Abstract
Background
Relapse to drug use after a period of abstinence is a persistent problem in the treatment of cocaine dependence. Physical activity decreases cocaine self-administration in laboratory animals and is associated with a positive prognosis in human substance-abusing populations. The purpose of this study was to examine the effects of long-term access to a running wheel on drug-primed and cue-induced reinstatement of cocaine-seeking behavior in male and female rats.
Methods
Long-Evans rats were obtained at weaning and assigned to sedentary (no wheel) and exercising (access to wheel) groups for the duration of the study. After 6 weeks, rats were implanted with intravenous catheters and trained to self-administer cocaine for 14 days. After training, saline was substituted for cocaine and responding was allowed to extinguish, after which cocaine-primed reinstatement was examined in both groups. Following this test, cocaine self-administration was re-established in both groups for a 5-day period. Next, a second period of abstinence occurred in which both cocaine and the cocaine-associated cues were withheld. After 5 days of abstinence, cue-induced reinstatement was examined in both groups.
Results
Sedentary and exercising rats exhibited similar levels of cocaine self-administration, but exercising rats responded less than sedentary rats during extinction. In tests of cocaine-primed and cue-induced reinstatement, exercising rats responded less than sedentary rats, and this effect was apparent in both males and females.
Conclusions
These data indicate that long-term access to a running wheel decreases drug-primed and cue-induced reinstatement, and that physical activity may be effective at preventing relapse in substance-abusing populations.
Keywords: cocaine, exercise, extinction, reinstatement, self-administration, sex differences
1. Introduction
Physical activity reduces drug self-administration in laboratory animals and is associated with positive outcomes in substance abuse treatment programs. In laboratory rats, concurrent access to a running wheel decreases cocaine self-administration (Cosgrove et al., 2002), and a history of wheel running during adolescence is associated with lower cocaine-maintained breakpoints on a progressive ratio schedule of reinforcement (Smith et al., 2008). In clinical populations, participation in activity-based activities is associated with higher abstinence rates and a better prognosis in contingency management programs (Weinstock et al., 2008). Physical activity also reduces self-rated measures of anxiety and depression and increases measures of self-esteem and self-efficacy (Dunn et al., 2005; Manger and Motta, 2005; Fillipas et al., 2006; Muller et al., 2006), all of which are predictive of treatment success. Although the mechanisms by which physical activity produces these effects are not known, such findings have led investigators to propose that physical activity may facilitate recovery and be an effective intervention in substance abuse treatment programs (Smith et al., 2008; Weinstock et al., 2008).
A major and persistent obstacle to long-term recovery is relapse to drug use after a period of abstinence. It is estimated that up to 70% of recovering substance abusers relapse within one year after initiating treatment (Carroll et al., 1994). Although multiple variables contribute to the likelihood of relapse, recent research has focused on two major factors: environmental stimuli associated with drug use (i.e., cues) and direct exposure to the addictive substance itself. In the laboratory, the reinstatement procedure is used to model relapse to drug-seeking behavior after exposure to drug-paired stimuli (i.e., cue-induced reinstatement) and non-contingent drug administration (i.e., drug-primed reinstatement). Tests of cue-induced and drug-primed reinstatement are typically preceded by a period of time during which responding is extinguished by withholding the drug and/or drug-related cues. An increase in responding after cue or non-contingent drug exposure is seen as mimicking a return to drug use after a period of abstinence, thus modeling the cardinal feature of relapse in substance-abusing populations (see Katz and Higgins, 2003 for review and discussion). Interventions that decrease responding in the reinstatement procedure have potential efficacy to decrease the likelihood of relapse in recovering addicts, and the predictive validity of this procedure has been demonstrated for several treatment interventions (Epstein et al., 2006). Recently, Zlebnik et al. (2010) reported that concurrent access to a running wheel serves as an alternative non-drug reinforcer to decrease drug-primed reinstatement in female rats. Similarly, Lynch et al. (2010) recently reported that access to a running wheel during a 14-day period of forced abstinence decreases cue-induced reinstatement in male rats. These studies provide promising evidence that physical activity may be an effective means to prevent relapse in substance-abusing populations.
The purpose of the present study was to characterize the effects of long-term access to a running wheel (i.e., 6+ weeks) on cocaine-primed and cue-induced reinstatement of drug-seeking behavior. In this study, laboratory rats were assigned to sedentary and exercising conditions at weaning and remained in these conditions until the end of the study. Previous studies indicate that female rats run more than males (Eikelboom and Mills, 1988), have higher rates of drug-primed reinstatement than males (Lynch and Carroll, 2000), and are more sensitive to interventions that decrease drug-seeking behavior than males (Carroll et al., 2004). Consequently, both males and females were examined in the present study.
2. Methods
2.1. Animals
Male and female Long-Evans rats were obtained at weaning (∼21 days) from Charles River Laboratories (Raleigh, NC). Upon arrival, rats were divided into sedentary and exercising conditions. Sedentary rats were housed in standard polycarbonate cages (interior dimensions: 50 × 28 × 20 cm); exercising rats were housed in polycarbonate cages of equal dimensions and with a running wheel affixed to the interior of the cage. Cages with locked or inactive wheels were not used for the sedentary control group because rodents climb in locked running wheels (Koteja et al., 1999), potentially comprising the primary experimental manipulation of the study (i.e., physical activity). Except during self-administration sessions, exercising rats had free access to their running wheels throughout the study. Voluntary wheel running in this manner leads to sustained enhancements in aerobic capacity, increasing absolute and relative VO2 max by 20-30% (Yano et al., 1997). All rats were housed individually in a large colony room on a 12-hr light/12-hr dark cycle (lights on 0700). Except during initial lever-press training (see below), food and water were freely available in the home cages. Estrous phase was not monitored. All subjects were maintained in accordance with the guidelines of the Animal Care and Use Committee of Davidson College and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animals Resources, 1996). Rats that lost catheter patency were removed from the study and not replaced. Catheter patency was assumed lost when saline flowed from the base of the catheter when flushed or when a loss of righting reflex was not observed following a methohexital infusion. Of the 32 rats that began the study, 28 rats completed all phases of the experiment: sedentary females (n = 7); exercising females (n = 7); sedentary males (n = 7); exercising males (n = 7).
2.2. Apparatus
All test sessions were conducted in sound-attenuating operant conditioning chambers (interior dimensions: 31 × 24 × 21 cm) from Med Associates, Inc. (St. Albans, VT). Each chamber contained two response levers located on the front wall, two white stimulus lights located above the levers, a food receptacle located between the levers, an audio speaker, and one house light located on the rear wall. A food pellet dispenser was located behind the front wall and an infusion pump was mounted outside the chamber. Drug infusions were delivered through a Tygon tube protected by a stainless steel spring and attached to a counterbalanced swivel suspended above the chamber. Experimental events were programmed and data were collected through software and interfacing from Med Associates, Inc. The left lever was designated the active lever for all rats.
Running wheels were stainless steel (interior diameter: 35 cm) and obtained from Harvard Apparatus, Inc. (Holliston, MA, USA). Mechanical switches were affixed to each wheel and counted each revolution on a digital counter. Wheel revolutions were recorded weekly prior to surgery and daily during the period of behavioral testing.
2.3. Lever Press Training
Five weeks after arrival, all rats were food restricted to 90% of their free-feeding body weight and trained to lever press on a fixed ratio 1 (FR1) schedule of reinforcement. On this schedule, each lever press delivered a 45 mg food pellet to the food receptacle. Each session continued until 40 reinforcers were obtained or 2 hours elapsed, whichever occurred first. If any rat did not acquire the lever-press response by the third day, the response was shaped by the experimenter using the method of successive approximations. Once a rat earned the maximum number of reinforcers for three consecutive days, lever press training was discontinued and the rat was placed back on unrestricted feed. All rats met this criterion within seven days, and no differences were observed between groups.
2.4. Surgery
Six weeks after arrival, rats were anesthetized with a combination of ketamine (100 mg/kg, ip) and xylazine (8.0 mg/kg, ip) and surgically implanted with intravenous catheters (CamCaths, Cambridge, UK) according to methods described previously (Smith et al., 2008; 2009). All rats were allowed to recover for 3 days before beginning self-administration training.
2.5. Cocaine Self-Administration and Extinction (Cocaine)
All self-administration sessions were conducted during the light phase of the light-dark cycle so as not to interfere with nocturnal running. Three days after surgery, rats were placed in the operant conditioning chambers and connected to the infusion pumps via Tygon tubing. Each session began with illumination of the house light and the white stimulus light above the left response lever. Throughout the session, lever presses were reinforced on an FR1 schedule of reinforcement. On this schedule, each response produced an infusion of 0.5 mg/kg cocaine and a distinct audiovisual stimulus. Immediately after each lever press, the infusion pump activated for 2.5 to 4.0 seconds (based on body weight), a tone sounded for 5 seconds, and the stimulus light above the response lever turned off for 20 seconds to signal a timeout period in which cocaine was not available. Aside from the 20-second timeout after each lever press, no limit was placed on the number of infusions that could be earned. Test sessions terminated automatically after 2 hours elapsed and continued in this manner for 14 consecutive days.
Following 14 days of cocaine self-administration, saline was substituted for cocaine for 7 consecutive days. During these sessions, each response produced an infusion of saline and the accompanying audiovisual stimulus (i.e., tone on; stimulus light off; activation of syringe pump) on an FR1 schedule of reinforcement. All other experimental events were identical to those present during the cocaine self-administration sessions. Pilot tests conducted prior to the study revealed that responding decreased by approximately 80% to less than 10 infusions per session within 7 days under these conditions; consequently, a period of 7 days was selected for extinction testing.
2.6. Cocaine-Primed Reinstatement
After 7 days, cocaine-primed reinstatement was examined during three test sessions conducted over the next 5 days. On the first, third, and fifth day, rats were injected with either saline (1.0 ml/kg, ip) or cocaine (15 or 30 mg/kg, ip) 10 minutes prior to being placed into the operant conditioning chamber. During test sessions, all experimental events were identical to those present during the saline substitution sessions. Specifically, each response produced an infusion of saline and the audiovisual stimulus on an FR1 schedule of reinforcement. The order of saline- and cocaine-priming tests was counterbalanced across rats. Saline substitution sessions (with no accompanying intraperitoneal injections) separated the priming tests on the second and fourth day of testing.
2.7. Cocaine Self-Administration and Extinction (Cocaine + Cues)
After cocaine-primed reinstatement testing, cocaine self-administration was re-established in all rats. During these sessions, each response produced 0.5 mg/kg/infusion cocaine and the audiovisual stimulus. Sessions terminated after 2 hours and continued for 5 consecutive days.
Following 5 days of cocaine self-administration, extinction testing began in which the infusion pumps were turned off and the audiovisual stimulus associated with each response was removed. During these sessions, the white stimulus light above the response lever remained on throughout the session, the tone was never sounded, and lever presses were recorded but had no programmed consequences. Extinction sessions terminated automatically after 2 hours elapsed. Pilot tests conducted prior to the study revealed that responding decreased by approximately 80% to less than 10 responses per session within 5 days under these conditions; consequently, a period of 5 days was selected for extinction testing.
2.8. Cue-Induced Reinstatement
After 5 days of extinction, cue-induced reinstatement was examined in a single test session. In this test, saline was substituted for cocaine, and the session began with a 5-second, non-contingent delivery of the audiovisual stimulus that was previously paired with cocaine (i.e., tone on; stimulus light off; activation of syringe pump). For the remainder of the session, each lever press produced the audiovisual stimulus for 5 seconds, including activation of the infusion pump, but cocaine was not delivered.
2.9. Data Analysis
All self-administration and extinction data were analyzed via three-way ANOVA, with sex (male vs. female) and condition (sedentary vs. exercise) serving as between-subject factors and session serving as a within-subject factor. Cocaine-primed reinstatement data were examined via three-way ANOVA, with sex and condition serving as between-subject factors and dose serving as a within-subject factor. Cue-induced reinstatement data were analyzed via two-way ANOVA, with sex and condition serving as between-subject factors. Wheel running data were analyzed via two-way ANOVA, with sex serving as a between-subject factor and time (week) serving as a within-subject factor. Post-hoc tests were conducted where appropriate using either the Holm-Bonferroni or Tukey-Kramer method for multiple comparisons. Effect sizes for the exercise manipulation were determined using Cohen's d. For exercising rats, Pearson product-moment correlations (with corrections to the alpha value for multiple comparisons) were used to determine the correlation between wheel running (rev/day) and responding during the self-administration, extinction, and reinstatement tests.
3. Results
3.1. Body Weight
Body weights did not differ between sedentary and exercising rats at the beginning (week 7) or end (week 12) of self-administration testing (Table 1). Males weighed more than females at both time points [main effect of sex: F (1, 24) = 236.432; p < .001], and both sexes gained weight over the course of the study [main effect of time: F (1, 24) = 176.450; p < .001]. Greater weight gains were seen in males than females over this five-week time period [time × sex interaction: F (1, 24) = 21.761; p < .001].
Table 1.
Mean (SEM) body weights a at the beginning (week 7) and end (week 12) of self-administration testing.
| Sex/Condition | Week 7 | Week 12 |
|---|---|---|
| Female | ||
| Sedentary | 210.86 (5.28) | 272.71 (5.46) |
| Exercise | 217.57 (5.37) | 280.43 (6.14) |
| Male | ||
| Sedentary | 310.14 (7.11) | 403.29 (10.27) |
| Exercise | 305.29 (10.27) | 421.43 (10.59) |
all data expressed in grams
3.2. Wheel Running
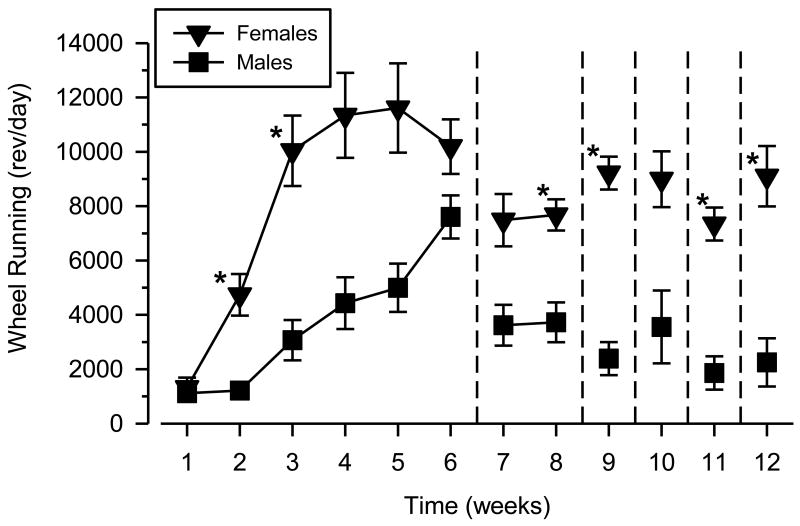
Differences in wheel running were observed between females and males over the course of the study (Fig. 1). Females increased their running at a faster rate than males, attained greater maximal levels of running than males, and reached these maximal levels at an earlier time point than males. During the week prior to surgery and self-administration training, females averaged 10,190 rev/day (range: 7428 to 15,045 rev/day) and males averaged 7604 rev/day (range: 4528 to 10,005 rev/day). Wheel running in both sexes decreased markedly following catheter implantation and the beginning of cocaine self-administration. Consistent with these observations, a repeated-measures ANOVA revealed a main effect of time [F (11, 143) = 16.507; p < .001], a main effect of sex [F (1, 13) = 225.919; p < .001], and a significant time × sex interaction [F (11, 143) = 4.065; p < .001]. Post-hoc tests revealed that females ran more than males during weeks 2-3, weeks 8-9, and weeks 11-12.
Fig. 1.
Wheel running over the course of the study in female and male rats. Left axis depicts wheel running expressed as the mean number of wheel revolutions per day (rev/day); horizontal axis depicts time expressed in “weeks” of 5- to 7-day intervals. Vertical reference lines after weeks 6, 8, 9, 10, and 11 indicate transitions between different experimental events: home cage and running wheel acclimation (weeks 1-6); cocaine self-administration (weeks 7-8); saline substitution (week 9); cocaine-primed reinstatement (week 10); cocaine self-administration (week 11); extinction testing and cue-induced reinstatement (week 12). Asterisks (*) indicate significant differences from male rats.
3.3. Cocaine Self-Administration and Extinction (Cocaine)
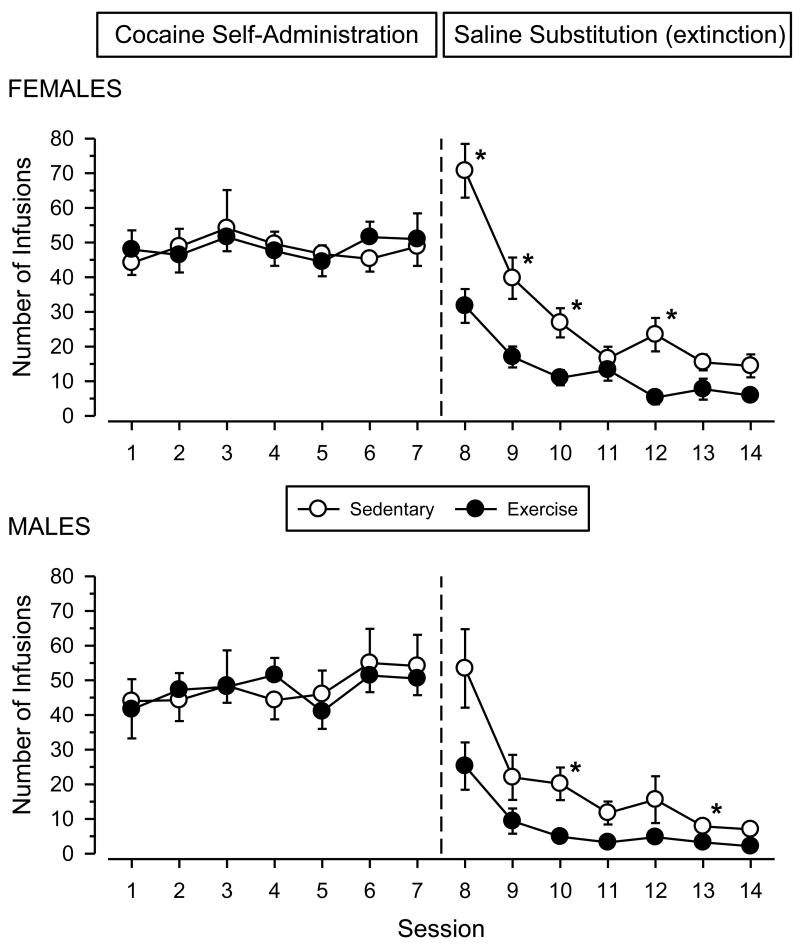
All rats responded on the first day of self-administration training (data not shown), and stable patterns of responding were evident in all groups by the second week of testing (Fig. 2). During the last 7 days of cocaine-self-administration, all groups averaged between 41 and 55 infusions per session (82 to 110 mg/kg cocaine per session) with no increasing or decreasing trends across sessions. Analysis of individual event records revealed stereotypical patterns of responding characterized by an initial “load up” phase, followed by a steady rate of responding with regular post-reinforcement pauses (data not shown). Repeated-measures ANOVA revealed no significant main effects or interactions.
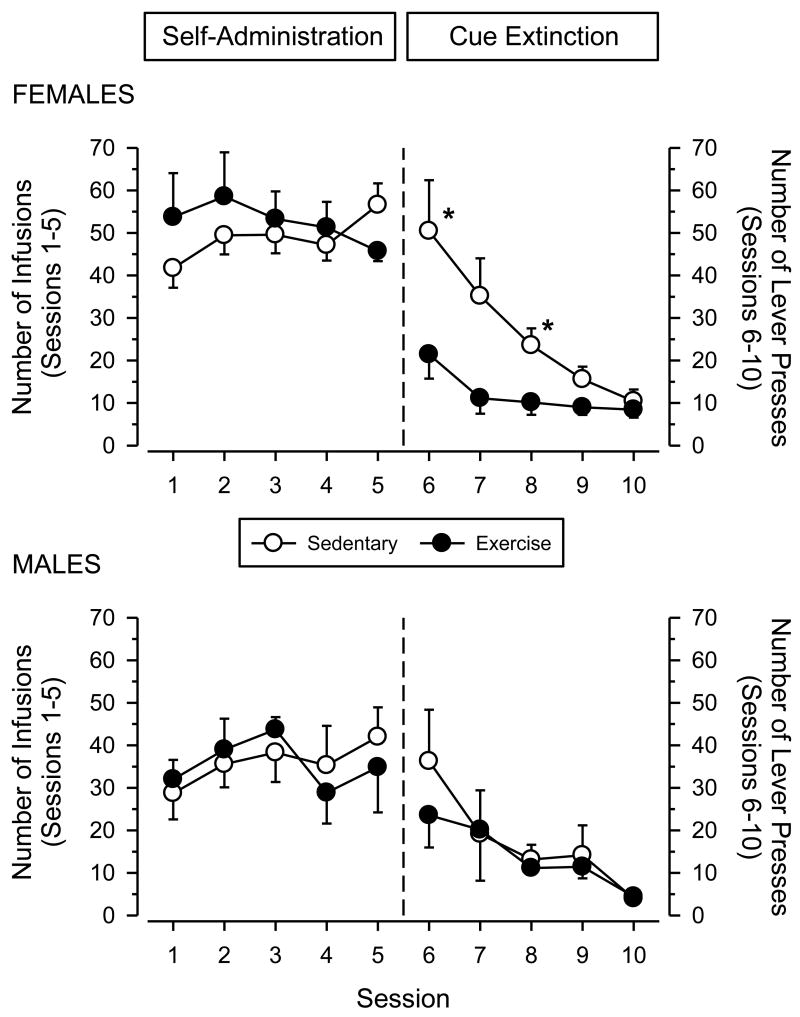
Fig. 2.
Data from self-administration sessions (Sessions 1-7) and saline substitution sessions (Sessions 8-14) in female (upper panel) and male (lower panel) rats. Vertical axes depict number of infusions obtained; horizontal axes indicate session number. Vertical reference line indicates transition between cocaine self-administration and extinction testing. Asterisks (*) indicate significant differences from exercising rats.
Responding decreased significantly in all groups when saline was substituted for cocaine [main effect of session: F (6, 150) = 45.366; p < .001]. Responding during extinction differed markedly across groups, with females responding more than males [main effect of sex: F (1, 25) = 10.738; p = .003] and sedentary rats responding more than exercising rats [main effect of condition: F (1, 25) = 36.593; p < .001]. For both sexes, responding decreased more rapidly in exercising rats than sedentary rats [session × condition interaction: F (6, 150) = 6.291; p < .001].
3.4. Cocaine-Primed Reinstatement
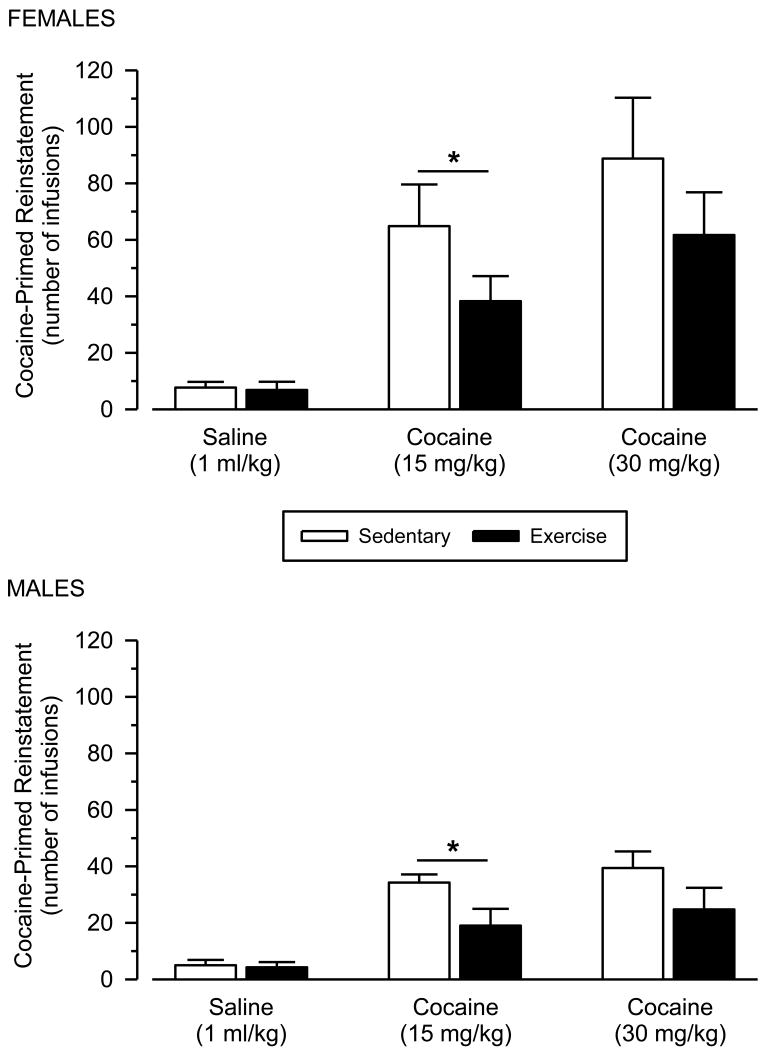
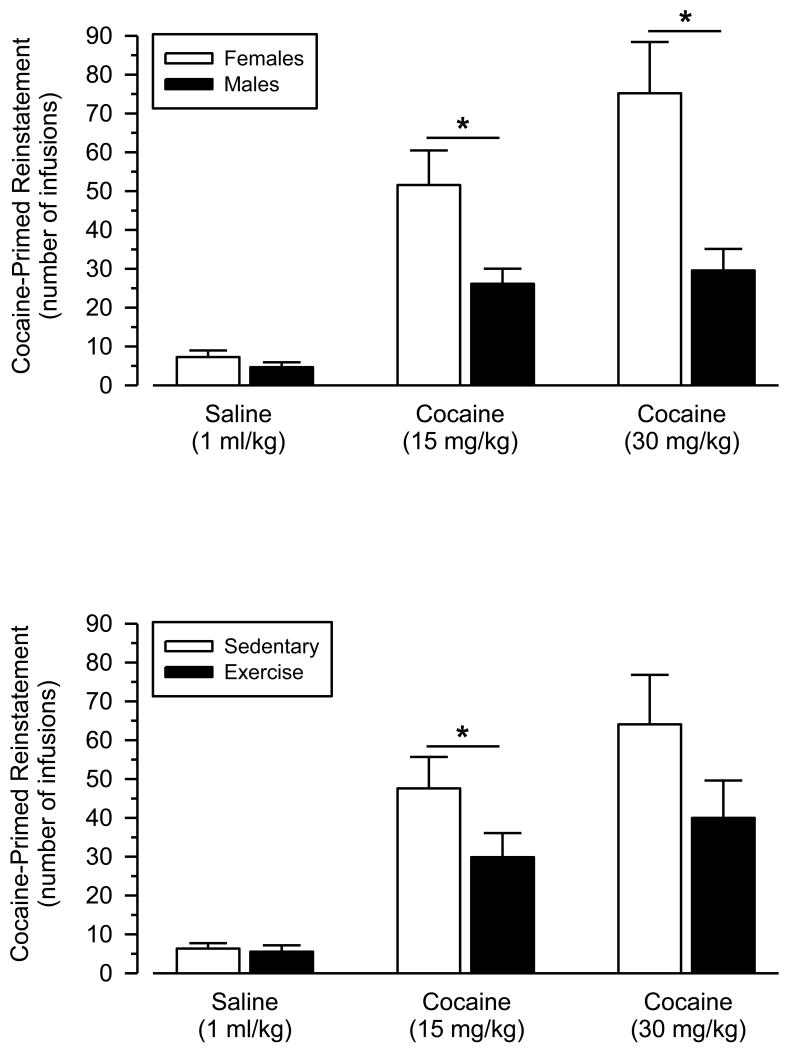
In the cocaine-primed reinstatement tests, no significant differences in responding were observed after saline administration (Fig. 3 and 4). In contrast, cocaine dose-dependently reinstated responding in all groups [main effect of dose: F (1, 25) = 12.704; p = .002]. Cocaine-primed reinstatement was significantly greater in female rats than male rats [main effect of sex: F (1, 25) = 14.241; p = .001], and this effect was particularly robust at the high dose of cocaine [dose × sex interaction: F (1, 25) = 5.240; p = .031]. Reinstatement responding was also significantly greater in sedentary rats than exercising rats [main effect of condition: F (1, 25) = 5.073; p = .033]. Effect sizes of wheel running were large and similar between males and females at both the low (males: d = 1.17; females: d = 0.90) and high (males: d = 0.78; females: d = 0.79) priming doses. Responding during saline substitution sessions on the second and fourth days (i.e., between the reinstatement tests) was uniformly low and similar to that observed after the saline priming injection (data not shown).
Fig. 3.
Cocaine-primed reinstatement in female (upper panel) and male (lower panel) rats. Vertical axes depict number of saline infusions obtained; horizontal axes indicate priming dose of cocaine or saline. Asterisks (*) indicate significant differences.
Fig. 4.
Cocaine-primed reinstatement in female and male rats when collapsed across condition (upper panel) and in sedentary and exercising rats when collapsed across sex (lower panel). Vertical axes depict number of saline infusions obtained; horizontal axes indicate priming dose of cocaine or saline. Asterisks (*) indicate significant differences.
3.5. Cocaine Self-Administration and Extinction (Cocaine + Cues)
Following the cocaine-primed reinstatement tests, cocaine self-administration was re-established in all groups for 5 days (Fig. 5). Responding was stable in all groups during this period, but females responded significantly more than males throughout the 5 days of testing [main effect of sex: F (1, 24) = 10.095; p = .004]. Daily averages for females ranged between 42 and 59 infusions per session (84 and 158 mg/kg cocaine per session), whereas daily averages for males ranged between 29 and 44 infusions per session (58 and 88 mg/kg cocaine per session). A four-way ANOVA incorporating occasion (week 8 vs. week 12) as an additional factor revealed a significant sex × occasion interaction [F (1, 24) = 11.267; p = .003], with males decreasing their level of responding from the first to second series of cocaine self-administration sessions (compare left panels of Fig. 2 and Fig. 5).
Fig. 5.
Data from self-administration sessions (Sessions 1-5) and extinction sessions with all cocaine-associated cues withheld (Sessions 6-10) in female (upper panel) and male (lower panel) rats. Left axes depict number of infusions obtained (Sessions 1-5); right axes depict number of lever presses (Sessions 6-10). Horizontal axes indicate session number. Vertical reference line indicates transition between cocaine self-administration and extinction testing. Asterisks (*) indicate significant differences from exercising rats.
After 5 days of cocaine self-administration, cocaine and all audiovisual cues associated with cocaine delivery were removed. Responding decreased significantly in all groups during this period [main effect of session: F (4, 96) = 13.405; p < .001]. Sedentary rats responded significantly more than exercising rats during the 5 days of testing [main effect of condition: F (1, 24) = 4.904; p = .037], and there was a trend for sedentary rats to extinguish responding at a slower rate than exercising rats [session × condition interaction: F (4, 96) = 2.150; p = .081]. No significant sex differences were observed.
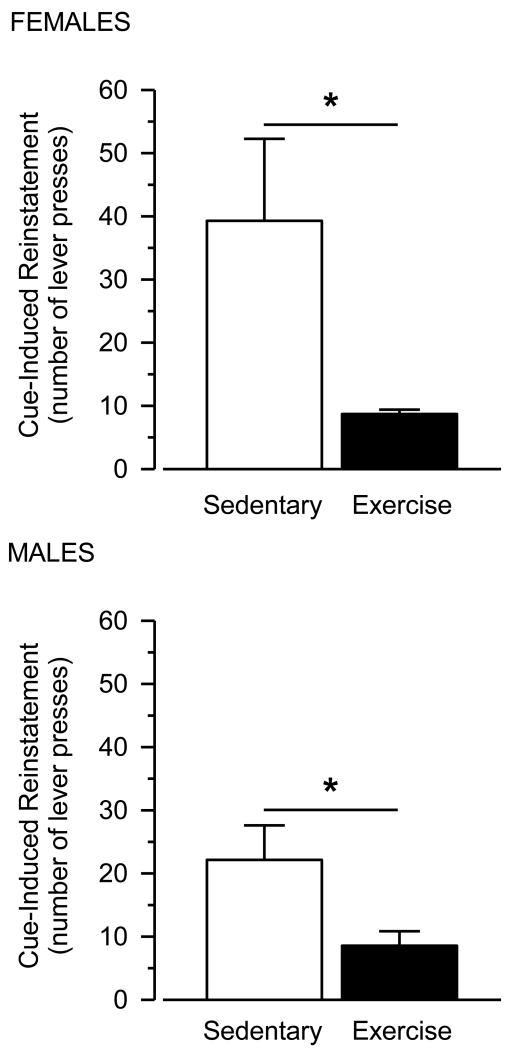
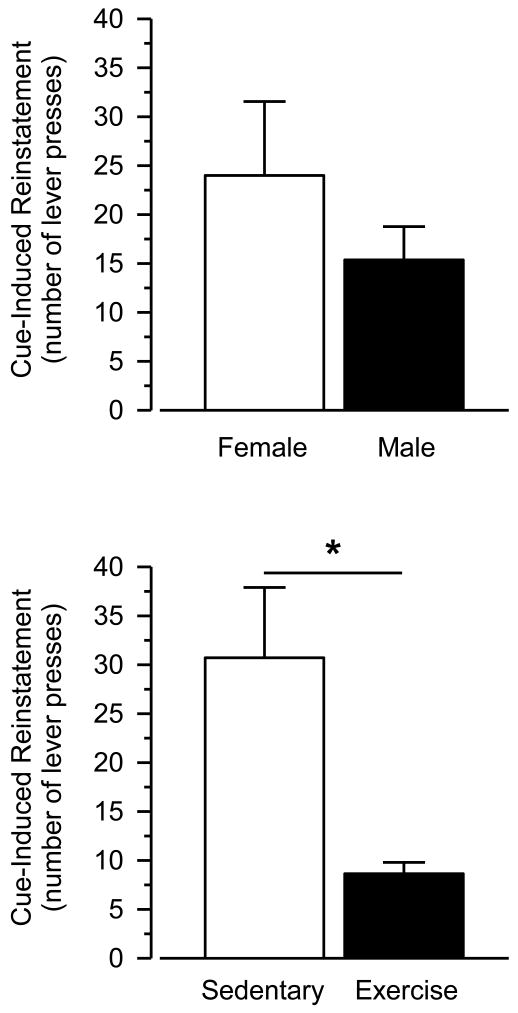
3.6. Cue-Induced Reinstatement
Reintroduction of the cocaine-associated cues reinstated responding in all groups (Fig. 6 and 7). Numerically, females responded more than males in the cue-induced reinstatement test, but this effect was not statistically significant. Reinstatement responding was greater in sedentary rats than exercising rats, and this effect was statistically significant [main effect of condition: F (1, 24) = 9.544; p = .005]. Similar to that observed in the cocaine-primed reinstatement tests, the effect sizes were large and similar between males and females (males: d = 1.23; females: d = 1.26)
Fig. 6.
Cue-induced reinstatement in female (upper panel) and male (lower panel) rats. Vertical axes depict number of lever presses. Asterisks (*) indicate significant differences.
Fig. 7.
Cue-induced reinstatement in female and male rats when collapsed across condition (upper panel) and in sedentary and exercising rats when collapsed across sex (lower panel). Vertical axes depict number of lever presses. Asterisk (*) indicates significant difference.
3.7. Correlation between Wheel Running and Reinstatement Responding
Pearson product-moment correlations were used to determine the relationship between wheel running during the various phases of the experiment (weeks 1-6, weeks 7-12, weeks 1-12, week 10, week 12) and responding during the self-administration tests, the extinction tests, the cocaine-primed reinstatement tests, and the cue-induced reinstatement test. No significant correlations were found for either males or females.
4. Discussion
The principal finding of this study is that long-term access to a running wheel decreased drug-primed and cue-induced reinstatement in both male and female rats. Access to a running wheel also significantly reduced extinction responding, and this effect was apparent when cocaine-associated cues were present during the extinction sessions and when the cues were withheld. All of these effects were observed under conditions in which sedentary and exercising rats displayed no differences in cocaine self-administration sessions, suggesting that the differences observed in the reinstatement and extinction tests were not due to non-specific factors related to pharmacological history or drug sensitivity.
Relapse to drug use after a period of abstinence is one of the biggest obstacles to the long-term recovery of individuals with a history of substance abuse, and even brief exposure to the abused substance may be sufficient to trigger relapse in vulnerable individuals (see Carroll and Comer, 1996; de Wit, 1996 for discussion). Recently, Zlebnik et al. (2010) reported that concurrent access to a running wheel decreases drug-primed reinstatement in female rats. In that study, a running wheel was concurrently available during the reinstatement sessions, and access to exercise was manipulated by locking or unlocking the wheel. The present study extends those findings by showing that a history of wheel running is sufficient to decrease drug-primed reinstatement, even if a running wheel is not concurrently available during the period of drug exposure and operant responding.
Consistent with previous studies (e.g., Comer et al., 1993; Schenk and Partridge, 1999), cocaine-primed reinstatement was more robust when a higher dose of cocaine (30 mg/kg) was used as the prime. Although access to a running wheel numerically attenuated the effects of cocaine-primed reinstatement at both doses, this effect was more robust at the lower dose of cocaine (15 mg/kg). Higher doses of cocaine are generally less sensitive to interventions that decrease drug-seeking behavior during the maintenance of drug self-administration (see LeSage et al., 1999), and a similar phenomenon may be at work in these reinstatement tests. Regardless, exercising rats responded almost 40% less than sedentary rats following the high dose of cocaine, suggesting that wheel running was efficacious in this dose condition.
Exposure to drug-associated cues reliably increases subjective measures of craving in human substance abusers (Childress et al., 1988; Robbins et al., 1997; Sinha et al., 2000) and is considered to be a contributing factor to relapse in recovering populations (see O'Brien et al., 1998; Weiss et al., 2001; Sinha and Li, 2007 for discussion). Lynch et al. (2010) recently reported that 2-hour daily access to a running wheel during a 14-day period of forced abstinence decreased responding in a within-session extinction/cue-induced reinstatement procedure. Only male rats were examined in that study, and the present investigation extends those findings by showing that access to a running wheel also reduces cue-induced reinstatement in females.
In addition to the effects observed in the reinstatement tests, access to a running wheel also significantly reduced responding during extinction. Although there is some debate about how responding during extinction should be interpreted, it is often viewed as a measure of drug-seeking behavior elicited by a context that was previously paired with drug administration (e.g., Crombag and Shaham, 2002). If such is the case, then the protective effects of wheel running are not limited to cocaine-primed and cue-induced reinstatement, but extend to responding elicited by the broader context (i.e., context-induced reinstatement). The finding that wheel running was consistent across all three procedures suggests that common behavioral processes are likely involved.
We did not see any effects of access to a running wheel on either the acquisition or maintenance of cocaine self-administration. All subjects were trained to lever press using food reinforcement prior to catheter implantation, and all responded (i.e., acquired) on the first day in which cocaine was available. We have consistently failed to see any effects of wheel running on FR schedules of reinforcement under short-access (1-2 hour sessions) conditions (e.g., Smith et al., 2008). Protective effects of wheel running on measures of drug-seeking behavior are typically observed when sessions are extended to at least 6 hours (e.g., Cosgrove et al., 2002), when response requirements are increased (e.g., Smith et al., 2008), or after a history of forced abstinence (e.g., Zlebnick et al., 2010; Lynch et al., 2010; present study). Future studies examining the effects of access to a running wheel on the acquisition of drug self-administration are needed to determine whether wheel running is effective as a preventative intervention.
Environmental enrichment modulates the positive-reinforcing effects of cocaine (Smith et al., 2009) and reduces cue-induced (but not cocaine-primed) reinstatement (Chauvet et al., 2009; Thiel et al., 2009). Running wheels are frequently used in environmental enrichment studies as enriching stimuli, and it is possible that simply having access to running wheels (rather than running per se) was responsible for the reduction in cue-induced reinstatement. Lynch et al. (2010) reported that cue-induced reinstatement was lower in male rats with functional running wheels compared to male rats with locked running wheels, suggesting that access to a wheel without the opportunity to run is not sufficient to reduce cocaine-seeking behavior in reinstatement procedures. Nevertheless, physical activity is one component of environmental enrichment (van Praag et al., 2000), and the current study is consistent with previous studies reporting that enriched environments decrease drug-seeking behavior (Stairs and Bardo, 2009).
One aim of this study was to compare the effects of access to a running wheel on cocaine-seeking behavior in males and females. Hormonal fluctuations due to the estrous cycle influence both wheel running and cocaine self-administration. For instance, wheel running peaks on the night of estrous in intact females (Steiner et al., 1982; Kent et al., 1991), and ovariectomy reduces physical activity and wheel running (Hertrampf et al., 2006). Similarly, cocaine self-administration, extinction responding, and cocaine-primed reinstatement are greater during estrous than non-estrous (Roberts et al., 1989; Feltenstein and See, 2007), and ovariectomy markedly reduces cocaine-seeking in reinstatement tests (Larson and Carroll, 2007). Females in the present study had higher rates of wheel running than males, responded to a greater extent in the drug-primed reinstatement test than males, and responded more during extinction in the presence of drug-associated cues than males. Unfortunately, the study was not powered to detect significant condition × sex interactions of small to moderate size, and conclusions regarding potential sex differences (or lack thereof) in the effects of wheel running must be viewed with caution. In both the cocaine-primed and cue-induced reinstatement procedures, wheel running reduced responding in males and females, and the effect sizes were similar between the two sexes. Although additional studies with larger numbers of subjects will be needed to determine if males and females are differentially sensitive to these effects, it is notable that access to running wheels reduced responding in males even though they ran less than females during all stages of the experiment.
Given the growing number of studies demonstrating the protective effects of physical activity on measures of drug-seeking behavior, the question of its mechanism of action is increasingly relevant. Running increases central monoamine concentrations (Meeusen and De Meirleir, 1995) and chronic running increases the density of dopamine D2 receptors in the striatum (Gilliam et al., 1984; MacRae et al., 1987). This latter effect is particularly relevant given that reductions in D2 receptor density are associated with greater levels of cocaine self-administration in laboratory animals (Morgan et al., 2002) and are postulated to contribute to a lack of control over drug intake in humans (Volkow et al., 2009). Wheel running also elevates the expression of the neuropeptide galanin (Holmes et al., 2006), a potent inhibitor of norepinephine release in locus coeruleus neurons. The locus coeruleus, the principal noradrenergic nucleus in the central nervous system, is activated during times of stress and may mediate relapse to drug-seeking behavior through the release of central norepinephine. Indeed, norepinephine signaling is critical for both stress-induced and drug-primed reinstatement in laboratory models (Erb et al., 2000; Leri et al., 2002; Zhang and Kosten, 2005). Additionally, wheel running increases gliogenesis in the prefrontal cortex of rats (Mandyam et al., 2007), and physical activity has positive effects on prefrontal-dependent behavior in humans (Small et al., 2006; Yanagisawa et al., 2010). Deficits in prefrontal functioning are believed to play a contributing role in various transitional stages of the addictive process (Goldstein and Volkow, 2002) and may play a role in relapse to drug-seeking behavior (Van den Oever et al., 2010). Recently, Lynch et al. (2010) reported that wheel running reduced cue-induced reinstatement in male rats, and that cocaine-seeking was positively correlated with levels of phosphorylated extracellular signal-regulated kinase (pERK). Importantly, they found that pERK levels were significantly decreased by wheel running, suggesting that wheel running may prevent neuroadaptations in the prefrontal cortex that contribute to cocaine-seeking behavior.
If drug-primed and cue-induced reinstatement procedures are valid models of relapse, then the present findings suggest that physical activity would be an effective intervention in drug abuse treatment programs. We know of no randomized clinical trials that have specifically tested this hypothesis in treatment-seeking cocaine users, but several findings from clinical and at-risk populations deserve mention. For instance, inpatient treatment programs that incorporated physical activity as part of a comprehensive intervention strategy led to decreases in depression and anxiety risk factors associated with relapse (Frankel and Murphy, 1974; Palmer et al., 1988). Similarly, a wellness program emphasizing physical fitness for incarcerated drug offenders produced improvements in self-esteem, health awareness, and relapse prevention skills (Peterson and Johnstone, 1995). Finally, in a contingency management program for recovering substance abusers, participation in activity-based activities was associated with a better prognosis and higher abstinence rates (Weinstock et al., 2008). Coupled with the present data, such findings argue for the expanded use of activity-related interventions in relapse prevention programs.
Acknowledgments
The authors thank Dr. Scott Tonidandel for statistical advice and guidance, Amy Sullivan for expert animal care and maintenance, and the National Institute on Drug Abuse for generously supplying the study drug.
Role of Funding Source: Financial support for this study was provided by US Public Service Grants DA14255 and DA027485 (to M.A.S). Additional support was provided by the Howard Hughes Medical Institute (Grant 52006292), the Duke Endowment, and Davidson College. These organizations had no further role in the design of the study; in the collection, analysis and interpretation of the data; in writing the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Contributors: Mark Smith served as principal investigator of the study and was responsible for writing the manuscript. Michael Pennock, Katherine Walker, and Kimberly Lang contributed to the design of the project and were responsible for all data collection. All authors approved the manuscript prior to submission.
Conflict of Interest: The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Carroll ME, Comer SD. Animal models of relapse. Exp Clin Psychopharmacol. 1996;4:11–18. [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin F. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence. Delayed emergence of psychotherapy effects. Arch Gen Psychiatry. 1994;51:989–997. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology. 2009;34:2767–2778. doi: 10.1038/npp.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, McLellan AT, O'Brien CP. Conditioned craving and arousal in cocaine addiction: a preliminary report. NIDA Res Monogr. 1988;81 [PubMed] [Google Scholar]
- Comer SD, Lac ST, Curtis LK, Carroll ME. Effects of buprenorphine and naltrexone on reinstatement of cocaine-reinforced responding in rats. J Pharmacol Exp Ther. 1993;267:1470–1477. [PubMed] [Google Scholar]
- Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacol Biochem Behav. 2002;73:663–671. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- de Wit H. Priming effects with drugs and other reinforcers. Exp Clin Psychopharmacol. 1996;4:5–10. [Google Scholar]
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28:1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Eikelboom R, Mills R. A microanalysis of wheel running in male and female rats. Physiol Behav. 1988;43:625–630. doi: 10.1016/0031-9384(88)90217-x. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89:183–189. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillipas S, Oldmeadow LB, Bailey MJ, Cherry CL. A six-month, supervised, aerobic and resistance exercise program improves self-efficacy in people with human immunodeficiency virus: a randomised controlled trial. Aust J Physiother. 2006;52:185–190. doi: 10.1016/s0004-9514(06)70027-7. [DOI] [PubMed] [Google Scholar]
- Frankel A, Murphy J. Physical fitness and personality in alcoholism. Canonical analysis of measures before and after treatment. Q J Stud Alcohol. 1974;35:1272–1278. [PubMed] [Google Scholar]
- Gilliam PE, Spirduso WW, Martin TP, Walters TJ, Wilcox RE, Farrar RP. The effects of exercise training on [3H]-spiperone binding in rat striatum. Pharmacol Biochem Behav. 1984;20:863–867. doi: 10.1016/0091-3057(84)90008-x. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertrampf T, Degen GH, Kaid AA, Laudenbach-Leschowsky U, Seibel J, Di Virgilio AL, Diel P. Combined effects of physical activity, dietary isoflavones and 17beta-estradiol on movement drive, body weight and bone mineral density in ovariectomized female rats. Planta Med. 2006;72:484–487. doi: 10.1055/s-2006-931579. [DOI] [PubMed] [Google Scholar]
- Holmes PV, Yoo HS, Dishman RK. Voluntary exercise and clomipramine treatment elevate prepro-galanin mRNA levels in the locus coeruleus in rats. Neurosci Lett. 2006;408:1–4. doi: 10.1016/j.neulet.2006.04.057. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Kent S, Hurd M, Satinoff E. Interactions between body temperature and wheel running over the estrous cycle in rats. Physiol Behav. 1991;49:1079–1084. doi: 10.1016/0031-9384(91)90334-k. [DOI] [PubMed] [Google Scholar]
- Koteja P, Garland T, Jr, Sax JK, Swallow JG, Carter PA. Behaviour of house mice artificially selected for high levels of voluntary wheel running. Anim Behav. 1999;58:1307–1318. doi: 10.1006/anbe.1999.1270. [DOI] [PubMed] [Google Scholar]
- Larson EB, Carroll ME. Estrogen receptor beta, but not alpha, mediates estrogen's effect on cocaine-induced reinstatement of extinguished cocaine-seeking behavior in ovariectomized female rats. Neuropsychopharmacology. 2007;32:334–345. doi: 10.1038/sj.npp.1301249. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Stafford D, Glowa JR. Preclinical research on cocaine self-administration: environmental determinants and their interaction with pharmacological treatment. Neurosci Biobehav Rev. 1999;23:717–741. doi: 10.1016/s0149-7634(99)00015-9. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology. 2000;148:196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Piehl KB, Acosta G, Peterson AB, Hemby SE. Aerobic exercise attenuates reinstatement of cocaine-seeking behavior and associated neuroadaptations in the prefrontal cortex. Biol Psychiatry. 2010;68:774–777. doi: 10.1016/j.biopsych.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae PG, Spirduso WW, Walters TJ, Farrar RP, Wilcox RE. Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolites in presenescent older rats. Psychopharmacology. 1987;92:236–240. doi: 10.1007/BF00177922. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Eisch AJ, Richardson HN, Koob GF. Methamphetamine self-administration and voluntary exercise have opposing effects on medial prefrontal cortex gliogenesis. J Neurosci. 2007;27:11442–11450. doi: 10.1523/JNEUROSCI.2505-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger TA, Motta RW. The impact of an exercise program on posttraumatic stress disorder, anxiety, and depression. Int J Emerg Ment Health. 2005;7:49–57. [PubMed] [Google Scholar]
- Meeusen R, De Meirleir K. Exercise and brain neurotransmission. Sports Med. 1995;20:160–188. doi: 10.2165/00007256-199520030-00004. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Muller SM, Dennis DL, Gorrow T. Emotional well-being of college students in health courses with and without an exercise component. Percept Mot Skills. 2006;103:717–725. doi: 10.2466/pms.103.3.717-725. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Palmer J, Vacc N, Epstein J. Adult inpatient alcoholics: physical exercise as a treatment intervention. J Stud Alcohol. 1988;49:418–421. doi: 10.15288/jsa.1988.49.418. [DOI] [PubMed] [Google Scholar]
- Peterson M, Johnstone BM. The Atwood Hall Health Promotion Program, Federal Medical Center, Lexington, KY. Effects on drug-involved federal offenders. J Subst Abuse Treat. 1995;12:43–48. [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O'Brien CP. Relationships among physiological and self-report responses produced by cocaine-related cues. Addict Behav. 1997;22:157–167. doi: 10.1016/s0306-4603(96)00007-x. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Cocaine-seeking produced by experimenter-administered drug injections: dose-effect relationships in rats. Psychopharmacology. 1999;147:285–290. doi: 10.1007/s002130051169. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O'Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology. 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Small GW, Silverman DH, Siddarth P, Ercoli LM, Miller KJ, Lavretsky H, Wright BC, Bookheimer SY, Barrio JR, Phelps ME. Effects of a 14-day healthy longevity lifestyle program on cognition and brain function. Am J Geriatr Psychiatry. 2006;14:538–545. doi: 10.1097/01.JGP.0000219279.72210.ca. [DOI] [PubMed] [Google Scholar]
- Smith MA, Iordanou JC, Cohen MB, Cole KT, Gergans SR, Lyle MA, Schmidt KT. Effects of environmental enrichment on sensitivity to cocaine in female rats: importance of control rates of behavior. Behav Pharmacol. 2009;20:312–321. doi: 10.1097/FBP.0b013e32832ec568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Schmidt KT, Iordanou JC, Mustroph ML. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend. 2008;98:129–135. doi: 10.1016/j.drugalcdep.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stairs DJ, Bardo MT. Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacol Biochem Behav. 2009;92:377–382. doi: 10.1016/j.pbb.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M, Katz RJ, Carroll BJ. Detailed analysis of estrous-related changes in wheel running and self-stimulation. Physiol Behav. 1982;28:201–204. doi: 10.1016/0031-9384(82)90127-5. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Pentkowski NS, Neisewander JL. Anti-craving effects of environmental enrichment. Int J Neuropsychopharmacol. 2009;12:1151–1156. doi: 10.1017/S1461145709990472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Oever MC, Spijker S, Smit AB, De Vries TJ. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci Biobehav Rev. 2010;35:276–284. doi: 10.1016/j.neubiorev.2009.11.016. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine's role in drug abuse and addiction. Neuropharmacology 56 Suppl. 2009;56 1:3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock J, Barry D, Petry NM. Exercise-related activities are associated with positive outcome in contingency management treatment for substance use disorders. Addict Behav. 2008;33:1072–1075. doi: 10.1016/j.addbeh.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Dan I, Tsuzuki D, Kato M, Okamoto M, Kyutoku Y, Soya H. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. Neuroimage. 2010;50:1702–1710. doi: 10.1016/j.neuroimage.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Yano H, Yano L, Kinoshita S, Tsuji E. Effect of voluntary exercise on maximal oxygen uptake in young female Fischer 344 rats. Jpn J Physiol. 1997;47:139–141. doi: 10.2170/jjphysiol.47.139. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Kosten TA. Prazosin, an alpha-1 adrenergic antagonist, reduces cocaine-induced reinstatement of drug-seeking. Biol Psychiatry. 2005;57:1202–1204. doi: 10.1016/j.biopsych.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Gliddon LA, Carroll ME. Reduction of extinction and reinstatement of cocaine seeking by wheel running in female rats. Psychopharmacology. 2010;209:113–125. doi: 10.1007/s00213-010-1776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]