Abstract
The serum- and glucocorticoid-inducible kinase 1 (SGK1) is known to regulate a wide variety of cellular processes, including renal sodium retention and cell survival. Angiotensin II (Ang II) is one of the many signaling molecules capable of regulating SGK1 expression, and is also known to impact cell survival. Here, we examined the role of SGK1 in Ang II-mediated cell survival. We hypothesized that Ang II protects cells from apoptosis by upregulating and activating SGK1. To test this, we examined the effects of Ang II stimulation on SGK1 expression and downstream signaling. We also examined the effects of Ang II treatment and siRNA-mediated SGK1 knockdown on apoptosis after serum starvation. We found that after 2 h of Ang II treatment, SGK1 mRNA expression was increased approximately 2- fold. This induction was sensitive to reductions in intracellular calcium levels after pretreatment with BAPTA-AM, but insensitive to the L-type calcium channel blocker verapamil. SGK1 induction was also sensitive to the tyrosine kinase inhibitor genistein. Ang II treatment also caused a rapid increase in the level of phosphorylation of SGK1 at Ser422 and Thr256, and Ser422 phosphorylation was rapamycin-sensitive. We found that Ang II treatment was protective against serum starvation-induced apoptosis, and this protective effect was significantly blunted when SGK1 was silenced via siRNA. Lastly, Ang II induced FOXO3A phosphorylation in an SGK1-dependent manner, thereby reducing the pro-apoptotic actions of FOXO3A. Overall, these results indicate that Ang II upregulates and activates SGK1, leading to increased cell survival via multiple, non-redundant mechanisms.
Keywords: Angiotensin II, SGK1, cell survival, FOXO3A, apoptosis
1. Introduction
The serum and glucocorticoid inducible kinase 1 (SGK1) is a ubiquitously expressed serine/threonine kinase that regulates a wide variety of ion channels and transporters. SGK1 plays a key role in renal sodium retention and potassium excretion by regulating ENaC, ROMK, and Na+-K+-ATPase activity [1]. The expression of SGK1 is controlled by a variety of stimuli, including numerous hormones and cytokines [1]. Among its many functions, SGK1 is known to have anti-apoptotic effects through regulation of transcription factors such as FOXO3A [2]. SGK1 expression is elevated during wound healing and in several fibrotic diseases, including diabetic nephropathy and cardiac fibrosis [1]. Additionally, SGK1 has been shown to promote cell survival in breast cancer cells, and contributes to glucocorticoid-induced chemotherapy resistance in breast cancer [3–4].
Angiotensin II (Ang II) is the principle signaling molecule in the renin-angiotensin-aldosterone system (RAAS). It acts through the Ang II Type 1 (AT1) receptor to facilitate cell growth and pro-survival effects in a variety of cell types [5]. It has been shown that Ang II induces SGK1 expression in murine fibroblasts, causing increased expression of connective tissue growth factor (CTGF) [6]. This pathway is believed to contribute to the pro-fibrotic effects of Ang II. It has also been demonstrated that Ang II has a protective effect against apoptosis in fibroblast-like synoviocytes [7]. Additionally, Ang II has been shown to play a role in a number of cancers due to its proliferative effects via the AT1 receptor [8–10]. However, whether Ang II can directly act through SGK1 in order to promote cell survival is unknown.
In the current study, we investigated the role of SGK1 in Ang II-mediated cell survival. Here, we hypothesized that Ang II would protect fibroblast-derived cells from serum starvation-induced apoptosis by upregulating and/or activating SGK1. To investigate this, we examined SGK1 mRNA expression, phosphorylation, downstream signaling, and apoptosis in a fibroblast-derived cell line in response to Ang II. We found that Ang II increased SGK1 mRNA and protein levels and increased the phosphorylation levels of SGK1 protein at Thr256 and Ser422. The Ang II-mediated increase in SGK1 mRNA levels required intracellular calcium mobilization and tyrosine kinase activation while the phosphorylation of SGK1 at Ser422 was mTOR-dependent. The consequence of Ang II-mediated SGK1 expression/activation was phosphorylation of FOXO3A and a subsequent increase in cell survival. As such, these results are significant in that they demonstrate a novel role of SGK1 in Ang II-mediated cell survival.
2. Materials and Methods
2.1 Cell Lines
The fibrosarcoma-derived cells stably expressing the AT1 receptor have been previously described [11]. Cells were cultured in DMEM supplemented with 10% FBS, penicillin, streptomycin, Zeocin, G418 and L-glutamine at 37°C and 5% CO2. For serum starvation, cells were cultured in DMEM supplemented with 0.5% BSA (wt/vol), penicillin, streptomycin, and L-glutamine at 37°C and 5% CO2.
2.2 Ang II, Inhibitor Compounds, and Cell Treatments
Ang II (Sigma) was stored in ddH2O at −20°C at a stock concentration of 10 μM. BAPTA-AM (Enzo Life Sciences) and PD98059 (Calbiochem) were stored at −20°C in DMSO at a stock concentration of 5 mM. Verapamil (Enzo Life Sciences), Genistein (Sigma), and Go6983 (Tocris) were stored at −20°C in DMSO at a stock concentration of 10 mM. Rapamycin (Sigma) was stored at −20°C in ethanol at a stock concentration of 500 μM.
For mRNA and protein analyses, cells were serum starved overnight and were then treated with a 100 nM concentration of Ang II for the indicated periods of time. In drug pre-treatment experiments, cells were serum starved overnight and pre-treated with the following inhibitors prior to Ang II stimulation: BAPTA-AM (50 μM, 10 min), verapamil (10 μM, 5 min), PD98059 (50 μM, 1h), Go6983 (10 μM, 1h), genistein (100 μM, 1h), or rapamycin (0.75 – 25 nM, 1h). After appropriate inhibitor pre-treatment, the cells were treated with 100 nM Ang II and processed as described in each figure.
2.3 Quantitative RT-PCR
For quantitative RT-PCR, RNA was extracted using an RNeasy mini kit (Qiagen) according to the manufacturer's protocol. 2 μg of each RNA sample was reverse transcribed into cDNA in a final reaction volume of 20 μl using a high capacity cDNA reverse transcription kit (Applied Biosystems). SGK1 mRNA levels were determined using the TaqMan gene expression assay Hs00178612_m1 (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase expression was determined as an internal loading control using the assay Hs02758991_g1 (Applied Biosystems). Real time PCR was performed with TaqMan universal PCR Master Mix (Applied Biosystems) in a final reaction volume of 20 μl in a StepOne real time PCR system according to the manufacturer's protocol.
2.4 Western blotting
Cells were lysed in radioimmunoprecipitation assay buffer on ice and protein concentration was determined using a Bradford assay (Bio-Rad). Approximately 50 μg of soluble protein was separated by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked using 5% milk/TBS-T for 1 h. The following primary antibodies were obtained from Santa Cruz and were used at a 1:500 dilution: pSGK1 (Ser422), pSGK1 (Thr256), pFOXO3A (Thr32), and FOXO3A. The β-actin primary antibody (Cell Signaling Technology) and the total SGK1 antibody (Sigma) were also used at a 1:500 dilution. An HRP-conjugated anti-rabbit secondary antibody (GE Healthcare) was used at 1:4000. Bands were visualized using the enhanced chemiluminescence system (Western Lightning Ultra; PerkinElmer Life Sciences).
2.5 Transfection with siRNA
Cells were transfected using DharmaFECT4 transfection reagent (Dharmacon) and a 25 nM concentration of SmartPool siRNA targeting SGK1 or a non-targeting SmartPool siRNA (Dharmacon). Cells were plated in a 6-well plate and incubated with transfection reagents for 6 h in antibiotic-free media. Cells were then allowed to recover overnight in complete media prior to experiments.
2.6 TUNEL staining
After siRNA transfection and recovery, cells were transferred to 8-well chamber slides for TUNEL assay. Cells were subsequently serum starved for 48 h in the presence or absence of 100 nM Ang II. Cells were fixed in 1% paraformaldehyde at room temperature for 10 min, washed 2× with PBS, and post-fixed with ethanol:acetic acid (2:1) for 5 min at −20°C. TUNEL staining was performed using the ApopTag Fluorescein In Situ Apoptosis Detection Kit (Millipore) according to the manufacturer's protocol. Images were obtained using an Olympus fluorescent microscope at 20× magnification.
2.7 Statistics
Statistical evaluation was performed using Student's t-test. Conditions were considered statistically significant when p < 0.05.
3. Results
3.1 Ang II increases SGK1 mRNA and protein levels in a calcium- and tyrosine kinase-dependent manner
Aldosterone is known to increase SGK1 expression within kidney epithelial cells leading to increased Na+ retention [1]. Ang II has been shown to similarly increase SGK1 expression in fibroblasts, but the cellular outcome is increased expression of the pro-fibrotic marker CTGF, rather than increased Na+ reabsorption [6]. These results indicate that SGK1 mediates distinct cellular outcomes in response to different stimuli. In the case of Ang II-mediated SGK1 activation in fibroblasts, the specific signaling pathways that mediate this effect and the overall impact on cell fate have not previously been determined. Therefore, in order to provide some fundamental understanding of these critical events, we employed a fibrosarcoma-derived cell line that stably expresses the AT1 receptor and examined Ang II/SGK1 signaling within this system.
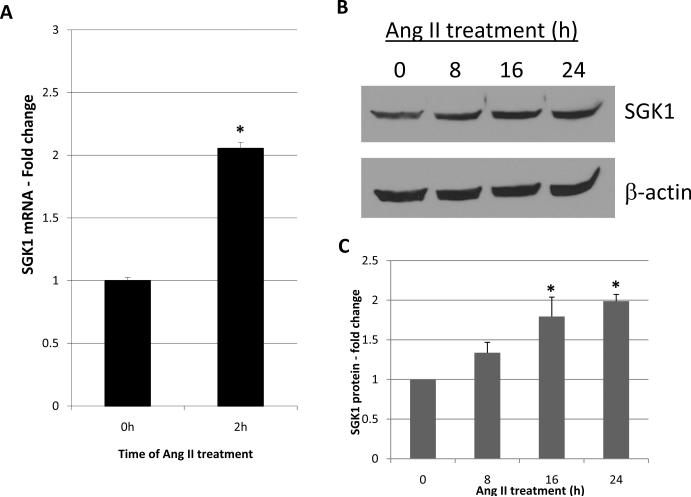
The creation and characterization of these cells has been previously published [11]. In short, they robustly express the AT1 receptor, but lack AT2 receptor expression. In addition to being highly responsive to Ang II, they readily express SGK1. We first wanted to determine if Ang II treatment can increase SGK1 mRNA levels in these cells as it does in cultured fibroblasts. For this, the cells were treated with Ang II for 2 h and SGK1 mRNA levels were examined by quantitative RT-PCR. We found that 2 h of Ang II treatment increased SGK1 mRNA levels by 2-fold (Figure 1A). To determine if SGK1 protein levels were also increased, cells were treated with Ang II for 0, 8, 16, and 24 h and SGK1 protein levels were analyzed by Western blot. We found that SGK1 protein levels were significantly increased at 16 and 24 h with a peak increase of ~2-fold observed at the 24 h time point (Figure 1B and C). Overall, these results indicate that Ang II increases SGK1 mRNA and protein levels in these cells. Furthermore, the results are virtually identical to those observed in human and mouse fibroblasts [6].
Figure 1.
Increase in SGK1 expression in response to Ang II. A, Cells were serum starved overnight and treated with Ang II for 0 or 2h and SGK1 mRNA was quantified by qRT-PCR. n=6. *, p<0.05 vs. 0 h control. B, Cells were serum starved overnight and treated with Ang II for 0, 8, 16, or 24 h and SGK1 protein levels were analyzed by Western blot. C, SGK1 protein levels were quantified using densitometry and normalized to β-actin. n=3. *, p<0.05 vs. 0 h control.
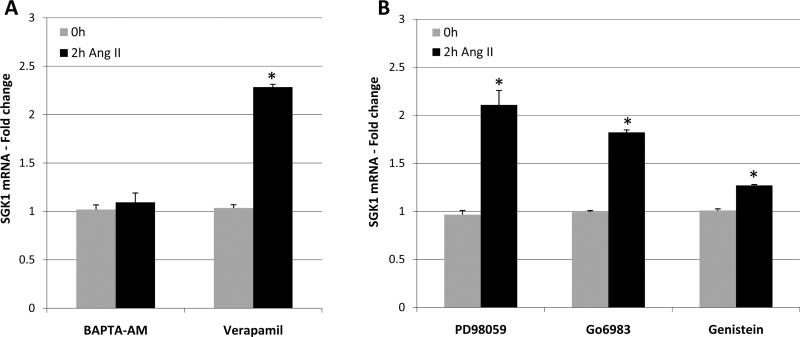
SGK1 mRNA expression is regulated by a variety of signaling pathways including PKC, MAPK, and calcium [1]. In order to examine the mechanism(s) by which Ang II increases SGK1 mRNA levels, we took a pharmacological approach whereby cells were pre-treated with various inhibitors prior to Ang II stimulation. Specifically, cells were pretreated with the indicated inhibitors and then treated with Ang II for 0 or 2h. SGK1 mRNA levels were then examined by quantitative RT-PCR. We found that pretreatment with the intracellular calcium chelator BAPTA-AM, completely blocked the ability of Ang II to increase SGK1 mRNA levels, while the L-type calcium channel blocker verapamil was without effect (Figure 2A). We also found that the ability of Ang II to increase SGK1 mRNA levels was insensitive to the MEK inhibitor PD98059, was minimally sensitive to the PKC inhibitor Go6983, and was very sensitive to the tyrosine kinase inhibitor genistein (Figure 2B). When taken together, these results indicate that the ability of Ang II to increase SGK1 mRNA levels requires intracellular calcium mobilization and tyrosine kinase activation.
Figure 2.
Ang II increases SGK1 mRNA levels via mobilization of intracellular calcium and tyrosine kinase activation. A, Cells were serum starved overnight and pre-treated with BAPTA-AM (50 μM, 10 min) or verapamil (10 μM, 5 min) prior to treatment with Ang II for 0 or 2 h. SGK1 mRNA was quantified via qRT-PCR. n=3. *, p<0.05 vs. 0 h control. B, Cells were serum starved overnight and pre-treated with PD98059 (50 μM, 1 h), Go6983 (10 μM, 1 h), or genistein (100 μM, 1 h) prior to treatment with Ang II for 0 or 2 h. SGK1 mRNA was quantified using qRT-PCR. n=3. *, p<0.05 vs. 0 h control.
3.2 Ang II causes an increase in SGK1 phosphorylation at Thr256 and causes an mTOR-dependent increase in SGK1 phosphorylation at Ser422
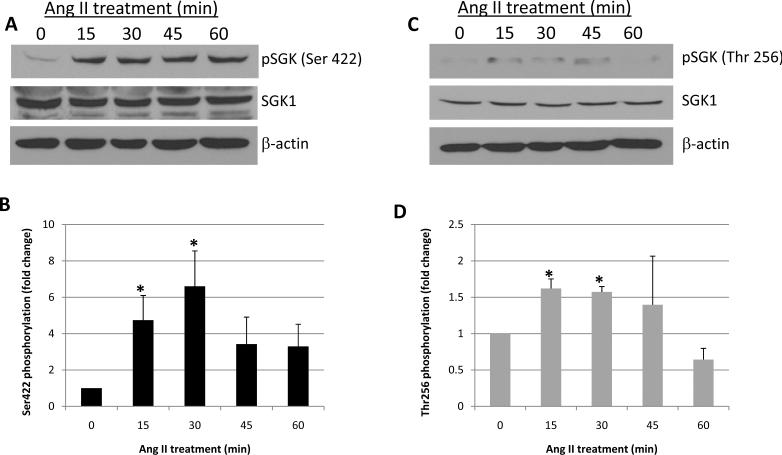
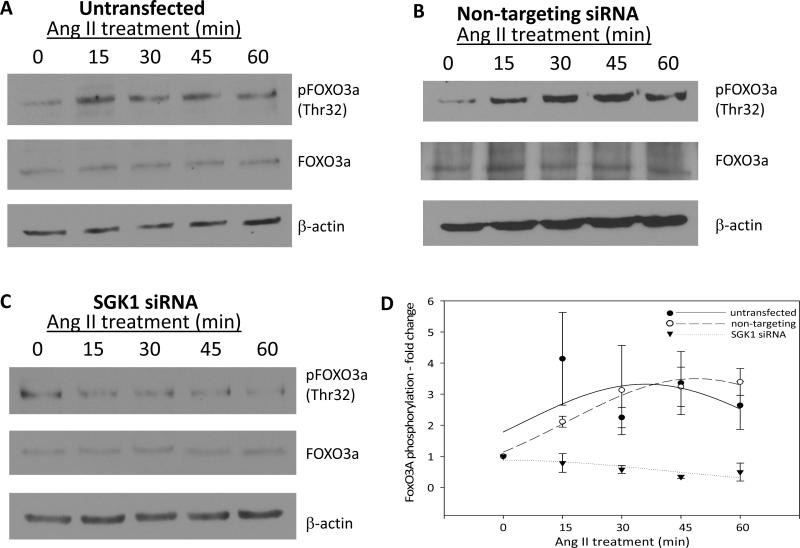
In addition to increased mRNA and protein levels, SGK1 enzymatic activity can be augmented via post translational modification of the protein. Specifically, activation of SGK1 requires phosphorylation at Ser422 in the hydrophobic motif and at Thr256 within the activation loop [1]. Currently, little is known about the direct effects of Ang II on the phosphorylation state of SGK1. Therefore, to determine if Ang II treatment has an impact on the phosphorylation of SGK1 at these critical sites, cells were treated with Ang II for 0, 15, 30, 45, and 60 min and SGK1 phosphorylation was examined by Western blot using phospho-specific antibodies. Figure 3A shows a representative phospho-Ser22 SGK1 blot and Figure 3B shows densitometric quantification of blots. The data indicate that Ang II treatment significantly increased the phosphorylation of Ser422 with peak phosphorylation occurring at the 30 min time point. With respect to Thr256 phosphorylation, Figure 3C shows a representative phospho-Thr256 SGK1 blot while Figure 3D shows the quantification of all blots. We similarly observed a significant increase in Thr256 phosphorylation with peak phosphorylation occurring at the 15–30 min time points. Thus, these results indicate that in addition to increasing SGK1 mRNA and protein levels, Ang II treatment activates SGK1 protein by increasing its phosphorylation at Ser422 and Thr256.
Figure 3.
Ang II increases the phosphorylation of SGK1 at Ser422 and Thr256. Cells were serum starved overnight and then treated with Ang II for the indicated times and SGK1 phosphorylation at Ser422 (A and B) and Thr256 (C and D) was examined by Western blot. Phosphorylation was quantified using densitometry and normalized to total SGK1 protein levels. n=3. *, p<0.05 vs. 0 min control.
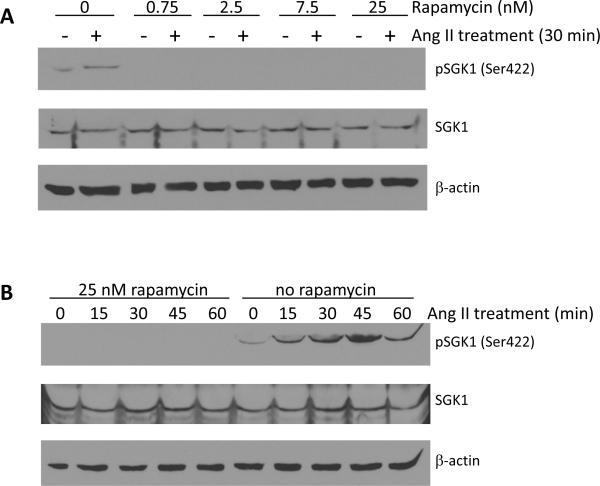
It is well established that SGK1 Thr256 phosphorylation is PDK1-dependent, so we did not examine this mechanism in our cell model [12]. However, it has previously been shown by various groups that either mTOR complex 1 (mTORC1) or mTOR complex 2 (mTORC2) can directly phosphorylate SGK1 at Ser422 [13–15]. In order to determine if the Ang II-mediated phosphorylation of SGK1 at Ser422 is mTORC1- or mTORC2-dependent, cells were pre-treated with rapamycin prior to Ang II stimulation and phosphorylation of Ser422 was again examined by phospho-specific Western blot analysis. Figure 4A shows that pretreatment for 1 h, with increasing concentrations of rapamycin, completely blocked the ability of Ang II to increase SGK1 Ser422 phosphorylation after 30 min of treatment, the time that normally induces maximal phosphorylation at Ser422 (Figure 3A and 3B). Additionally, a 1 h pretreatment with 25 nM rapamycin completely blocked Ser422 phosphorylation over a wide range of Ang II treatment points (Figure 4B). The concentrations of rapamycin and the duration of treatment used in these experiments are below the threshold of those shown to inhibit mTORC2 activity in vitro [16–18]. Therefore, the ability of rapamycin to block Ang II-mediated phosphorylation at Ser422 indicates that this signaling process is mTORC1-dependent.
Figure 4.
The Ang II-mediated phosphorylation of SGK1 at Ser422 is mTOR-dependent. A, Cells were serum starved overnight and then pre-treated with rapamycin (0 – 25 nM, 1 h) prior to treatment with Ang II for 0 or 30 min. n=3. B, Cells were serum starved overnight and then pre-treated with rapamycin (25 nM, 1 h) prior to treatment with Ang II for 0–60 min. n=3.
3.3 Ang II protects cells from serum-induced apoptosis in an SGK1-dependent manner
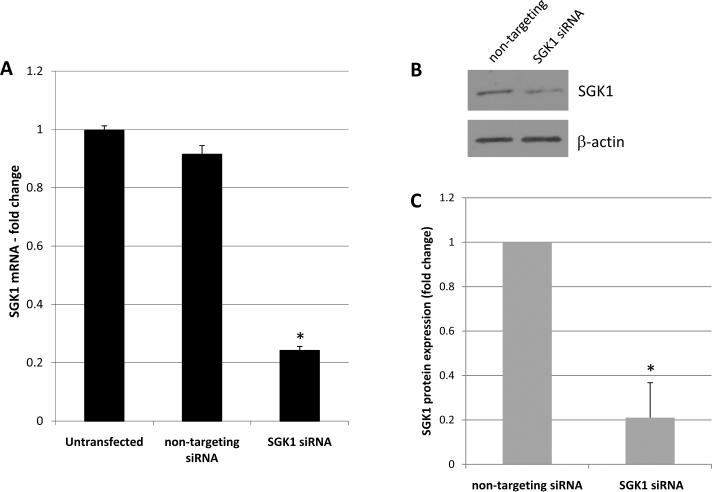
The AT1 receptor typically mediates pro-growth and pro-cell survival signaling [5]. Furthermore, SGK1 itself has been directly implicated in cell survival [2]. Given the ability of Ang II to activate SGK1 in this system, we wanted to determine if Ang II mediates cell survival, and if so, whether this effect is SGK1-dependent. To determine this, we elected to use an SGK1 siRNA knockdown approach in cells that would be serum starved in the presence or absence of Ang II and then examined for survivability. To demonstrate the feasibility of this approach, cells were either left untransfected or transfected with non-targeting siRNA or SGK1 targeted siRNA. Two days later, the levels of SGK1 mRNA were determined via quantitative RT-PCR. We found that while transfection of the non-targeting siRNA was largely without effect, transfection of the SGK1 siRNA reduced SGK1 mRNA levels by ~80% (Figure 5A). To determine if this decrease in SGK1 mRNA translated to reduced levels of SGK1 protein, whole cell protein lysates were examined for SGK1 protein levels via Western blot analysis. Figure 5B shows a representative Western blot and Figure 5C shows the quantification of all blots. We found that transfection of SGK1 siRNA decreased SGK1 protein levels by ~80% when compared to non-targeting transfected controls. When taken together, the data in Figure 5 demonstrate that siRNA targeting can effectively reduce SGK1 mRNA and protein levels in these cells.
Figure 5.
Confirmation of SGK1 knockdown. A, Cells were left untransfected or transfected with non-targeting or SGK1-targeting siRNA and SGK1 mRNA levels were then quantified by qRT-PCR. n=4. *, p<0.05 vs. untransfected and non-targeting siRNA. B, Cells were transfected with non-targeting or SGK1-targeting siRNA and SGK1 protein levels were examined by Western blot. C, SGK1 protein levels were quantified using densitometry and normalized to β-actin. n=2. *, p<0.05 vs. non-targeting siRNA.
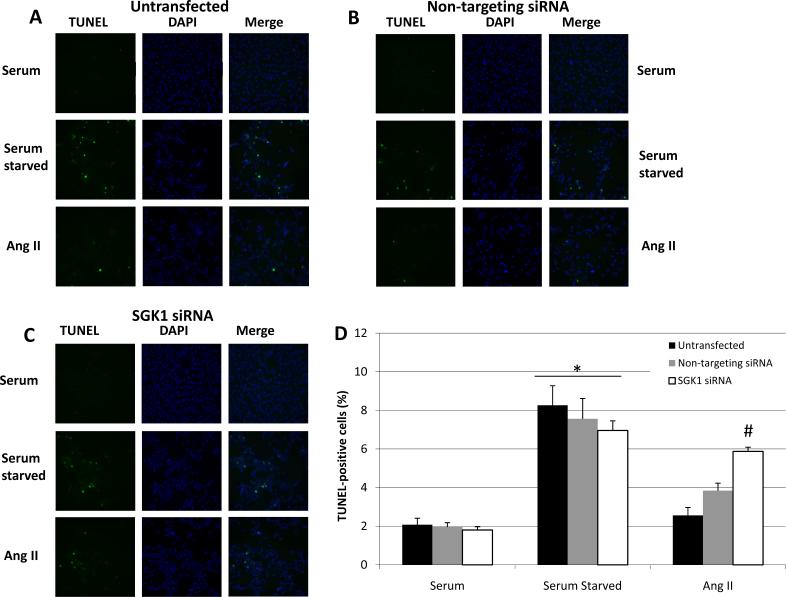
We next wanted to determine the role of SGK1 in Ang II-mediated cell survival. Specifically, cells were transfected as indicated and then serum starved in the presence or absence of 100 nM Ang II for 48 h, at which time cell survival was determined via TUNEL staining. TUNEL detects apoptotic cells and its presence correlates inversely with cell survival. We found that in untransfected cells, serum starvation caused a significant increase in the percentage of TUNEL-positive cells and Ang II significantly prevented this increase (Figure 6A). This result indicates that Ang II is capable of protecting these cells from serum starvation-induced apoptosis. When cells were transfected with the non-targeting control siRNA, a similar pattern was observed whereby serum starvation increase TUNEL staining and Ang II reversed this effect (Figure 6B). However, cells transfected with SGK1-targeting siRNA had a high level of apoptosis under serum-starved conditions and Ang II-treatment was unable to reverse this effect (Figure 6C). To quantify these results, the percentages of TUNEL-positive cells were determined and plotted as a function of treatment condition (Figure 6D). The data indicate that when compared to serum-containing conditions, serum starvation significantly increased the percentage of TUNEL positive cells in untransfected, non-targeting transfected, and SGK1 siRNA transfected cells. Furthermore, Ang II was able to improve cell survivability (ie, decrease apoptosis) in the untransfected and non-targeting transfected cells, but not in the SGK1 siRNA transfected cells, thereby indicating a critical role of SGK1 in Ang II protection against serum starvation-induced apoptosis.
Figure 6.
Ang II protects cells from starvation-induced apoptosis and this is SGK1-dependent. Cells were left untransfected (A), transfected with non-targeting siRNA (B), or transfected with SGK1-targeting siRNA (C) and then serum starved for 48 h in the presence or absence of 100 nM Ang II. Cells were analyzed for apoptosis by TUNEL assay. TUNEL-positive cells are green and the DAPI nuclear counter stain is blue. D, The percentage of TUNEL-positive cells was quantified and plotted as a function of condition. n=4. *, p<0.05 vs. serum containing groups. #, p<0.05 vs. untransfected Ang II-treated group.
3.4 Ang II induces FOXO3A phosphorylation in an SGK1-dependent manner
One mechanism by which SGK1 regulates cell survival is through the phosphorylation of FOXO3A [2]. This transcription factor activates transcription of pro-apoptotic genes, and its phosphorylation has an inhibitory effect by excluding it from the nucleus. Therefore, we wanted to determine if Ang II regulates cell survival by inducing SGK1-dependent phosphorylation of FOXO3A. To determine this, untransfected cells were treated with Ang II for 0, 15, 30, 45, and 60 min and FOXO3A phosphorylation at Thr32 was examined by Western blot. We found that Ang II caused an increase in FOXO3A phosphorylation after 15 min and this persisted through the 60 min time point (Figure 7A). To determine if this effect was SGK1-dependent, cells were transfected with either non-targeting or SGK1-targeting siRNA and then treated with Ang II. We found that in cells transfected with non-targeting siRNA, the results closely mirrored those seen in the untransfected cells as phosphorylation of FOXO3A at Thr32 was significantly increased after 15 min of Ang II treatment (Figure 7B). However, in cells transfected with SGK1-targeting siRNA, there was no increase in FOXO3A phosphorylation at Thr32 after Ang II treatment (Figure 7C). All blots were quantified and the relative phosphorylation levels of FOXO3A were plotted as a function of Ang II treatment time (Figure 7D). The results indicate that Ang II induces FOXO3A phosphorylation in an SGK1-dependent manner. Thus, FOXO3A phosphorylation may be a potential mechanism by which Ang II and SGK1 mediate cell survival in these cells.
Figure 7.
Ang II induces FOXO3A phosphorylation in an SGK1-dependent manner. Cells were left untransfected (A), transfected with non-targeting siRNA (B), or transfected with SGK1-targeting siRNA (C) and then serum starved overnight prior to Ang II treatment for 0–60 min. FOXO3A phosphorylation at Thr32 was examined by Western blot. D, FOXO3A phosphorylation was quantified using densitometry and normalized to total FOXO3A protein. n=3 for untransfected and n=2 for non-targeting and SGK1-targeting siRNA.
4. Discussion
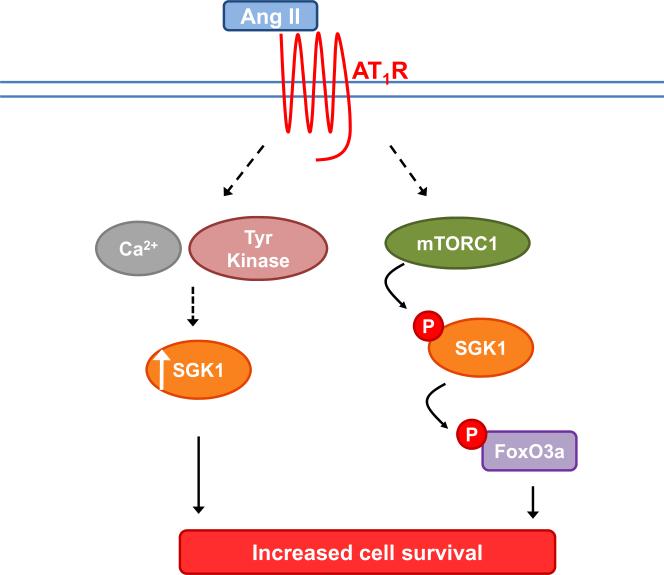
Ang II signaling via the AT1 receptor has numerous biological effects, one of which is cell survival. Some mechanisms by which Ang II/AT1 signaling can regulate cell survival include activation of NFκB, p38/MAPK, and PDK1-AKT signaling pathways [5]. The current study reveals that SGK1 plays a critical role in Ang II-mediated cell survival in fibroblast-derived cells. Figure 8 represents a working model of the proposed signaling pathways involved in this Ang II-mediated cell survival. Ang II, acting through the AT1 receptor, leads to increased levels of SGK1 via calcium- and tyrosine kinase-dependent mechanisms (Figure 2). Additionally, Ang II causes phosphorylation of SGK1 at Ser422 in an mTOR-dependent manner (Figures 3 and 4). Activated SGK1 then phosphorylates FOXO3A on Thr32 (Figure 7). The combined effect of increased SGK1 mRNA/protein levels and its concomitant activation, lead to an overall increase in cell survival (Figure 6).
Figure 8.
Proposed signaling pathway for the role of SGK1 in Ang II-mediated cell survival. Ang II acts via the AT1 receptor to 1) increase SGK1 mRNA and SGK1 protein levels via calcium and tyrosine kinase-dependent mechanisms and 2) increase SGK1 and FOXO3A phosphorylation levels via an mTORC1-dependent mechanism. The combined effect of increased SGK1 mRNA levels, increased SGK1 protein levels, and activation of existing pools of SGK1 protein via post translational phosphorylation, is an increase in overall cell survival.
These results may have a number of physiological and/or pathophysiological implications as SGK1 and Ang II are involved in many of the same cell signaling events and clinical pathologies. For example, SGK1 and Ang II are both involved in the progression of numerous fibrotic diseases including renal, hepatic and cardiac fibrosis [1, 19–23]. Additionally, it has been shown that Ang II contributes to hepatic myofibroblast survival during liver fibrosis [24]. It is therefore possible that some of the pro-survival effects of Ang II in hepatic myofibroblasts may be mediated by SGK1 signaling. Ang II and SGK1 have also been independently found to promote cell survival in breast cancer cells, and both SGK1 and the AT1 receptor are involved in multiple myeloma [4, 8, 10, 25]. Given these overlapping roles in such a wide range of pathologies, it is possible that Ang II and SGK1 signaling contribute concurrently in a number of these cases.
Many of the studies examining Ang II-mediated SGK1 expression and function have focused on the actions of the RAAS, and therefore have concentrated more on aldosterone release and subsequent SGK1 upregulation. However, the current study focuses on aldosterone-independent effects of Ang II on SGK1 expression and signaling. We have shown that Ang II impacts both the phosphorylation of SGK1 at activating sites and the expression of SGK1 mRNA/protein, leading to an overall increase in cell survival. Therefore, these results add to the body of literature on Ang II signaling and demonstrate that the Ang II/AT1 signaling axis can influence cell survival through multiple, independent pathways.
Interestingly, we found that phosphorylation of SGK1 at Ser422 in response to Ang II was dependent on mTORC1 activity. In order exclude the possibility of mTORC2 inhibition contributing to this effect, we treated with rapamycin at concentrations and durations that are not shown to inhibit mTORC2 in cell culture models [16–18]. Various reports in the literature have shown that Ser422 of SGK1 can be phosphorylated by mTORC2, and fewer groups have shown that mTORC1 is capable of phosphorylating this residue [13–15]. However, no reports have focused specifically on Ser422 phosphorylation in response to Ang II. Therefore, the results found here indicate that the involvement of mTORC1 and/or mTORC2 may vary depending on the initial stimulus. It was recently found that Ang II activates mTOR via the AT1 receptor, leading to tubulointerstitial fibrosis in a rat model, which further supports the proposed model of AngII/AT1R/mTOR-mediated activation of SGK1 [26].
In this study, we employed a fibrosarcoma cell line expressing the AT1 receptor and lacking the AT2 receptor [11]. This was beneficial, as it allowed for isolation of Ang II signaling via the AT1 receptor alone, and thus did not require additional confirmation with the use of AT1 or AT2 receptor blockers. Also, we used serum starvation as the model of apoptosis induction. This strategy was favorable as it allowed for examination of the effects of Ang II signaling on apoptosis in the absence of other growth factors or stimuli. Nevertheless, it would be interesting in future studies to investigate the role of these Ang II/AT1R/SGK1 signaling events in cell survival in other models, such as drug-induced apoptosis. Additionally, it would be interesting to investigate the physiological role of this signaling axis in disease models.
5. Conclusions
In summary, we have shown that Ang II promotes cell survival through upregulation and activation of SGK1. Ang II accomplishes this by increasing the mRNA/protein levels of SGK1 via calcium and tyrosine kinase-dependent signaling and also by increasing the phosphorylation of the protein via an mTOR-dependent mechanism. SGK1 then promotes cell survival by mediating the phosphorylation of FOXO3A at Thr32. Overall, these studies describe novel signaling mechanisms that allow for Ang II-mediated cell survival.
Highlights
-
>
Ang II promotes cell survival through upregulation and activation of SGK1
-
>
Ang II accomplishes this by increasing the mRNA/protein levels of SGK1 via calcium and tyrosine kinase-dependent signaling and also by increasing the phosphorylation of the protein via an mTOR-dependent mechanism
-
>
SGK1 then promotes cell survival by mediating the phosphorylation of FOXO3A at Thr32
-
>
These studies describe novel signaling mechanisms that allow for Ang II-mediated cell survival.
Acknowledgements
We thank Doug Smith for assistance with immunofluorescence imaging. This work was supported by National Institutes of Health Award R01-HL67277, a University of Florida Opportunity Fund Award, and a University of Florida/Moffitt Cancer Center Collaborative Initiative Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. Physiol Rev. 2006;86:1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- [2].Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Mol Cell Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang L, Cui R, Cheng X, Du J. Cancer Res. 2005;65:457–464. [PubMed] [Google Scholar]
- [4].Wu W, Chaudhuri S, Brickley DR, Pang D, Karrison T, Conzen SD. Cancer Res. 2004;64:1757–1764. doi: 10.1158/0008-5472.can-03-2546. [DOI] [PubMed] [Google Scholar]
- [5].Mehta PK, Griendling KK. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- [6].Hussain A, Wyatt AW, Wang K, Bhandaru M, Biswas R, Avram D, Foller M, Rexhepaj R, Friedrich B, Ullrich S, Muller G, Kuhl D, Risler T, Lang F. Kidney Blood Press Res. 2008;31:80–86. doi: 10.1159/000119703. [DOI] [PubMed] [Google Scholar]
- [7].Pattacini L, Casali B, Boiardi L, Pipitone N, Albertazzi L, Salvarani C. Rheumatology (Oxford) 2007;46:1252–1257. doi: 10.1093/rheumatology/kem092. [DOI] [PubMed] [Google Scholar]
- [8].Haznedaroglu IC, Beyazit Y. J Renin Angiotensin Aldosterone Syst. 2010;11:205–213. doi: 10.1177/1470320310379876. [DOI] [PubMed] [Google Scholar]
- [9].Uemura H, Hoshino K, Kubota Y. Curr Cancer Drug Targets. 2011;11:442–450. doi: 10.2174/156800911795538101. [DOI] [PubMed] [Google Scholar]
- [10].Zhao Y, Chen X, Cai L, Yang Y, Sui G, Fu S. J Cell Physiol. 2010;225:168–173. doi: 10.1002/jcp.22209. [DOI] [PubMed] [Google Scholar]
- [11].Sandberg EM, Ma X, VonDerLinden D, Godeny MD, Sayeski PP. J Biol Chem. 2004;279:1956–1967. doi: 10.1074/jbc.M303540200. [DOI] [PubMed] [Google Scholar]
- [12].Kobayashi T, Cohen P. Biochem J. 1999;339(pt.2):319–328. [PMC free article] [PubMed] [Google Scholar]
- [13].García-Martínez JM, Alessi DR. Biochem J. 2008;416:375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- [14].Hong F, Larrea MD, Doughty C, Kwiatkowski DJ, Squillace R, Slingerland JM. Mol Cell. 2008;30:701–711. doi: 10.1016/j.molcel.2008.04.027. [DOI] [PubMed] [Google Scholar]
- [15].Lu M, Wang J, Jones KT, Ives HE, Feldman ME, Yao LJ, Shokat KM, Ashrafi K, Pearce D. J Am Soc Nephrol. 2010;21:811–818. doi: 10.1681/ASN.2009111168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jacinto E, Loewith R, Schmidt A, Lin S, Rüegg MA, Hall A, Hall MN. Nat Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- [17].Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- [18].Barilli A, Visigalli R, Sala R, Gazzola GC, Parolari A, Tremoli E, Bonomini S, Simon A, Closs EI, Dall'Asta V, Bussolati O. Cardiovasc Res. 2008;78:563–571. doi: 10.1093/cvr/cvn024. [DOI] [PubMed] [Google Scholar]
- [19].Vallon V, Wyatt AW, Klingel K, Huang DY, Hussain A, Berchtold S, Friedrich B, Grahammer F, Belaiba RS, Görlach A, Wulff P, Daut J, Dalton ND, Ross J, Flögel U, Schrader J, Osswald H, Kandolf R, Kuhl D, Lang F. J Mol Med (Berl) 2006;84:396–404. doi: 10.1007/s00109-005-0027-z. [DOI] [PubMed] [Google Scholar]
- [20].Lang F, Klingel K, Wagner CA, Stegen C, Warntges S, Friedrich B, Lanzendorfer M, Melzig J, Moschen I, Steuer S, Waldegger S, Sauter M, Paulmichl M, Gerke V, Risler T, Gamba G, Capasso G, Kandolf R, Hebert SC, Massry SG, Broër S. Proc Natl Acad Sci U S A. 2000;97:8157–8162. doi: 10.1073/pnas.97.14.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pereira RM, dos Santos RA, da Costa Dias FL, Teixeira MM, Simões e Silva AC. World J Gastroenterol. 2009;15:2579–2586. doi: 10.3748/wjg.15.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ruiz-Ortega M, Ruperez M, Esteban V, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. Nephrol Dial Transplant. 2006;21:16–20. doi: 10.1093/ndt/gfi265. [DOI] [PubMed] [Google Scholar]
- [23].Billet S, Aguilar F, Baudry C, Clauser E. Kidney Int. 2008;74:1379–1384. doi: 10.1038/ki.2008.358. [DOI] [PubMed] [Google Scholar]
- [24].Oakley F, Teoh V, Ching ASG, Bataller R, Colmenero J, Jonsson JR, Eliopoulos AG, Watson MR, Manas D, Mann DA. Gastroenterology. 2009;136:2334–2344. e2331. doi: 10.1053/j.gastro.2009.02.081. [DOI] [PubMed] [Google Scholar]
- [25].Fagerli UM, Ullrich K, Stühmer T, Holien T, Köchert K, Holt RU, Bruland O, Chatterjee M, Nogai H, Lenz G, Shaughnessy JD, Mathas S, Sundan A, Bargou RC, Dörken B, Børset M, Janz M. Oncogene. 2011;30:3198–3206. doi: 10.1038/onc.2011.79. [DOI] [PubMed] [Google Scholar]
- [26].Whaley-Connell A, Habibi J, Panfili Z, Hayden MR, Bagree S, Nistala R, Hyder S, Krueger B, Demarco V, Pulakat L, Ferrario CM, Parrish A, Sowers JR. Am J Nephrol. 2011;34:115–125. doi: 10.1159/000329327. [DOI] [PMC free article] [PubMed] [Google Scholar]