Abstract
Objective
To measure the circulating concentrations of nitric oxide (NO) adducts with NO bioactivity following inhaled NO therapy in infants with pulmonary hypertension.
Study design
In this single center study five sequential blood samples were collected from infants with pulmonary hypertension before, during and after therapy with iNO (n=17). Samples were collected from a control group of hospitalized infants without pulmonary hypertension (n=16) and from healthy adults for comparison (n=12).
Results
After beginning iNO (20 ppm) whole blood nitrite increased about two-fold within two hours (P<0.01). Whole blood nitrate increased to four-fold higher than baseline during treatment with 20ppm iNO (P<0.01). S-nitrosohemoglobin (SNO-Hb) increased measurably after beginning iNO (P<0.01) whereas iron nitrosyl hemoglobin and total Hb-bound NO-species compounds did not change.
Conclusion
Treatment of pulmonary hypertensive infants with iNO results in increases in nitrite, nitrate, and SNO-Hb in circulating blood. We speculate that these compounds may be carriers of NO bioactivity throughout the body and account for peripheral effects of iNO in the brain, heart and other organs.
Keywords: newborn, nitric oxide
Inhalation of low doses of nitric oxide gas has become widely accepted in the treatment of persistent pulmonary hypertension in newborn infants. It was initially thought that the effects of inhaled NO (iNO) would be confined to the lung because NO that diffuses into the blood is rapidly metabolized with a half-life of milliseconds,[1] a rate that would preclude the transport of free NO in the blood. However, there is now evidence that inhalation of NO gas at concentrations used to treat pulmonary hypertension results in peripheral vasodilation [2–4] and protects against ischemic injury in systemic organs such as the heart [5–8] and liver [9]. Likewise, preterm newborns receiving iNO had neuroprotective effects attributable to NO even in the absence of pulmonary disease [10]. The evidence for systemic effects of iNO has generated much interest in the potential bioactivity of various metabolites of NO capable of circulating in the blood. Known metabolites include nitrate formed by the reaction of NO with oxyhemoglobin, nitrite formed by the oxidation of NO in plasma, nitrosothiols formed by the binding of NO to thiols, and iron nitrosyl adducts such as iron nitrosylhemoglobin formed by the binding of NO to the heme centers of deoxyhemoglobin. Even though each of these NO metabolites are stable enough to circulate throughout the body, they are also potential sources of NO. We test the hypothesis that iNO therapy increases concentrations of these circulating NO-bioactive molecules in newborn infants with pulmonary hypertension. Furthermore, as insufficient production of NO by pulmonary endothelial nitric oxide synthase (eNOS), due to low concentrations of the substrate L-arginine, has been proposed as a significant cause of pulmonary hypertension in the newborn [11, 12], blood L-arginine concentrations were compared between infants with pulmonary hypertension and infants without pulmonary disease.
Methods
The study was a non-randomized, patient control, single-center study conducted at Loma Linda University Children's Hospital, Neonatal Intensive Care Unit (NICU). The protocol was approved by the Institutional Review Board and written informed consent was obtained from a parent or legal guardian or from the study subject (for the adult group).
- Three groups of subjects were studied: infants with pulmonary hypertension (the study group), infants of the same age without pulmonary disease (the non-hypertensive group), and normal healthy adults for comparative purposes.
The pulmonary hypertensive group consisted of term or late preterm infants requiring iNO therapy for pulmonary hypertension. Pulmonary hypertension was diagnosed by either echocardiography or a preductal/postductal oxygen saturation difference of at least 5% and an oxygenation index (OI) of ≥ 25. Oxygenation index was calculated as the mean airway pressure multiplied by the fraction of inspired oxygen and 100, divided by the partial pressure of arterial oxygen (PaO2) in Torr. Echocardiographic evidence of pulmonary hypertension consisted of a pulmonary artery pressure more than two-thirds of the systemic systolic pressure, as indicated by tricuspid valve regurgitation and right-to-left shunt across the ductus or the atrial septum. The non-hypertensive infant group consisted of term or late preterm infants without evidence of pulmonary hypertension but had indwelling catheters for clinical monitoring. The adult group consisted of healthy, normotensive, non-smoking adults of both sexes.
Infants were excluded from the study if they had culture-proven or clinical evidence of sepsis, defined as a white cell count of more than 30,000 or less than 5,000 cells per cubic millimeter, a ratio of immature to total neutrophils of more than 0.2, and a serum C-reactive protein concentration of more than 3 μg·ml−1 (normal <1 μg·ml−1). Infants were also excluded if they had congenital heart disease, intrauterine growth restriction, chromosomal abnormalities, or required extracorporeal membrane oxygenation. In addition, infants with hypotension requiring vasopressor support were excluded from the control group.
Sample Collection and Handling
A single one-milliliter blood sample was collected from non-hypertensive infants more than twelve hours after birth, and from adult subjects in the morning following an 8-hour fast. One-milliliter samples were collected from infants with pulmonary hypertension prior to initiation of iNO, 1 to 2 h after initiation of iNO at 20 ppm, immediately prior to weaning from 20 ppm, immediately prior to weaning from 5 ppm, and 24 hours after cessation of iNO. The time of collection of the final three samples varied with respect to the time of initiating iNO due to inter-patient variations in the duration of iNO treatment.
Blood samples were collected from infants via indwelling arterial catheters, and from adults via direct venipuncture. The samples were separated immediately into two aliquots: 400 μl was added to 100 μl of a nitrite stabilization solution [13] and stored at −70 C until assayed for nitrite and nitrate. The remaining 600 μl was centrifuged for one minute at 10,000 rcf. The plasma was removed and stored at −70 C for subsequent measurement of L-arginine concentrations. The red blood cell pellet was stored at −70 C for measurement of SNO-Hb concentrations.
Arterial blood gases and pH were determined by amperometric electrode, and hemoglobin concentration and oxyhemoglobin saturation were determined spectrophotometrically (ABL-825, Radiometer, Copenhagen, Denmark).
Nitrite and Nitrate Determination
Nitrite was measured using a triiodide reductive chemiluminescence technique as previously described [14]. The assay quantifies nitrite concentrations above 10 nM with a precision of ± 5 nM. Nitrate (NO3−) was measured by incubation of 205 μl of sample with 5 μl nitrate reductase enzyme (10 U/mL solvent, Roche, Indianapolis IN) in 10 μl of 1M HEPES buffer solution (Fisher Scientific, Pittsburgh PA), 10 μl of 0.1 mM flavin adenine dinuclueotide disodium salt hydrate (Sigma Aldrich, St Louis MO), and 20 μl of 1mM nicotinamide adenine dinucleotide phosphate-oxidase tetrasodium salt (Roche, Indianapolis IN) at 37°C for 45 min to convert all the nitrate to nitrite, which was then measured by triiodide chemiluminescence.
Erythrocytic Hb-bound NO adducts
Assays of RBC SNO-Hb, HbFeNO, and total Hb-bound NO were performed using photolysis-chemiluminescence (PC) [15]. Washed red blood cells were lysed and Hb was desalted using a G-25 fine Sephadex column. Hb-bound NO was measured by PC assay in the presence (HbFeNO) and absence (total Hb-bound NO) of HgCl2, which selectively cleaves SNO bonds. SNO-Hb is the difference between total Hb-NO and HbFeNO. The specificity of this technique was demonstrated previously and the assay is insensitive to nitrite and nitrate.
Plasma L-arginine assay
Plasma arginine concentrations were measured using an isotope-dilution gas chromatograph/mass spectrometry (GC/MS) method as previously described [16].
Statistical analysis
Using Prism v5.0c for Macintosh (Graphpad Software, La Jolla, CA), the significance of changes in nitrite, nitrate, SNO and L-arginine concentrations over time were tested using one way analysis of variance (ANOVA) with post-hoc analysis (Bonferroni test). The influence of arterial PO2, pH, HbO2%, and time on nitrite concentration was examined using step-wise multiple linear regression (DATAMSTR, courtesy of R.A. Brace, UC San Diego, CA, USA). Significance of differences in baseline levels of nitrite, nitrate, and L-arginine concentrations between infants with pulmonary hypertension, non-hypertensive infants, and adults was tested using t-tests. P values < 0.05 were considered statistically significant.
Results
Nineteen patients were enrolled in the pulmonary hypertension group. Two of these infants died while being treated with iNO, one prior to the first 20 ppm sample, and one who was placed on extracorporeal membrane oxygenation (ECMO) prior to the first 20 ppm sample, and their data have been excluded from the results (Table). Sixteen infants were enrolled in the control group with complete data for each of them. Of the 16 control infants, 7 had gastroschisis, 5 had respiratory distress, one had hyperbilirubinemia which was treated with ultraviolet light, one had pneumonia, and two had Rh incompatibility, one of which received an exchange transfusion approximately 14 hours prior to sample collection. There were no differences between the two groups with respect to weight or age. The fractional inspired oxygen concentration (FiO2) was significantly higher in the infants with pulmonary hypertension compared with the control group. Infants in the pulmonary hypertensive group received iNO therapy for an average of 7.3 ± 1.3 days. Twelve subjects were enrolled in the adult group.
Table l.
Patient demographics
| Control Infants | iNO Infants | ||
|---|---|---|---|
| N (males) | 16 (9) | 17 (12) | |
| weight (kg) | 2.8 ± 0.2 | 3.00 ± 0.1 | n.s. |
| age (days) | 1.4 ± 0.5 | 1.4 ± 0.5 | n.s. |
| gestational age at birth (weeks) | 37.3 ± 0.4 | 36.9 ± 0.6 | n.s. |
| baseline FiO2 | 0.25 ± 0.02 | 0.95 ± 0.03 | p<0.001 |
| baseline hematocrit (%) | 41.4 ± 1.8 | 44.2 ± 24 | n.s. |
| Days on iNO | n/a | 7.3 ± 1.3 | n/a |
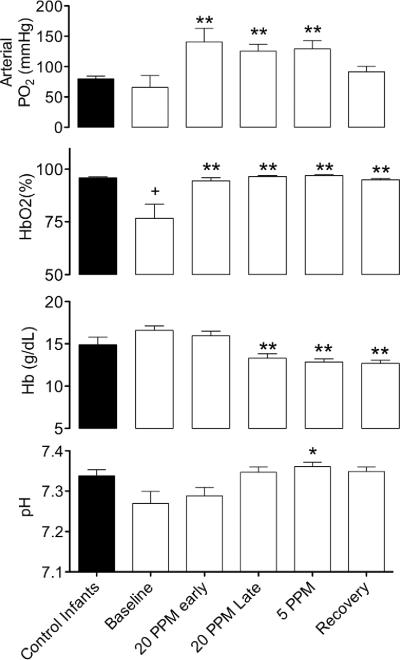
Arterial PO2 (PaO2), oxyhemoglobin saturation (HbO2%) hemoglobin concentration, and pH of the control infants and the infants with pulmonary hypertension over the course of treatment with iNO are shown in Figure 1. Oxyhemoglobin saturations were significantly higher in the control infants compared with infants with pulmonary hypertension prior to initiation of iNO therapy (93 ± 2% vs 77 ± 7%, respectively). PaO2 increased from a baseline of 65 ± 20 to 141 ± 22 Torr two hours after treatment had begun, and remained significantly elevated until the recovery period. Likewise, arterial HbO2 increased from a baseline of 77 ± 7 to 94 ± 2% in the same period and remained significantly increased for the remainder of the study. Hemoglobin concentrations fell, coincident with the administration of intravenous fluids and phlebotomy losses, from baseline levels of 16.6 ± 0.6 g/dl to 13.3 ± 0.5 g/dl immediately prior to the initiation of weaning from iNO (20 ppm, late period), and remained significantly lower than baseline levels for the remainder of the study. Arterial pH tended to increase from a baseline level of 7.27 ± 0.03, and was significantly higher at the 5-ppm time point (7.36 ± 0.01).
Figure 1.
Changes in blood gases and hemoglobin concentration during treatment of pulmonary hypertension in infants. After beginning to breathe 20 ppm iNO arterial oxygen tension and oxyhemoglobin saturation increase significantly and the increase persists during treatment and weaning. Hemoglobin levels decrease and arterial pH increases during weaning (*=significant difference from baseline, p<0.05; *=significant difference from baseline, p<0.01, 1-way ANOVA with Bonferroni post test). Hemoglobin oxygen saturation (HbO2) is significantly lower in patients with pulmonary hypertension compared with controls (+=p<0.05, unpaired t-test).
Nitrite and nitrate
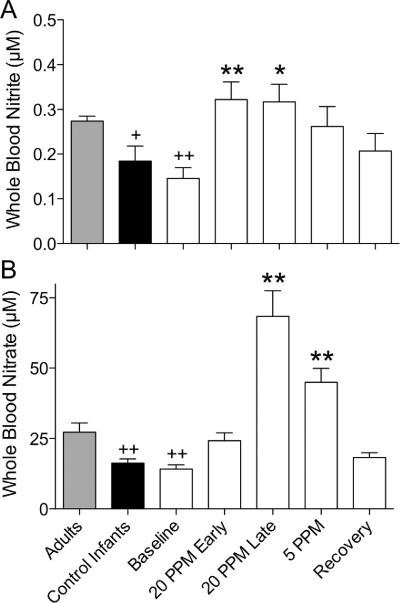
Nitrite concentrations were significantly higher in adults (0.27 ± 0.01 μM) compared with both control infants (0.18 ± 0.03 μM) and infants with pulmonary hypertension (0.15 ± 0.02 μM), but levels in the two infant groups were not significantly different (Figure 2). Similarly, whole blood nitrate concentrations of control infants (16 ± 1 μM) and infants with pulmonary hypertension (14 ± 1 μM) did not differ, but both were significantly lower than adult concentrations (27 ± 3 μM).
Figure 2.
Changes in concentrations of nitrite and nitrate in whole blood during treatment with iNO. (A) Soon after administration of NO, nitrite concentrations about double compared with baseline (*=p<0.05, **=p<0.01, 1-way ANOVA with Bonferroni post test). (B) Nitrate levels also increase, but more slowly, reaching about 4-fold higher than control values while breathing 20 ppm NO; the increase persists during weaning. Nitrite and nitrate concentrations are both lower in newborns compared with normal healthy adults (+=p<0.05, +=p<0.01, 1-way ANOVA), even though there is no significant difference between baseline levels of newborns with pulmonary hypertension and controls.
Within two hours following initiation of iNO therapy, whole blood nitrite concentrations increased above baseline levels by more than 2-fold to and remained significantly higher than baseline levels until iNO had been lowered to 5 ppm during the weaning protocol. Nitrate concentrations increased nearly four-fold during iNO therapy, reaching statistical significance just prior to weaning from 20 ppm and remaining elevated until the recovery period. Both nitrite and nitrate concentrations had returned to baseline values within 24 hours following cessation of iNO therapy.
Nitrosothiols
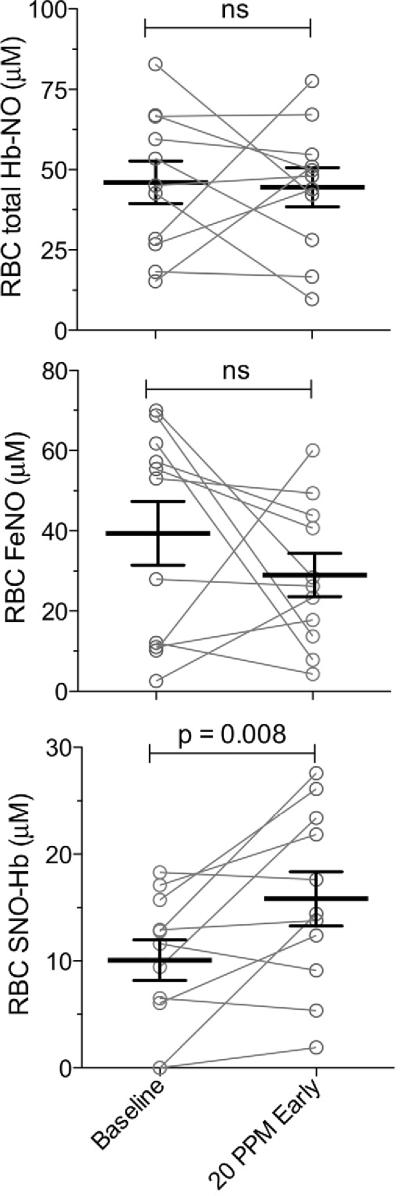
Erythrocyte concentrations of total NO, iron nitrosyl NO, and red blood cell SNO-Hb are shown in Figure 3 for infants with pulmonary hypertension during baseline and after two hours of treatment with 20 ppm iNO. There were no significant differences for total NO or iron nitrosyl NO. In contrast, erythrocyte SNO-Hb concentrations increased significantly within the first two hours of treatment with iNO.
Figure 3.
After beginning NO inhalation SNO-Hb increases measurably (53% change; p< 0.008, paired t-test). Iron nitrosyl-Hb levels do not increase within the red cells, nor do total Hb-bound NO levels as measured by photolysis-chemiluminescence +/− mercury as described.
SNO-Hb formation and stability is favored when hemoglobin is in the R-state conformation [17]. Thus, we performed multivariate linear regression analysis of SNO-Hb concentrations against arterial pH, PO2, and HbO2%, all of which affect hemoglobin conformation. This analysis yielded no significant effect of any of these variables on SNO-Hb concentrations, perhaps due to the effect of other uncontrolled factors, such as endogenous NO synthesis and uptake of exogenous NO, influencing overall SNO-Hb concentrations to a greater degree than the hemoglobin allosteric effect. Indeed, if the analysis was restricted to only the samples collected after the infants were receiving iNO treatment, when the patients were more stabilized and comprised a more homogenous group, SNO-Hb concentrations were significantly affected by changes in arterial PO2 (r2=0.389, p=0.016), and by the combination of pH, PO2 and HbO2 (r2=0.46, p=0.038).
Plasma L-Arginine
There was no significant difference between plasma L-arginine concentrations in infants with pulmonary hypertension and control infants (Figure 4). Plasma L-arginine concentrations increased over the course of treatment with iNO, reaching significance when the 5 ppm sample was collected . The increase in plasma L-arginine concentrations was co-incident with a three-fold increase (from 0.34±0.12 to 1.2±0.9 g·kg−1·day−1) in the amount of L-arginine being administered to the infants in parenteral nutrition.
Figure 4.
Plasma levels of L-arginine at different times during treatment of pulmonary hypertension with iNO. There is no significant difference between baseline plasma L-arginine concentrations of controls and infants with pulmonary hypertension (unpaired t-test). Plasma arginine concentrations increase over the course of iNO therapy, reaching statistical significance at the 5 ppm sampling time point (*=p<0.05, 1-way ANOVA with Bonferroni post test).
Discussion
The extrapulmonary effects of iNO have generated significant interest because the various bioactive metabolites of NO may transmit NO bioactivity from the lung to other parts of the body. In this study we measured a two-fold increase in blood nitrite concentrations and a four-fold increase in nitrate concentrations. Within erythrocytes we also found increased concentrations of SNO-Hb but not of iron-nitrosyl Hb or total erythrocyte Hb-NO concentrations. In addition, in contrast to previous reports, we did not find evidence that would support a deficiency of circulating L-arginine as a cause of newborn pulmonary hypertension.
Although once considered inert at physiological concentrations, studies have demonstrated that both nitrite and nitrate play a role in cardiovascular homeostasis and take part in responses to hypoxic and ischemic stress [18]. Increasing blood nitrite concentrations from physiological levels (0.15 to 0.45 μM [19, 20]) to ~0.8 to 20 μM has been reported to provide protection in animal models of myocardial [21, 22], hepatic [22], renal [23], and cerebral ischemia [24]. The mechanism for this effect remains a topic of active investigation, but may involve the conversion of nitrite to a more bioactive nitrogen oxide species, a reaction which can occur non-enzymatically at low pH or catalytically by reaction with a number of metal-containing proteins (See review [18]). There is also evidence that nitrite may be converted directly to SNO-Hb by reaction with hemoglobin [25, 26]. Many mechanisms have been proposed for the cytoprotective effects of NO and nitrosothiols, and are reviewed elsewhere [27].
Blood nitrite concentrations were significantly lower in infants with and without pulmonary hypertension compared with adults. Whether this newborn-adult difference is due to a normal progression during maturation or caused by other factors associated with hospitalization cannot be determined from the present study.
In response to iNO therapy, blood nitrite concentrations about doubled but remained in the normal range for humans, raising the question whether increases would be high enough to have systemic effects. In mice, intraperitoneal doses of nitrite too small to cause measurable increases in plasma nitrite concentrations protected against hepatic infarct [22]. In humans, less than two-fold increases in plasma nitrite concentrations decreases systolic blood pressure [28, 29]. Thus increases in blood nitrite concentrations as occur in response to iNO, though not pronounced, are in the range that has been found to have systemic effects.
Although nitrate is thought to be inert in mammalian cells, it is actively transported into the saliva by the salivary glands where it is converted to nitrite by nitrate-reducing bacteria [30]. As a result, increasing plasma nitrate concentrations are associated with increases in plasma nitrite [28, 29], decreases in mean systemic blood pressure [28, 29], and increased exercise tolerance [29, 31]. Thus, although adult studies demonstrate systemic effects of nitrate and increased concentrations were observed in the current study, nitrate would seem unlikely to be of importance here because the infants were receiving antibiotics that would have disrupted the normal oral flora and paralytic agents that would have limited swallowing in these intubated infants.
The red blood cell plays a dichotomous role in the regulation of NO homeostasis. On one hand, NO reacts rapidly with oxyhemoglobin to form nitrate [1], and as a result the red blood cell is often considered an irreversible NO scavenger. NO can also be protected from oxidation, however, by forming reversible adducts with the sulfhydryl group of a cysteine residue in the beta subunit of hemoglobin to form S-nitro-hemoglobin (SNO-Hb) [32]. NO also reacts with the heme iron of deoxyhemoglobin to form iron-nitrosyl-hemoglobin (HbFeNO) [33]. Preserved in either of these forms, NO is stable enough to be carried throughout the circulation, making both species candidates for the transport of iNO from the lungs to the rest of the body.
Iron-nitrosyl hemoglobin is normally present in blood at low- to mid-micromolar concentrations [34], but has been reported to increase significantly in response to treatment with 80 ppm iNO in both humans [4] and adult sheep [35]. In contrast, the current work found no significant increase in HbFeNO concentrations in response to iNO therapy (Figure 3). The reason for this difference may be the lower dose of NO (20 ppm) administered to the infants in this study. Thus the present findings do not point to a role for HbFeNO as a carrier of NO activity.
Previous reports of higher SNO-Hb concentrations in arterial compared with venous blood led to the hypothesis that SNO-Hb is influenced by hemoglobin conformation such that it is produced in the lungs as hemoglobin assumes the R-state (oxygenated) and that NO is then released in hypoxic tissues as hemoglobin shifts to the T-state (deoxygenated) [15, 17, 32]. Confirmation of the arterial-venous differences in SNO-Hb concentration has proven controversial [4, 34, 36], partially due to the use of triiodide chemiluminescence assay techniques which are prone to artifactual underestimation of SNO-Hb [37]. Although the current study did not directly compare arterial and venous SNO-Hb concentrations, the effect of hemoglobin conformation on SNO-Hb stability is supported by the finding of a significant direct relationship, at the first 20ppm time point, between SNO-Hb concentrations and arterial PO2, pH, and HbO2, all of which would favor the SNO-Hb-stable R conformation.
The SNO-Hb concentrations (~10 μM in the erythrocyte) for neonatal blood using mercury, coupled photolysis methodology, are somewhat higher than previous measurements with this method in adult blood (2~3 μM in arterial erythrocytes) [15, 38]. The elevated SNO-Hb concentrations in neonatal arterial samples may result from a relative abundance of fetal hemoglobin, as SNO-Hb is is favored when hemoglobin is in a high O2-affinity R-state.
Plasma concentrations of L-arginine were lower in infants with pulmonary hypertension compared with controls, suggesting hypoargininemia may limit endothelial NO production and thus play a role in the disease [11, 12, 39, 40]. Thus we had anticipated lower nitrite and L-arginine concentrations in infants with pulmonary hypertension, but found rather that neither plasma nitrite nor L-arginine was significantly reduced. Furthermore, baseline plasma L-arginine concentrations in the current study (98 ± 26 μM) were notably higher than those previously reported in infants with pulmonary hypertension (~20 to 32 μM [11, 12]), and were comparable with levels in normal adults (114 ± 27 μM [41]). The difference in findings could be due to parenteral L-arginine administration during the baseline period. Nonetheless, the fact that pulmonary hypertension was still present in the patients despite normal plasma L-arginine concentrations suggests that L-arginine deficiency was not a prominent cause of pulmonary hypertension in this patient cohort.
Due to ethical and technical restrictions the current study compares controls and infants with pulmonary hypertension only at the baseline time point. Therefore, changes in nitrite, nitrate, and SNO-Hb that may occur normally after birth were not measured, and may have been superimposed on those changes resulting from iNO therapy. In addition, whereas the current study provides only observational data regarding the effect of inhaled iNO on circulating concentrations of NO species, it is not adequately powered to demonstrate any correlation between the observed changes and biomarkers that may explain the peripheral effects of iNO. Rather, the study demonstrates the extent to which concentrations of these adducts increase during iNO therapy, providing a useful benchmark for dose selection in future interventional studies that might test the benefit of increasing the circulatory transport of NO bioactivity.
Rather than free NO itself, more stable NO adducts are likely to carry NO bioactivity through the circulation and mediate the peripheral effects of iNO. These adducts include nitrite in the plasma and SNO-Hb in the red cells, with iron nitrosyl hemoglobin appearing less likely. Increasing their concentrations may be considered as a useful strategy to provide protection against tissue hypoxia and ischemia in peripheral organs. The safety and dose-dependent effectiveness of these metabolites remains to be more fully established.
Acknowledgments
The authors acknowledge the expert technical assistance of Shannon L. Bragg.
Supported by NIH (grant HL095973 to A.B.) and VA (grant BX-000281-01 to T.M.).
Abbreviations
- ANOVA
analysis of variance
- eNOS
endothelial nitric oxide synthase
- FiO2
fractional inspired oxygen
- HbFeNO
iron nitrosylated hemoglobin
- Hb
hemoglobin
- HbO2
oxyhemoglobin
- iNO
inhaled nitric oxide
- NICU
neonatal intensive care unit
- NO
nitric oxide
- OI
oxygenation index
- PaO2
arterial oxygen tension
- PC
photolysis chemiluminescence
- ppm
parts per million
- SNO
Hb- nitrosylated hemoglobin
- TFFA
trifluroacetic anhydride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Bibliography
- [1].Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, et al. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–83. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- [2].Fox-Robichaud A, Payne D, Hasan SU, Ostrovsky L, Fairhead T, Reinhardt P, et al. Inhaled NO as a viable antiadhesive therapy for ischemia/reperfusion injury of distal microvascular beds. The Journal of Clinical Investigation. 1998;101:2497–505. doi: 10.1172/JCI2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Troncy E, Francoeur M, Salazkin I, Yang F, Charbonneau M, Leclerc G, et al. Extra-pulmonary effects of inhaled nitric oxide in swine with and without phenylephrine. Br J Anaesth. 1997;79:631–40. doi: 10.1093/bja/79.5.631. [DOI] [PubMed] [Google Scholar]
- [4].Cannon RO, 3rd, Schechter AN, Panza JA, Ognibene FP, Pease-Fye ME, Waclawiw MA, et al. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. The Journal of Clinical Investigation. 2001;108:279–87. doi: 10.1172/JCI12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Neviere R, Guery B, Mordon S, Zerimech F, Charre S, Wattel F, et al. Inhaled NO reduces leukocyte-endothelial cell interactions and myocardial dysfunction in endotoxemic rats. American Journal of Physiology. 2000;278:H1783–90. doi: 10.1152/ajpheart.2000.278.6.H1783. [DOI] [PubMed] [Google Scholar]
- [6].Liu X, Huang Y, Pokreisz P, Vermeersch P, Marsboom G, Swinnen M, et al. Nitric oxide inhalation improves microvascular flow and decreases infarction size after myocardial ischemia and reperfusion. J Am Coll Cardiol. 2007;50:808–17. doi: 10.1016/j.jacc.2007.04.069. [DOI] [PubMed] [Google Scholar]
- [7].Nagasaka Y, Fernandez BO, Garcia-Saura MF, Petersen B, Ichinose F, Bloch KD, et al. Brief periods of nitric oxide inhalation protect against myocardial ischemia-reperfusion injury. Anesthesiology. 2008;109:675–82. doi: 10.1097/ALN.0b013e318186316e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hataishi R, Rodrigues AC, Neilan TG, Morgan JG, Buys E, Shiva S, et al. Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia-reperfusion injury. American Journal of Physiology. 2006;291:H379–84. doi: 10.1152/ajpheart.01172.2005. [DOI] [PubMed] [Google Scholar]
- [9].Lang JD, Jr., Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, et al. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. The Journal of Clinical Investigation. 2007;117:2583–91. doi: 10.1172/JCI31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mestan KK, Marks JD, Hecox K, Huo D, Schreiber MD. Neurodevelopmental outcomes of premature infants treated with inhaled nitric oxide. The New England Journal of Medicine. 2005;353:23–32. doi: 10.1056/NEJMoa043514. [DOI] [PubMed] [Google Scholar]
- [11].Pearson DL, Dawling S, Walsh WF, Haines JL, Christman BW, Bazyk A, et al. Neonatal pulmonary hypertension--urea-cycle intermediates, nitric oxide production, and carbamoyl-phosphate synthetase function. The New England Journal of Medicine. 2001;344:1832–8. doi: 10.1056/NEJM200106143442404. [DOI] [PubMed] [Google Scholar]
- [12].Vosatka RJ, Kashyap S, Trifiletti RR. Arginine deficiency accompanies persistent pulmonary hypertension of the newborn. Biol Neonate. 1994;66:65–70. doi: 10.1159/000244091. [DOI] [PubMed] [Google Scholar]
- [13].Pelletier MM, Kleinbongard P, Ringwood L, Hito R, Hunter CJ, Schechter AN, et al. The measurement of blood and plasma nitrite by chemiluminescence: pitfalls and solutions. Free Radic Biol Med. 2006;41:541–8. doi: 10.1016/j.freeradbiomed.2006.05.001. [DOI] [PubMed] [Google Scholar]
- [14].Blood AB, Power GG. In vitro and in vivo kinetic handling of nitrite in blood: effects of varying hemoglobin oxygen saturation. American Journal of Physiology. 2007;293:H1508–17. doi: 10.1152/ajpheart.01259.2006. [DOI] [PubMed] [Google Scholar]
- [15].McMahon TJ, Moon RE, Luschinger BP, Carraway MS, Stone AE, Stolp BW, et al. Nitric oxide in the human respiratory cycle. Nature Medicine. 2002;8:711–7. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- [16].Kayali Z, Herring J, Baron P, Franco E, Ojogho O, Smith J, et al. Increased plasma nitric oxide, L-arginine, and arginase-1 in cirrhotic patients with progressive renal dysfunction. J Gastroenterol Hepatol. 2009;24:1030–7. doi: 10.1111/j.1440-1746.2008.05757.x. [DOI] [PubMed] [Google Scholar]
- [17].Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, et al. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–7. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- [18].Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, et al. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–31. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- [19].Wagner DA, Schultz DS, Deen WM, Young VR, Tannenbaum SR. Metabolic fate of an oral dose of 15N-labeled nitrate in humans: effect of diet supplementation with ascorbic acid. Cancer Res. 1983;43:1921–5. [PubMed] [Google Scholar]
- [20].Lundberg JO, Weitzberg E. NO-synthase independent NO generation in mammals. Biochem Biophys Res Commun. 2010;396:39–45. doi: 10.1016/j.bbrc.2010.02.136. [DOI] [PubMed] [Google Scholar]
- [21].Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35:790–6. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- [22].Dejam A, Hunter CJ, Pelletier MM, Hsu LL, Machado RF, Shiva S, et al. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood. 2005;106:734–9. doi: 10.1182/blood-2005-02-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, et al. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol. 2005;1:290–7. doi: 10.1038/nchembio734. [DOI] [PubMed] [Google Scholar]
- [24].Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004;101:13683–8. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. The Journal of Clinical Investigation. 2005;115:1232–40. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tripatara P, Patel NS, Webb A, Rathod K, Lecomte FM, Mazzon E, et al. Nitrite-derived nitric oxide protects the rat kidney against ischemia/reperfusion injury in vivo: role for xanthine oxidoreductase. J Am Soc Nephrol. 2007;18:570–80. doi: 10.1681/ASN.2006050450. [DOI] [PubMed] [Google Scholar]
- [27].Jung KH, Chu K, Ko SY, Lee ST, Sinn DI, Park DK, et al. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke. 2006;37:2744–50. doi: 10.1161/01.STR.0000245116.40163.1c. [DOI] [PubMed] [Google Scholar]
- [28].Angelo M, Singel DJ, Stamler JS. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc Natl Acad Sci U S A. 2006;103:8366–71. doi: 10.1073/pnas.0600942103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nagababu E, Ramasamy S, Rifkind JM. S-nitrosohemoglobin: a mechanism for its formation in conjunction with nitrite reduction by deoxyhemoglobin. Nitric Oxide. 2006;15:20–9. doi: 10.1016/j.niox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- [30].Sun J, Murphy E. Protein S-nitrosylation and cardioprotection. Circ Res. 2010;106:285–96. doi: 10.1161/CIRCRESAHA.109.209452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–90. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, et al. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1121–31. doi: 10.1152/ajpregu.00206.2010. [DOI] [PubMed] [Google Scholar]
- [33].Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, et al. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nature Medicine. 1995;1:546–51. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- [34].Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 2007;191:59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- [35].Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–6. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- [36].Cassoly R, Gibson Q. Conformation, co-operativity and ligand binding in human hemoglobin. J Mol Biol. 1975;91:301–13. doi: 10.1016/0022-2836(75)90382-4. [DOI] [PubMed] [Google Scholar]
- [37].Gladwin MT, Ognibene FP, Pannell LK, Nichols JS, Pease-Fye ME, Shelhamer JH, et al. Relative role of heme nitrosylation and beta-cysteine 93 nitrosation in the transport and metabolism of nitric oxide by hemoglobin in the human circulation. Proc Natl Acad Sci U S A. 2000;97:9943–8. doi: 10.1073/pnas.180155397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Takahashi Y, Kobayashi H, Tanaka N, Sato T, Takizawa N, Tomita T. Nitrosyl hemoglobin in blood of normoxic and hypoxic sheep during nitric oxide inhalation. The American Journal of Physiology. 1998;274:H349–57. doi: 10.1152/ajpheart.1998.274.1.H349. [DOI] [PubMed] [Google Scholar]
- [39].Moore EG, Gibson QH. Cooperativity in the dissociation of nitric oxide from hemoglobin. The Journal of Biological Chemistry. 1976;251:2788–94. [PubMed] [Google Scholar]
- [40].Gladwin MT, Wang X, Reiter CD, Yang BK, Vivas EX, Bonaventura C, et al. S-Nitrosohemoglobin is unstable in the reductive erythrocyte environment and lacks O2/NO-linked allosteric function. The Journal of Biological Chemistry. 2002;277:27818–28. doi: 10.1074/jbc.M203236200. [DOI] [PubMed] [Google Scholar]
- [41].Hausladen A, Rafikov R, Angelo M, Singel DJ, Nudler E, Stamler JS. Assessment of nitric oxide signals by triiodide chemiluminescence. Proc Natl Acad Sci U S A. 2007;104:2157–62. doi: 10.1073/pnas.0611191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].McMahon TJ, Ahearn GS, Moya MP, Gow AJ, Huang YC, Luchsinger BP, et al. A nitric oxide processing defect of red blood cells created by hypoxia: deficiency of S-nitrosohemoglobin in pulmonary hypertension. Proc Natl Acad Sci U S A. 2005;102:14801–6. doi: 10.1073/pnas.0506957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].McCaffrey MJ, Bose CL, Reiter PD, Stiles AD. Effect of L-arginine infusion on infants with persistent pulmonary hypertension of the newborn. Biol Neonate. 1995;67:240–3. doi: 10.1159/000244170. [DOI] [PubMed] [Google Scholar]
- [44].Castillo L, DeRojas-Walker T, Yu YM, Sanchez M, Chapman TE, Shannon D, et al. Whole body arginine metabolism and nitric oxide synthesis in newborns with persistent pulmonary hypertension. Pediatr Res. 1995;38:17–24. doi: 10.1203/00006450-199507000-00004. [DOI] [PubMed] [Google Scholar]
- [45].Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. Journal of American Medical Association. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]