9.(a) Kovacic P. Med Hypotheses. 2007;69:1105. doi: 10.1016/j.mehy.2007.01.085. [DOI] [PubMed] [Google Scholar]; (b) Kovacic P. Med Hypotheses. 2006;67:115. doi: 10.1016/j.mehy.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 10.(a) Martín VS, Woodward SS, Katsuki T, Yamada Y, Ikeda M, Sharpless KB. J Am Chem Soc. 1981;103:6237. [Google Scholar]; (b) Slater JW, Zechnich AD, Haxby DG. Drugs. 1999;57:31. doi: 10.2165/00003495-199957010-00004. [DOI] [PubMed] [Google Scholar]
- 11.(a) Straub H, Koehling R, Speckmann EJ. Brain Res. 1994;658:119. doi: 10.1016/s0006-8993(09)90017-8. [DOI] [PubMed] [Google Scholar]; (b) Ashton D, Reid K, Willems R, Marrannes R, Wauguier A. Drug Develop Res. 1986;8:396. [Google Scholar]
- 12.Gibson JR, Manna VK, Salisbury J. J Int Med Res. 1989;17:28. [PubMed] [Google Scholar]
- 13.Wipf P, Kendall C, Stephenson CRJ. J Am Chem Soc. 2003;125:761. doi: 10.1021/ja028092a. [DOI] [PubMed] [Google Scholar]
- 14.Esquivias J, Arrayás RG, Carretero JC. J Org Chem. 2005;70:7451. doi: 10.1021/jo0511602. [DOI] [PubMed] [Google Scholar]; (b) Esquivias J, Arrayás RG, Carretero JC. Angew Chem Int Ed. 2006;45:629. doi: 10.1002/anie.200503305. [DOI] [PubMed] [Google Scholar]
- 15.González AS, Arrayás RG, Carretero JC. Org Lett. 2006;8:2977. doi: 10.1021/ol060866v. [DOI] [PubMed] [Google Scholar]; (b) Nakamura S, Sano H, Nakashima H, Kubo K, Shibata N, Toru T. Tetrahedron: Asymmetry. 2007;48:5565. [Google Scholar]
- 16.Charette AB, Boezio AA, Côté A, Moreau E, Pytkowicz J, Desrosiers J-N, Legault C. Pure Appl Chem. 2005;77:1259. [Google Scholar]
- 17.Porter JR, Traverse JF, Hoveyda AH, Snapper ML. J Am Chem Soc. 2001;123:984. doi: 10.1021/ja003747y. [DOI] [PubMed] [Google Scholar]
- 18.Zani L, Alesi S, Cozzi PG, Bolm C. J Org Chem. 2006;71:1558. doi: 10.1021/jo052273o. [DOI] [PubMed] [Google Scholar]
- 19.Nakamaru S, Sato N, Sugimoto M, Toru T. Tetrahedron: Asymmetry. 2004;15:1513. [Google Scholar]
- 20.Mancheńo OG, González AS, Arrayás RG, Carretero JC. J Am Chem Soc. 2004;126:456. doi: 10.1021/ja038494y. [DOI] [PubMed] [Google Scholar]
- 21.Hanada M, Sugawara K, Koko K, Toda S, Nishiyama Y, Tomita K, Yamamoto H, Konishi M, Oki T. J Antibiot. 1992;45:1746. doi: 10.7164/antibiotics.45.1746. [DOI] [PubMed] [Google Scholar]; (b) Soaresa CO, Alvesa MJM, Becharaa EJH. Free Radic Med. 2011;50:1760. doi: 10.1016/j.freeradbiomed.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 22.Kabalka GW, Shoup TM, Goudgaon NM. Tetrahedron Lett. 1989;30:1483. [Google Scholar]
- 23.Pak CS, Lim DS. Synth Commun. 2001;31:2209. [Google Scholar]
- 24.Arrayás RG, Cabrera S, Carretero JC. Org Lett. 2005;7:219. doi: 10.1021/ol0478133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.