Abstract
Infant guinea pigs exhibit a 2-stage response to maternal separation: an initial active stage, characterized by vocalizing, and a second passive stage marked by depressive-like behavior (hunched posture, prolonged eye-closure, extensive piloerection) that appears to be mediated by proinflammatory activity. Recently we found that pups showed an enhanced (i.e., sensitized) depressive-like behavioral response during repeated separation. Further, core body temperature was higher during the beginning of a second separation compared to the first, suggesting a more-rapid stress-induced febrile response to separation the second day, though the possibility that temperature was already elevated prior to the second separation could not be ruled out. Therefore, the present study examined temperature prior to, and during, 2 daily separations. We also examined the temperature response to a third separation conducted 3 days after the second, and assessed the effect of repeated separation on plasma cortisol levels. Core temperature did not differ just prior to the separations, but showed a more-rapid increase and then decline during both a second and third separation than during a first. Temperature responses were not associated with changes in motor activity. Depressive-like behavior was greater during the second and third separations. Pups separated a first time showed a larger plasma cortisol response at the conclusion of separation than did animals of the same age separated a third time. In all, the results indicate that the sensitization of depressive-like behavior during repeated separations over several days is accompanied by a more-rapid febrile response that may be related to a reduction of glucocorticoid suppression.
Keywords: maternal separation, depressive behavior, depression, fever, core body temperature, cortisol, hypothalamic-pituitary-adrenal, HPA, sensitization, guinea pig
1. Introduction
When children or infant monkeys are separated from their mothers for a protracted period, an initial stage of active distress is often followed by a second passive, depressive-like stage of response [34, 40, 46, 50]. Over the years, it has become clear that prolonged separation and other forms of attachment disruption in children (e.g., abuse, neglect, death of a parent) also increases the vulnerability for developing a depressive or anxiety disorder at a later age [1, 4, 9, 45]. In nonhuman primates, seemingly parallel long-term effects of early separation on later behavior have been observed [15]. The mechanism through which attachment disruption confers vulnerability for later psychopathology remains unknown, but has been hypothesized to involve a sensitization process—for instance of corticotropin-releasing factor (CRF) secretion or amygdala activity—so that reaction to later loss and other adverse circumstances is magnified as a result of the early attachment disruption [14, 16, 19].
Guinea pigs are laboratory rodents that display good evidence for a specific filial attachment process, as well as similarities to primates in their responses to social separation [20, 32, 44]. Of particular interest here, guinea pigs exhibit a two-stage, active/passive behavioral response to maternal separation procedures. Upon isolation in a novel enclosure, pups initially vocalize at a high rate and tend to move about the enclosure. But after an hr or so, they quiet and assume a hunched or crouched posture, with eyes closed and piloerection over most of the body [26]. These effects do not occur if the mother is with the pup in the test enclosure. It appears that the stressor of being separated from the mother in an unfamiliar environment activates proinflammatory signaling that mediates the passive stage.
In recent years, it has become clear that stressors can often induce a systemic proinflammatory reaction (i.e., “stress-induced sickness”) [36] that promotes fever and behaviors that appear to support fever (e.g., hunched posture, piloerection) or conserve energy (e.g., sleepiness) [18]. Three lines of evidence support the hypothesis that the passive response in guinea pigs is mediated by proinflammatory signaling stimulated by the stressor of the separation procedure: (a) pups injected with lipopolysacchride (LPS), which elicits a potent proinflammatory reaction, exhibited high levels of crouching, eye-closure, and piloerection [24]; (b) any of three anti-inflammatory compounds reduced the passive behavioral response to separation [29, 43, 47]; and, (c) separation induced two signs of systemic inflammation—an increase in the expression of the proinflammatory cytokine tumor-necrosis factor alpha in spleen [22] and an increase in core temperature [23, 24], suggesting fever. These findings are of particular interest in light of mounting evidence that proinflammatory processes contribute to forms of human depressive illness [12], and may be a major mechanism through which stressors precipitate depressive episodes [41].
There is increasing support for the notion that behaviors induced by proinflammatory activity in laboratory animals share common features with depression. For instance, proinflammatory stimulation in rats and mice increases depressive-like behavior in conventional depression testing paradigms such as tail suspension and the sucrose preference test of anhedonia [12]. Mice deficient in proinflammatory signaling show reduced impact of stress on sucrose consumption and other depression-related measures [17], and antidepressant medication reduces some proinflammatory-induced behavioral change [54]. Thus, in addition to the face validity deriving from the similarity of the second stage of separation in guinea pigs and primates, there is sufficient reason to consider that the behavior of separated guinea pigs might inform our understanding of how early separation and other forms of early stress increase vulnerability for later depression.
We recently observed that the passive behavioral response of guinea pigs sensitizes with repeated separations. Pups exhibited an increase in passive behavior during a second 3-hr separation that followed a first separation by 1 or 4 days [23, 28]. Further, central infusion of interleukin-10—a cytokine with anti-inflammatory properties—before an initial separation blocked the increase in passive behavior during a separation the following day [28]. This finding suggests that proinflammatory activity not only mediates the initial response to separation, but also may contribute to the sensitization of the behavioral response with repeated separations. It may be, for instance, that the proinflammatory reaction is enhanced with repeated separations, which in turn, promotes the sensitization of the depressive-like behavior [21]. This possibility is consistent with another recent finding that core body temperature was higher during the initial portion of a second daily, 3-hr separation, and lower in the latter portion than was observed on the previous day [23]. This suggested a shorter-latency febrile response during the second separation. However, because temperature was examined only during the separation, it is unclear whether the initial rise in temperature on the second day was due to an actual elevation in the temperature response during the second separation or to a difference between days in temperature at the time the second separation began (e.g., a persistent elevation in basal temperature or an anticipatory response to subtle environmental cues on the second day). Further, because temperature was observed only on two consecutive days, it is unclear whether the increased thermogenic response was a transitory phenomenon or a more-persistent effect.
Therefore in the present study, we used a telemetry device to measure temperature for a 1-hr period just prior to, as well as during, three, 3-hr separations. Tests occurred on two consecutive days as well as 3 days after the second. Control measures were included to differentiate febrile responses from other changes in core temperature (e.g., activity-induced thermogenic responses). We again assessed sensitization of the passive behavioral response and, unlike our earlier study of behavior over this time span, included age-matched controls separated a first time at the same mean age as pups undergoing their third separation. Finally, because of the hypothesized role of proinflammatory activity in the sensitization process, together with the well-established suppressive action of glucocorticoids on inflammatory responses, we also evaluated the effect of single versus repeated separations on plasma cortisol. Our separation procedure reliably activates the hypothalamic-pituitary-adrenal (HPA) axis of guinea pig pups [27, 37], but HPA activity has not previously been examined in the context of this sensitization paradigm.
2. Method
2.1 Animals
Albino guinea pigs (Cavia porcellus) of the Hartley strain were bred and housed in our laboratory. Mother and litter were maintained together for the duration of the experiment in a cage (73 × 54 × 24 cm), which had a wire front and sawdust bedding. Food and water were continuously supplied. The colony room was maintained on a 12-hr light:dark cycle, with lights on at 07:00 hr. All procedures were conducted in accordance with the Wright State University Animal Care and Use Committee.
Three groups of pups were used for this study. The SEP1 and SEP3 groups received surgery to implant a telemetry device and were administered one and three, 3-hr separation tests, respectively, with the last test of SEP3 occurring at the same approximate age as the single test of SEP1. For SEP3, pups were separated on two consecutive days, and then again three days later (i.e. Days 1, 2, and 5). The SURG group received the same surgery to implant a telemetry device, but unlike the other groups, did not undergo separation testing. SURG pups served as a control for assessing the effect of the separation procedure on plasma cortisol levels. Each group was comprised of 11 or 12 pups with approximately equal numbers of males and females (SEP1: 6 males, 6 females; SEP3: 6 males, 6 females; SURG: 6 males, 5 females). No more than one pup from a litter was assigned to any condition. When a SEP1 and SEP3 pup were drawn from the same litter, they were always separated on different days.
2.2 Surgery and Telemetry
Telemetry probes (PD 4000 Emitter from Mini-Mitter Company) were surgically implanted into the abdominal cavity under isoflurane anesthesia (2–4%) using aseptic procedures between 16 and 19 days of age. Pups were treated with 0.05 mg/kg atropine (intraperitoneal) to reduce secretory activity. Buprenorphine (0.015 mg/0.05 ml) was given subcutaneously immediately following surgery and again 24 hr later. At least three days intervened between surgery and any experimental manipulation.
Core temperature was measured by the probe, which sent signals to a receiver plate (54.6 × 27.9 cm) placed under the cage during testing. The telemetry device also signaled movement of the pup. Temperature and motor activity data were recorded using computer software (Vital View) of the manufacturer, which collects data in 3-min bins.
2.3 Testing Procedure
Because of constraints involved in scheduling 4-hr tests with a single receiver plate, testing occurred over a range of several days. That is, for SEP3 the first test was between 21 and 25 days of age, with subsequent tests occurring exactly 1 and 4 days after the first. Pups in the other two groups were tested over a similar range at the oldest age, so that the average age of SEP3 pups at the time of their final test and blood sample collection (27.3 days) approximated the ages of pups tested and/or sampled in the SEP1 (27.7 days) and SURG (27.8 days) groups. The “pre-separation” phase of testing was designed to continuously monitor the core temperature of the pup in the home cage without the potential confound of passive heat exchange from physical contact with mother or littermates. To begin this phase, mother and litter were moved to a new home cage that was identical to the previous cage except for a white, plastic, mesh divider that separated its width into 45-cm and 28-cm sections. Mother and litter were placed into the larger section to allow habituation to the presence of the divider for at least 1 hr. The cage was then moved to a nearby table with the receiver plate under the smaller section of the cage. The subject pup was placed in the section over the receiver plate, while the mother and littermates remained on the other side. This prevented any influence on temperature data due to huddling with littermates or mother, but still permitted visual, auditory and olfactory contact between the subject pup and the other animals.
Immediately following the pre-separation phase, the pup was transported in a carrying cage to the adjoining testing room (~ 30 s) where it was placed alone into an empty, clear, plastic test cage (47 × 24 × 20 cm) positioned completely over the receiver plate to begin the “separation” phase. Temperature and activity were recorded for the next 3 hr. Additional behaviors were scored during Min 0–30, 60–90, and 150–180 from behind one-way glass by an observer trained to at least 85% inter-observer reliability. The number of whistle vocalizations (the primary behavior of the active stage) was tallied using a hand-held counter. A microphone positioned over the cage broadcast the sound to the headphones of the observer. In addition, the observer scored the number of 1-min intervals in which pups exhibited each of three passive, depressive-like behaviors characteristic of separated pups: (1) a distinctive crouched stance with feet pulled close to the body—this sometimes transitions into lying on the cage floor with the trunk supporting the body; (2) complete or near complete closure of at least one eye for at least 1 s; and, (3) piloerection over most of the body. The measure used in this study (full passive response) was the number of 1-min intervals in which all three passive behaviors were displayed [43].
2.4 Blood Collection and Hormone Determination
Immediately after a pup’s final separation or removal from the home cage (SURG group), it was exposed for 1 min to CO2 and decapitated. Trunk blood was collected on heparin and centrifuged for 20 min at 1,935 g. Separated plasma was stored at −20°C until assayed. As in previous studies, a commercial cortisol kit (Siemens “Coat-a-Count”) was used with plasma diluted 1:5. Intra-assay variability was calculated to be < 3%. All samples were collected within 2 min of the onset of disturbance.
2.5 Data Analysis
To further reduce temperature and activity data, analyses were based on 15-min time blocks. Data during the pre-separation and separation periods were analyzed separately with analysis of variance (ANOVA) procedures. When sphericity for within-subject comparisons was judged to be problematic by the Mauchley test, the Huynh-Feldt correction factor was used. Significant effects were followed with tests for simple main effects [53] and Newman-Keuls paired comparisons tests, as appropriate. Dunnett’s procedure was used to further assess significant changes in temperature over the course of separation. Paired-t-tests compared data from the last time-block of the pre-separation period with that from the first time block of the separation period. A significance level of p < 0.05 (two-tailed) was adopted throughout. There were no statistical outliers on any measure. During the pre-separation phase, several pups breached the mesh barrier to reunite with mother and littermates, resulting in loss of three subjects for some analyses of temperature and activity. In addition, three blood samples were missing, resulting in sample sizes of 11 for SEP1 and 10 for SEP3 for cortisol analysis.
3. Results
3.1 Core Temperature
3.1.1 Pre-separation
Changes in core temperature prior to repeated separation tests in the SEP3 group were assessed with a 2 (Gender) × 3 (Test) × 4 (Time Block) ANOVA, with the last two factors treated as repeated measures. No significant effects were obtained. Of interest here, the smallest numerical difference among tests was seen in the last time block of the pre-separation phase (Fig. 1, top panel). To examine temperature prior to a first and third separation in pups of the same age, a 2 (Group) × 2 (Gender) × 4 (Time Block) repeated measures ANOVA compared the temperature of pups in the SEP1 group prior to their separation with the temperature of pups in SEP3 prior to their third separation. The ANOVA yielded only a significant Group × Gender interaction, F (1, 19) = 6.78, p < 0.05. Core temperature tended to be higher prior to the first than the third separation for males (SEP1 = 39.24 +/− 0.06; SEP3 = 39.04 +/− 0.06), and higher prior to the third than the first separation for females (SEP1 = 39.08 +/− 0.06; SEP3 = 39.19 +/− 0.06). However, simple main effect tests indicated that neither of these individual differences was significant. As seen in Figure 1(bottom panel), pre-separation temperature of pups again tended to converge in the latter time blocks of the pre-separation period.
Fig. 1.
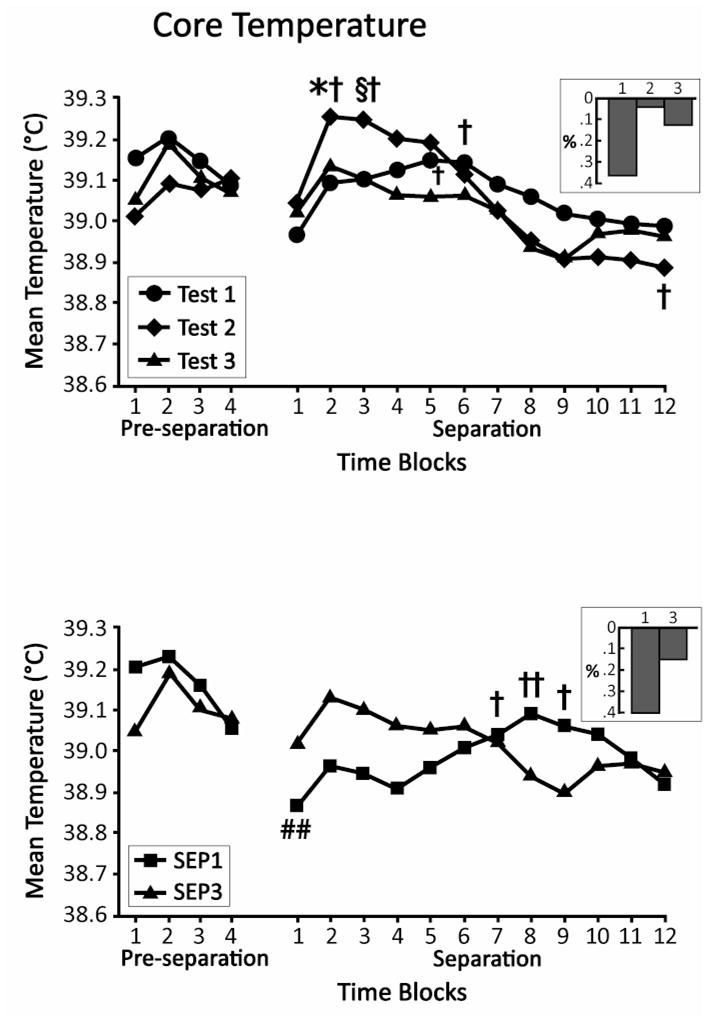
Mean core body temperature of SEP3 pups before and during their three separation tests (top) and of SEP1 pups during their separation test relative to the third separation test of SEP3, which occurred at a comparable age (bottom). On the left of each figure is shown temperature during the four, 15-min time blocks of the pre-separation period. On the right, temperature during the 12, 15-min blocks of the separation period is depicted. * p < 0.05 vs Test 1; § p < 0.05 vs Tests 1 and 3; † p < 0.05 vs Time Block 1; †† p < 0.01 vs Time Block 1; ## p < 0.01 vs Time Block 4 of pre-separation. Insets depict per cent change from Time Block 4 of pre-separation to Time Block 1 of separation.
3.1.2. Separation
A 2 (Gender) × 3 (Test) × 12 (Time Block) repeated measures ANOVA of changes in core temperature during repeated separations of the SEP3 pups yielded a significant Test × Time Block interaction, F (22, 220) = 1.89, p < 0.05. Simple main effects tests at each Time Block revealed differences across tests for Time Blocks 2 and 3 (p’s < 0.05). Newman-Keuls tests (p < 0.05) showed that temperature was higher during the second separation than during the first for Time Block 2, and higher during the second than during the first or third for Time Block 3 (Fig. 1, top panel). To evaluate fluctuations in temperature during the 3 hr of each individual separation, Dunnett’s tests compared the first to subsequent 15-min time blocks. During the first separation, core temperature was significantly higher during the 61st to the 75th minutes of separation (Time Blocks 5 & 6) than during the initial 15 min (p’s < 0.05). In comparison, during the second separation, temperature rose more rapidly, with significant elevations during Time Blocks 2 and 3 (16–45 min of separation; p’s < 0.05) and then showed a greater subsequent decline, which differed significantly from Time Block 1 during the final 15 min of separation (p < 0.05). During the third separation, there were no significant fluctuations relative to the initial 15 min.
For comparison of the difference in temperature during a first and third separation in pups of the same age (SEP1 vs SEP3), a 2 (Group) × 2 (Gender) × 12 (Time Block) repeated measures ANOVA yielded a significant main effect of Gender, F (1, 20) = 6.35, p < 0.05 (males > females), and a significant Group × Time Block interaction, F (3.7, 74.7) = 3.09, p < 0.05 (Huynh-Feldt correction), indicating that the pattern observed in the bottom panel of Figure 1 of relatively higher temperature during the initial portion of the separation period, and lower temperature in the later portion, in pups separated a third time as compared to those separated a first time at the same age, was statistically reliable. However, simple main effect tests indicated that the groups did not differ significantly at any single time block. Differences between the groups did emerge when examining changes in temperature over time. Whereas there were no fluctuations in temperature relative to the first 15 min for those pups separated a third time, those pups first isolated at this later age showed significant elevations during Time Blocks 7 (p < 0.05), 8 (p < 0.01), and 9 (p < 0.05). That is, pups first separated at this older age showed an even more gradual elevation in core temperature than pups first separated several days earlier.
3.1.3 Transition from pre-separation to separation
Unexpectedly, the temperature of pups appeared to drop between the end of pre-separation testing and the beginning of separation. To evaluate whether these apparent changes were statistically reliable, t-tests for paired samples compared body temperature during the last 15 min of pre-separation with first 15 min of separation. These comparisons were significant for SEP1 pups, t (11) = 3.23, p < 0.01, and approached significance for the first separation of SEP3 pups, t (11) = 2.08, p = 0.061(Fig. 1).
3.2 Activity
3.2.1. Pre-separation
The primary purpose for evaluating activity counts was to determine whether or not any changes in temperature were associated with motor activity of the pup. Activity was high during the early portion of the pre-separation period, and then generally declined (Fig. 2). This observation was verified by the ANOVAs for multiple separations of SEP3 pups as well as for the first and third separations of SEP1 and SEP3 pups. Both yielded only a significant effect of Blocks, F (3, 21) = 4.24, p < 0.05 and F (1, 18) = 20.78, p < 0.01, respectively.
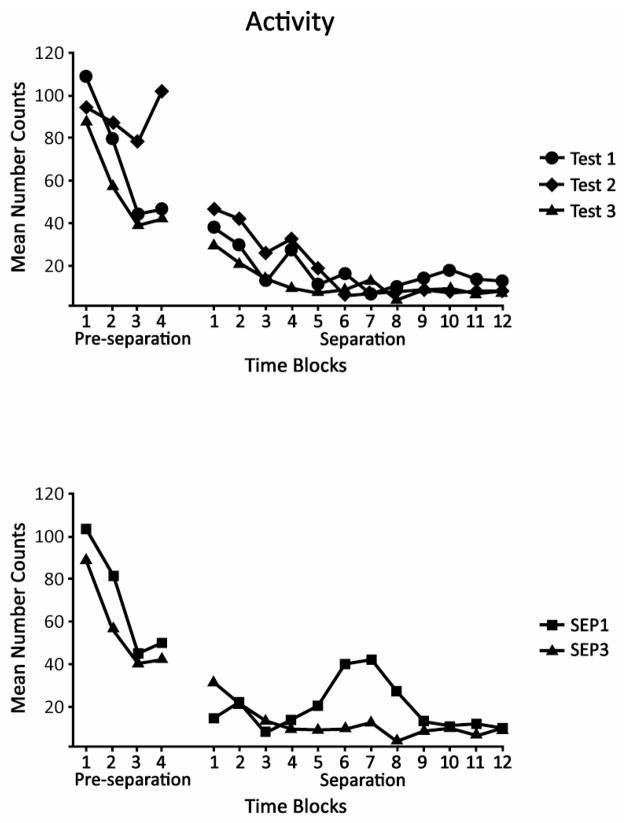
Fig. 2.
Mean activity counts of SEP3 pups before and during their three separation tests (top) and of SEP1 pups during their separation test relative to the third separation test of SEP3, which occurred at a comparable age (bottom). On the left of each figure is shown activity during the four, 15-min time blocks of the pre-separation period. On the right, activity during the 12, 15-min intervals of the separation period is depicted. There were no significant differences among Tests 1–3 or between SEP1 and SEP3.
3.2.2. Separation
During separation, activity—unlike core temperature—did not vary across the separations of SEP3 (Fig. 2, top panel). The only significant outcomes were a main effect of Blocks, F (1, 10) = 7.20, p < 0.01, and Gender × Blocks interaction, F (1, 10) = 2.10, p < 0.05. Whereas pups of both genders showed a reduction in activity across blocks, the decline was more rapid for females than for males (data not shown). For the comparison of SEP1 and SEP3 there were no significant effects (Fig. 2, bottom panel). In sum, changes in activity during separation were not associated with changes in core temperature.
3.2.3. Transition from pre-separation to separation
Because activity counts, like body temperature, showed numerical declines from the end of pre-separation to the beginning of separation, differences from the last 15 min of pre-separation to the first 15 min of separation were again examined with t-tests. These comparisons were not significant, though the difference for the SEP1 group approached significance, t (10) = 2.08, p = 0.064 (Fig. 2, bottom panel).
3.3. Passive Response
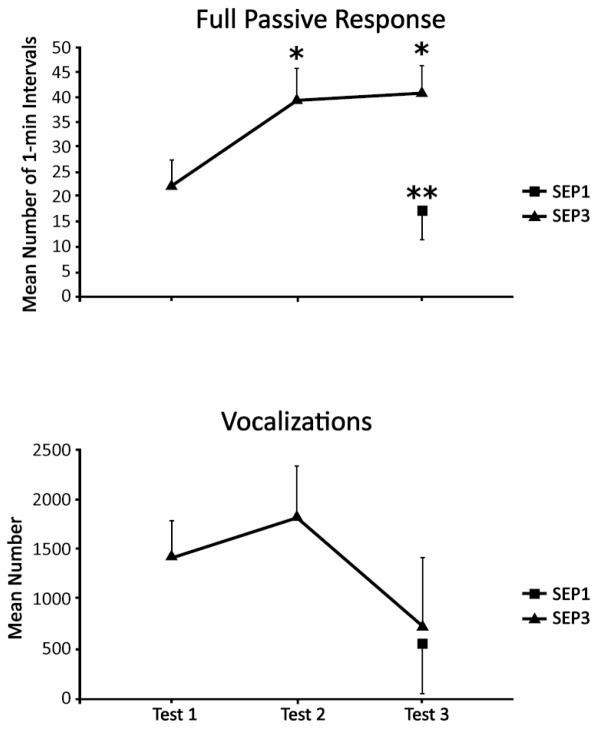
To compare the full passive response in the SEP3 group across separations, a 2 (Gender) × 3 (Test) ANOVA resulted in a significant effect of Test, F (2, 20) = 6.24, p < 0.01. Newman-Keuls paired-comparisons showed that pups exhibited more of the full passive response during the second and third separations than they did during the first (p’s <0.05; Fig. 3, top panel). The 2 (Group) × 2 (Gender) ANOVA comparing the groups separated a first (SEP1) or third (SEP3) time at the same age yielded a significant effect of Group, F (1, 20) = 11.47, p < 0.01, indicating more passive behavior in pups separated a third time, thus confirming the sensitization effect.
Fig. 3.
Mean number of 1-min intervals that pups in the SEP3 group exhibited the full passive response (top) and the mean number of vocalizations of SEP3 pups (bottom) during each of their three separations. Values for SEP1 pups (tested at the oldest age) are shown for comparison. Vertical lines represent standard errors of the means. * p < 0.05 vs Test 1; ** p < 0.01 vs SEP3
3.4 Vocalizations
The mean level of vocalizing diminished by the third separation (Fig. 3, bottom panel). However, the ANOVA for repeated separations showed that this effect only approached significance (p < 0.07), reflecting considerable variation in this measure. Similarly, the 2 (Group) × 2 (Gender) ANOVA comparing first and third separations on the same day yielded no significant effects, indicating that the vocalizations of the 27-day-old animals were comparable during a first and third separation.
3.5 Cortisol
For cortisol, a 3 (Group) × 2 (Gender) ANOVA produced one significant effect, a main effect of Group, F (2, 26) = 19.38, p < 0.01. Newman-Keuls paired comparisons showed that while both a first and third separation produced cortisol elevations relative to a non-separated group receiving the same surgery, the response to the first separation was greater than that to the third. Specifically, values for SEP1 were greater than those for either SEP3 (p < 0.01) or SURG (p < 0.01), and cortisol levels for SEP3 were greater than those for SURG (p < 0.05; Fig. 4).
Fig. 4.
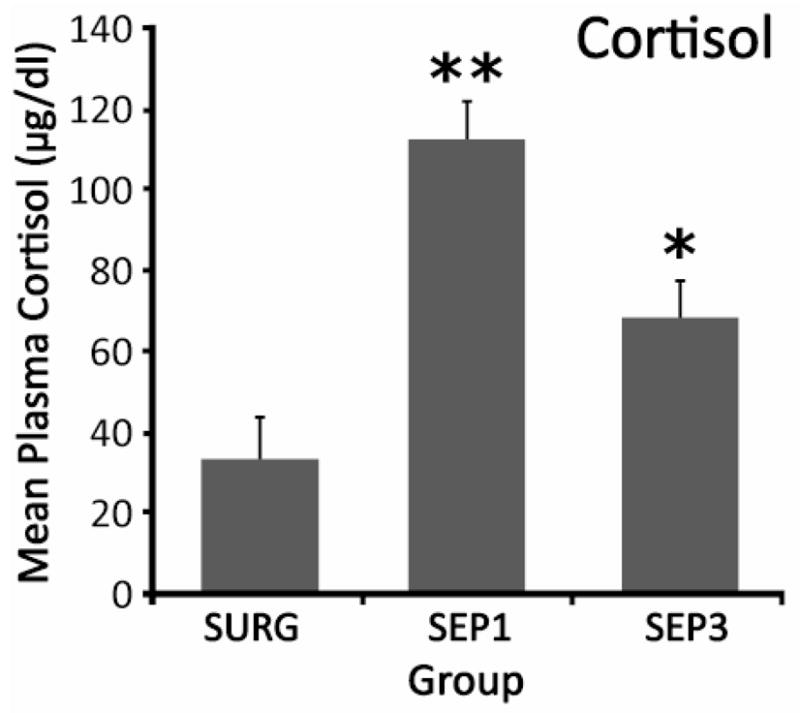
Mean plasma cortisol levels of pups of the three groups. Vertical lines indicate standard errors of the means. * p < 0.05 vs SURG; **p < 0.01 vs SURG and SEP3
4. Discussion
In the present study, separated guinea pig pups exhibited behaviors characteristic of both the initial active phase of separation (vocalizing) and the depressive-like passive stage that typically follows (crouching, eye-closure, piloerection). As in a recent study [28], pups showed increased levels of depressive-like behavior during both a second, 3-hr separation that occurred 24 hr after the first, as well as during a third separation another 3 days later. Unlike the earlier study, we included age-matched controls, which confirmed that the elevated level of depressive-like behavior at the oldest age was due to repeated separations, as opposed to increasing age. In contrast to the passive behavior, vocalizing tended to decrease over separations, though this effect appeared to be due to increasing age. This dissociation is consistent with the view that the separation vocalizations of guinea pig pups represent anxiety-like, rather than depression-like, behavior [31,55]. Because we have hypothesized that the sensitization of the passive, depressive-like behavior is mediated by enhanced proinflammatory activity, and because proinflammatory signaling initiates fever, the primary purpose of the present study was to examine the relation between depressive-like behavior and fever over repeated separations.
Fever is a regulated increase in core body temperature (i.e. elevated “set point”) as distinguished from, for instance, a rise in temperature due to proximity to a heat source or an inability to dissipate heat following exercise. Exposure to stressors can induce a febrile response driven by proinflammatory activity [36]. However, stressors also produce typically brief elevations in body temperature (stress-induced hyperthermia) that may be mediated by other factors, such as increased sympathetic output [51]. In the present experiment, the procedure for measuring temperature prior to separation was designed to avoid any confound due to physical contact with the heat sources afforded by the mother and littermates. These pre-separation measurements were conducted over an hr in anticipation of an initial stress-induced hyperthermia that would resolve toward base levels by the end of the pre-separation session. Instead we found no indication that a baseline measurement was ever reached; rather levels during pre-separation approximated those observed in guinea pig pups following LPS injection in a prior study [7]. Core temperature during the pre-separation period may have been influenced not only by stress-induced hyperthermia, but also by the exertion of the pups as they attempted to regain access to their mother and siblings. This interpretation is supported by the higher activity counts, particularly during the early portion of the pre-separation period, our own informal observations of the pups’ behavior at the mesh barrier, and by the loss of data of several pups that managed to breach the barrier to re-join their cage mates. Further evidence that core temperature during all pre-separation time blocks was elevated over resting body temperature comes from an earlier study in which we simply removed 3-week-old pups from the home cage or the separation environment and measured their temperature with a rectal thermometer [24]. In that study, temperature during an initial separation was elevated at 90 min, but not 180 min, over home cage levels. This is what one would expect if the pre-separation values of core temperature of pups of comparable age and experience in the present experiment (SEP3 pups during their first separation) were, in fact, elevated over resting levels, and help confirm that peak temperature levels during separation in the present study reflect actual increases over basal temperature.
In our previous published study using the telemetry device to continuously monitor temperature during separation [23], pups separated for the first time showed a gradual increase in core temperature, whereas temperature during separation the next day was significantly higher during the first 45 min and then declined more rapidly. Thus, it was not possible to determine if the difference was due to an actual change in response to separation, or to a difference at the time separation began, such as an elevated basal temperature or an anticipatory response to subtle environmental cues the second day. Because such effects have been reported in the literature [13, 42], resolution of the question was a priority. In the present experiment, we saw patterns of core temperature change during separation on 2 consecutive days that were very similar to those of the previous study: a slower increase during the first separation and significantly higher values during the early portion of separation on Day 2 compared to Day 1. Importantly, these effects were obtained even though core temperature values were equivalent during the last time block of the pre-separation period. Thus, we conclude that the elevation during the early portion of the second separation represents an actual change in response to the second separation. This conclusion is bolstered by the observation that in the present experiment, as opposed to the earlier one, differences between the first and second separation were not statistically significant during the first time block. These findings also fit nicely with other published work showing that prior stressor exposure leads to a more-rapid febrile response during later immune challenge [33].
During the third separation, occurring 3 days after the second, we found evidence for the same differential pattern of response relative to the first separation. When compared to age-matched controls separated a first time, the pattern of higher temperature during the early portion of the separation followed by lower temperature during the latter portion again was significant, though seemingly somewhat less distinct. Again there were no differences between these groups during the last time block of the pre-separation period. This persistence of the short-latency temperature rise during repeated separations shows that the effect on temperature is more than just a transient change during the first 24 hr.
Moreover, correspondence between this pattern of temperature change and the increase in passive behavior observed during second and third separations suggests these responses may be functionally linked. Our earlier finding that administration of an anti-inflammatory prior to an initial separation prevented sensitization of the full passive response during separation the following day [28] implicates proinflammatory processes in the behavioral sensitization. The current findings provide additional support for this interpretation. That is, a more-rapid febrile response during the second and third separations may indicate a more-rapid proinflammatory response, which may have then led to more passive behavior occurring during the 3-hr separation test. Prior studies in rats have shown that naturally varying levels of cytokines in brain are predictive of more-rapid febrile responses to novel environment exposure [3]. While we have yet to demonstrate differences in central proinflammatory activity associated with prior separations, the present findings provide strong indirect support for a role for proinflammatory cytokines/processes in mediating sensitized behavioral responses to repeated separation.
We also found that cortisol levels at the conclusion of a third separation were lower than levels following a first separation. While these results are very preliminary (e.g., cortisol responses were not measured following a second separation; only one time point was assessed), they suggest that reduced glucocorticoid suppression of proinflammatory processes during subsequent separations may help account for the more-rapid febrile response and increased passive behavior. In rats and mice, lasting effects of early separation on glucocorticoid levels and other measures often appear to be mediated by the treatment of the pups by the mother upon return to the home cage [e.g.,30,38]. In the precocial guinea pig, by contrast, active maternal care rarely is observed beyond the first week postpartum [35], even following separation and reunion [25]. Thus, one can conclude with relative certainty that effects observed in the later separations of the current study were not due to the mother’s care-taking activities following earlier separations.
In laboratory rodents, procedures that induce a proinflammatory reaction during infancy have been found to have a variety of effects on proinflammatory activity, as well as behavioral and endocrine stress responses, at later ages. While these effects are variable [21], later proinflammatory activity and responses to stressors often have been found to be enhanced [e.g., 5, 6, 8, 49, 52]. The results of the present experiment build on those of other studies with guinea pigs [e.g., 21, 28] that together suggest proinflammatory activity not only underlies the depressive-like response to an initial separation, but also can promote increased depressive-like responses to later separations. Further, there is now a substantial literature indicating that proinflammatory activity is a mediator of forms of depressive illness in adulthood [2, 12], while other human studies have demonstrated that early adversity is associated with later enhanced proinflammatory activity and depression [10, 11, 39]. In total, there is mounting evidence that part of the means by which filial attachment disruption and other forms of early adverse experience increase vulnerability for the development of depressive illness in later life may involve the interaction of neural and endocrine systems with proinflammatory processes.
Highlights.
Depressive-like behavior sensitized during repeated maternal separations
Repeated separation produced more-rapid, stress-induced febrile response
Behavioral sensitization and accelerated febrile response persisted at least 3 days
Cortisol levels decreased with repeated separation
Proinflammatory signaling may mediate sensitization
Acknowledgments
The authors thank Cohen Carlisle, Chris Fitch, and Vincent Alexander for technical assistance. This work was supported by grant MH068228 from the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, Troudart T, Bloch M, Heresco-Levy U, Lerer B. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4:163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- 2.Anisman H. Cascading effects of stressors and inflammatory immune system activation: implications for major depression. J psychiatry Neurosci. 2009;34:4–20. [PMC free article] [PubMed] [Google Scholar]
- 3.Barnum CJ, Blandino P, Jr, Deak T. Social status modulates basal IL-1 concentrations in the hypothalamus of pair-housed rats and influences certain features of stress reactivity. Brain Behav Immun. 2008;22:517–527. doi: 10.1016/j.bbi.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Bernet CZ, Stein MB. Relationship of childhood maltreatment to the onset and course of major depression in adulthood. Depress Anxiety. 1999;9:169–174. [PubMed] [Google Scholar]
- 5.Bilbo SD, Newsum NJ, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Differential effects of neonatal handling on early life infection-induced alterations in cognition in adulthood. Brain Behav Immun. 2007;21:332–342. doi: 10.1016/j.bbi.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Bilbo SD, Wieseler J, Barrientos RM, Watkins LR, Maier SF. Rats infected early in life with bacteria exhibit exaggerated fever, sickness behavior, and lack of endotoxin tolerance in adulthood. Brain Behav Immun. 2008;22 (Suppl):3–4. [Google Scholar]
- 7.Blatteis CM. Postnatal development of pyrogenic sensitivity in guinea pigs. J Appl Physiol. 1975;39:251–257. doi: 10.1152/jappl.1975.39.2.251. [DOI] [PubMed] [Google Scholar]
- 8.Breivik T, Stephan M, Brabant GE, Straub RH, Pabst R, von Horsten S. Postnatal lipopolysacchride-induced illness predisposes to periodontal disease in adulthood. Brain Behav Immun. 2002;6:421–438. doi: 10.1006/brbi.2001.0642. [DOI] [PubMed] [Google Scholar]
- 9.Brown GW, Harris T, Copeland JR. Depression and loss. Brit J Psychiatry. 1977;130:1–18. doi: 10.1192/bjp.130.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychoparmacol. 2010;35:2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–416. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Rev Neurosci. 2008;9:46–57. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deak T, Meriwether JL, Fleshner M, Spencer RL, Abouhamze A, Moldawer LL, Grahn RE, Watkins LR, Maier SF. Evidence that brief stress may induce the acute phase response in rats. Am J Physiol. 1997;273:R1998–R2004. doi: 10.1152/ajpregu.1997.273.6.R1998. [DOI] [PubMed] [Google Scholar]
- 14.Gillespie CF, Nemeroff CB. Corticotropin-releasing factor and the psychobiology of early-life stress. Cur Dir Psychol Sci. 2007;16:85–89. [Google Scholar]
- 15.Gilmer WS, McKinney WT. Early experience and depressive disorders: human and non-human primate studies. J Affect Disord. 2003;75:97–113. doi: 10.1016/s0165-0327(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 16.Gold PW, Goodwin FK, Chrousos GP. Clinical and biochemical manifestations of depression: relation to the neurobiology of stress (Part 2) New Eng J Med. 1988;319:413–420. doi: 10.1056/NEJM198808183190706. [DOI] [PubMed] [Google Scholar]
- 17.Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13:717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- 18.Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- 19.Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biol Psychiatry. 1999;46:1509–1522. doi: 10.1016/s0006-3223(99)00224-3. [DOI] [PubMed] [Google Scholar]
- 20.Hennessy MB. Enduring maternal influences in a precocial rodent. Dev Psychobiol. 2003;42:225–336. doi: 10.1002/dev.10095. [DOI] [PubMed] [Google Scholar]
- 21.Hennessy MB, Deak T, Schiml-Webb PA. Early attachment-figure separation and increased risk for later depression: potential mediation by proinflammatory processes. Neurosci Biobehav Rev. 2010;34:782–790. doi: 10.1016/j.neubiorev.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hennessy MB, Deak T, Schiml-Webb PA, Barnum CJ. Immune influences on behavior and endocrine activity in early-experience and maternal separation paradigms. In: Czerbska MT, editor. Psychoneuroendocrinology Research Trends. Hauppauge, NY: Nova Science Publishers; 2007. pp. 293–319. [Google Scholar]
- 23.Hennessy MB, Deak T, Schiml-Webb PA, Carlisle CW, O’Brien E. Maternal separation produces, and a second separation enhances, core temperature and passive behavioral responses in guinea pig pups. Physiol Behav. 2010;100:305–310. doi: 10.1016/j.physbeh.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hennessy MB, Deak T, Schiml-Webb PA, Wilson SE, Greenlee TM, McCall E. Responses of guinea pig pups during isolation in a novel environment may represent stress-induced sickness behaviors. Physiol Behav. 2004;81:5–13. doi: 10.1016/j.physbeh.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Hennessy MB, Jenkins R. A descriptive analysis of nursing behavior in the guinea pig (Cavia porcellus) J Comp Psychol. 1994;108:23–28. doi: 10.1037/0735-7036.108.1.23. [DOI] [PubMed] [Google Scholar]
- 26.Hennessy MB, Long SJ, Nigh CK, Williams WT, Nolan DJ. Effects of peripherally administered corticotropin-releasing factor (CRF) and a CRF antagonist: Does peripheral CRF activity mediate behavior of guinea pig pups during isolation? Behavioral Neuroscience. 1995;109:1137–1145. doi: 10.1037//0735-7044.109.6.1137. [DOI] [PubMed] [Google Scholar]
- 27.Hennessy MB, Moorman L. Factors influencing cortisol and behavioral responses to maternal separation in guinea pigs. Behav Neurosci. 1989;103:378–385. doi: 10.1037//0735-7044.103.2.378. [DOI] [PubMed] [Google Scholar]
- 28.Hennessy MB, Paik KD, Caraway JD, Schiml-Webb PA, Deak T. Proinflammatory activity and the sensitization of depressive-like behavior during maternal separation. Behav Neurosci. 2011;125:426–433. doi: 10.1037/a0023559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hennessy MB, Schiml-Webb PA, Miller EE, Maken DS, Bullinger KL, Deak T. Anti-inflammatory agents attenuate the passive responses of guinea pig pups: Evidence for stress-induced sickness behavior during maternal separation. Psychoneuroendocrinol. 2007;32:508–515. doi: 10.1016/j.psyneuen.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hennessy MB, Vogt J, Levine S. Strain of mother determines long-term effects of early handling: Evidence for maternal mediation. Physiol Psychol. 1982;10:153–157. [Google Scholar]
- 31.Hodgson RA, Higgins GA, Guthrie DH, Lu SX, Pond AJ, Mullins DE, Guzzi MF, Parker EM, Varty GB. Comparison of the V1b antagonist SSR149415 and the CRF1 antagonist, CP-154,526, in rodent models of anxiety and depression. Pharmacol Biochem Behav. 2007;86:431–440. doi: 10.1016/j.pbb.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 32.Jäckel M, Trillmich F. Olfactory individual recognition of mothers by young guinea pigs (Cavia porcellus) Ethol. 2003;109:197–208. [Google Scholar]
- 33.Johnson JD, O’Connor KA, Hansen MK, Watkins LR, Maier SF. Effects of prior stress on LPS-induced cytokine and sickness responses. Am J Physiol. 2003;284:R422–R432. doi: 10.1152/ajpregu.00230.2002. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman IC, Rosenblum LA. The reaction to separation in infant monkeys: Anaclitic depression and conservation withdrawal. Psychosom Med. 1967;29:648–675. doi: 10.1097/00006842-196711000-00010. [DOI] [PubMed] [Google Scholar]
- 35.König B. Maternal activity budget during lactation in two species of Caviidae (Cavia porcellus and Galea musteloides) Zeit Tierpsychol. 1985;68:215–230. [Google Scholar]
- 36.Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- 37.Maken DS, Weinberg J, Cool D, Hennessy MB. An investigation of the effects of maternal separation and novelty on central mechanisms mediating pituitary-adrenal activity in infant guinea pigs. Behav Neurosci. 2010;124:800–809. doi: 10.1037/a0021465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- 39.Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol Sci. 2010;21:848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mineka S, Suomi SJ. Social separation in monkeys. Psych Bull. 1978;85:1376–1400. [PubMed] [Google Scholar]
- 41.Miura H, Ozaki N, Sawada M, Isobe K, Ohta T, Nagatsu T. A link between stress and depression: shift in the balance between the kynurenine and serontonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress. 2008;11:198–209. doi: 10.1080/10253890701754068. [DOI] [PubMed] [Google Scholar]
- 42.Oka T, Oka K, Hori T. Mechanisms and mediators of psychological stress-induced rise in core temperature. Psychosom Med. 2001;63:476–486. doi: 10.1097/00006842-200105000-00018. [DOI] [PubMed] [Google Scholar]
- 43.Perkeybile AM, Schiml-Webb PA, O’Brien E, Deak T, Hennessy MB. Anti-inflammatory influences on behavioral, but not cortisol, responses during maternal separation. Psychoneuroendocrinol. 2009;34:1101–1108. doi: 10.1016/j.psyneuen.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pettijohn TF. Attachment and separation distress in the infant guinea pig. Dev Psychobiol. 1979;12:73–91. doi: 10.1002/dev.420120109. [DOI] [PubMed] [Google Scholar]
- 45.Reinherz HZ, Giaconia RM, Carmola Hauf AM, Wasserman MS, Silverman AB. Major depression in the transition to adulthood: risks and impairments. J Abnorm Psychol. 1999;108:500–510. doi: 10.1037//0021-843x.108.3.500. [DOI] [PubMed] [Google Scholar]
- 46.Robertson J. Nursing Times. Apr 18, 1953. Some responses of young children to the loss of maternal care; pp. 382–386. [Google Scholar]
- 47.Schiml-Webb PA, Deak T, Greenlee TM, Maken DS, Hennessy MB. Alpha-melanocyte stimulating hormone reduces putative stress-induced sickness behaviors in isolated guinea pig pups. Behav Brain Res. 2006;168:326–330. doi: 10.1016/j.bbr.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 48.Schulkin J, McEwen BS, Gold PW. Allostasis, Amygdala, and Anticipatory Angst. Neurosci Biobeh Rev. 1994;18:385–396. doi: 10.1016/0149-7634(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 49.Shanks N, Larocque S, Meaney MJ. Neonatal endotoxin exposure alters the development of the hypothalamic-pituitary-adrenal axis: early illness and later responsivity to stress. J Neurosci. 1995;15:376–384. doi: 10.1523/JNEUROSCI.15-01-00376.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spitz RA. Anaclitic depression: an inquiry into the genesis of psychiatric conditions in early childhood: II Psychoanal Study. Child. 1946;2:313–342. [PubMed] [Google Scholar]
- 51.Vinkers CH, Groenink L, van Bogaert MJV, Westphal KGC, Kalkman CJ, van Oorschot R, Oosting RS, Olivier B, Korte SM. Stress-induced hyperthermia and infection-induced fever: two of a kind? Physiol Behav. 2009;98:37–43. doi: 10.1016/j.physbeh.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Walker FR, March J, Hodgson DM. Endotoxin exposure in early life alters the development of anxiety-like behaviour in the Fischer 344 rat. Behav Brain Res. 2004;154:63–69. doi: 10.1016/j.bbr.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 53.Winer BJ. Statistical Principles in Experimental Design. New York: McGraw-Hill; 1971. [Google Scholar]
- 54.Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- 55.Yokoyama F, Yamauchi M, Oyama M, Okuma K, Onozawa K, Nagayama T, Shinei R, Ishikawa M, Sato Y, Kakui N. Anxiolytic-like profiles of histamine H3 receptor agonists in animal models of anxiety: a comparative study with antidepressants and benzodiazepine anxiolytic. Psychopharmacol. 2009;205:177–187. doi: 10.1007/s00213-009-1528-1. [DOI] [PubMed] [Google Scholar]