Abstract
The nuclear factor-κB (NF-κB) transcription factor functions as a crucial regulator of cell survival and chemoresistance in pancreatic cancer. Recent studies suggest that tocotrienols, which are the unsaturated forms of vitamin E, are a promising class of anti-cancer compounds that inhibit the growth and survival of many cancer cells, including pancreatic cancer. Here, we show that tocotrienols inhibited NF-κB activity and the survival of human pancreatic cancer cells in vitro and in vivo. Importantly, we found the bioactivity of the 4 natural tocotrienol compounds (α-, β-, δ-, and γ-tocotrienol) to be directly related to their ability to suppress NF-κB activity in vitro and in vivo. The most bioactive tocotrienol for pancreatic cancer, δ-tocotrienol, significantly enhanced the efficacy of gemcitabine to inhibit pancreatic cancer growth and survival in vitro and in vivo. Moreover, we found that δ-tocotrienol augmentation of gemcitabine activity in pancreatic cancer cells and tumors is associated with significant suppression of NF-κB activity and the expression of NF-κB transcriptional targets [Bcl-XL, X-linked inhibitor of apoptosis (XIAP), and survivin]. Our study represents the first comprehensive pre-clinical evaluation of the activity of natural vitamin E compounds in pancreatic cancer. Given these results, we are conducting a phase I trial of δ-tocotrienol in patients with pancreatic cancer utilizing pancreatic tumor cell survival and NF-κB signaling components as intermediate biomarkers. Our data also support future clinical investigation of δ-tocotrienol to augment gemcitabine activity in pancreatic cancer.
Keywords: gemcitabine, δ-tocotrienol, pancreatic cancer, apoptosis, NF-κB, chemoresistance
Introduction
Pancreatic cancer is a leading cause of cancer mortality with <5% of patients surviving 5 years after diagnosis (1). Gemcitabine is a mainstay treatment for pancreatic cancer (2). However, tumor resistance to gemcitabine therapy is common, making this a critical challenge for pancreatic cancer management.
Pro-survival signaling mediated by the transcription factor nuclear factor-κB (NF-κB) is a key player in gemcitabine resistance in pancreatic cancer (3–11). Therefore, targeting dysregulated NF-κB signaling is an important strategy to improve the efficacy of gemcitabine therapy and thereby improve outcomes for patients with pancreatic cancer. Several studies have combined natural compounds that inhibit NF-κB, such as genistein, curcumin, fisetin, and green tea, with gemcitabine to investigate synergy in treating pancreatic cancer (11–14). However, translation of these studies to the clinic has been challenging due to the low bioavailability of some of these natural compounds in humans.
Tocotrienols, a group of four (α-, β-, δ-, and γ-tocotrienol) unsaturated naturally occurring vitamin E compounds (Figure 1A), have received increasing attention for their potential as nontoxic dietary anti-cancer agents (15, 16). Our group recently showed that oral administration of 100 mg/kg/day of δ-tocotrienol to mice resulted in levels that were 10 times higher in pancreas than in subcutaneously implanted tumor tissue, suggesting that these compounds will have reasonable bioavailability for pancreatic tumor intervention (17). In this study, we investigated the potential of the four natural tocotrienols to inhibit pancreatic cancer and NF-κB activation in vitro and in vivo. In addition, we investigated the potential of the most bioactive tocotrienol to augment gemcitabine activity in vitro and in vivo.
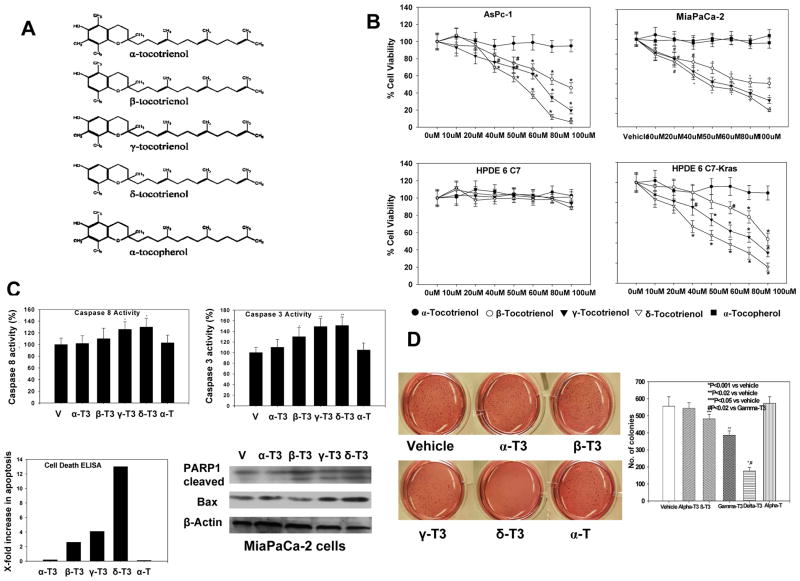
Figure 1.
A, Chemical structures of tocotrienols and tocopherol. B, Effect of tocotrienols on pancreatic cancer cell proliferation (MTT assay). Points, means; bars, standard error (SE) (n = 3–5, *P < 0.001, #P < 0.01). C, Effect of tocotrienols on caspase 3 and 8 activities in MiaPaCa-2 cells (top left and right). Bars, SE (n = 3, *P < 0.05 **P < 0.01). Bottom left: effect of tocotrienols on pancreatic cancer apoptotic cell death (ELISA) in MiaPaCa-2 cells (n = 3). Bottom right: Western blot of PARP-1 cleavage and pro-apoptotic Bax protein expression in MiaPaCa-2 cells (n = 3). D, Effect of tocotrienols on pancreatic cancer malignant transformation assay in MiaPaCa-2 cells. δ-Tocotrienol significantly inhibited malignant transformation compared with vehicle (*P < 0.001) and β- or γ-tocotrienol treatment (#P < 0.02). In contrast, no effect was observed with α-tocotrienol or α-tocopherol treatment. Bars, SE (n = 3).
MATERIALS AND METHODS
Chemicals and cell lines
All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. Tocotrienols (α, β, γ, and δ) and α- and δ-tocopherol were obtained from Davos Life Ltd (Helios, Singapore). Gemcitabine-HCl was purchased from Eli Lilly and Company (Indianapolis, IN). L-glutamine, penicillin, streptomycin, and HEPES buffer were purchased from Mediatech (Herndon, VA). Fetal bovine serum (FBS) was purchased from HyClone (Logan, UT). Dulbecco’s modified minimal essential medium (DMEM), RPMI 1640, keratinocyte serum-free medium, sodium pyruvate, trypsin-EDTA, and phosphate-buffered saline (PBS) were purchased from Invitrogen (Carlsbad, CA). Ethanol (100%) was purchased from Aaper Alcohol and Chemical (Shelbyville, KY). Pancreatic cancer cell lines MiaPaCa-2 and AsPc-1 were obtained from ATCC (Manassas, VA). Human pancreatic ductal epithelial cells (HPDE6 C7) and HPDE6 C7-KRas cells were gifts from Dr. Tsao, Ontario Cancer Institute, University of Toronto. These cells were authenticated by morphologic, cell proliferation, and Mycoplasma tests, as recommended in ATCC Technical Bulletin No. 8 (2007).
Cell culture and growth
HPDE6 C7 and HPDE6 C7-KRas cells were grown in keratinocyte growth media (Invitrogen) supplemented with human EGF and bovine pituitary extract. Mia PaCa-2 and AsPc-1 cells were grown in monolayers with DMEM and RPMI media, respectively, supplemented with 10% FBS, 2 mM L-glutamine, penicillin (50 IU/mL), and streptomycin (50 mg/mL). RPMI medium was also supplemented with 10−2 M HEPES buffer and 10−3 M sodium pyruvate. Cells were cultured at 37°C in a humidified atmosphere of 5% CO2-95% O2.
Cell proliferation MTT assay
Cells were seeded in 96-well plates at a density of 3,000 cells per well and allowed to attach overnight. Cells were then incubated for 72 hours with various concentrations of α, β, γ, and δ-tocotrienol and α-tocopherol (10−5 to 10−4 M). In other experiments, cells were incubated for 72 hours with gemcitabine and δ-tocotrienol (10−5 to 10−4 M) and their combination or ethanol (<5%) in PBS as vehicle control. Media were aspirated and replaced with 20 μL of 1 mg/mL MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] and incubated for 2–4 hours at 37°C in a humidified atmosphere of 5% CO2. Media were aspirated, 200 μL of DMSO were added to each well, plates were incubated for 5 minutes with shaking, and absorbance was read at 540 nm.
Isobologram analysis
Gemcitabine and δ-tocotrienol were combined at ratios of 1:1 and 1:2 of their IC50 values to plot the isobologram using fraction effects and combination index (CI) through ClacuSyn software (Biosoft, Cambridge, UK). Constant ratio (IC50 ratio) of the two-drug combination is used. The dose range (different fraction of the IC50 values) and effects (proliferation) are entered in ClacuSyn, which displays the parameters (Dm, m, and r) as well as isobologram using CI, where Dm is the median-effect dose signifying the potency, m is measurement of the sigmoidicity (shape) of the dose-effect curve, and r is linear correlation coefficient of the median-effect plot. The software analyzes the quantitative measure of the degree of drug interactions in terms of additive effect (CI = 1), synergism (CI <1), or antagonism (CI >1), based on a published method (18).
Trypan blue dye exclusion assay
Treated cells were trypsinized and washed with PBS, and cell suspension (50 μL) was mixed with 50 μL of Trypan blue dye and incubated for 2 minutes at room temperature. A 10-μL volume was loaded onto a hemacytometer, and cells were scored as live or dead based on Trypan blue exclusion.
Cell death assay
Cytoplasmic DNA fragments and histone were detected in cells using Cell Death Detection ELISA kit from Roche Diagnostic (Indianapolis, IN).
Colonogenic survival (anchorage-independent) growth assay
The standard soft agar colony formation assay was performed in MiaPaca-2 cells. Cells were seeded at a density of 5,000/well in 12-well plates in 0.3% agar over a 0.6% bottom agar layer. Colonies were fed with growth media and α, β, γ, and δ-tocotrienol or α/δ-tocopherol (5× 10−5 M), and colony formation growth was observed for 14 days. In other experiments, colonies were fed with growth media and gemcitabine (2× 10−5 M) and δ-tocotrienol (5× 10−5 M) and their combination, and colony formation growth was observed for 14 days. Colonies were photographed after overnight incubation with 1 mg/mL MTT in the wells. Colonies were counted under stereomicroscope and compared with controls. Experiments were done at least twice, each in triplicate.
NF-κB (p65) binding activity assay
NF-κB binding activities in nuclear and cytosolic fractions of MiaPaca-2 cells were determined using the Trans AM ELISA kit from Active Motif (Carlsbad, CA), according to manufacturer’s instructions.
Caspase enzyme activity assay
Treated cells were lysed with lysis buffer (0.5 mM Tris/pH 8.0, 5 mM EDTA/pH 8, 0.15 mM NaCl, 0.5% NP-40). The enzymatic activities of caspase 3, 8, and 9 were determined using specific fluorogenic substrates. The liberated fluorescent 7-amido-4-methyl-coumarin groups were quantified using a multi-well plate VersaFluorTM fluorometer at 355-nm excitation and 460-nm emission wavelengths (Bio-Rad).
Cell lysis and protein determination
Cells were washed three times in cold PBS (pH 7) and then lysed in mammalian-protein extraction reagent lysis buffer (Pierce, Rockford, IL) containing EDTA and protease inhibitor cocktail. Protein concentration was determined using BCA reagents (Pierce) according to manufacturer’s instructions.
Western blot analysis
Extracted proteins from cells and tumor tissues (40 μg) were resolved on 12.5% SDS-PAGE running gel and 5% stacking gel. Proteins were then electrotransferred onto nitrocellulose membranes. After blocking in 5% nonfat powdered milk for 1 hour, membranes were washed and treated with antibodies to CK18, PARP-1, NF-κB subunits (p65/p50), IκBα, p-IκBα, Bcl-XL, XIAP, survivin, and β-actin (1:1,000 and 1:5,000) overnight at 4°C (Axxora, San Diego, CA; Santa Cruz Biotechnology, Santa Cruz, CA; Cell Signaling, Danvers, MA). After they were washed, blots were incubated with horseradish peroxidase-conjugated secondary antibody IgG (1:5,000 and 1:10,000) for 1 hour at room temperature. Washed blots were then treated with SuperSignal West Pico chemiluminescent substrate (Pierce) for positive antibody reaction. Membranes were exposed to X-ray film (Kodak) for visualization and densitometric quantization of protein bands using AlphaEaseFC software (Alpha Innotech).
Animals and treatments
Female NIH SCID nude mice (4–5 weeks old, 20–25 g) were obtained from Charles River (Wilmington, MA) and kept in our Center’s animal facility for 1 week for quarantine. Mice (n = 25) were injected with AsPc-1 cells (one million) with Matrigel (100 μL) to both flanks. When tumor volume reached 100 mm3, mice were randomly divided into 5 treatment groups: 1) normal controls (100 μL oral gavage of ethanol extracted olive oil (vehicle) twice daily for 4 weeks, 2) α-tocotrienol treated, 3) β-tocotrienol treated, 4) γ-tocotrienol treated, and 5) δ-tocotrienol treated. Tocotrienols were administered at 200 mg/kg oral gavage twice daily for 4 weeks. In other experiments, mice (n = 20) were injected with AsPc-1 cells (one million) with Matrigel (100 μL) to both flanks. When tumor volume reached 100 mm3, mice were randomly divided into 4 treatment groups: 1) normal controls (100 μL oral gavage of ethanol extracted olive oil (vehicle) twice daily for 4 weeks, 2) δ-tocotrienol treated (200 mg/kg oral gavage twice daily for 4 weeks), 3) gemcitabine treated (100 mg/kg IP twice a week for 4 weeks), and 4) δ-tocotrienol + gemcitabine treated (200 mg/kg oral gavage daily plus gemcitabine at 100 mg/kg IP twice a week for 4 weeks). Tumor volumes were measured every other day. Animal studies were approved by our Institutional Laboratory Animal Care and Use Committee and were conducted per the guidelines of the National Institutes of Health.
Statistical analysis
Data are expressed as means ± SEM and were analyzed statistically using unpaired t-tests or one-way analysis of variance (ANOVA) where appropriate. ANOVA was followed by Duncan’s multiple range tests using SAS statistical software for comparison between different treatment groups. Significance was set at P < 0.05.
RESULTS
Effect of tocotrienols on growth and survival of pancreatic cancer cells in vitro
Using the MTT assay, we found that natural tocotrienols have variable inhibitory effects on human pancreatic cancer cells, with δ-tocotrienol and γ-tocotrienol exhibiting the most significant inhibitory effects (cell viability reduced up to 60% with 50 μM) and β-tocotrienol having a moderate inhibitory effect (cell viability reduced up to 40% with 50 μM) (Figure 1B). Both α-tocotrienol and saturated vitamin E (α-tocopherol) did not show any significant inhibitory effects. In contrast, treatment of HPDE6 C7 cells resulted in essentially no effect on cell viability. HPDE6 C7-Kras cells were sensitive to the effects of some of the tocotrienols, with 50 μM tocotrienol resulting in 55%, 30%, 15%, and 0% growth inhibition for δ-, γ-, β-, and α-tocotrienol, respectively.
To further assess whether the loss of viability could in part be due to apoptosis, we evaluated the effects of tocotrienols on apoptosis using histone-DNA ELISA, caspase 3, and caspase 8 activity as well as Western immunoblotting for PARP-1 and the pro-apoptotic protein Bax in MiaPaCa-2 cells. Figure 1C shows a significant increase in apoptotic cells, closely paralleling the loss of viable cells following treatment with 50 μM tocotrienol. Caspase 3 and 8 activity was also significantly increased after treatment of MiaPaCa-2 cells with δ-tocotrienol and γ-tocotrienol. Western immunoblotting revealed that tocotrienol treatment resulted in the appearance of a cleaved active component of PARP-1 and induction of Bax in MiaPaCa-2 cells treated with δ-, γ-, and β-tocotrienol 50 μM for 72 hours.
Tocotrienols also influenced colony formation of human pancreatic cancer cells in soft agar, which is one of the best in vitro indicators of malignant behavior. As shown in Figure 1D, treatment of MiaPaCa-2 cells with 50 μM tocotrienol resulted in significantly fewer and smaller colonies than shown in cells treated with vehicle control or the saturated form of vitamin E. The relative potency of the tocotrienols in inhibiting colony formation was δ-tocotrienol (65%), γ-tocotrienol (30%), β-tocotrienol (13%), and α-tocotrienol (2%).
Effect of tocotrienols on growth and survival of pancreatic cancer cells in vivo
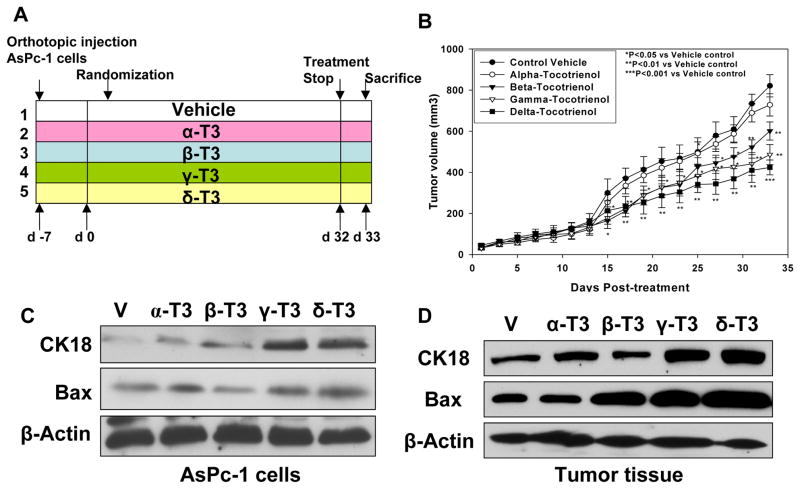
Based on our in vitro studies, which strongly support differential effects of natural tocotrienols on pancreatic cancer cells, we examined the effects of tocotrienol on the growth of AsPc-1 human pancreatic cancer xenografts in mice. Such studies have never been documented in vivo to the best of our knowledge. A mouse dose of 200 mg/kg IG tocotrienol twice a day was selected, based on our previously published report (17). Treatment started after randomization and continued per experimental protocol for 34 days. Figure 2B shows a gradual increase in tumor volume in the control and α-tocotrienol groups. Tumor volumes in the β-, δ-, and γ-tocotrienol groups were also significantly lower from day 15 (P < 0.05) to end of treatment (P < 0.01 for β- and γ-tocotrienol and P < 0.001 for δ-tocotrienol). On day 34, δ-tocotrienol significantly reduced tumor volume by 50% (P < 0.001), γ-tocotrienol reduced tumor volume by 42% (P < 0.01), and β-tocotrienol reduced tumor volume by 32% (P < 0.01). In contrast, α-tocotrienol did not significantly decrease tumor growth.
Figure 2.
A, Effect of in vivo tocotrienols on AsPc-1 pancreatic tumor growth in SCID nude mice. B, Tumor volume in δ-, γ-, and β-tocotrienol groups was significantly decreased compared with vehicle (V). Points, means; bars, SE (n = 10, *P < 0.05, **P < 0.01, ***P < 0.001). C, Expression of apoptosis marker CK18 and pro-apoptotic Bax protein in AsPc-1 cells (n = 3) by Western blot. D, CK18 and Bax expression in mouse tumor tissues (n = 5) by Western blot.
We next examined expression of cell apoptosis markers CK18 and Bax in AsPC-1 cells in vitro and in tumor tissues. As shown in Figures 2C and 2D, γ- and δ-tocotrienol significantly induced expression of CK18 and Bax.
Effect of tocotrienols on NF-κB activation in pancreatic cancer cells
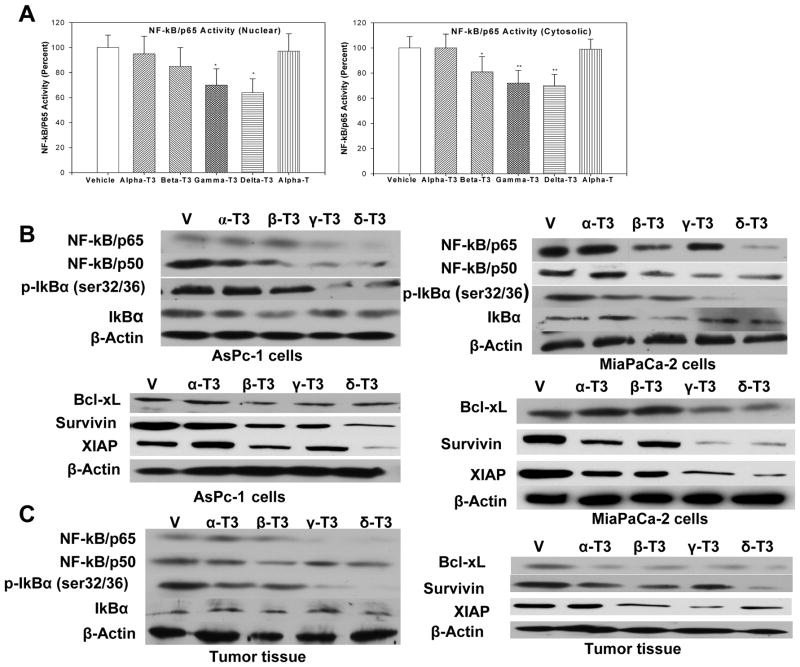
Because previous studies have shown that γ-tocotrienol can suppress constitutive NF-κB activation in pancreatic cancer cells (19), the possibility that tocotrienol bioactivity in pancreatic cancer is related to NF-κB suppression was considered. As illustrated in Figure 3A, tocotrienol treatment suppressed constitutive NF-κB activation in a tocotrienol compound-dependent manner. The extent of NF-κB activity inhibition was found to be significant for γ- and δ-tocotrienol in the nuclear extract and for β-, γ-, and δ-tocotrienol in the cytosolic extract. Interestingly, α-tocotrienol and α-tocopherol had no effect on NF-κB activity. These results were further confirmed by immunoblotting experiments in AsPc-1 and MiaPaCa-2 cells (Figure 3B) and in tumor tissues of mice xenografted with AsPc-1 cells (Figure 3C). A crucial step in the activation of NF-κB proteins is the phosphorylation of IκBα freeing NF-κB complexes to translocate to the nucleus where they induce target gene expression, including pro-survival factors such as Bcl-XL, survivin, and XIAP. As shown in Figures 3B and 3C, δ-tocotrienol consistently suppressed NF-κB/p65 and phosphorylated IκBα expression in pancreatic cancer cells and tumor tissues, consistent with down-regulation of Bcl-XL, survivin, and XIAP in pancreatic cancer cells and tumor tissues. Consistent with a previous report, γ-tocotrienol also suppressed NF-κB proteins and their target genes (19). However, α- and β-tocotrienol did not suppress the NF-κB proteins, p-IκBα or Bcl-XL, survivin, and XIAP, in a consistent fashion.
Figure 3.
A, Effect of tocotrienols on nuclear and cytosolic NF-kB/p65 binding activity in MiaPaCa-2 cells. β-, γ-, and δ-Tocotrienol significantly decreased p65 binding to DNA in the cytosol, whereas γ- and δ-tocotrienol significantly decreased p65 binding to DNA in the nucleus compared to vehicle. Bars, SE (n = 3, *P < 0.05, **P < 0.02). B, Effect of tocotrienols on NF-κB subunits, IκBα phosphorylation, and NF-κB-associated gene products in AsPc-1 and MiaPaCa-2 cells (n = 3) by Western blot. C, Effect of tocotrienol treatment on p65 and p50 subunit, p-IκBα, and anti-apoptotic protein expression in mouse tumor tissue (n = 5) by Western blot.
δ-Tocotrienol augments inhibition of pancreatic cancer cell proliferation by gemcitabine
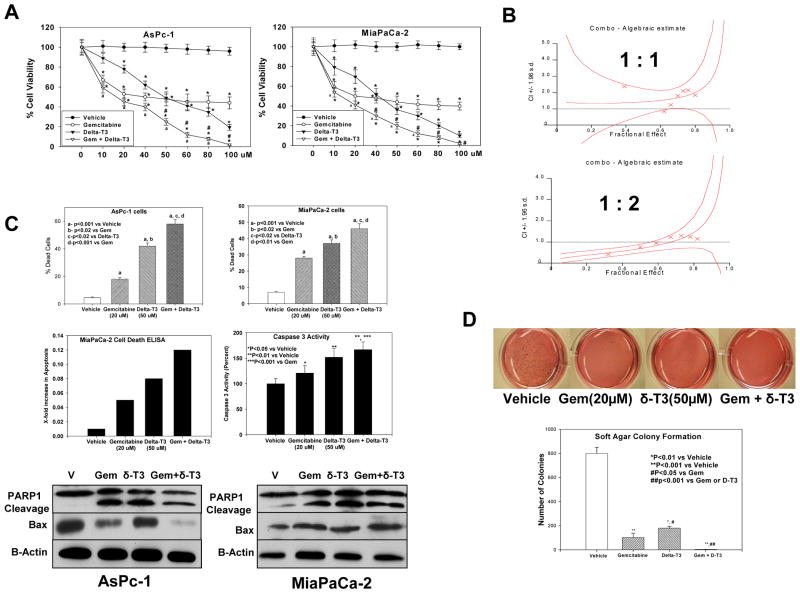
Recently, γ-tocotrienol was reported to augment gemcitabine activity and to inhibit NF-κB activation in pancreatic cancer cells (19). Because we observed that both γ and δ-tocotrienol significantly inhibited NF-κB activation, we investigated whether δ-tocotrienol augmented gemcitabine inhibition of pancreatic cancer growth. The effects of δ-tocotrienol and gemcitabine alone and in combination on AsPc-1 and MiaPaCa-2 cell proliferation were determined by MTT. Cells were treated with vehicle, δ-tocotrienol, gemcitabine, or δ-tocotrienol + gemcitabine at (0–100 μM) for 72 hours (Figure 4A). At 10–100 μM, δ-tocotrienol + gemcitabine significantly augmented gemcitabine inhibition of cell proliferation. Furthermore, to confirm synergism, we determined combination index (CI) values for two combination treatment groups. Our results show that cells treated with gemcitabine + δ-tocotrienol at a ratio of 1:1 had additive/antagonistic loss of cell viability (CI = 1.32), whereas cells treated with gemcitabine + δ-tocotrienol at 1:2 showed synergistic loss of cell viability (CI = 0.89) (Figure 4B). We further investigated the effects of gemcitabine and δ-tocotrienol alone and in combination on anchorage-independent growth of MiaPaCa-2 cells using soft agar colony formation assay (Figure 4D). Gemcitabine (20 μM) alone inhibited colony formation by 84%; δ-tocotrienol (50 μM) alone inhibited colony formation by 67%. Gemcitabine (20 μM) plus δ-tocotrienol (50 μM) resulted in 99% inhibition of anchorage-independent growth.
Figure 4.
A, Gemcitabine and δ-tocotrienol significantly (*P < 0.001) decreased proliferation of AsPc1 and MiaPaCa2 cells compared with vehicle, with the combination being more effective in inhibiting cell proliferation than either drug alone (#P < 0.01 and aP < 0.01). B, Effect of gemcitabine + δ-tocotrienol (1:1) and gemcitabine + δ-tocotrienol (1:2) as shown in isobologram with combination index in MiaPaCa-2 cells. Points, means; bars, SE (n = 3–5). C, Effect of gemcitabine and δ-tocotrienol alone and in combination on pancreatic cancer survival (Trypan blue) in AsPc-1 and MiaPaCa-2 cells. δ-Tocotrienol induced greater cell death than vehicle control (aP < 0.001) or gemcitabine alone (bP < 0.02). However, the combination resulted in greater effect on cell death than vehicle alone (aP < 0.001) or gemcitabine or δ-tocotrienol alone (cP < 0.02 and dP < 0.01). Bars (means) ± SE (n = 3–5). Apoptosis-inducing activity was 3-, 1-, and 6-fold for δ-tocotrienol, gemcitabine, and the combination, respectively, compared with vehicle. Bars, means (n = 3). Caspase 3 activity in MiaPaCa-2 cells treated with δ-tocotrienol was significantly increased (**P < 0.01) versus gemcitabine (*P<0.05) and vehicle. However, the combination resulted in greater caspase-3 activity than with vehicle (**P < 0.01) or with gemcitabine alone (***P < 0.001). Bars (means) ± SE (n = 3). Western blots show PARP-1 cleavage and pro-apoptotic Bax protein expression in MiaPaCa-2 and AsPc-1 cells (n = 3). D, Effects of gemcitabine and δ-tocotrienol alone and in combination on pancreatic cancer malignant transformation in MiaPaCa-2 cells. Gemcitabine is more effective in inhibiting colony formation (**P < 0.001) than δ-tocotrienol (*P < 0.01; #P < 0.02). However, the combination resulted in almost complete inhibition of the malignant transformation compared with vehicle (**P < 0.001) or with gemcitabine or δ-tocotrienol alone (##P < 0.001). Bars (means) ± SE (n = 3).
δ-Tocotrienol augments gemcitabine-induced apoptosis in pancreatic cancer cells
We next compared whether enhanced cytotoxicity by gemcitabine + δ-tocotrienol was mediated by apoptosis. Figure 4C shows that, relative to single-agent gemcitabine, combination with δ-tocotrienol elicited a significantly (P < 0.001, P < 0.01) higher percent of cell death in pancreatic cancer cell lines, suggesting that the loss of viable cells is due to the induction of cell death pathway. We confirmed this by measuring the fold increase in apoptosis in MiaPaCa-2 cells using the cell death-ELISA and the caspase 3 activity assay. Whereas treatment of MiaPaCa-2 cells with gemcitabine and δ-tocotrienol as single agents increased apoptosis, gemcitabine + δ-tocotrienol significantly augmented the fold increase in apoptosis. Caspase 3 activity was also observed to be significantly elevated (P < 0.001) in the combination treatment group. PARP-1, a 116-kDa nuclear poly(ADP-ribose) polymerase 1, is one of the main cleavage targets of caspase 3 in vivo, serving as a marker of cells undergoing apoptosis (20). Our data confirm induction of apoptosis by gemcitabine, δ-tocotrienol, and the combination of the two agents in AsPc-1 and MiaPaCa-2 cells.
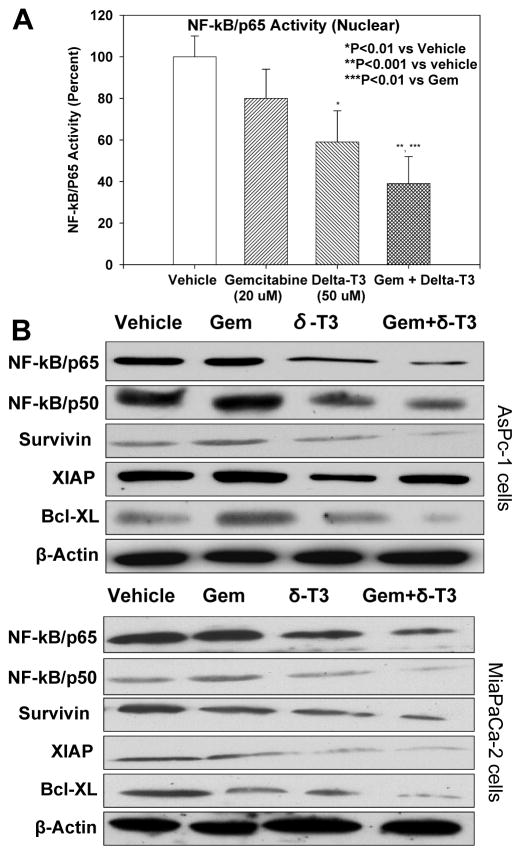
δ-Tocotrienol down-regulates constitutively activated NF-κB in gemcitabine-treated pancreatic cancer cells
MiaPaCa-2 cells were exposed to 50 μM δ-tocotrienol, 20 μM gemcitabine, or their combination for 72 hours, and their nuclear extracts were subjected to NF-κB DNA binding activity assay (ELISA). We found that gemcitabine alone did not significantly affect NF-κB DNA binding activity (Figure 5A). Interestingly, treatment with δ-tocotrienol significantly inhibited binding activity, with combined gemcitabine and δ-tocotrienol significantly inhibiting NF-κB DNA binding activity more than either drug alone. These results were further confirmed by Western immunoblotting in AsPc-1 and MiaPaCa-2 cells. As shown in Figure 5B, expression levels of NF-κB/p65 and NF-κB/p50 proteins were significantly depleted in cells treated with δ-tocotrienol and in those treated with gemcitabine + δ-tocotrienol. Additionally, we assessed the anti-apoptotic molecules Bcl-XL, survivin, and XIAP. Relative to control and gemcitabine alone, Bcl-XL, survivin, and XIAP expression levels were down-regulated in cells exposed to δ-tocotrienol alone and in cells exposed to gemcitabine + δ-tocotrienol. These results strongly suggest that the augmentation of gemcitabine activity in pancreatic cancer cells by δ-tocotrienol is in part due to the inactivation of NF-κB and its downstream genes by δ-tocotrienol.
Figure 5.
A, Effect of gemcitabine and δ-tocotrienol alone and in combination on NF-κB/p65 DNA binding activity in nuclear compartment of MiaPaCa-2 cells. Gemcitabine was refractory to p65 binding to DNA, whereas δ-tocotrienol significantly decreased p65 binding to DNA (*P < 0.01) compared to vehicle. However, the combination resulted in greater inhibition of p65 binding to DNA than vehicle (**P < 0.001) or gemcitabine alone (***P < 0.01). Bars (means) ± SE (n = 3). B, Expression of NF-κB subunit and NF-κB-regulated anti-apoptotic proteins (n = 3).
δ-Tocotrienol enhances the in vivo therapeutic effects of gemcitabine in a pancreatic tumor model in SCID nude mice
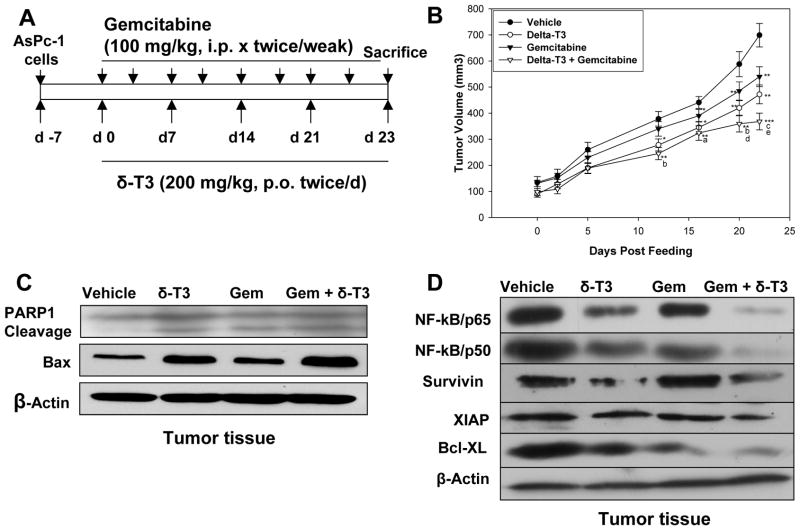
Based on our in vitro results, we designed studies to determine the effects of δ-tocotrienol and gemcitabine in a human model of pancreatic tumor in SCID nude mice (Figure 6A). The dose of δ-tocotrienol was determined from our previous studies of δ-tocotrienol pharmacokinetics in mice (17); our in vitro data indicated that a 2:1 ratio of δ-tocotrienol + gemcitabine was synergistic in inhibiting pancreatic cancer growth.
Figure 6.
A, Effect of gemcitabine and δ-tocotrienol alone and in combination on in vivo AsPc-1 tumor growth in mice. B, Gemcitabine and δ-tocotrienol alone significantly inhibited growth of AsPc-1 tumor (tumor volume) in vivo compared to vehicle (*P < 0.05, **P < 0.01, ***P < 0.001). However, the combination resulted in greater inhibition of tumor growth than shown with vehicle (*P < 0.05, **P < 0.01, ***P < 0.001), gemcitabine alone (aP < 0.05, bP < 0.02, cP < 0.01), and δ-tocotrienol alone (cP < 0.01, dP < 0.05, eP < 0.01). Points (means); bars ± SE (n = 8–10). C, Apoptotic protein expression in AsPc-1 mouse tumor tissue by Western blot. Both gemcitabine and δ-tocotrienol increased PARP-1 cleavage and Bax protein expression. However, the combination resulted in greater increase in PARP-1 cleavage and Bax protein expression than shown with vehicle (n = 4–5). D, Expression of apoptotic protein, NF-κB subunits, and anti-apoptotic protein in AsPc-1 mouse tumor tissues by Western blot (n = 4–5).
AsPC-1 cells were implanted in the subcutaneous tissue of flanks of SCID nude mice. Mice were randomly assigned to the 4 treatment groups (see Materials and Methods), with treatment continued per experimental protocol for 23 days. Animals were euthanized on the last day of the therapy. To determine the effects of treatment on tumor development, we assessed tumor volume on days 0–23 of treatment. As shown in Figure 6B, on day 12, tumor volumes in the δ-tocotrienol alone and δ-tocotrienol + gemcitabine groups were significantly lower than tumor volumes in vehicle and gemcitabine alone treatment groups (P < 0.01 and P < 0.001). From treatment day 17, tumor volumes in mice treated with δ-tocotrienol + gemcitabine were significantly reduced compared with that shown in mice treated with gemcitabine or δ-tocotrienol alone. The results are in accordance with the in vitro results.
We next examined the cleavage of PARP-1 protein, a marker of apoptosis, in the tumor tissues of mice as well as the expression of the pro-apoptotic protein Bax. As shown in Figure 6C, δ-tocotrienol, gemcitabine, and δ-tocotrienol + gemcitabine induced PARP-1 cleavage in mouse tumors. Bax expression was significantly induced in the δ-tocotrienol alone and the gemcitabine + δ-tocotrienol tumors compared to vehicle and gemcitabine alone.
Effect of δ-tocotrienol + gemcitabine treatment on NF-κB activation and NF-κB-regulated gene products
We further investigated whether the effects of combining δ-tocotrienol and gemcitabine on tumor growth in mice were associated with inhibition of NF-κB activation. Our Western blot results clearly show that NF-κB/p65 and NF-κB/p50 proteins and the NF-κB transcriptional targets Bcl-XL, survivin, and XIAP were moderately down-regulated by δ-tocotrienol alone. Similar to our in vitro studies, gemcitabine alone did not affect the expression of NF-κB or NF-κB-regulated gene products. However, similar to our in vitro studies, constitutively active NF-κB was almost completely suppressed in tumor samples of mice treated with δ-tocotrienol + gemcitabine (Figure 6D). Tumors also revealed down-regulation of important NF-κB-regulated pro-apoptotic proteins (Bcl-XL, survivin, and XIAP).
DISCUSSION
Our results clearly demonstrate that the four natural tocotrienols have different abilities to inhibit cancer growth and survival. We show that δ- and γ-tocotrienol consistently inhibited cancer growth and survival in vitro and in vivo. In contrast, α- and β-tocotrienol and α-tocopherol do not inhibit growth and survival of pancreatic cancer cells. Our data agree with the results reported by Hussein and Mo (21) who showed that δ-tocotrienol suppressed the proliferation of Panc-1, MiaPaCa-2, and BxPc-3 human pancreatic cancer cells. Our results are also in agreement with Kunnumakkara and colleagues (19) who reported that γ-tocotrienol inhibited the growth and survival of Panc-1, MiaPaCa-2, and BxPc-3 human pancreatic cancer cells. Our data provide the first report of a direct comparison of the effects of all four of the vitamin E tocotrienol compounds on human pancreatic cancer cells in vitro and in vivo. The results indicate that δ-tocotrienol is the most bioactive tocotrienol against human pancreatic cancer cells and provide the rationale for selecting δ-tocotrienol as the lead tocotrienol compound for further studies of the use of tocotrienols for pancreatic cancer prevention and treatment.
Our in vitro and in vivo results demonstrate that the bioactivity of tocotrienols in pancreatic cancer cells was directly related to inhibition of NF-κB activity. Our results agree with Kunnumakkara et al (19) who reported that γ-tocotrienol inhibited NF-κB activity in vitro and in vivo. We show for the first time that δ-tocotrienol also inhibited NF-κB activity and the expression of NF-κB regulated gene products. In contrast, α- and β-tocotrienol had no significant effect on NF-κB activity. These results strongly suggest that the bioactivity of tocotrienols against cancer cells is due in part to inhibition of the activity of the transcription factor NF-κB. Since numerous studies have shown that inhibition of NF-κB can enhance gemcitabine activity in pancreatic cancer cells (3, 8, 9, 19, 22), we investigated whether δ-tocotrienol could enhance the activity of gemcitabine against human pancreatic cancer. We found that δ-tocotrienol augmented gemcitabine inhibition of cell viability, as well as induction of apoptosis in pancreatic cancer cells. We also found that δ-tocotrienol augmented gemcitabine inhibition of growth of human pancreatic AsPc-1 tumors in SCID nude mice. Augmentation of gemcitabine activity by δ-tocotrienol is associated with inhibition of constitutively active NF-κB and NF-κB-regulated gene products. Our results are in agreement with Kunnumakkara et al (19) who demonstrated that γ-tocotrienol inhibition of constitutively active NF-κB and NF-κB-regulated gene products was associated with γ-tocotrienol potentiation of gemcitabine-induced apoptosis, as well as γ-tocotrienol potentiation of the effects of gemcitabine against human pancreatic tumors in nude mice.
Several mechanisms by which tocotrienols exert their anti-cancer effects have been reported. Suppression of inflammatory transcription factor NF-κB, which is involved in tumorigenesis, and inhibition of HMG-CoA reductase, mammalian DNA polymerase, and certain protein kinases are potential targets to tocotrienols (16). In this study, we found that the in vitro and in vivo anti-pancreatic cancer activity of tocotrienols can be attributed to inhibition of NF-κB signaling. Additional studies are being designed to clarify the mechanisms of inhibition of NF-κB signaling by δ-tocotrienol in pancreatic cancer cells. One possibility is that δ-tocotrienol inhibits the degradation of the protein that inhibits NF-κB, the inhibitor of NF-κB (IκB). IκB sequesters NF-κB in the cytoplasm, and phosphorylation of IκB makes it a target for ubiquitination. The added ubiquitin molecules render IκB for degradation by proteosomes. We observed that δ-tocotrienol treatment resulted in a decrease of phosphorylated IκB. Whether δ-tocotrienol affects the kinase that phosphorylates IκB is unknown.
In summary, this is the first report evaluating the effects of all of the natural vitamin E tocotrienol compounds on pancreatic cancer cells. Our results show that δ- and γ-tocotrienol inhibited NF-κB activity, cell growth, cell survival, and tumor growth in nude mice. We further show that δ-tocotrienol augmented gemcitabine activity in vitro and in vivo. These results suggest that inhibition of NF-κB signaling by δ-tocotrienol may be an effective approach for the prevention and treatment of pancreatic cancer. Our findings suggest evaluation of NF-κB signaling compounds as an endpoint biomarker in our ongoing phase I trial of δ-tocotrienol in patients with pancreatic tumors (ClinicalTrials.gov NCT00985777).
Acknowledgments
The study was supported in part by National Cancer Institute/USPHS grant 1RO1 CA-129227-01A1 and DAVOS-69-15099-99-01.
We thank Rasa Hamilton (Moffitt Cancer Center) for editorial assistance.
ABBREVIATIONS
- ANOVA
analysis of variance
- CI
combination index
- CK18
cytokeratine-18
- DMSO
dimethyl sulfoxide
- DMEM
Dulbecco’s modified minimal essential medium
- EDTA
ethylene diamine tetra-acetic acid
- ELISA
enzyme-linked immunosorbant assay
- FBS
fetal bovine serum
- Gem
gemcitabine
- HPDE6 C7
human pancreatic ductal epithelial cells
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NF-κB
nuclear factor kappa B
- p-IκBα
phosphorylated inhibitor of kappa B alpha
- PARP1
poly-ADP riboxyl transferase 1
- PBS
phosphate-buffered saline
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- T3
tocotrienol
- T
tocopherol
- XIAP
X-linked inhibitor of apoptosis
Footnotes
Conflicts of interest: Drs. Malafa and Sebti are named as inventors on US Patent “Delta-Tocotrienol Treatment and Prevention of Pancreatic Cancer (June 26, 2007; OTML docket number 06A069) but do not have financial interest in the companies that have licensed this patent.
References
- 1.Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007;110:738–44. doi: 10.1002/cncr.22852. [DOI] [PubMed] [Google Scholar]
- 2.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 3.Arlt A, Gehrz A, Muerkoster S, Vorndamm J, Kruse ML, Folsch UR, et al. Role of NF-kappaB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22:3243–51. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- 4.Bai J, Sui J, Demirjian A, Vollmer CM, Jr, Marasco W, Callery MP. Predominant Bcl-XL knockdown disables antiapoptotic mechanisms: tumor necrosis factor-related apoptosis-inducing ligand-based triple chemotherapy overcomes chemoresistance in pancreatic cancer cells in vitro. Cancer Res. 2005;65:2344–52. doi: 10.1158/0008-5472.CAN-04-3502. [DOI] [PubMed] [Google Scholar]
- 5.Liu WS, Yan HJ, Qin RY, Tian R, Wang M, Jiang JX, et al. siRNA directed against survivin enhances pancreatic cancer cell gemcitabine chemosensitivity. Dig Dis Sci. 2009;54:89–96. doi: 10.1007/s10620-008-0329-4. [DOI] [PubMed] [Google Scholar]
- 6.Shrikhande SV, Kleeff J, Kayed H, Keleg S, Reiser C, Giese T, et al. Silencing of X-linked inhibitor of apoptosis (XIAP) decreases gemcitabine resistance of pancreatic cancer cells. Anticancer Res. 2006;26:3265–73. [PubMed] [Google Scholar]
- 7.Wang CY, Guttridge DC, Mayo MW, Baldwin AS., Jr NF-kappaB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol. 1999;19:5923–9. doi: 10.1128/mcb.19.9.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong R, Sun B, Wang SJ, Pan SH, Wang G, Chen H, et al. An experimental study of gemcitabine inducing pancreatic cancer cell apoptosis potentiated by nuclear factor-kappa B P65 siRNA. Zhonghua Wai Ke Za Zhi. 2010;48:128–33. [PubMed] [Google Scholar]
- 9.Pan X, Arumugam T, Yamamoto T, Levin PA, Ramachandran V, Ji B, et al. Nuclear factor-kappaB p65/relA silencing induces apoptosis and increases gemcitabine effectiveness in a subset of pancreatic cancer cells. Clin Cancer Res. 2008;14:8143–51. doi: 10.1158/1078-0432.CCR-08-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harikumar KB, Kunnumakkara AB, Sethi G, Diagaradjane P, Anand P, Pandey MK, et al. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. Int J Cancer. 2010;127:257–68. doi: 10.1002/ijc.25041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–61. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 12.Mahon PC, Baril P, Bhakta V, Chelala C, Caulee K, Harada T, et al. S100A4 contributes to the suppression of BNIP3 expression, chemoresistance, and inhibition of apoptosis in pancreatic cancer. Cancer Res. 2007;67:6786–95. doi: 10.1158/0008-5472.CAN-07-0440. [DOI] [PubMed] [Google Scholar]
- 13.Kurbitz C, Heise D, Redmer T, Goumas F, Arlt A, Lemke J, et al. Epicatechin gallate and catechin gallate are superior to epigallocatechin gallate in growth suppression and anti-inflammatory activities in pancreatic tumor cells. Cancer Sci. 2011;102:728–34. doi: 10.1111/j.1349-7006.2011.01870.x. [DOI] [PubMed] [Google Scholar]
- 14.Murtaza I, Adhami VM, Hafeez BB, Saleem M, Mukhtar H. Fisetin, a natural flavonoid, targets chemoresistant human pancreatic cancer AsPC-1 cells through DR3-mediated inhibition of NF-kappaB. Int J Cancer. 2009;125:2465–73. doi: 10.1002/ijc.24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyazawa T, Shibata A, Sookwong P, Kawakami Y, Eitsuka T, Asai A, et al. Antiangiogenic and anticancer potential of unsaturated vitamin E (tocotrienol) J Nutr Biochem. 2009;20:79–86. doi: 10.1016/j.jnutbio.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal BB, Sundaram C, Prasad S, Kannappan R. Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol. 2010;80:1613–31. doi: 10.1016/j.bcp.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Husain K, Francois RA, Hutchinson SZ, Neuger AM, Lush R, Coppola D, et al. Vitamin E delta-tocotrienol levels in tumor and pancreatic tissue of mice after oral administration. Pharmacology. 2009;83:157–63. doi: 10.1159/000190792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther. 2001;298:865–72. [PubMed] [Google Scholar]
- 19.Kunnumakkara AB, Sung B, Ravindran J, Diagaradjane P, Deorukhkar A, Dey S, et al. Gamma-tocotrienol inhibits pancreatic tumors and sensitizes them to gemcitabine treatment by modulating the inflammatory microenvironment. Cancer Res. 2010;70:8695–705. doi: 10.1158/0008-5472.CAN-10-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 21.Hussein D, Mo H. d-delta-Tocotrienol-mediated suppression of the proliferation of human PANC-1, MIA PaCa-2, and BxPC-3 pancreatic carcinoma cells. Pancreas. 2009;38:e124–36. doi: 10.1097/MPA.0b013e3181a20f9c. [DOI] [PubMed] [Google Scholar]
- 22.Kong R, Sun B, Jiang H, Pan S, Chen H, Wang S, et al. Downregulation of nuclear factor-kappaB p65 subunit by small interfering RNA synergizes with gemcitabine to inhibit the growth of pancreatic cancer. Cancer Lett. 2010;291:90–8. doi: 10.1016/j.canlet.2009.10.001. [DOI] [PubMed] [Google Scholar]