Abstract
Mitochondria contribute to neuronal function not only via their ability to generate ATP, but also via their ability to buffer large Ca2+ loads. This review summarizes evidence that mitochondrial Ca2+ sequestration is especially important for sustaining the function of vertebrate motor nerve terminals during repetitive stimulation. Motor terminal mitochondria can sequester large amounts of Ca2+ because they have mechanisms for limiting both the mitochondrial depolarization and the increase in matrix free [Ca2+] associated with Ca2+ influx. In mice expressing mutations of human superoxide dismutase −1 (SOD1) that cause some cases of familial amyotrophic lateral sclerosis (fALS), motor terminals degenerate well before the death of motor neuron cell bodies. This review presents evidence for early and progressive mitochondrial dysfunction in motor terminals of mutant SOD1 mice (G93A, G85R). This dysfunction would impair mitochondrial ability to sequester stimulation-associated Ca2+ loads, and thus likely contributes to the early degeneration of motor terminals.
Keywords: Motor nerve terminal, Superoxide dismutase 1, Mitochondria, Calcium, Amyotrophic lateral sclerosis
Mitochondrial Ca2+ sequestration and extrusion are important for sustaining motor terminal function during and after repetitive nerve stimulation
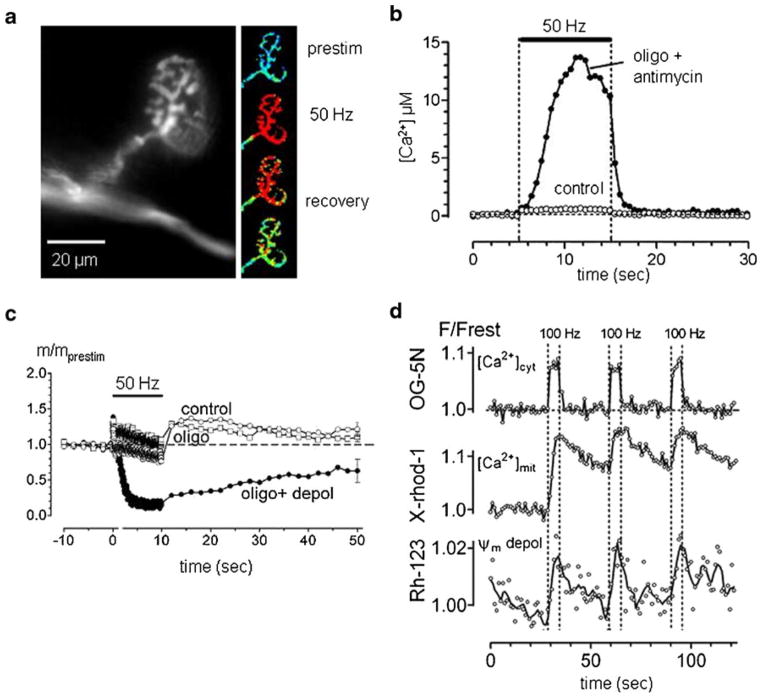
When action potentials invade vertebrate motor nerve terminals at rates ≥20 Hz, much of the Ca2+ that enters is temporarily sequestered by mitochondria (David et al. 1998; David 1999; David and Barrett 2003). In mouse motor terminals (Fig. 1a) this mitochondrial Ca2+ uptake is temperature-sensitive: uptake at room temperature is much less than that at near-physiological temperatures (≥30 °C, David and Barrett 2000). Mitochondrial Ca2+ sequestration is so powerful that after <50 impulses the rate of rise of spatially-averaged cytosolic [Ca2+] becomes very slow (Fig. 1b,d), even though the sustained endplate potential (EPP) demonstrates that Ca2+ continues to enter the terminal (Fig. 1c). We know that much of this Ca2+ is entering mitochondria because mitochondrial [Ca2+] increases during nerve stimulation (Fig. 1d), and because when mitochondrial Ca2+ uptake is inhibited by agents that depolarize the membrane potential across the inner mitochondrial membrane (Ψm), cytosolic [Ca2+] rises to much higher levels during stimulation (Fig. 1b). This effect of mitochondrial depolarizing agents is attributable to inhibition of mitochondrial Ca2+ uptake rather than to inhibition of ATP synthesis, since oligomycin, which inhibits ATP synthesis without depolarizing mitochondria, does not alter stimulation-induced cytosolic [Ca2+] responses. Agents that inhibit Ca2+ uptake into endoplasmic reticulum also have little effect on stimulation-induced cytosolic [Ca2+] responses in healthy motor terminals (David and Barrett 2003).
Fig. 1.
Mitochondrial regulation of cytosolic [Ca2+] is important for maintaining neuromuscular transmission at mouse motor nerve terminals. a. fluorescence micrograph of motor terminal ionophoretically injected with a Ca2+ indicator. The online version of the small pictures at right shows the response to 50 Hz stimulation of the motor axon; the intensity of the fluorescence of the Ca-indicator (normalized to pre-stimulation values) is encoded on a pseudo-color scale, where blue, green, yellow and red represent increasing [Ca2+] elevations. b. elevation of cytosolic [Ca2+] induced by stimulation (horizontal bar) under control conditions and following inhibition of mitochondrial Ca2+ uptake by depolarizating Ψm with antimycin (inhibits complex III of the ETC; Oligomycin blocks Complex V, the ATP synthase. c. changes in EPP quantal content (m/mprestim) during and following stimulation under control conditions, in oligomycin, and following Ψm depolarization (depol). Ψm depolarization enhances depression and eliminates post-tetanic potentiation. d. Changes in cytosolic [Ca2+], mitochondrial matrix [Ca2+] and Ψm evoked by 3 bouts of stimulation at 100 Hz, monitored (respectively) by changes in the fluorescence of ionophoretically injected Oregon Green (OG)-5 N, X-rhod-1 loaded into mitochondria, and rhodamine (Rh)-123. Each trace comes from a different preparation of the levator auris longus muscle, whose contractions were blocked with d-tubocurarine or μ-conotoxin GIIIA. Records are normalized to pre-stimulation values. Parts B and C adapted from David and Barrett (2003); part D from Nguyen et al. (2009)
Mitochondrial Ca2+ uptake is functionally important, because when this uptake is inhibited by depolarizing mitochondria, the EPP depresses much more rapidly during tetanic stimulation (Fig. 1c). Depression is accelerated because the greatly increased cytosolic [Ca2+] stimulates so much asynchronous transmitter release that the pool of vesicles available for producing synchronous release (i.e., the EPP) becomes depleted (David and Barrett 2003; Talbot et al. 2003).
Uptake of Ca2+ into the mitochondrial matrix is passive, occurring via a channel/uniporter (Kirichok et al. 2004) down an electrochemical gradient (−150 to −200 mV, inside negative) created by proton extrusion via complexes I, III and IV of the electron transport chain (ETC, reviewed by Gunter and Pfeiffer 1990; Nicholls and Ferguson 2002). Extrusion of Ca2+ from normally polarized mitochondria requires energy; in motor terminal mitochondria this extrusion occurs mainly via a Na+/Ca2+ exchanger which is pharmacologically distinct from the Na+/Ca2+ exchanger in the plasma membrane. The Ca2+ extruded from mitochondria following tetanic stimulation aids in the recovery from post-tetanic depression of the EPP; when the mitochondrial Na+/Ca2+ exchanger is blocked with CGP-37157, EPP amplitude recovers much more slowly (García-Chacón et al. 2006). This finding supports Tang and Zucker’s (1997) hypothesis that mitochondrial Ca2+ extrusion is important for post-tetanic potentiation.
Regulation of matrix [Ca2+] and of Ψm permits mitochondria to sequester large Ca2+ loads
Entry of calcium into small structures like mitochondria would be expected to dissipate rapidly the electrochemical gradient favoring Ca2+ entry, both by elevating matrix [Ca2+] and by depolarizing Ψm. Thus it might seem amazing that motor terminal mitochondria continue to sequester Ca2+ so effectively during trains of action potentials. One mechanism that permits continued Ca2+ sequestration is a powerful Ca2+ buffering system in the mitochondrial matrix that apparently involves reversible formation of insoluble complexes containing Ca and phosphate (Nicholls and Chalmers 2004; phosphate is transported into the matrix electroneutrally, in exchange for matrix OH−). The power of this unconventional buffering system is manifest during repetitive stimulation: the concentration of free [Ca2+], monitored using a fluorescent indicator loaded into the matrix, rises to a plateau level of 1–2 μM, which is sustained as long as stimulation continues (Fig. 1d; David et al. 2003), even though Ca2+ continues to enter the terminal (as evidenced by continuing EPPs) and continues to enter mitochondria (since cytosolic [Ca2+] does not increase above a plateau level). This powerful matrix buffering system is also evident in mitochondria isolated from brain, provided that the Ca2+ load is administered gradually (Nicholls and Chalmers 2004).
Another mechanism permitting sustained Ca2+ uptake into mitochondria is their ability to maintain Ψm. Recordings of stimulation-induced changes in Ψm in motor terminal mitochondria reveal that tetanic stimulation (100 Hz, 5 s) depolarizes Ψm by <5 mV, with baseline Ψm rapidly restored when stimulation stops (Fig. 1d; Nguyen et al. 2009). Experiments that included measurements of a transient, stimulation-induced decrease in NADH fluorescence in motor terminal mitochondria suggest that the main reason for the relatively small change in Ψm is acceleration of ETC activity (proton extrusion helps counteract the depolarization produced by Ca2+ influx, Talbot et al. 2007). Work on isolated mitochondria has demonstrated that the rate of proton extrusion via the ETC is limited by development of the large negative Ψm, and that even small Ψm depolarizations can increase ETC activity (reviewed by Nicholls and Ferguson 2002; Johnson-Cadwell et al. 2007).
Thus mechanisms first described in isolated mitochondria that limit the increase in matrix [Ca2+] and help counteract Ψm depolarization help explain why motor terminal mitochondria are so effective in sequestering large, stimulation-induced Ca2+ loads. Other mechanisms operative in motor neurons may allow motor terminal mitochondria to take up Ca2+ at lower, more physiological levels of cytosolic [Ca2+] than mitochondria in other cell types. For example, spinal motor neurons are strongly immunoreactive for spermidine/spermine (Laube et al. 2002), which reduces the [Ca2+] at which isolated mitochondria begin to take up Ca2+ (reviewed by Salvi and Toninello 2004).
Mitochondrial dysfunction may contribute to early motor terminal death in mutant SOD1 mice
Both of the mechanisms that allow mitochondria to sequester large amounts of Ca2+ depend on mitochondrial metabolism: formation of matrix Ca-phosphate complexes is favored by the alkaline matrix pH (Nicholls and Chalmers 2004) established by proton extrusion via the ETC, and preservation of Ψm during Ca2+ influx depends on the ability to accelerate ETC activity (see above). Thus diseases that impair mitochondrial function will impair mitochondrial Ca2+ sequestration. Such impairment would be expected to be especially injurious to motor nerve terminals, since they are small structures that are exposed to large Ca2+ loads during repetitive stimulation of the motor nerve.
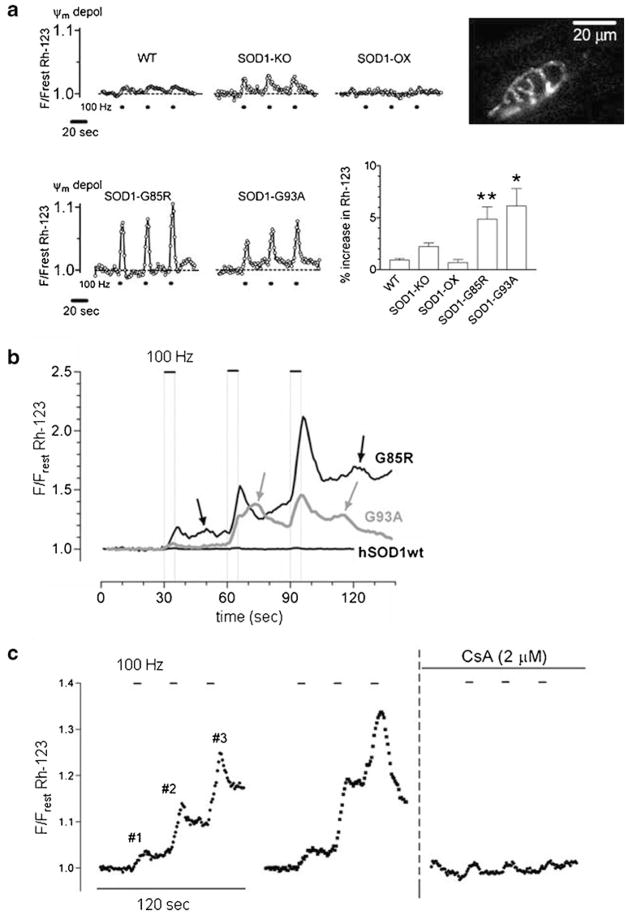
To explore this idea, we assayed mitochondrial function in motor terminals of transgenic mice that express either the G93A or G85R mutation of human SOD1, both of which produce fALS via a toxic gain of function (Gurney et al. 1994; Chiu et al. 1995; Bruijn et al. 1997). In multiple mutant SOD1 mouse models morphological signs of degeneration become detectable in motor terminals and axons well before the degeneration of motor neuron cell bodies, especially for motor terminals innervating fast, fatiguable muscle fibers (Frey et al. 2000; Fischer et al. 2004; reviewed by Gordon et al. 2004; Murray et al. 2010). Mutant SOD1 alters expression of many mitochondria-associated proteins in spinal cord (Li et al. 2010), and early defects in mitochondrial ability to take up Ca2+ have been noted in mitochondria isolated from the spinal cord of fALS mice (Damiano et al. 2006). Figure 2a presents evidence that mitochondria in the motor terminals of these mice also have an impaired response to stimulation-induced Ca2+ loads. In both G93A and G85R mutant SOD1 mice the average Ψm depolarization recorded in response to nerve stimulation was significantly greater than that recorded in wild-type (wt) mice even at early, presymptomatic ages. This greater Ψm depolarization in mutant SOD1 terminals would be expected to reduce mitochondrial ability to sequester Ca2+ loads. This difference was not due to differences in dismutase activity, because (1) the G93A mutation retains dismutase activity but the G85R mutation does not (Valentine et al. 2005), and (2) stimulation-induced Ψm depolarizations were not significantly different from wt in mice lacking SOD1 activity or in mice that express both normal mouse and normal human SOD1 (Fig. 2a).
Fig. 2.
Stimulation-induced Ψm depolarizations increase in motor terminals of mutant SOD1 mice, with transient opening of the mPTP in symptomatic fALS mice. a. Representative traces comparing Ψm depolarizations (monitored as increase in Rh-123 fluorescence) in motor terminals of wild-type (WT) mice, mice lacking SOD1 (SOD1-KO), mice that express normal human SOD1 (in addition to mouse SOD1, SOD1-OX), and presymptomatic mice expressing the fALS-causing G85R and G93A mutations of human SOD1. Dots below the traces indicate stimulus trains (each 100 Hz, 5 s; same pattern as in Fig. 1d). Bar graph plots averaged results for the first stimulus train, indicating that fALS motor terminals show a ~5-fold greater fluorescence increase than WT terminals. The inset micrograph illustrates regions of an Rh-123-loaded presymptomatic G85R mouse motor terminal whose fluorescence increased during stimulation (difference between resting and stimulated images). b. Superimposed traces illustrating the larger, summating, stimulation-induced Ψm depolarizations recorded in motor terminals of older, symptomatic G85R and G93A mice. On this scale the smaller Ψm depolarizations in a motor terminal expressing normal human SOD1 (hSOD1wt) are nearly undetectable. Arrows indicate additional, asynchronous Ψm depolarizations. c. The large Ψm depolarizations recorded in a symptomatic G93A motor terminal are reduced by cyclo-sporin A (CsA), which inhibits mPTP opening. Part A adapted from Nguyen et al. (2009); parts B and C from Nguyen et al. (2011)
Consistent with the hypothesis that mitochondrial dysfunction contributes importantly to motor terminal degeneration, Fig. 2b shows that stimulation-induced Ψm depolarizations become larger as fALS mice become older and symptomatic (note the change in vertical scale between Fig. 2a and b). In the presymptomatic mice of Fig. 2a the Ψm depolarizations produced by three consecutive trains of stimulation are similar, but in the older, symptomatic mice of Fig. 2b the Ψm depolarizations decay more slowly and become progressively larger for later stimulus trains. Terminals from older fALS mice also display asynchronous Ψm depolarizations localized to subregions of the terminal (Nguyen et al. 2011). Cyclo-sporin A, which inhibits opening of the mitochondrial permeability transition pore (mPTP), reduces the amplitude of the large stimulation-evoked Ψm depolarizations in symptomatic fALS mice (Fig. 2c), and also blocks the asynchronous depolarizations. Synaptic mitochondria are more susceptible than non-synaptic mitochondria to Ca2+-induced mPTP opening (Naga et al. 2007).
Measurements of Ψm thus suggest that disease progression in fALS motor terminals has at least two mitochondrial components. An early-manifesting component impairs the ability of the ETC to accelerate during the Ψm depolarization caused by Ca2+ influx. A later-manifesting component increases the likelihood that stimulation-induced Ca2+ influx will produce (reversible) opening of the mPTP. An additional mitochondrial dysfunction is suggested by the work of Vila et al. (2003), which demonstrates reduced ability to limit the elevation of matrix [Ca2+] in terminals of symptomatic SOD1-G93A mice. Shi et al. (2010) review abnormal interactions between mutant SOD1s and mitochondria that might underlie these problems. All of the above-mentioned mitochondrial dysfunctions would be expected to reduce net mitochondrial uptake of stimulation-induced Ca2+ loads. The resultant elevation of cytosolic [Ca2+] would increase the likelihood of damage to motor terminals and axons due to activation of Ca2+-dependent proteases such as the calpains. This mechanism may help explain why motor terminals innervating fast, fatiguable limb muscles are the first to degenerate in fALS mice, since these motor neurons typically innervate large motor units and discharge in high-frequency bursts of action potentials (reviewed by Burke 2004), two characteristics that would increase the peak stimulation-induced Ca2+ load delivered to the motor neuron. Differences in cytosolic Ca2+ buffering ability, which would also influence the motor neurons’ ability to handle Ca2+ loads, have also been linked to differential susceptibility of motor neurons in fALS mice (reviewed by Grosskreutz et al. 2010).
Studies of motor terminals like those described above were performed in preparations in which muscle activation was blocked (e.g., by tubocurarine); thus all the stimulation-induced changes originate from mitochondria within motor neurons. (In contrast, only a small fraction of mitochondria isolated from spinal cord originates from motor neurons.) Other advantages of studying mitochondrial function in motor terminals are that the mitochondria are in situ and are exposed to physiologically-delivered Ca2+ loads. A disadvantage of this preparation is that, because motor terminal mitochondria constitute only a tiny fraction of the total mitochondria in a neuromuscular preparation, it is difficult to study the function of resting motor terminal mitochondria or to perform biochemical assays routinely performed in isolated mitochondrial preparations.
Studies summarized above provide evidence that mutant SOD1-induced dysfunction of mitochondria that impairs their ability to handle stimulation-induced Ca2+ loads may contribute to the early motor terminal degeneration documented in fast muscles of fALS mice. If so, then treatments to enhance/preserve mitochondrial function might help to preserve motor terminals, thereby preserving the functional contact between motor neurons and skeletal muscles. Such peripherally-directed treatments might complement more centrally-directed treatments to preserve the motor neuron cell body, forming an effective combination therapy to slow disease progression in ALS patients.
Acknowledgments
The authors gratefully acknowledge research support from the National Institutes of Health (NINDS R01s 12404 and 58888; R21 49374), the Muscular Dystrophy Association (4344, 186832), the Amyotrophic Lateral Sclerosis Association (1348), and the University of Miami Miller School of Medicine.
References
- Bruijn LI, Becher MW, Lee MK, Anderson KL, Jemkins NA, Copeland NG, et al. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- Burke RE. In: Myology. 3. Engel AG, Franzini-Armstrong D, editors. McGraw-Hill; New York: 2004. [Google Scholar]
- Chiu AY, Zhai P, Dalcanto MC, Peters TM, Kwon YW, Prattis SM, et al. Mol Cell Neurosci. 1995;6:349–362. doi: 10.1006/mcne.1995.1027. [DOI] [PubMed] [Google Scholar]
- Damiano M, Starkov AA, Petri S, Kipiani K, Kiaei M, Mattiazzi M, et al. J Neurochem. 2006;96:1349–1361. doi: 10.1111/j.1471-4159.2006.03619.x. [DOI] [PubMed] [Google Scholar]
- David G. J Neurosci. 1999;19:7495–7506. doi: 10.1523/JNEUROSCI.19-17-07495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Barrett EF. J Neurosci. 2000;20:7290–7296. doi: 10.1523/JNEUROSCI.20-19-07290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Barrett EF. J Physiol. 2003;548:425–438. doi: 10.1113/jphysiol.2002.035196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Barrett JN, Barrett EF. J Physiol. 1998;509:59–65. doi: 10.1111/j.1469-7793.1998.059bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Talbot J, Barrett EF. Cell Calcium. 2003;33:197–206. doi: 10.1016/s0143-4160(02)00229-4. [DOI] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, et al. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. J Neurosci. 2000;20:2534–2542. doi: 10.1523/JNEUROSCI.20-07-02534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Chacón L, Nguyen K, David G, Barrett EF. J Physiol. 2006;574:663–675. doi: 10.1113/jphysiol.2006.110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R, Hegedus J, Tam SL. Neurol Res. 2004;26:174–185. doi: 10.1179/016164104225013806. [DOI] [PubMed] [Google Scholar]
- Grosskreutz J, Van Den Bosch L, Keller BU. Cell Calcium. 2010;47:165–174. doi: 10.1016/j.ceca.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Pfeiffer DR. Am J Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, et al. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Johnson-Cadwell LI, Jekabsons MB, Wang A, Polster BM, Nicholls DG. J Neurochem. 2007;101:1619–1631. doi: 10.1111/j.1471-4159.2007.04516.x. [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- Laube G, Bernstein HG, Wolf G, Veh RW. J Comp Neurol. 2002;444:369–386. doi: 10.1002/cne.10157. [DOI] [PubMed] [Google Scholar]
- Li Q, Van de Velde C, Israelson A, Xie J, Bailey AO, Dong M-Q, Chun S-J, Roy T, Winer L, Yates JR, Capaldi RA, Cleveland DW, Miller TM. Proc Natl Acad Sci USA. 2010;107:21146–21151. doi: 10.1073/pnas.1014862107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray LM, Talbot K, Gillingwater TH. Neuropathol Appl Neurobiol. 2010;36:133–156. doi: 10.1111/j.1365-2990.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- Naga KK, Sullivan PG, Geddes JW. J Neurosci. 2007;27:7469–7475. doi: 10.1523/JNEUROSCI.0646-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, García-Chacón LE, Barrett JN, Barrett EF, David G. Proc Natl Acad Sci USA. 2009;106:2007–2011. doi: 10.1073/pnas.0810934106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, Barrett JN, García-Chacón LE, David G, Barrett EF. Neurobiol Dis. 2011;42:381–390. doi: 10.1016/j.nbd.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Chalmers S. J Bioenerg Biomembr. 2004;36:277–281. doi: 10.1023/B:JOBB.0000041753.52832.f3. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Ferguson SJ. Bioenergetics. Vol. 3. Academic; London: 2002. [Google Scholar]
- Salvi M, Toninello A. Biochim Biophys Acta. 2004;1661:113–124. doi: 10.1016/j.bbamem.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Shi P, Gal J, Kwinter DM, Liu X, Zhu H. Biochim Biophys Acta. 2010;1802:45–51. doi: 10.1016/j.bbadis.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot JD, David G, Barrett EF. J Neurophysiol. 2003;90:491–502. doi: 10.1152/jn.00012.2003. [DOI] [PubMed] [Google Scholar]
- Talbot JD, Barrett JN, Barrett EF, David G. J Physiol. 2007;579:783–798. doi: 10.1113/jphysiol.2006.126383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Zucker RS. Neuron. 1997;18:483–491. doi: 10.1016/s0896-6273(00)81248-9. [DOI] [PubMed] [Google Scholar]
- Valentine JS, Doucette PA, Zittinpotter S. Annu Rev Biochem. 2005;74:563–593. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- Vila L, Barrett EF, Barrett JN. J Physiol. 2003;549:719–728. doi: 10.1113/jphysiol.2003.041905. [DOI] [PMC free article] [PubMed] [Google Scholar]