Abstract
Although the incidence of human monkeypox has greatly increased in Central Africa over the last decade, resources for surveillance remain extremely limited. We conducted a geospatial analysis using existing data to better inform future surveillance efforts. Using active surveillance data collected between 2005 and 2007, we identified locations in Sankuru district, Democratic Republic of Congo (DRC) where there have been one or more cases of human monkeypox. To assess what taxa constitute the main reservoirs of monkeypox, we tested whether human cases were associated with (i) rope squirrels (Funisciurus sp.), which were implicated in monkeypox outbreaks elsewhere in the DRC in the 1980s, or (ii) terrestrial rodents in the genera Cricetomys and Graphiurus, which are believed to be monkeypox reservoirs in West Africa. Results suggest that the best predictors of human monkeypox cases are proximity to dense forests and associated habitat preferred by rope squirrels. The risk of contracting monkeypox is significantly greater near sites predicted to be habitable for squirrels (OR = 1.32; 95% CI 1.08–1.63). We recommend that semi-deciduous rainforests with oil-palm, the rope squirrel’s main food source, be prioritized for monitoring.
Electronic supplementary material
The online version of this article (doi:10.1007/s10393-010-0355-5) contains supplementary material, which is available to authorized users.
Keywords: monkeypox, orthopoxvirus, smallpox vaccination, epidemiology, active surveillance, human transmission
Introduction
Monkeypox virus (Family, Poxviridae: genus, Orthopoxvirus) is a zoonotic DNA virus that causes serious smallpox-like illness in humans and has an estimated 10% mortality rate (Jezek and Fenner, 1988). The World Health Organization (WHO) has designated monkeypox virus as the most important poxvirus that infects humans since smallpox was declared eradicated in 1980 (Jezek et al., 1987). Humans can contract monkeypox through direct contact with infected wildlife or other humans (Khodakevich et al., 1987a). From the time when it was first discovered in humans in 1970, most cases of monkeypox were reported in the Democratic Republic of Congo (DRC; formerly Zaire). The virus may have increased its natural geographic distribution substantially, with human cases recently reported in the Republic of Congo and Sudan, though distinguishing monkeypox spread from underreporting remains difficult due to inadequate surveillance. Factors that complicate monkeypox surveillance in Central Africa include logistic difficulties in reaching cases in remote locations and decreased case reporting by health care providers during recent years (Rimoin et al., 2010). As a result of a human-assisted exportation event, 71 people in the US were infected in a multi-state outbreak caused by rodents imported as pets from West Africa in 2003 (Reed et al., 2004). Currently, there is no high resolution distribution map showing the predicted occurrence of monkeypox. Because human-to-human transmission appears to be limited, most monkeypox cases are closely associated with spillover transmission from animal reservoirs, so the geographic range of human monkeypox will be influenced strongly by the preferred habitat of the reservoir species.
Here we conduct a geospatial analysis of surveillance data to assess whether terrestrial or arboreal rodents are more important as reservoirs of monkeypox virus. The former were implicated in the US outbreak, whereas the latter tested positive for monkeypox in northern Zaire in the 1980s (see below). In addition, we construct a high resolution (1 km2) map of the ecological niche of monkeypox virus in Sankuru district, DRC. Previous niche models of the virus (Levine et al., 2007) did not examine reservoirs and covered a larger area of Africa at a coarser scale (100 km2). Finer-scale maps (1 km2) of Sankuru can better assist public health decision-makers in designing future monkeypox surveillance and control efforts.
Like all infectious diseases, human monkeypox cases arise from the interaction of the three corners of the epidemiological triangle: the agent, host, and environment (Supplementary Material Fig. 3; Lilienfeld and Stolley, 1994; in the triangle metaphor, disease vectors are considered part of the environment). Although the human hosts of the virus are the subject of ongoing field studies, the lack of roads, delays in the reporting of suspected cases, and the lasting effects of past and ongoing armed conflict in Central Africa make monitoring the human disease challenging. To complement and support these efforts, our study focuses on the environmental corner of the epidemiological triangle (note that because we focus on human monkeypox, nonhuman reservoirs are treated as part of the environment). We use satellite-based remote sensing, which provides biophysical data systematically over inaccessible geographic areas (Jensen, 2007), to analyze the association between human monkeypox cases and aspects of the biological and physical environment. The biological aspects of the environment examined in our study are human population density, reservoir occurrences, and vegetation. Furthermore, we consider two aspects of the physical/nonbiological environment, climate and topography.
In addition to biotic and abiotic factors, the geographic area occupied by a pathogen is controlled by its migration capacity (Soberon and Peterson, 2005; Soberon, 2007). Including migration capacity into a niche model requires developing a biogeographical hypothesis; for example, in a study carried out at the national scale, it might be postulated that the species is unlikely to colonize areas outside of the provinces where the species has historically been recorded (Soberon, 2010). Although our study analyzes a single province and does not formulate a biogeographic hypothesis about the migration of monkeypox, we acknowledge that extending the modeling approach utilized here to incorporate migration hypotheses would be important for estimating the distribution of monkeypox throughout Central Africa.
Fundamental to predicting the spatial distribution of human cases is an understanding of reservoir species. On the one hand, ecological studies and serologic testing in Zaire in the 1980s indicated that the virus circulates in arboreal rodents and nonhuman primates, but detected no antibodies against monkeypox virus in terrestrial rodents (reviewed in Jezek and Fenner, 1988). On the other hand, these studies were conducted 400 km north of Sankuru in Equateur province, so the extent to which their findings apply to Sankuru is unclear. Moreover, during the US outbreak, terrestrial and arboreal rodents imported from Ghana both tested positive for monkeypox virus. These findings led us to examine whether terrestrial rodents or arboreal rodents in Sankuru are important reservoirs. We analyzed the four taxa that were monkeypox-positive in the US outbreak: African dormice (two species), giant pouched rats, and rope squirrels. (Dormice and pouched rats are terrestrial rodents, but rope squirrels are arboreal.) For each taxon, we constructed a model of its potential habitat in Sankuru (see Supplementary Material Section 1.4). Next, we assessed whether human monkeypox cases in Sankuru depended on climate, because previous work has found a relationship between precipitation and monkeypox occurrences (Levine et al., 2007). Finally, we used regression analysis to quantify the extent to which the reservoir distributions and other ecological variables explained the occurrence of human monkeypox cases. To test the robustness of this approach, we repeated the analysis using a nonparametric technique based on fundamentally different assumptions.
The overarching goal of this study is to use remote sensing and epidemiological surveillance data to identify ecological risk factors for contracting human monkeypox. A novel contribution of our analysis is that it reveals the possible reservoir taxa for predicting human monkeypox cases. By quantifying the relative importance of different rodent reservoirs, our results help delineate the current spatial extent of the monkeypox virus and help predict where it may spread in the future. Our analysis yields two tangible products that will help public health authorities monitor monkeypox more effectively: (i) a spatial model that estimates the probability of occurrence of human monkeypox across the Sankuru district at the 1 km2 resolution, and (ii) an assessment of which environmental variables are statistically significant for predicting monkeypox cases. Although our study found that vegetation variables and mammalian reservoirs were more important for determining the distribution of the monkeypox than climate, in general, the role of climate is likely to depend on the spatial scale of the analysis. Furthermore, although we did not detect an association between monkeypox and climate at a fine spatial scale, the distributions of other pathogens in the DRC, such as plague, are affected by climate at a fine spatial resolution (Eisen et al., 2010).
Methods
Study Region
We analyzed the distribution of monkeypox virus in Sankuru district, Kasai-Oriental Province, DRC where the most comprehensive surveillance data is currently available. Sankuru is located in the Cuvette Centrale of the Congo River, site of one of the world’s largest rainforest tracts, between the Sankuru and Lomami Rivers (Fig. 1; Bwangoy et al., 2010). The habitat is composed of closed-canopy, semi-deciduous and evergreen forest (Fig. 2; Arino et al., 2008). Sankuru has a total area of 105,378 km2 and a human population of approximately one million (Vijayaraj et al., 2007). The economy of rural Sankuru is largely based on agriculture and hunting, resulting in close contact between people and wildlife. The majority of settlements are surrounded by agricultural areas and are 3 to 5 km from the forest (Jezek and Fenner, 1988). Most protein is obtained from bushmeat, principally monkeys and rodents (Colyn et al., 1987).
Figure 1.
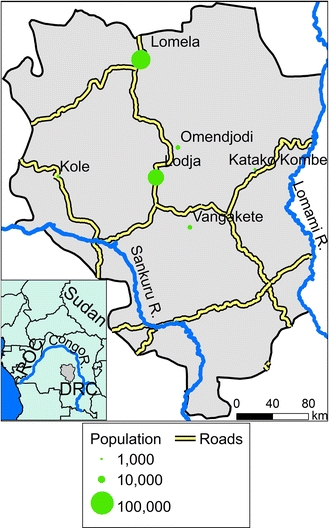
Sankuru district, Democratic Republic of Congo. Main panel: Sankuru’s major towns and roads. Inset: The regional context of monkeypox epidemics in Central Africa. Since 1970, the largest number of human monkeypox cases has been reported in the Congo River basin in the Democratic Republic of Congo (DRC), but monkeypox has recently been identified in the Republic of Congo (ROC) and Sudan. The gray polygon in the central DRC is the Sankuru district
Figure 2.
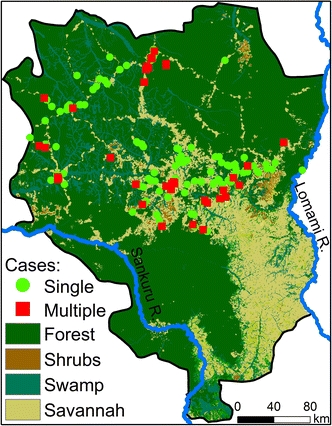
Georeferenced human monkeypox cases in Sankuru (2005–2007). Cases occur predominantly in human settlements located near closed-canopy, semi-deciduous or evergreen forest. These data represent the cases detected through active surveillance for which latitudes and longitudes were available. There are 156 sites, at the 1 km2 scale, with at least one confirmed case of human monkeypox. This spatial resolution was chosen so that our analysis would approximate the scale of individual villages, but the processing of the satellite images would remain computationally tractable. Such processing becomes more difficult with an increasingly fine scale
Human Monkeypox Samples
Active surveillance was conducted from November 2005 to December 2007 in collaboration with DRC Ministry of Health and local health workers. Trained field teams monitored the district’s Health Zones and examined suspected cases of human monkeypox, defined as fever followed by papulovesicular rash. For each suspected case, at least two (and in most instances, three) types of data were collected. First, biological samples were collected from suspected cases by conducting a physical exam, during which scabs, vesicle fluid, and blood were acquired. Second, an extensive questionnaire was used to collect clinical and epidemiological data, as well as information about animal exposure (data not shown). Third, we recorded Geographic Information Systems (GIS) data on the latitude and longitude of most suspected cases (where the infected person resided). Our analysis does not distinguish human-to-human from wildlife-to-human transmission. Samples were stored at -20°C and sent to the Bundeswehr Institute of Microbiology and the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) for laboratory diagnosis following established protocols. These protocols are published elsewhere (Rimoin et al., 2007).
Human monkeypox cases were confirmed at 156 geographic sites in Sankuru. The number of confirmed cases was greater than 156, but our GIS model required aggregating the data to the 1 km2 scale. In some instances, this resulted in distinct cases being treated as a single geographic site for modeling purposes. (This study analyzes the occurrence or nonoccurrence of monkeypox, rather than the number of monkeypox cases per site.) Additionally, a small number of suspected cases lacked GIS data. Given the incomplete GIS information, not all reported cases could be assigned exact point coordinates. Thus, our analysis included 201 confirmed human monkeypox cases at 156 geographic sites.
Environmental Variables
We used four types of environmental factors to predict cases of human monkeypox: climate (n = 19 variables), vegetation (n = 7), human population density, and habitat suitability for rodent reservoirs of monkeypox (n = 4). The climate variables consisted of temperature and precipitation, including both the annual mean and measures of seasonal variation (Supplementary Material Table 4, and Supplementary Material Section 1.1 and references therein). Our climate data were based on interpolating from weather stations. We confirmed that the number of stations did not bias the predictions by verifying that our climate data correlate with satellite-based precipitation estimates, which do not depend on weather stations on the ground (Pearson’s r = 0.74, P < 2.2 × 10−16). The vegetation variables represent tree density and the roughness of the canopy (Supplementary Material Table 5, and Supplementary Material Section 1.3). Sections 1.2 and 1.4 of the Supplementary Material describe the population density data and reservoir models.
Data Reduction
Our overall goal was to construct a regression model for predicting the probability of human monkeypox at each 1 km2 site in Sankuru. However, the 31 environmental variables were highly correlated, which can lead to inaccurate maximum likelihood estimates of the parameters of a regression model (Aguilera et al., 2006). To address this, we converted the 31 original variables to uncorrelated principal components (hereafter “PCs,” see Fig. 3; Wilks, 1995). We decided how many of the PCs to retain, as candidate variables for the regression analysis, by calculating the number of components that cumulatively explained at least 70% of the variance in original variables, and by constructing a scree plot (Everitt, 2005). Both approaches gave the same result.
Figure 3.
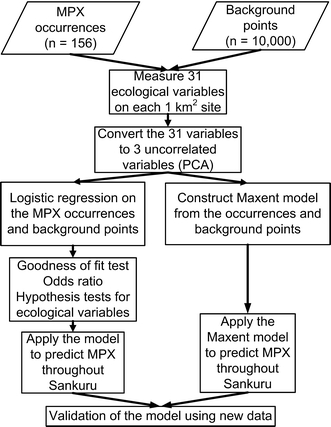
Stages of the analysis. The ecological variables were measured by analyzing satellite images of Sankuru (for an overview of this methodology, see Jensen, 2007). Section 1.7 of the Supplementary Material describes the model validation. MPX, monkeypox; PCA, principal components analysis
In particular, the PC analysis simplified the original 31 predictor variables into three uncorrelated variables that collectively explained 71.2% of the variance in the original 31 (Table 1; Supplementary Material Figs. 1 and 2). (Utilizing additional PCs that explained more than 99% of the variance did not improve the fit of the model to our data [χ2 = 24.77, df = 16, P > 0.05] but such an approach has been useful in other niche modeling studies [Peterson et al., 2008]). We interpret the first PC, which explained 45% of the variance in the 31 original variables, as precipitation in lowlands that contain habitat for the two terrestrial African dormouse species. This is because the first PC assigns positive weights to lower elevation sites that have high precipitation, as well as a high probability of being in the dormouse’s ecological niche (since the two dormice are roughly collinear, this PC is a measure of both species). Exploratory data analysis indicated that analyzing each dormouse separately yielded similar results (see Supplementary Material Section 1.5). The second PC (variance explained: 14.8%) represents forest density and habitat suitability for arboreal rope squirrels. Thus, PC1 and PC2 represent both habitat suitability for monkeypox reservoirs and other ecological variables, such as climate and vegetation. The third PC is primarily a measure of temperature (variance explained: 11.4%). None of the PCs that we retained assigned a large positive weight to the giant pouched rat, another terrestrial species. Thus, the subsequent analysis of the first three PCs only considers two taxa, dormice and rope squirrels. However, this does not appear to be a serious shortcoming because the pouched rat is less important for explaining human monkeypox cases than the rope squirrel (see “Ecological Determinants of Human Monkeypox Cases” in the Results section). Although PC1 explained the most variance in the original environmental variables (45%), PC1 did not emerge as the most important predictor of monkeypox occurrences (see below).
Table 1.
Variables Used to Predict Human Monkeypoxa
| Principal component | % Variation explained | Variables with large positive weights | Variables with large negative weights |
|---|---|---|---|
| Precipitation in lowlands with African dormice | 45 | Total annual precipitation | Temperature diurnal range |
| Precipitation of the wettest month | Temperature annual range | ||
| Precipitation of the driest month | Precipitation seasonality | ||
| Precipitation of the driest quarter | Elevation | ||
| Precipitation of the coldest quarter | |||
| Probability that the site is in niche of the African dormouse (G. crassicaudatus) | |||
| Density of evergreen forest with rope squirrels | 14.8 | Percent tree cover | Standard deviation of scatterometer backscatter |
| Mean scatterometer backscatter | |||
| Maximum leaf area index | |||
| Maximum NDVI | |||
| Mean wet season NDVI | |||
| Probability that the site is in the niche of the rope squirrel | |||
| Temperature | 11.4 | Annual mean temperature | None |
| Maximum temperature of the warmest month | |||
| Mean temperature of the wettest quarter | |||
| Mean temperature of the driest quarter | |||
| Mean temperature of the warmest quarter | |||
| Mean temperature of the coldest quarter |
NDVI, normalized difference vegetation index
aThree uncorrelated variables constructed through principal components analysis
Statistical Models for Predicting Human Monkeypox
To ensure that our results were robust to the assumptions underlying our statistical analyses, two distinct statistical models were used to estimate the probability of human monkeypox occurrence throughout Sankuru: logistic regression and Maxent. In both models, the dependent variable was the occurrence (see “Human Monkeypox Samples” in Methods section) or nonoccurrence of human monkeypox. Following established modeling practices (Elith et al., 2006), we selected 10,000 sites at random throughout Sankuru to serve as pseudo-absences (i.e., assumed nonoccurrences). In both models, the independent variable was PC2 (see below).
In the initial multivariate logistic regression model (Jewell, 2004), the independent variables were the three PCs (Table 1). Next, we tested if using fewer than three independent variables would result in a more parsimonious model. We identified the best model using stepwise variable selection, which is a hybrid of forward and backward selection (Montgomery et al., 2006). The use of a distinct approach, the selection of the model that minimized Akaike’s information criterion (AIC), yielded the same results. We assessed the importance of the PCs by computing the Akaike weight for each one, which provides a ranking in terms of how important the PC is for predicting monkeypox.
Since logistic regression assumes that the dependent variable represents occurrences and true absences but our data consisted of monkeypox occurrences and pseudo-absences, we repeated the analysis using Maxent, which only requires occurrence and pseudo-absence data (Phillips and Dudik, 2008). We used logistic regression, in addition to Maxent, because the latter does not provide hypothesis tests to assess the significance of the predictor variables or χ2 statistics that can be used for stepwise selection. Thus, we compared a method that may be more familiar to epidemiologists, and that has more developed tests of variable importance (logistic regression), to a newer ecological niche modeling technique with limited tests of variable importance (Maxent). A potential benefit of using newer ecological niche modeling methods is that they can accommodate more complex patterns in the response of a reservoir to heterogeneities in the landscape (reviewed in Peterson, 2006).
Results
Ecological Niches of Monkeypox Reservoirs
The models of the distributions of the four monkeypox reservoirs were accurate when validated by calculating the area under the receiver operating curve (AUC) on a withheld subset of 25% of the occurrence data (Table 2). The AUC is the probability that our model will correctly classify a site as suitable or unsuitable habitat for a reservoir when the model is tested on new data (Rosner, 2006; McPherson and Jetz, 2007). However, these results should be interpreted cautiously because the manner in which Maxent calculates the AUC includes areas of receiver operating characteristic space that are not directly relevant to niche modeling (Lobo et al., 2008; Peterson et al., 2008). Furthermore, our models of dormice habitat were highly concordant with published models (Supplementary Material Fig. 5; Holden and Levine, 2009). Land cover was the most important variable for determining rope squirrel habitat (Supplementary Material Table 3). We validated the rope squirrel niche model in two ways. First, we constructed a new model based on four new rope squirrel occurrences in Sankuru, from the Global Biodiversity Information Facility database, using the technique of Peterson et al. (2006) for predicting disease distributions with little data. The null hypothesis of no agreement between the initial rope squirrel model and the new model was rejected (χ 2 = 21332.93, df = 4, P < 0.0001), suggesting that the initial model was accurate. Second, we implemented a small sample size test (Pearson et al., 2007). The null hypothesis that the rope squirrel model performed no better than random was rejected (P = 1.83 × 10−4).
Table 2.
Rodent Reservoir Data Used to Predict Human Monkeypoxa,b
| Reservoir species or genus | Common name | Records | AUC |
|---|---|---|---|
| Cricetomys species | Giant pouched rat | 21 | 0.808 |
| Graphiurus crassicaudatus | African dormouse | 32 | 0.934 |
| Graphiurus lorraineus | African dormouse | 26 | 0.924 |
| Funisciurus species | Rope squirrel | 21 | 0.856 |
AUC, area under the receiver operating curve
aFour variables
bThe predictive accuracy of the reservoir models was assessed by computing the AUC for each reservoir. The AUC ranges from 0 to 1, with 0.5 indicating a random prediction. Species distribution models with an AUC greater than 0.7 are considered useful, and those with an AUC greater than 0.9 are considered excellent (McPherson and Jetz, 2007). The AUC was calculated on a withheld test set comprised of 25% of the data for each reservoir
Ecological Determinants of Human Monkeypox Cases
The most important ecological variables for determining the ecological niche of the monkeypox virus were forest vegetation and the presence of the rope squirrel. Both the stepwise procedure and the AIC analysis indicated that the best logistic regression model consisted of only one predictor variable, PC2, which represents the density of forest containing rope squirrel habitat (Supplementary Material Table 1). The fit of the best model to the data was highly significant (Hosmer–Lemeshow χ2 = 38.17, P < 0.0001). Forest with rope squirrel habitat was also the major ecological driver of monkeypox occurrences, according to Maxent’s nonparametric tests of variable importance. PC2 was the most important variable based on the relative contribution to the Maxent model (81.9%). Furthermore, removing PC2 from the Maxent model decreased the accuracy of the model more than removing any other variable. Finally, a Maxent model comprised of only PC2 was more accurate than a model comprised of any other single variable.
We further validated the regression model by separating the data into a test and training set, and measuring the absolute deviation between the observed and predicted probabilities. The error of the regression model was, at most, 7% when we repeated this procedure 1,000 times (Supplementary Material Section 1.6). We also carried out spatial subdivision of the data into test and training sets to account for spatial autocorrelation, which involves using points in the on-diagonal quadrants to train the model and using the off-diagonal quadrants to test the model (for details about spatial subsetting, see Peterson et al., 2007; Williams et al., 2008). Models trained on the on-diagonal sites predicted monkeypox occurrences in the off-diagonal sites significantly better than expected at random (one-tailed binomial tests, P < 0.001). We also validated the Maxent model by carrying out four replicate analyses each with a unique set of testing localities. The mean AUC was excellent (0.924) and variance around the mean was small (4.4 × 10−4), suggesting that the Maxent models of monkeypox in Sankuru are robust.
The null hypothesis of no effect of forest density and squirrel habitat suitability on human monkeypox cases was rejected (Wald χ2 = 7.2, P = 0.0073). In particular, the odds of contracting human monkeypox are 32% greater near dense forests with rope squirrels in Sankuru than in nonforested sites (OR = 1.32; 95% CI 1.08–1.63). PC1 (precipitation in lowlands) and PC3 (temperature) were not significant for explaining human monkeypox, because they did not meet the P = 0.05 threshold for inclusion in the regression model. PC2 had an Akaike weight of 0.8, which indicates that there is strong evidence that forest density and rope squirrel habitat suitability are important. Moreover, the weight of evidence in support of PC2 was twice as large as the weight of any other PC (Supplementary Material Table 2).
Spatial Distribution of Risk
According to the logistic regression model, sites in the ecological niche of the monkeypox virus are located predominantly in northern and western Sankuru in the vicinity of primary forest (Figs. 4 and 5a). For example, the probability of human monkeypox is predicted to be high in forest-dominated subdistricts, such as Kole and Lomela (Fig. 5b). Conversely, we predict fewer human monkeypox cases in the subdistricts of Katako Kombe and Lubefu, which have extensive savannah and less habitat for forest-dwelling rope squirrels. The risk of monkeypox outbreaks appears to be greatest in central Sankuru, insofar as this area has the highest human population density as well as the greatest number of sites that are predicted to be in the virus’ ecological niche (Fig. 5c). The use of pseudo-absences did not appear to have a strong influence on our predictions about the spatial distribution of monkeypox in Sankuru. When we repeated the analysis using Maxent, a method that is designed to accommodate pseudo-absences, there was significant similarity between the resulting maps and the predictions based on logistic regression (weighted kappa test: Z = 167.8, P < 0.0001; Supplementary Material Section 1.7).
Figure 4.
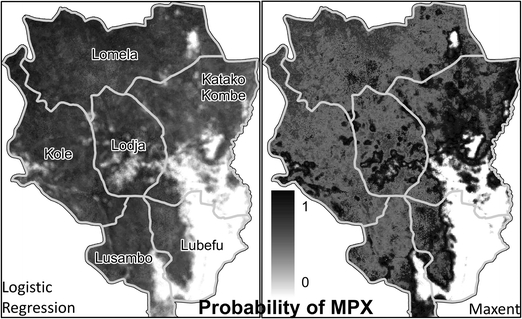
Different modeling approaches provide similar predictions about the geographic distribution of human monkeypox (MPX) in Sankuru. The polygons outlined in gray are subdistricts within Sankuru. Section 1.7 of the Supplementary Material describes how we quantified the degree of similarity between logistic regression and Maxent
Figure 5.
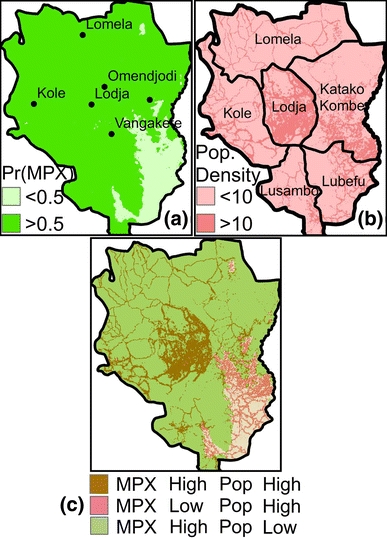
Risk maps of human monkeypox in Sankuru. (a) Probability that sites in Sankuru are in the ecological niche of monkeypox (MPX), according to the logistic regression model. Figure 4 shows the predicted probabilities prior to the application of the 0.5 threshold. (b) Human population per km2. The polygons outlined in black are subdistricts within Sankuru. (c) Human population density overlaid on ecological suitability for the virus. “MPX High” means the probability of an MPX case is estimated to be greater than 0.5. Sites with human population densities greater than 10 people/km2 are labeled “Pop High.” Defining “High” and “Low” using other thresholds gave similar results
Discussion
Evaluation of the Importance of Rope Squirrels As Potential Monkeypox Reservoirs
This study began with the broad goal of using remote sensing and surveillance data to identify ecological risk factors for monkeypox. After the data reduction stage of the analysis, we focused more narrowly on whether an arboreal rodent, the rope squirrel, was a more important monkeypox reservoir in Sankuru than terrestrial rodents (African dormice). Our results support the hypothesis that the risk of human monkeypox is significantly greater in forests that contain suitable habitat for the rope squirrel. Our finding that the presence of rope squirrels is correlated with virus spillover to humans in Sankuru is consistent with the fact that rope squirrels are the only natural host that have been demonstrated to transmit the monkeypox virus directly to humans in Africa (reviewed in Essbauer et al., 2010). Squirrels are abundant near villages in the rural DRC, constituting about 23% of the total number of game animals hunted in the vicinity of settlements where monkeypox was reported in the 1980s (Khodakevich et al., 1987a; Jezek and Fenner, 1988). Since rope squirrels are among the most frequently consumed bushmeat species in rural areas of the DRC (Colyn et al., 1987), and the virus can survive in the organs of dead rope squirrels for at least 7 h at ambient temperature (Khodakevich et al., 1987b), there are many opportunities for villagers to acquire the virus through contact with rope squirrels. Rope squirrels may be a particularly potent source of monkeypox infections because they occupy areas frequented by older children who are unvaccinated against smallpox.
Several pieces of evidence suggest that the monkeypox virus could be sustained in rural Sankuru by circulating in rope squirrel populations. In 1985, the monkeypox virus was isolated from a wild rope squirrel in Zaire in a forested area near Sankuru. This squirrel was in the active stage of the illness and had poxvirus skin eruptions (Khodakevich et al., 1986). Rope squirrels also had the highest seroprevalence among species studied in the 1980s (Khodakevich et al., 1987a). Moreover, rope squirrels can transmit the virus to one another via respiratory droplets, vomit, and feces (Khodakevich et al., 1988). Additionally, since the monkeypox virus is not excreted for a prolonged time after infection, and does not persist in tissues for more than 4 weeks, the maintenance of the virus in nature requires an abundant and iteroparous reservoir (Khodakevich et al., 1987b; Jezek and Fenner, 1988). Rope squirrels have these life history traits, reaching densities of up to 500 individuals per square kilometer in the lower stratum of semi-deciduous forests near human settlements in rural areas of the DRC (Khodakevich et al., 1987a).
A limitation of this study is that we analyzed a relatively small number of candidate reservoir species. For example, we did not include nonhuman primates or sun squirrels in the genus Heliosciurus. Both of these taxa showed evidence of past infection with poxvirus during serological surveys in Zaire in the 1980s (Khodakevich et al., 1987a, 1988; Jezek and Fenner, 1988). However, these studies found seroprevalence to monkeypox virus in nonhuman primates to be an order of magnitude less than in rope squirrels, which suggests that these primates could be an occasional source of infection for humans but are not likely to be the sole reservoir in which the virus circulates continuously. Additionally, the reported seroprevalence rates for rope squirrels were greater than those for sun squirrels. Thus, although examining other small mammal species might identify additional reservoirs as important, we do not expect the analysis of a more comprehensive set of taxa to invalidate our findings about the importance of rope squirrels in Sankuru.
Seroprevalence studies of local animals suggest that numerous animal species have antibodies to monkeypox virus, suggesting that there may be more than one reservoir species implicated in the transmission cycle (Jezek and Fenner, 1988). Investigation of other wildlife species that share habitat with rope squirrels is an important avenue for future research. When assessing the importance of potential monkeypox reservoirs, we only considered rodents that tested positive for monkeypox during the US outbreak in 2003 or in Zaire in the 1980s. A more comprehensive approach, which we intend to pursue in future research, would be to consider mammals that have tested positive, and mammals that have not been tested but could possibly be important monkeypox reservoirs because their distributions coincide with the distribution of African poxviruses (Peterson et al., 2004). This comprehensive approach could be used to prioritize mammal clades to be tested in future trapping expeditions. Moreover, although our study supports the role of rope squirrels as monkeypox reservoirs in the DRC, this analysis is not incompatible with the hypothesis that terrestrial rodents are the main reservoir of the virus in West Africa.
Assessment of the Role of Climate in Determining Human Monkeypox Cases
Previous work analyzed the effect of climate on the distribution of monkeypox at the continental scale and utilized a spatial resolution of 100 km2 (Levine et al., 2007). At this spatial scale, Levine et al. (2007) found climate to be a significant predictor of monkeypox occurrences (though they did not assert that climate was more important than other ecological variables). On other hand, the hypothesis that human monkeypox occurrences depend primarily on climatic variables is not supported by our data for Sankuru, to the extent that our best model did not include either precipitation or temperature as predictors of human monkeypox cases. However, our conclusions and those of Levine et al. (2007) are not incompatible, insofar as the two studies utilized different spatial grain sizes and extents. For example, climate may be an important driver of monkeypox outbreaks at the continental scale but have limited predictive power at the district scale. Indeed, the present study and that of Levine et al. (2007) are complementary, in that the latter characterizes the distribution of monkeypox across Africa rather than making detailed predictions within the DRC, whereas our study only examines Sankuru, DRC, but at a spatial scale of 1 km2, which allows our analysis to get closer to the mechanism of monkeypox outbreaks and the spatial scale of individual villages.
We reduced the 31 original variables to three uncorrelated PCs but did not account for different functional groups within the variables. An alternative approach would be to construct one PC to represent each functional group (for example, one temperature PC, one precipitation PC, etc.). This approach would have made it easier to interpret the PCs and may have provided a more straightforward assessment of the contribution of climate to the distribution of monkeypox. However, we opted not to pursue this approach because it would not ensure that the temperature PC is uncorrelated with the precipitation PC. Our logistic regression required the explanatory variables to be uncorrelated in order to test their statistical significance.
Implications for Monkeypox Surveillance and Control
The spatial modeling approach developed herein has the potential to help public health decision-makers effectively utilize the limited resources available for monkeypox surveillance and control. Our study implies that rope squirrels should be assigned greater priority for monitoring in the DRC than the other taxa considered in the regression model, African dormice and the pouched rat. Rope squirrels were also the most important reservoirs when eight other potential reservoirs were analyzed, including species not considered here, such as pangolins, porcupines, and nonhuman primates (H. A. Thomassen, personal communication). Additionally, our results suggest that surveillance activities should be renewed or intensified in semi-deciduous forests in Sankuru because they contain oil-palm (Elaeis guineensis), which is an important food source for the rope squirrel (Khodakevich et al., 1987a, b, 1988). Renewed surveillance efforts could confirm that the rope squirrel, which was implicated as a reservoir in the 1980s, remains important today. However, the design of effective monkeypox control measures in Sankuru will require additional animal sampling to validate that rope squirrels are a reservoir and disconfirm that other mammals in the same habitat are equally important reservoirs. If future field work confirms that the Funisciurus squirrels are the most important monkeypox reservoir in Sankuru, then monkeypox control measures that could be explored include distributing educational materials to increase residents’ awareness of pox lesions on animals and to encourage avoidance of sick wildlife. Further work is required to understand how ecological factors, reservoir population dynamics, and human behavior combine to influence the risk of cross-species transmission (Lloyd-Smith et al., 2009).
We focused our analysis on Sankuru because that is the only DRC district that has had recent active surveillance, and hence is the only region for which sufficiently detailed data are available. However, our approach is generalizable and could be implemented to yield testable predictions about human monkeypox in a larger area. For example, the regression coefficients that we estimated for Sankuru could be applied to remote-sensed data for the entire Congo Basin, and the resulting predictions could be validated via future field sampling, though, as noted in the Introduction, this would require developing hypotheses about the capacity of the virus to migrate.
Conclusions
Proximity to dense forest with rope squirrels is a highly significant risk factor for human monkeypox in Sankuru district, DRC, greatly increasing the odds of monkeypox occurrence. This is compatible with the finding that the rope squirrel was the most important monkeypox reservoir in northern Zaire in the 1980s (reviewed in Jezek and Fenner, 1988). We recommend further targeted studies to confirm our prediction that the rope squirrel remains an important monkeypox reservoir in Sankuru today. Future surveillance could prioritize oil-palm forests that are rope squirrel habitat for increased monitoring, and should measure poxvirus seroprevalence in other species that share the same habitat.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was made possible by the generous support of the Faucett Family Foundation. We respectfully thank the DRC Ministry of Health and local health workers who were responsible for specimen collection and case investigation. Additional support for this study was provided by the National Institutes of Health, National Institute of Child Health and Human Development, Bethesda, MD, USA, by the joint National Science Foundation–National Institutes of Health Ecology of Infectious Diseases Program (grant number EF-0430146), by the RAPIDD program of the Science and Technology Directorate, Department of Homeland Security, by the Fogarty International Center, National Institutes of Health, and by the National Institute of Allergy and Infectious Diseases (grant number EID-1R01AI074059-01). We thank two anonymous reviewers for comments that improved the manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Aguilera AM, Escabias M, Valderrama MJ. Using principal components for estimating logistic regression with high-dimensional multicollinear data. Computational Statistics & Data Analysis. 2006;50:1905–1924. doi: 10.1016/j.csda.2005.03.011. [DOI] [Google Scholar]
- Arino O, Bicheron P, Achard F, Latham J, Witt R, Weber J-L. GlobCover: the most detailed portrait of Earth. European Space Agency Bulletin. 2008;136:25–31. [Google Scholar]
- Bwangoy JRB, Hansen MC, Roy DP, De Grandi G, Justice CO. Wetland mapping in the Congo Basin using optical and radar remotely sensed data and derived topographical indices. Remote Sensing of Environment. 2010;114:73–86. doi: 10.1016/j.rse.2009.08.004. [DOI] [Google Scholar]
- Colyn MM, Dudu A, Mbaelele MMM. Exploitation du petit et moyen gibier des forets ombrophiles du Zaire. Nature et Faune. 1987;3:22–34. [Google Scholar]
- Eisen RJ, Griffith KS, Borchert JN, MacMillan K, Apangu T, Owor N, et al. Assessing human risk of exposure to plague bacteria in northwestern Uganda based on remotely sensed predictors. American Journal of Tropical Medicine and Hygiene. 2010;82:904–911. doi: 10.4269/ajtmh.2010.09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elith J, Graham CH, Anderson RP, Dudik M, Ferrier S, Guisan A, et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography. 2006;29:129–151. doi: 10.1111/j.2006.0906-7590.04596.x. [DOI] [Google Scholar]
- Essbauer S, Pfeffer M, Meyer H. Zoonotic poxviruses. Veterinary Microbiology. 2010;140:229–236. doi: 10.1016/j.vetmic.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BS. An R and S-PLUS Companion to Multivariate Analysis. Berlin: Springer; 2005. [Google Scholar]
- Holden ME, Levine RS. Systematic revision of Sub-Saharan African dormice (Rodentia: Gliridae: Graphiurus) Part II. Bulletin of the American Museum of Natural History. 2009;331:314–355. doi: 10.1206/582-9.1. [DOI] [Google Scholar]
- Jensen JR. Remote Sensing of the Environment: An Earth Resource Perspective. 2. Upper Saddle River, NJ: Pearson/Prentice Hall; 2007. [Google Scholar]
- Jewell NP. Statistics for Epidemiology. Boca Raton, FL: Chapman & Hall/CRC; 2004. [Google Scholar]
- Jezek Z, Fenner F. Human Monkeypox. Monographs in Virology. Basel, Switzerland: Karger; 1988. [Google Scholar]
- Jezek Z, Nakano JH, Arita I, Mutombo M, Szczeniowski M, Dunn C. Serological survey for human monkeypox infections in a selected population in Zaire. Journal of Tropical Medicine and Hygiene. 1987;90:31–38. [PubMed] [Google Scholar]
- Khodakevich L, Jezek Z, Kinzanzka K. Isolation of monkeypox virus from wild squirrel infected in nature. Lancet. 1986;1:98–99. doi: 10.1016/S0140-6736(86)90748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodakevich L, Jezek Z, Messinger D. Monkeypox virus: ecology and public health significance. Bulletin of the World Health Organization. 1988;66:747–752. [PMC free article] [PubMed] [Google Scholar]
- Khodakevich L, Szczeniowski M, Nambu-ma-Disu, Jezek Z, Marennikova S, Nakano J, et al. (1987a) The role of squirrels in sustaining monkeypox virus transmission. Tropical and Geographical Medicine 39:115–122 [PubMed]
- Khodakevich L, Szczeniowski M, Nambu-ma-Disu, Jezek Z, Marennikova S, Nakano J, et al. (1987b) Monkeypox virus in relation to the ecological features surrounding human-settlements in Bumba Zone, Zaire. Tropical and Geographical Medicine 39:56–63 [PubMed]
- Levine RS, Peterson AT, Yorita KL, Carroll D, Damon IK, Reynolds MG. Ecological niche and geographic distribution of human monkeypox in Africa. PLoS ONE. 2007;2:e176. doi: 10.1371/journal.pone.0000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienfeld DE, Stolley PD. Foundations of Epidemiology. 3. New York: Oxford University Press; 1994. [Google Scholar]
- Lloyd-Smith JO, George D, Pepin KM, Pitzer VE, Pulliam JRC, Dobson AP, et al. Epidemic dynamics at the human–animal interface. Science. 2009;326:1362–1367. doi: 10.1126/science.1177345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo JM, Jimenez-Valverde A, Real R. AUC: a misleading measure of the performance of predictive distribution models. Global Ecology and Biogeography. 2008;17:145–151. doi: 10.1111/j.1466-8238.2007.00358.x. [DOI] [Google Scholar]
- McPherson JM, Jetz W. Effects of species’ ecology on the accuracy of distribution models. Ecography. 2007;30:135–151. [Google Scholar]
- Montgomery DC, Peck EA, Vining GG. An Introduction to Linear Regression Analysis. 4. New York: Wiley; 2006. [Google Scholar]
- Pearson RG, Raxworthy CJ, Nakamura M, Peterson AT. Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. Journal of Biogeography. 2007;34:102–117. doi: 10.1111/j.1365-2699.2006.01594.x. [DOI] [Google Scholar]
- Peterson AT. Ecologic niche modeling and spatial patterns of disease transmission. Emerging Infectious Diseases. 2006;12:1822–1826. doi: 10.3201/eid1212.060373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AT, Carroll DS, Mills JN, Johnson KM. Potential mammalian filovirus reservoirs. Emerging Infectious Diseases. 2004;10:2073–2081. doi: 10.3201/eid1012.040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AT, Lash RR, Carroll DS, Johnson KM. Geographic potential for outbreaks of Marburg hemorrhagic fever. American Journal of Tropical Medicine and Hygiene. 2006;75:9–15. doi: 10.4269/ajtmh.2006.75.1.0750009. [DOI] [PubMed] [Google Scholar]
- Peterson AT, Papes M, Eaton M. Transferability and model evaluation in ecological niche modeling: a comparison of GARP and Maxent. Ecography. 2007;30:550–560. [Google Scholar]
- Peterson AT, Papes M, Soberon J. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecological Modelling. 2008;213:63–72. doi: 10.1016/j.ecolmodel.2007.11.008. [DOI] [Google Scholar]
- Phillips SJ, Dudik M. Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography. 2008;31:161–175. doi: 10.1111/j.0906-7590.2008.5203.x. [DOI] [Google Scholar]
- Reed KD, Melski JW, Graham MB, Regnery RL, Sotir MJ, Wegner MV, et al. The detection of monkeypox in humans in the Western Hemisphere. New England Journal of Medicine. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- Rimoin AW, Kisalu N, Kebela-Ilunga B, Mukaba T, Wright LL, Formenty P, et al. Endemic human monkeypox, Democratic Republic of Congo, 2001–2004. Emerging Infectious Diseases. 2007;13:934–937. doi: 10.3201/eid1306.061540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoin AW, Mulembakani PM, Johnston SC, Lloyd-Smith JO, Kisalu NK, Kinkela TL, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of Biostatistics. 6. Belmont, CA: Duxbury; 2006. [Google Scholar]
- Soberon J. Grinnellian and Eltonian niches and geographic distributions of species. Ecology Letters. 2007;10:1115–1123. doi: 10.1111/j.1461-0248.2007.01107.x. [DOI] [PubMed] [Google Scholar]
- Soberon J. Niche and area of distribution modeling: a population ecology perspective. Ecography. 2010;33:159–167. doi: 10.1111/j.1600-0587.2009.06074.x. [DOI] [Google Scholar]
- Soberon J, Peterson AT. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodiversity Informatics. 2005;2:1–10. [Google Scholar]
- Vijayaraj V, Bright EA, Bhaduri BL (2007) High resolution urban feature extraction for population mapping using high performance computing. In: Proceedings of IGARSS 2007, Barcelona, Spain: IEEE, pp 278–281
- Wilks DS. Statistical Methods in the Atmospheric Sciences: An Introduction. San Diego: Academic Press; 1995. [Google Scholar]
- Williams RAJ, Fasina FO, Peterson AT. Predictable ecology and geography of avian influenza (H5N1) transmission in Nigeria and West Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2008;102:471–479. doi: 10.1016/j.trstmh.2008.01.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


