Abstract
Objective
Animal studies have demonstrated that dietary supplementation with flaxseed oil inhibits colorectal cancer growth. Recent data indicate that walnuts have strong antiproliferative properties against colon cancer cells in vitro but no previous study has assessed the effects of walnuts in vivo or performed a joint evaluation of flaxseed oil and walnuts. The aim of the present study was to examine the effect of dietary walnuts on colorectal cancer in vivo and to comparatively evaluate their efficacy in relation to flaxseed oil.
Methods
HT-29 human colon cancer cells were injected in 6-wk-old female nude mice. After a 1-wk acclimation period, mice (n = 48) were randomized to diets containing ~ 19% of total energy from walnuts, flaxseed oil, or corn oil (control) and were subsequently studied for 25 d.
Results
Tumor growth rate was significantly slower in walnut-fed and flaxseed-fed mice compared with corn oil-fed animals (P < 0.05) by 27% and 43%, respectively. Accordingly, final tumor weight was reduced by 33% and 44%, respectively (P < 0.05 versus control); the differences between walnut and flaxseed diets did not reach significance. We found no differences among groups in metabolic and hormonal profile, serum antioxidant capacity, or inflammation (P > 0.05). However, walnuts and flaxseed oil significantly reduced serum expression levels of angiogenesis factors, including vascular endothelial growth factor (by 30% and 80%, respectively), and approximately doubled total necrotic areas despite smaller tumor sizes (P < 0.05 versus control). Dietary walnuts significantly decreased angiogenesis (CD34 staining; P = 0.017 versus control), whereas this effect did not reach significance in the flaxseed oil group (P = 0.454 versus control).
Conclusion
We conclude that walnuts in the diet inhibit colorectal cancer growth by suppressing angiogenesis. Further studies are needed to confirm our findings in humans and explore underlying mechanisms.
Keywords: Nuts, Linseed, Colon cancer, Xenograft, VEGF
Introduction
Colorectal cancer is the third most common type of cancer worldwide, with mortality being approximately one half that of the incidence [1]. There are several established risk factors for colorectal cancer, many of which, such as dietary habits, are modifiable [2]. It has been estimated that 30% to 50% of colorectal cancer in men and ~20% in women could be prevented by adoption of a prudent diet and other lifestyle changes [3,4]. Nevertheless, although a “Western” dietary pattern is typically associated with increased colorectal cancer risk [5], there is still much uncertainty regarding the putative protective role of individual foods and nutrients [2].
The results of several large-scale epidemiological studies in humans show an inverse association between nut and seed consumption and the incidence of colorectal cancer [6–9]. With one exception [10], animal studies examining the effect of flaxseed (linseed) supplementation on the formation of precancerous colorectal lesions [11–13] and the growth of chemically induced colorectal tumors [14–16] reported beneficial effects. However, the underlying mechanisms remain unclear and poorly described. Furthermore, the recent observations that walnuts retard the growth rate of breast cancer cells implanted in mice [17] and that walnut extracts have dose-dependent inhibitory effects on the growth of colon cancer cells in vitro [18] raise the possibility that dietary walnuts could also be beneficial against colorectal cancer in vivo.
The purpose of the present study, therefore, was to comparatively investigate the effect of isoenergetic amounts of walnuts and flaxseed oil in the diet on colorectal cancer growth rate in mice in vivo and to explore relevant underlying molecular mechanisms.
Materials and methods
Animals
Six-week-old female athymic nude (nu/nu) mice were obtained from Charles River Laboratories (Wilmington, MA, USA). They were acclimatized on the control diet for 10 d prior to implantation of tumor cells. Mice were individually numbered for unique identification and were housed, four per cage, in a barrier mouse facility (temperature controlled at 24°C, 12-h light/dark cycles) at the Beth Israel Deaconess Medical Center (BIDMC) Animal Research Facility (Boston, MA, USA). All animal use and handling were approved by the BIDMC Institutional Animal Care and Use Committee.
Tumor induction
HT-29 human colon cancer cells (American Type Culture Collection, Manassas, VA, USA) were cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Five million cells were injected subcutaneously in one flank of each mouse. Seven days after tumor cell injection, tumors had reached 3–5 mm in diameter. Injection of tumor cells into animals results in variable tumor sizes; to reduce variability in this experiment, outliers (25% of all mice with tumors in the highest and lowest quartiles) were excluded before diet randomization.
Dietary treatment
Seven days after tumor cell injection, 48 mice were randomized after stratification for body weight to either control, walnut, or flaxseed oil diets (n = 16 per group), which were prepared by Research Diets (New Brunswick, NJ, USA). The control diet was modeled after the standard AIN-76 diet for mice. The walnut diet was formulated to contain the equivalent of two servings of walnuts per day in humans, which provides 376 calories or 18.8% of a 2000 calorie/d diet; this amount of energy was substituted for corn oil (Welch, Holme & Clark, Newark, NJ, USA) in the control diet, or lignan-containing flaxseed oil (Barlean’s Organic Oils, Ferndale, WA, USA). The walnut diet was prepared from whole walnut kernels (kindly provided by the California Walnut Commission), including the brown husk but not the shell, which were finely ground in a food processor and immediately mixed with the remainder of the dry ingredients of the diet. The diets were pelleted and dried for 2 d in a temperature- and humidity-controlled room to remove excess water. Thereafter, diets were sterilized with 10–20 kGy of gamma irradiation (Cobalt 60) and stored in sealed bags at −20°C to prevent fat oxidation and bacterial growth. Mice had free access to water and were fed fresh food twice weekly. Mice were fed the experimental diets for 25 d, from day 7 after tumor cell injection until the end of the study on day 32.
Body weight and tumor growth
Body weight and tumor size were measured three times weekly. An electronic scale was used to weigh the mice, and a vernier caliper was used to measure tumor size. Tumor volume was calculated using the formula: volume = 0.5 × length × width × depth.
Sacrifice and tissue handling
The experiment was terminated after 25 d of diet treatment, when the tumor of at least one control-fed mouse reached the maximum allowable size of 1500 mm3 [17]. Blood was collected from the submandibular vein and the serum was frozen at −80°C until further analyses. Mice were sacrificed using carbon dioxide inhalation. The tumor, liver, and visceral fat were weighed upon removal. The tumors were dissected and a cross-section was fixed in 10% neutral buffered formalin followed by paraffin embedding; 4 µm-thick sections were placed on microscope slides for histological and immunohistochemical staining. Portions of the tumor were flash frozen in liquid nitrogen and stored at −80°C until protein extraction.
Determination of hormones, substrates, markers of inflammation, and serum antioxidant capacity
Commercially available ELISA kits were used for measuring serum concentrations of adiponectin, leptin, insulin, and C-reactive protein (all from ALPCO Diagnostics, Salem, NH, USA) and insulin-like growth factor (IGF) 1 (R&D Systems, Minneapolis, MN, USA). Free fatty acid concentration was determined with the NEFA-HR(2) enzymatic colorimetric assay (Wako Diagnostics, Richmond, VA, USA). Serum antioxidant capacity was assayed as previously described [17]. Glucose concentration was measured by using the LifeScan One Touch Ultra glucose meter (Johnson & Johnson, New Brunswick, NJ, USA).
Western blot analyses
Protein extraction was performed in lysis buffer containing 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 5 mM EDTA, 0.1 mM PMSF, 0.05% aprotinin, and 0.1% Igepal. SDS-PAGE (4–12%) was performed using 70 µg protein, blotted onto nitrocellulose membranes (Schleicher & Schuell, Keene, NH, USA). Antibodies against p-Erk (Tyr204, sc-7976), Erk (sc-93), p-AMPK (Thr 172, sc-101630), AMPK (sc-19126), p-STAT3 (tyr 705, sc-7993), STAT3 (sc-8019), p-Akt (Ser473, sc-101629), Akt (sc-5298), and actin (sc-69879), and secondary antibodies (anti-mouse, sc-2005; anti-rabbit, sc-2357; anti-goat, sc-2354) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against p-S6 (Ser 235/236, #2211S) and S6 (#2317) were purchased from Cell Signaling (Beverly, MA, USA). The membranes were blocked for 1 h in TBS containing 1% BSA (p-AMPK, AMPK, p-Akt, Akt, p-STAT3, STAT3) or 5% milk (p-Erk, Erk, p-S6, S6, p53, actin), and incubation with primary antibodies was performed in TBS-Tween containing 1% BSA or 5% nonfat dry milk, respectively. Following overnight incubation, blots were washed three times with TBS-Tween and then incubated with horseradish peroxidase-labeled secondary antibodies for 2 h. Enhanced chemiluminescence (Amersham, Arlington Heights, IL, USA) was used for detection. Measurement of signal intensity was performed using ImageJ processing and analysis software (National Institutes of Health, Bethesda, MD, USA).
Immunohistochemical analyses
Representative tumors from each diet group were chosen for further analyses. Hematoxylin and eosin (H&E) staining was performed by the Histology Core at BIDMC (Boston, MA, USA). Immunohistochemical staining for Ki67, CD34, and Mac2 was performed at the Immunohistochemical Core Facility at Brigham and Women’s Hospital (Boston, MA, USA). The number of Ki67-positive cells was counted in five representative images (×20) to evaluate proliferation (n = 4 per group). TUNEL staining was performed using Millipore’s Apoptag kit using tonsil as positive control to assess apoptosis (n = 4 per group). Mac2-positive cells were counted in five visual fields at ×40 magnification to evaluate tumor macrophage infiltration (n = 4 per group). To assess angiogenesis, areas of highest tumor vessel density were determined in each tumor and five representative images were taken at ×40. Images were evaluated using a horizontal grid by marking every point where CD34-stained vessels crossed the grid (n = 7 for the control and walnut groups, n = 8 for the flaxseed group). All analyses were performed in a blinded manner.
Angiogenesis proteome profile array
Vascular endothelial growth factor (VEGF) and IGF-binding proteins (IGFBP) -1, -2, and -3, as well as other angiogenesis-related proteins, were measured from pooled serum samples by using the semiquantitative Proteome Profiler Mouse Angiogenesis Array Kit (R&D Systems). X-ray films were developed using Pierce ECL (Thermo Scientific, Waltham, MA, USA) after 1-min exposure. Mean pixel density was measured for each spot and normalized to the positive control using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Relative differences of 25% or more from control values were considered significant.
Statistical analysis
Data were tested for normality by using the Shapiro-Wilk procedure. Non-normally distributed data were log-transformed for analysis, or ranked when no suitable transformation was possible. To evaluate differences in food intake, body weight, and tumor volume over the course of the study, multilevel modeling was used allowing for random intercepts and slopes among mice; fixed effects included time and diet and their interaction. One-way ANOVA followed by Tukey’s post-hoc test were used to evaluate differences among the three groups in tumor weight and all serum parameters at sacrifice. Results are presented as means and 95% confidence intervals for normally distributed variables, or medians and quartiles for ranked variables. Statistical significance was set at two-sided P values < 0.05. All analyses were performed with SPSS version 18.0 (SPSS, Chicago, IL, USA).
Results
Diet composition
The three experimental diets were isocaloric and isonutrient (Table 1), but differed in fatty acid composition (Table 2). The corn oil-based control diet had the highest amount of linoleic acid (18:2n-6) and the lowest amount of alpha-linolenic acid (ALA), and an n-6/n-3 ratio of 42.9:1. The walnut diet contained about as much linoleic acid as the control diet, but more ALA, and had a lower n-6/n-3 ratio of 6.4:1. The flaxseed oil diet contained less linoleic acid and more ALA than the other two diets and had an inversed n-6/n-3 ratio of 1:5.
Table 1.
Nutrient composition of the three experimental diets (g per 100 g of diet)
| Control | Walnut | Flaxseed oil | |
|---|---|---|---|
| Protein | 20.30 | 20.21 | 20.30 |
| Carbohydrate | 61.00 | 60.72 | 61.00 |
| Fat | 10.00 | 9.95 | 10.00 |
| Fiber | 5.00 | 4.98 | 5.00 |
| Casein | 20.00 | 18.33 | 20.00 |
| DL-methionine | 0.30 | 0.28 | 0.30 |
| Corn starch | 15.00 | 13.48 | 15.00 |
| Sucrose | 45.00 | 45.0 | 45.00 |
| Cellulose, BW200 | 5.00 | 4.81 | 5.00 |
| Mineral mix S10001 | 3.50 | 3.50 | 3.50 |
| Vitamin mix V10001 | 1.00 | 1.00 | 1.00 |
| Choline bitartrate | 0.20 | 0.20 | 0.20 |
| Corn oil | 10.00 | 2.76 | 0.00 |
| Walnuts | 0.00 | 11.10 | 0.00 |
| Flaxseed oil | 0.00 | 0.00 | 10.00 |
Table 2.
Fatty acid composition of the three experimental diets (g per 100 g of oil)
| Control | Walnut | Flaxseed oil | |
|---|---|---|---|
| Corn oil | 100.00 | 27.61 | 0.00 |
| Flaxseed oil | 0.00 | 0.00 | 100.00 |
| Walnut oil | 0.00 | 72.39* | 0.00 |
| C18, Stearic | 1.92 | 2.20 | 3.56 |
| C18:1, Oleic | 25.10 | 19.64 | 18.01 |
| C18:2, Linoleic | 60.58 | 59.92 | 14.03 |
| C18:3, Linolenic | 1.41 | 9.32 | 57.91 |
| C18:4, Stearidonic | 0.00 | 0.00 | 0.00 |
| Saturated | 12.90 | 11.10 | 9.60 |
| Monounsaturated | 25.10 | 19.70 | 17.20 |
| Polyunsaturated | 62.00 | 69.30 | 68.90 |
| n-3 (%) | 1.40 | 9.30 | 57.90 |
| n-6 (%) | 60.60 | 59.90 | 14.00 |
| n-6/n-3 ratio6 | 42.9:1 | 6.4:1 | 1:5 |
Walnuts are 65.22% fat; hence, 111 g of walnuts in the diet correspond to 72.39 g of walnut oil.
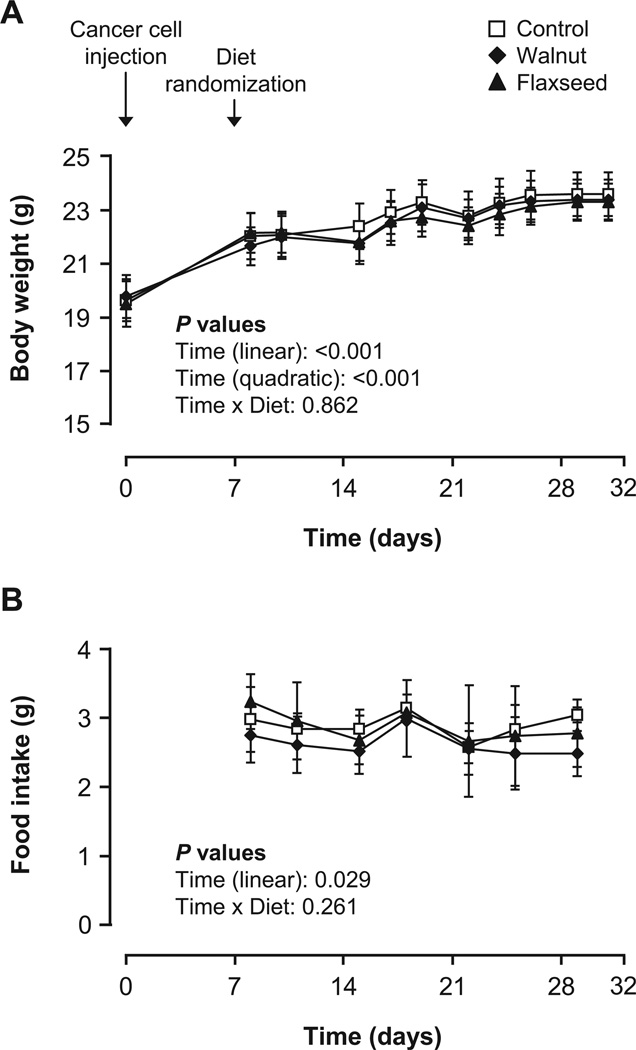
Walnuts and flaxseed oil do not affect food intake and body and organ weight
Body weight increased significantly over the course of the study (P < 0.001), but the rate of change decreased as time progressed (negative quadratic time coefficient, P < 0.001) (Fig. 1A). Accordingly, food intake slightly but significantly decreased (P = 0.029; Fig. 1B). There were no differences between groups in the observed changes in body weight and food intake (interaction of diet with time: P = 0.862 and P = 0.261, respectively). Final body weight (P = 0.838) and the relative weight of visceral fat pads (P = 0.797) and liver (P = 0.142) did not differ among control-, walnut-, and flaxseed oil-fed mice.
Fig. 1.
Body weight (A) and daily food intake (B) of mice fed the control, walnut, and flaxseed oil diets. Values are means and 95% confidence intervals. The body weight and food intake of mice were measured from the day of tumor cell injection (d0) to the day of sacrifice (d32). Mice were randomized to control, walnut, or flaxseed oil diets on d7 and sacrificed on d32. Body weight increased (P < 0.001) and food intake decreased (P = 0.029) over the course of the study. There were no significant differences among groups (P > 0.05).
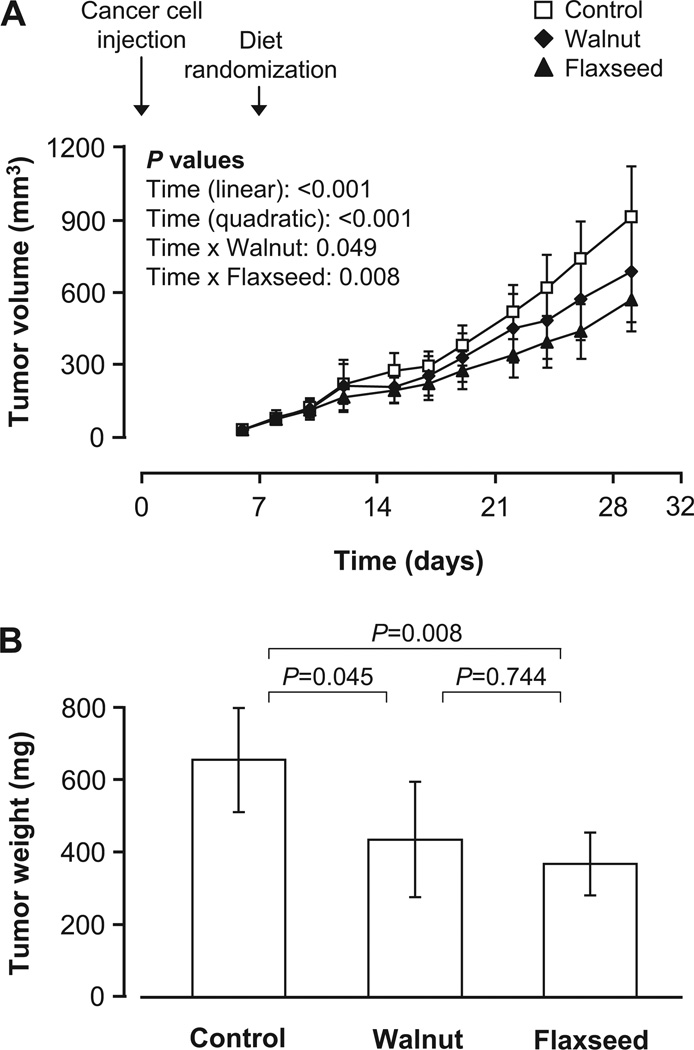
Walnuts and flaxseed oil inhibit colorectal cancer growth and lead to extensive central necrosis
Tumor size increased significantly during the observation period (P < 0.001) and the rate of growth increased as time progressed (positive quadratic time coefficient, P < 0.001). There were significant interactions between time and diet (P = 0.022), so that tumor growth rate was significantly slower in both the walnut (by ~27%, P = 0.049) and the flaxseed oil (by ~43%, P = 0.008) groups compared with the control group (Fig. 2A). Accordingly, final tumor weight was significantly reduced by ~33% (P = 0.045) and ~44% (P = 0.008) following consumption of walnuts and flaxseed oil, respectively, relative to the control diet (Fig. 2B).
Fig. 2.
Tumor growth rate (A) and final tumor weight (B) at autopsy in mice fed the control, walnut, and flaxseed oil diets. Values are means and 95% confidence intervals. Tumor volume was measured from the day of tumor cell injection (d0) to the day of sacrifice (d32). Mice were randomized to control, walnut, or flaxseed oil diets on d7 and sacrificed on d32. Walnut and flaxseed oil diets significantly retarded tumor growth rate compared with the control diet (P < 0.05), resulting in significantly reduced tumor weight at autopsy (P < 0.05). There were no significant differences between walnut and flaxseed oil groups (P > 0.05).
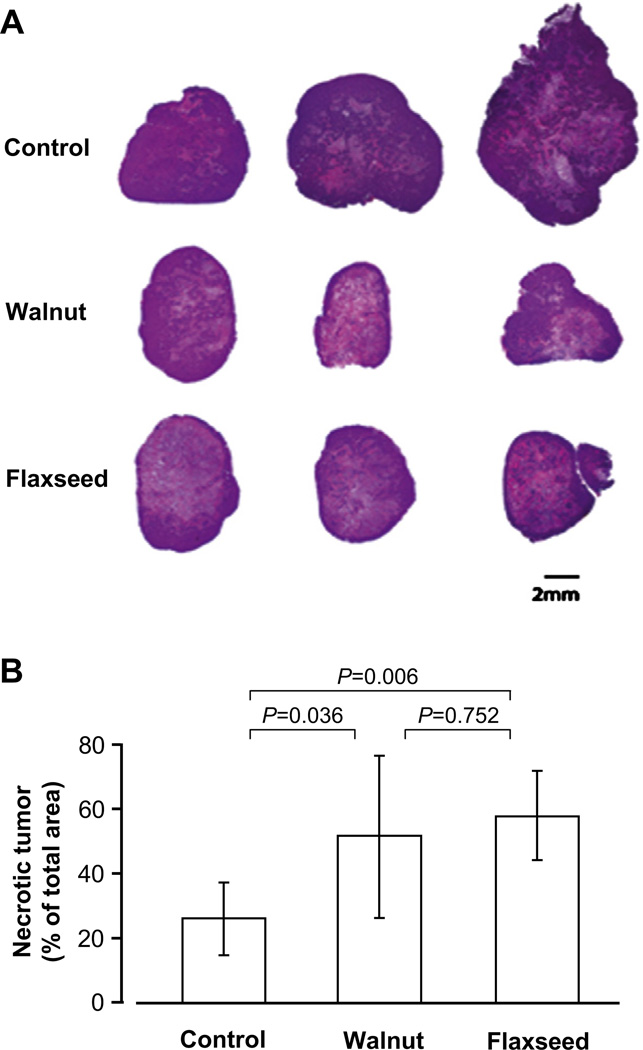
H&E staining revealed very large central necrotic areas despite smaller tumor sizes in both the walnut and the flaxseed oil diet groups, whereas tumors from the control diet group did not have large necrotic areas and showed much more viable tumor tissue despite overall larger size (Fig. 3A). Compared with control tumors, the total extent of necrosis was approximately double in tumors from mice on walnut and flaxseed oil diets (P < 0.05; Fig. 3B).
Fig. 3.
Hematoxylin and eosin (H&E) staining of representative tumors from mice fed the control, walnut, and flaxseed oil diets (A), and corresponding total necrotic areas (B). Values are means and 95% confidence intervals. Despite their smaller size, tumors from mice fed walnuts and flaxseed oil had extensive central necrosis and larger total necrotic areas compared with control tumors (P < 0.05). There were no significant differences between walnut and flaxseed oil groups (P > 0.05).
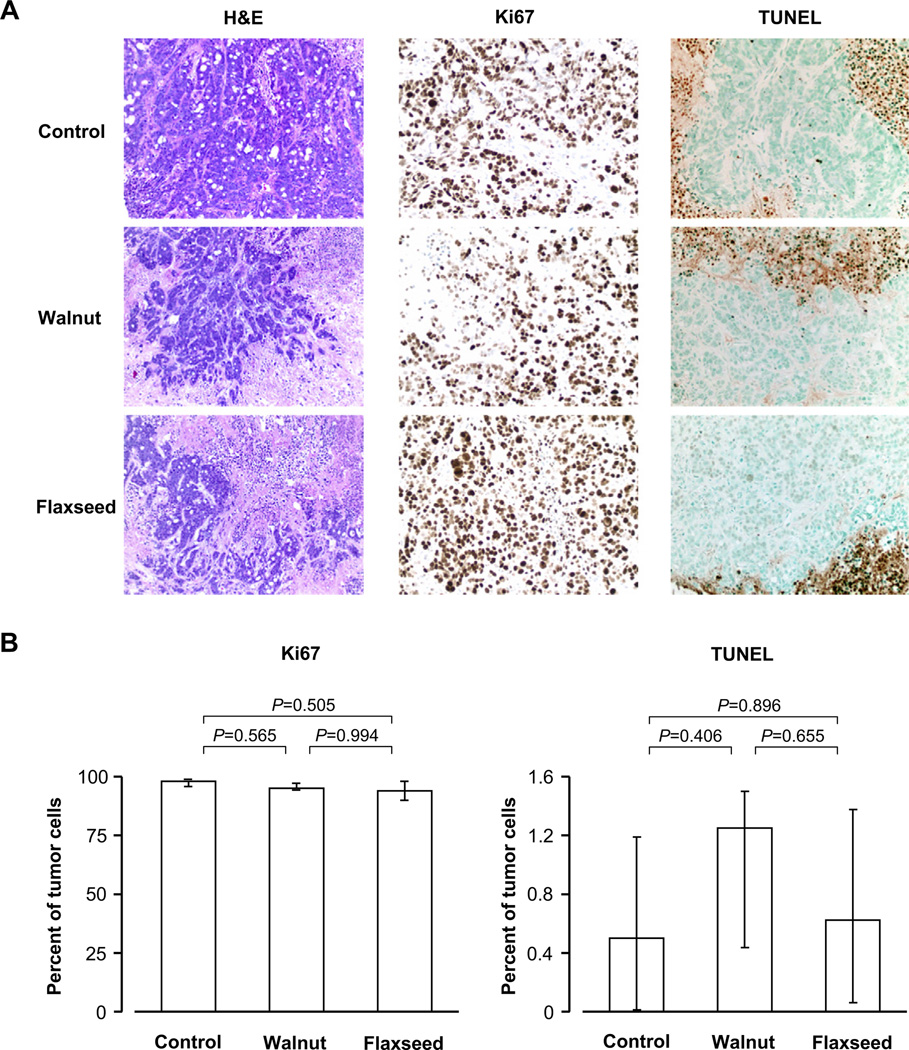
Walnuts and flaxseed oil do not affect apoptotic or proliferative pathways
Tumors from different groups had different amounts of necrosis and viable tumor tissue (H&E; Fig. 4A). Within the viable tumor tissue, Ki67 staining did not reveal any differences in proliferation among groups (P = 0.471, Fig. 4A and 4B). TUNEL staining revealed only very few (<5%) apoptotic cells within viable tumor areas without differences among groups (P = 0.425, Fig. 4A and 4B).
Fig. 4.
Representative areas from tumors of mice fed the control, walnut, and flaxseed oil diets, showing H&E staining and immunohistochemical staining for the proliferation marker Ki67 and the apoptotic marker TUNEL (A), and quantitative estimations of proliferation and apoptosis (B). Values are medians and quartiles. Vital tumor areas in control, walnut, and flaxseed oil diet groups did not differ in proliferation or apoptosis (almost all tumor cells were proliferating in all three groups, and there was hardly any apoptosis observed).
Western blot analyses revealed no significant differences in phosphorylation levels of Akt, Erk, AMPK, STAT3, or S6 (all P values > 0.2). We found no evidence of p53 expression (HT-29 cells are p53-deficient).
Walnuts and flaxseed oil have no effect on metabolic profile, serum antioxidant capacity, serum markers of inflammation, and mediators of apoptosis/proliferation
Consumption of the different diets did not significantly affect serum antioxidant capacity, or free fatty acid, glucose, leptin, adiponectin, and C-reactive protein concentrations (Table 3) and tumor macrophage infiltration (data not shown). Likewise, serum insulin and IGF-1 concentrations (Table 3) were not significantly different among groups.
Table 3.
Serum parameters in mice fed the three experimental diets
| Control | Walnut | Flaxseed oil | P value | |
|---|---|---|---|---|
| Antioxidant capacity (mM Trolox Eq)* | 1.1 (1.0, 1.3) | 1.1 (0.9, 1.2) | 1.2 (1.0, 1.4) | 0.718 |
| C-reactive protein (ng/mL)* | 11 (10, 11) | 11 (10, 13) | 11 (10, 13) | 0.535 |
| Adiponectin (µg/mL) | 58 (51, 66) | 67 (50, 84) | 61 (55, 67) | 0.477 |
| Leptin (ng/mL)* | 1.2 (0.9, 1.7) | 1.8 (1.4, 2.5) | 1.2 (0.9, 1.6) | 0.066 |
| Insulin (ng/mL)† | 0.42 (0.25, 0.77) | 0.39 (0.31, 0.73) | 0.46 (0.37, 0.80) | 0.814 |
| Glucose (mg/dL)† | 117 (113, 150) | 123 (103, 138) | 125 (98, 172) | 0.797 |
| Free fatty acids (mEq/L) | 0.65 (0.57, 0.74) | 0.69 (0.59, 0.78) | 0.71 (0.61, 0.81) | 0.656 |
| Insulin-like growth factor 1 (ng/mL)* | 77 (61, 96) | 64 (46, 88) | 78 (61, 99) | 0.477 |
Values are means and 95% confidence intervals, except for glucose and insulin concentrations (medians and quartiles). Symbols indicate datasets that were either *log-transformed or †ranked for analysis. There were no significant differences among groups
Walnuts and flaxseed oil reduce the expression of angiogenic proteins and walnuts inhibit angiogenesis
Tumor size usually correlates directly with the size of necrotic areas, due to increasingly insufficient oxygen supply in fast-growing tumors. However, H&E staining of the larger tumors in the control diet group displayed smaller necrotic areas, possibly indicating higher levels of angiogenic factors and increased angiogenesis. To test this possibility, we performed a serum proteome profiler array. Of 34 serum proteins analyzed, a reduction of 25% or more was observed in 11 (Table 4) following walnut and flaxseed oil feeding compared with corn oil feeding. In particular, VEGF was down-regulated to ~20% of the control values in flaxseed oil-fed animals and to ~70% in walnut-fed animals. Serum expression levels of IGFBP-1, IGFBP-2, and IGFBP-3 were not affected by walnut or flaxseed oil consumption (within ±25% of control values), suggesting that the reduction was specific to angiogenic factors (Table 4). Levels of angiogenic factors were overall lower and microvessel density was greater in the flaxseed oil than in the walnut group; it is thus possible that expression levels of angiogenic factors does not correlate with relative microvessel density in vital tumor areas but rather reflects the overall number of microvessels within a tumor.
Table 4.
Expression levels of angiogenic factors in mice fed the three experimental diets
| Factor | Control diet | Walnut diet | Flaxseed oil diet |
|---|---|---|---|
| Pos. control | 100 | 100 | 100 |
| Cyr61 | 100 | 73.3 | 43.5 |
| Endoglin | 100 | 50.7 | 43.4 |
| Fractalkine | 100 | 63.4 | 50.9 |
| MCP-1 | 100 | 68.8 | 51.4 |
| MIP-1alpha | 100 | 57.9 | 68.2 |
| Pentraxin-3 | 100 | 60.1 | 46.3 |
| SDF-1 | 100 | 67.3 | 36.4 |
| Serpin F1 | 100 | 68.3 | 65.6 |
| TIMP-1 | 100 | 42.4 | 30.1 |
| TIMP-4 | 100 | 71.5 | 67.7 |
| VEGF | 100 | 71.0 | 18.9 |
| Neg. control | n.d. | n.d. | n.d. |
| IGFBP-1 | 100 | 81.1 | 76.4 |
| IGFBP-2 | 100 | 108.1 | 113.5 |
| IGFBP-3 | 100 | 110.2 | 112.1 |
Data are expressed relative to the control value
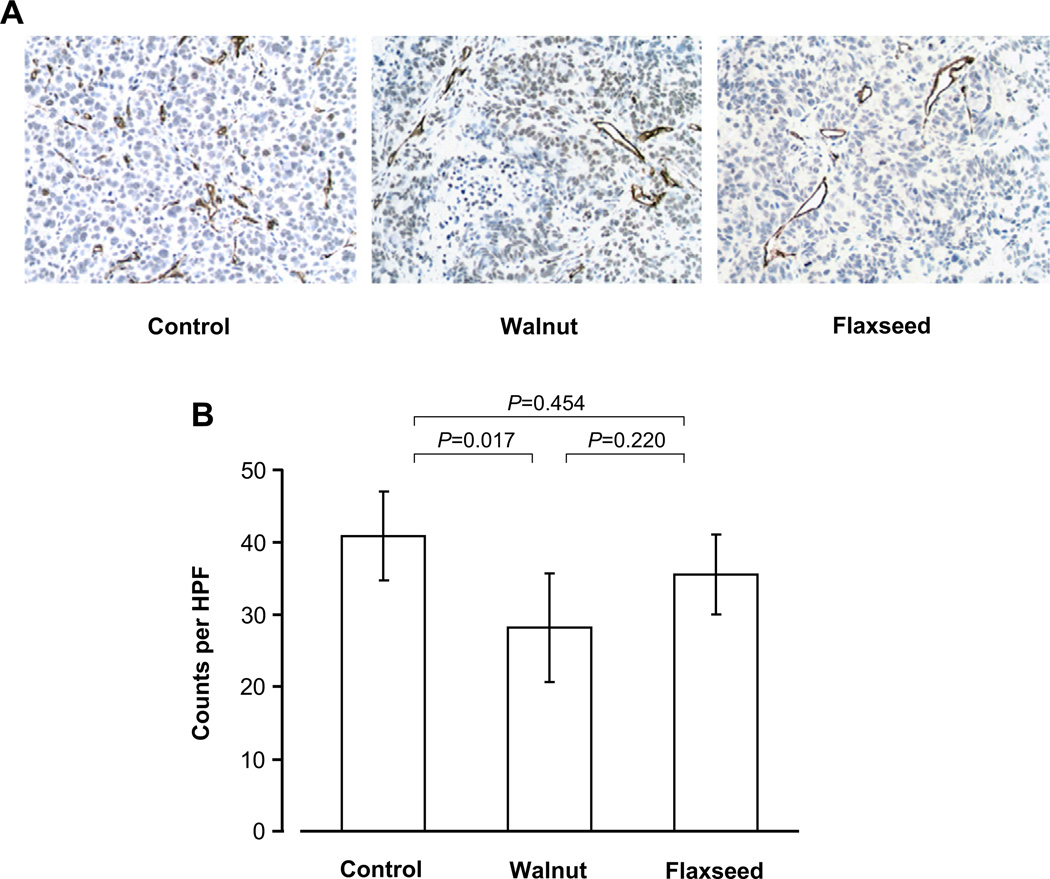
Tumors from mice fed the walnut and flaxseed oil diets had larger areas of central necrosis and smaller areas of vital tumor tissue where angiogenesis could be evaluated. Angiogenesis within viable tissue was assessed by CD34 staining in seven to eight tumors from each diet group. Despite considerable heterogeneity in staining density, angiogenesis was significantly different among diet groups (P = 0.023; Fig. 5). Compared with control-fed mice, angiogenesis was decreased in walnut-fed mice (P = 0.017) but not in flaxseed oil-fed mice (P = 0.454; Fig. 5).
Fig. 5.
Representative areas from tumors of mice fed the control, walnut, and flaxseed oil diets, showing CD34 staining (A) and quantitative estimations of angiogenesis (B). Values are means and 95% confidence intervals. Angiogenesis was significantly reduced in the walnut diet group compared to the control group, but the reduction in the flaxseed oil diet group did not reach statistical significance.
Discussion
In the present study, we examined the effects of dietary walnut and flaxseed oil supplementation on colorectal cancer growth and possible underlying mechanisms in vivo in mice. We found that isocaloric amounts of walnuts and flaxseed oil, compared with corn oil, inhibit colorectal cancer growth rate by 30% to 45%, and that tumor weight is decreased accordingly. We observed large central areas of necrosis in the tumors of sacrificed mice on both walnut and flaxseed diets, despite their smaller overall size and weight at autopsy, which is indicative of insufficient blood supply. We also found that walnuts and flaxseed oil intake reduces expression levels of several angiogenic proteins including VEGF, but does not affect mediators of apoptosis and proliferation in serum, such as insulin, IGF-1, and IGFBPs, or markers of inflammation and antioxidant capacity. Accordingly, angiogenesis within viable tissue was significantly reduced in walnut-fed mice. Our data suggest that consumption of walnuts may be beneficial against the progression of colorectal cancer by suppressing angiogenesis.
This is the first demonstration that dietary walnuts inhibit colorectal cancer growth in vivo. Moreover, compared with control tumors, tumors from walnut- and flaxseed oil-fed mice had larger central necrotic areas despite their smaller size. Our results are consistent with those from a recent study showing that walnuts retard the growth rate of breast cancer cells implanted between the scapulae of mice by 50% to 60% compared to an isocaloric control diet [17]. Similar to our findings, there was no effect of walnut consumption on the number of apoptotic fractions within the viable tumor area; however, proliferation was decreased by ~40% [17]. Another recent study has also observed an antiproliferative effect of walnut extracts against human colon cancer cells in vitro [18]. We found no differences among groups in apoptosis (TUNEL staining) and proliferation (Ki67 staining, expression of Akt, Erk, and S6 kinase), but these preliminary data were collected from only four available sections per group and thus need to be replicated in larger studies. Also, the limited amount of viable tissue in the border of tumors from walnut- and flaxseed oil-fed animals could have confounded our analyses and made it difficult to reliably detect changes at the time of sacrifice. More importantly, it is possible that angiogenesis and other mechanisms might have played a much more important role during earlier stages, i.e., as necrosis was developing over time and before its size reached the large necrotic areas seen at sacrifice. Future studies with larger numbers of mice need to focus on this question at different time points during tumor progression.
Typically, tumor size correlates directly with the extent of necrosis [19]; necrosis develops as a reaction to regional tissue hypoxia, resulting from diminished vascular supply due to rapid tumor growth [20]. The extensive central necrosis following walnut and flaxseed consumption is therefore indicative of a considerably impaired capacity to maintain sufficient nutrient and oxygen supply via the bloodstream, even at a much slower growth rate than control tumors. Consistent with this notion, we observed significant decreases in angiogenesis (CD34 staining) and serum expression levels of several angiogenic factors following walnut consumption. Although the role of several of these factors in colorectal cancer remains unclear, as some, e.g., TIMP-1, have been associated with both proangiogenic and antiangiogenic functions in other tissues [21,22], the reduction in VEGF may be important for the antitumorigenic effect of dietary walnuts, as VEGF is an established key factor regulating the development of new blood vessels, which facilitate colorectal cancer progression [2,23]. These observations collectively suggest that down-regulation of angiogenesis is involved in the antitumorigenic effect of dietary walnuts.
Similar to walnuts, we found that consumption of flaxseed oil suppresses the growth rate of established colorectal cancer in vivo, reduces VEGF, and leads to extensive central necrosis. This is in agreement with the results of all but one [10] previous studies. Dietary flaxseed supplementation reduces the formation of aberrant crypt foci, which are precursor lesions in the development of colon cancer [11–13], and also reduces the incidence, multiplicity, and size of colorectal cancer induced by injection of the chemical carcinogen azoxymethane in various animal models [14–16]. The underlying mechanisms are incompletely understood. Studies assessing the effect of flaxseed on other types of cancer have reported reductions in serum VEGF, as we did, but also angiogenesis and, subsequently, tumor growth [24, 25]; this has been associated with both up-regulated apoptosis and down-regulated proliferation [26,27]. Although we did not detect significant changes in these potential mechanisms, the reduction in serum angiogenic factors appeared to be greater overall in the flaxseed oil than the walnut diet group, but the reduction in angiogenesis (CD34 staining) did not reach significance. This could indicate that levels of angiogenic factors at sacrifice correlate more strongly with the overall number of microvessels rather than their density in the remaining vital tumor areas. This apparent discrepancy may also result from the fact that we sacrificed the animals at a stage where the low levels of VEGF had already led to extended necrosis, thereby limiting our analyses of microvessel density to very confined areas within the tumor. Alternatively, microvessel density at sacrifice reflects, as the final outcome, the aggregate effect of exposure to angiogenic factors over a prolonged period of time prior to sacrifice, and measurement of one angiogenic factor (VEGF) at one time point (i.e., sacrifice) may not necessarily reflect the aggregate effects of continuous exposure to levels of angiogenesis factors as accurately as measuring microvessel density.
The antitumorigenic effect of dietary walnuts and flaxseed oil was remarkably similar in qualitative terms and in no instance did the differences between the two experimental diets reach significance. Walnuts and flaxseed are particularly rich sources of ALA (C18:3n-3), which is the natural precursor of eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3). EPA and DHA have been shown to retard tumor growth and inhibit metastases in various in vitro and in vivo models of colorectal cancer [28–32]. Human studies provide evidence that dietary ALA supplementation by means of flaxseed oil and/or walnut consumption increases serum EPA and DHA concentrations [33–35], indicating conversion of ALA into longer chain fatty acids in vivo. Provided adequate amount and duration of consumption, dietary ALA may also reduce inflammation [35–37] and improve insulin resistance [38,39], i.e., two factors involved in colorectal cancer progression [2]. Flaxseed oil provides more than five times more ALA than walnuts [35] and, in our study, led to an inversed n-6/n-3 ratio in the diet (1:5), which was much different than that of the walnut diet (6.4:1). The qualitatively similar effects of dietary walnuts and flaxseed oil suggest that dietary ALA or the overall n-6/n-3 fatty acid ratio cannot be responsible for the observed inhibition of colorectal cancer growth seen in response to either of the two experimental treatments that differed significantly in terms of ALA content and n-6/n-3 ratio. It remains to be seen in future larger studies whether walnuts result in quantitatively possibly different effects than flaxseed oil on tumor size and growth on the basis of the hypothesis that increasing ALA concentrations would result in quantitatively more significant responses. Attesting to this hypothesis are recent data demonstrating that the putative growth-inhibitory and proapoptotic effects of ALA on cancer cells increase dose-dependently in vitro [40]; similar observations have been made in vivo regarding the antitumorigenic effect of flaxseed oil [26].
Finally, we cannot rule out the possibility that other, yet to be identified, dietary constituents may also be involved. Flaxseed is a very rich source of precursors of the mammalian lignans enterodiol and enterolactone (phytoestrogens), which may reduce cancer risk by preventing precancerous cellular changes and by reducing angiogenesis and metastasis [41]. This may be especially important for colorectal cancer, because the colon is the primary site of production of mammalian lignans from plant precursors [42]. In fact, pure enterolactone was shown to have antiproliferative and proapoptotic effects against human colon cancer cells in vitro and in vivo [43]. Walnuts also contain several compounds with putative anti-tumorigenic properties, such as ellagic acid, selenium, phytosterols, and dietary fiber [9,17]. It is also likely that several compounds act synergistically toward this end. It would, thus, be of interest to study further possible underlying mechanisms and to hopefully identify individual nutrients responsible for the observed inhibition of colorectal cancer growth.
Conclusion
In summary, we investigated the effects of dietary supplementation with isoenergetic amounts of walnuts or flaxseed oil on established colorectal cancer in mice in vivo and found significant inhibition of cancer growth rate and reduction in tumor weight, compared with a corn oil-based control diet. We also observed extensive central necrosis in tumors from walnut-fed and flaxseed oil-fed animals. Impaired angiogenesis appears to underlie the antitumorigenic effects of dietary walnuts, and possibly also flaxseed oil. It remains to be studied whether dietary consumption of walnuts has a similar beneficial effect on colon cancer in humans.
Acknowledgments
This work was supported by the National Institutes of Health (DK081913) and the American Institute for Cancer Research and the California Walnut Commission; sponsors had no input on interpretation or reporting of the findings.
References
- 1.World Cancer Research Fund/American Institute for Cancer Research. Washington, DC: AICR; 2007. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. [Google Scholar]
- 2.Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010;138:2029–2043. doi: 10.1053/j.gastro.2010.01.057. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkin DM, Olsen AH, Sasieni P. The potential for prevention of colorectal cancer in the UK. Eur J Cancer Prev. 2009;18:179–190. doi: 10.1097/CEJ.0b013e32830c8d83. [DOI] [PubMed] [Google Scholar]
- 4.Platz EA, Willett WC, Colditz GA, Rimm EB, Spiegelman D, Giovannucci E. Proportion of colon cancer risk that might be preventable in a cohort of middle-aged US men. Cancer Causes Control. 2000;11:579–588. doi: 10.1023/a:1008999232442. [DOI] [PubMed] [Google Scholar]
- 5.Fung T, Hu FB, Fuchs C, Giovannucci E, Hunter DJ, Stampfer MJ, et al. Major dietary patterns and the risk of colorectal cancer in women. Arch Intern Med. 2003;163:309–314. doi: 10.1001/archinte.163.3.309. [DOI] [PubMed] [Google Scholar]
- 6.Jenab M, Ferrari P, Slimani N, Norat T, Casagrande C, Overad K, et al. Association of nut and seed intake with colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2004;13:1595–1603. [PubMed] [Google Scholar]
- 7.Singh PN, Fraser GE. Dietary risk factors for colon cancer in a low-risk population. Am J Epidemiol. 1998;148:761–774. doi: 10.1093/oxfordjournals.aje.a009697. [DOI] [PubMed] [Google Scholar]
- 8.Yeh CC, You SL, Chen CJ, Sung FC. Peanut consumption and reduced risk of colorectal cancer in women: a prospective study in Taiwan. World J Gastroenterol. 2006;12:222–227. doi: 10.3748/wjg.v12.i2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez CA, Salas-Salvado J. The potential of nuts in the prevention of cancer. Br J Nutr. 2006;96 Suppl 2:S87–S94. doi: 10.1017/bjn20061868. [DOI] [PubMed] [Google Scholar]
- 10.van Kranen HJ, Mortensen A, Sorensen IK, van den Berg-Wijnands J, Beems R, Nurmi T, et al. Lignan precursors from flaxseed or rye bran do not protect against the development of intestinal neoplasia in ApcMin mice. Nutr Cancer. 2003;45:203–210. doi: 10.1207/S15327914NC4502_10. [DOI] [PubMed] [Google Scholar]
- 11.Jenab M, Thompson LU. The influence of flaxseed and lignans on colon carcinogenesis and beta-glucuronidase activity. Carcinogenesis. 1996;17:1343–1348. doi: 10.1093/carcin/17.6.1343. [DOI] [PubMed] [Google Scholar]
- 12.Serraino M, Thompson LU. Flaxseed supplementation and early markers of colon carcinogenesis. Cancer Lett. 1992;63:159–165. doi: 10.1016/0304-3835(92)90066-5. [DOI] [PubMed] [Google Scholar]
- 13.Williams D, Verghese M, Walker LT, Boateng J, Shackelford L, Chawan CB. Flax seed oil and flax seed meal reduce the formation of aberrant crypt foci (ACF) in azoxymethane-induced colon cancer in Fisher 344 male rats. Food Chem Toxicol. 2007;45:153–159. doi: 10.1016/j.fct.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Bommareddy A, Arasada BL, Mathees DP, Dwivedi C. Chemopreventive effects of dietary flaxseed on colon tumor development. Nutr Cancer. 2006;54:216–222. doi: 10.1207/s15327914nc5402_8. [DOI] [PubMed] [Google Scholar]
- 15.Bommareddy A, Zhang X, Schrader D, Kaushik RS, Zeman D, Matthees DP, et al. Effects of dietary flaxseed on intestinal tumorigenesis in Apc(Min) mouse. Nutr Cancer. 2009;61:276–283. doi: 10.1080/01635580802419764. [DOI] [PubMed] [Google Scholar]
- 16.Dwivedi C, Natarajan K, Matthees DP. Chemopreventive effects of dietary flaxseed oil on colon tumor development. Nutr Cancer. 2005;51:52–58. doi: 10.1207/s15327914nc5101_8. [DOI] [PubMed] [Google Scholar]
- 17.Hardman WE, Ion G. Suppression of implanted MDA-MB 231 human breast cancer growth in nude mice by dietary walnut. Nutr Cancer. 2008;60:666–674. doi: 10.1080/01635580802065302. [DOI] [PubMed] [Google Scholar]
- 18.Carvalho M, Ferreira PJ, Mendes VS, Silva R, Pereira JA, Jeronimo C, et al. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem Toxicol. 2010;48:441–447. doi: 10.1016/j.fct.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 19.De Jaeger K, Merlo FM, Kavanagh MC, Fyles AW, Hedley D, Hill RP. Heterogeneity of tumor oxygenation: relationship to tumor necrosis, tumor size, and metastasis. Int J Radiat Oncol Biol Phys. 1998;42:717–721. doi: 10.1016/s0360-3016(98)00323-x. [DOI] [PubMed] [Google Scholar]
- 20.Jain RK. Vascular and interstitial barriers to delivery of therapeutic agents in tumors. Cancer Metastasis Rev. 1990;9:253–266. doi: 10.1007/BF00046364. [DOI] [PubMed] [Google Scholar]
- 21.Elezkurtaj S, Kopitz C, Baker AH, Perez-Canto A, Arlt MJ, Khokha R, et al. Adenovirus-mediated overexpression of tissue inhibitor of metalloproteinases-1 in the liver: efficient protection against T-cell lymphoma and colon carcinoma metastasis. J Gene Med. 2004;6:1228–1237. doi: 10.1002/jgm.637. [DOI] [PubMed] [Google Scholar]
- 22.Kopitz C, Gerg M, Bandapalli OR, Ister D, Pennington CJ, Hauser S, et al. Tissue inhibitor of metalloproteinases-1 promotes liver metastasis by induction of hepatocyte growth factor signaling. Cancer Res. 2007;67:8615–8623. doi: 10.1158/0008-5472.CAN-07-0232. [DOI] [PubMed] [Google Scholar]
- 23.Winder T, Lenz HJ. Vascular endothelial growth factor and epidermal growth factor signaling pathways as therapeutic targets for colorectal cancer. Gastroenterology. 2010;138:2163–2176. doi: 10.1053/j.gastro.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Bergman Jungestrom M, Thompson LU, Dabrosin C. Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin Cancer Res. 2007;13:1061–1067. doi: 10.1158/1078-0432.CCR-06-1651. [DOI] [PubMed] [Google Scholar]
- 25.Dabrosin C, Chen J, Wang L, Thompson LU. Flaxseed inhibits metastasis and decreases extracellular vascular endothelial growth factor in human breast cancer xenografts. Cancer Lett. 2002;185:31–37. doi: 10.1016/s0304-3835(02)00239-2. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Power KA, Mann J, Cheng A, Thompson LU. Flaxseed alone or in combination with tamoxifen inhibits MCF-7 breast tumor growth in ovariectomized athymic mice with high circulating levels of estrogen. Exp Biol Med (Maywood) 2007;232:1071–1080. doi: 10.3181/0702-RM-36. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Chen J, Thompson LU. The inhibitory effect of flaxseed on the growth and metastasis of estrogen receptor negative human breast cancer xenografts is attributed to both its lignan and oil components. Int J Cancer. 2005;116:793–798. doi: 10.1002/ijc.21067. [DOI] [PubMed] [Google Scholar]
- 28.Hossain Z, Hosokawa M, Takahashi K. Growth inhibition and induction of apoptosis of colon cancer cell lines by applying marine phospholipid. Nutr Cancer. 2009;61:123–130. doi: 10.1080/01635580802395725. [DOI] [PubMed] [Google Scholar]
- 29.Huang YC, Jessup JM, Forse RA, Flickner S, Pleskow D, Anastopoulos HT, et al. n-3 fatty acids decrease colonic epithelial cell proliferation in high-risk bowel mucosa. Lipids. 1996;31 Suppl:S313–S317. doi: 10.1007/BF02637099. [DOI] [PubMed] [Google Scholar]
- 30.Iigo M, Nakagawa T, Ishikawa C, Iwahori Y, Asamoto M, Yazawa K, et al. Inhibitory effects of docosahexaenoic acid on colon carcinoma 26 metastasis to the lung. Br J Cancer. 1997;75:650–655. doi: 10.1038/bjc.1997.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jakobsen CH, Storvold GL, Bremseth H, Follestad T, Sand K, Mack M, et al. DHA induces ER stress and growth arrest in human colon cancer cells: associations with cholesterol and calcium homeostasis. J Lipid Res. 2008;49:2089–2100. doi: 10.1194/jlr.M700389-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki I, Iigo M, Ishikawa C, Kuhara T, Asamoto M, Kunimoto T, et al. Inhibitory effects of oleic and docosahexaenoic acids on lung metastasis by colon-carcinoma-26 cells are associated with reduced matrix metalloproteinase-2 and -9 activities. Int J Cancer. 1997;73:607–612. doi: 10.1002/(sici)1097-0215(19971114)73:4<607::aid-ijc24>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 33.Dodin S, Cunnane SC, Masse B, Lemay A, Jacques H, Asselin G, et al. Flaxseed on cardiovascular disease markers in healthy menopausal women: a randomized, double-blind, placebo-controlled trial. Nutrition. 2008;24:23–30. doi: 10.1016/j.nut.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Ros E, Nunez I, Perez-Heras A, Serra M, Gilabert R, Casals E, et al. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation. 2004;109:1609–1614. doi: 10.1161/01.CIR.0000124477.91474.FF. [DOI] [PubMed] [Google Scholar]
- 35.Zhao G, Etherton TD, Martin KR, West SG, Gillies PJ, Kris-Etherton PM. Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr. 2004;134:2991–2997. doi: 10.1093/jn/134.11.2991. [DOI] [PubMed] [Google Scholar]
- 36.Rallidis LS, Paschos G, Liakos GK, Velissaridou AH, Anastasiadis G, Zampelas A. Dietary alpha-linolenic acid decreases C-reactive protein, serum amyloid A and interleukin-6 in dyslipidaemic patients. Atherosclerosis. 2003;167:237–242. doi: 10.1016/s0021-9150(02)00427-6. [DOI] [PubMed] [Google Scholar]
- 37.Prasad K. Flaxseed and cardiovascular health. J Cardiovasc Pharmacol. 2009;54:369–377. doi: 10.1097/FJC.0b013e3181af04e5. [DOI] [PubMed] [Google Scholar]
- 38.Bloedon LT, Balikai S, Chittams J, Cunnane SC, Berlin JA, Rader DJ, et al. Flaxseed and cardiovascular risk factors: results from a double blind, randomized, controlled clinical trial. J Am Coll Nutr. 2008;27:65–74. doi: 10.1080/07315724.2008.10719676. [DOI] [PubMed] [Google Scholar]
- 39.Muramatsu T, Yatsuya H, Toyoshima H, Sasaki S, Li Y, Otsuka R, et al. Higher dietary intake of alpha-linolenic acid is associated with lower insulin resistance in middle-aged Japanese. Prev Med. 2010;50:272–276. doi: 10.1016/j.ypmed.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Kim JY, Park HD, Park E, Chon JW, Park YK. Growth-inhibitory and proapoptotic effects of alpha-linolenic acid on estrogen-positive breast cancer cells. Ann NY Acad Sci. 2009;1171:190–195. doi: 10.1111/j.1749-6632.2009.04897.x. [DOI] [PubMed] [Google Scholar]
- 41.Adolphe JL, Whiting SJ, Juurlink BH, Thorpe LU, Alcorn J. Health effects with consumption of the flax lignan secoisolariciresinol diglucoside. Br J Nutr. 2010;103:929–938. doi: 10.1017/S0007114509992753. [DOI] [PubMed] [Google Scholar]
- 42.Thompson LU. Experimental studies on lignans and cancer. Baillieres Clin Endocrinol Metab. 1998;12:691–705. doi: 10.1016/s0950-351x(98)80011-6. [DOI] [PubMed] [Google Scholar]
- 43.Danbara N, Yuri T, Tsujita-Kyutoku M, Tsukamoto R, Uehara N, Tsubura A. Enterolactone induces apoptosis and inhibits growth of Colo 201 human colon cancer cells both in vitro and in vivo. Anticancer Res. 2005;25:2269–2276. [PubMed] [Google Scholar]