Abstract
Alpha-synuclein (α-Syn) is a major component of Lewy bodies, abnormal protein aggregates that are present in neurons of patients with Parkinson’s disease and other neurological disorders. Despite intensive investigation, the in vivo role of α-Syn in physiological and pathological processes is not fully understood. This study addresses a current debate on the nuclear localization of α-Syn protein in the brain. To assess the specificity of various α-Syn antibodies, we compared their staining patterns in wild type mouse brains with that of the α-Syn knock-out mice. Among five different α-Syn antibodies tested here, two generated intensive nuclear staining throughout the normal mouse brain. However, nuclear staining by these two antibodies was also present in neurons of the α-Syn knock-out mice. This provides evidence that the nuclear signal is not specifically related to the presence of α-Syn, but rather results from the cross-reactivity of the two antibodies to some unknown antigens in neuronal nuclei. In mouse brain neurons, endogenous α-Syn proteins are primarily localized to neuronal processes and nerve terminals but present only at low levels in the cell bodies. This is different from a generally uniform distribution of exogenously expressed α-Syn in both cytoplasm and nuclei of heterologous cells, and suggests that the neuritic enrichment of α-Syn in neurons may be mediated by their specific interactions with certain structural or molecular components in the neuropil.
Keywords: α-synuclein, mouse brain, nuclear localization, antibodies
INTRODUCTION
Alpha-synuclein (α-Syn) is a small protein implicated in the pathogenesis of a number of neurodegenerative disorders including Parkinson’s disease (PD) (Recchia et al., 2004; Rampello et al., 2004; Wenning and Jellinger, 2005). Mutations in the α-Syn gene (A53T, A30P and E46K) cause autosomal-dominant hereditary PD (Polymeropoulos et al., 1997; Kruger et al., 1998; Spira et al., 2001; Zarranz et al., 2004). Recent genome-wide association studies also revealed the involvement of α-Syn gene variants in sporadic PD (Venda et al., 2010). Moreover, α-Syn is a major protein component of Lewy bodies (LB), eosinophilic cytoplasmic inclusions developed in brain neurons of PD and other disorders (Spillantini et al., 1997; Giasson et al., 2000b; Ma et al., 2003; Lin et al., 2004; Cantuti-Castelvetri et al., 2005).
α-Syn was initially identified from the electric ray Torpedo californica as a neuronal-specific protein which is localized to the presynaptic nerve terminal and nucleus (Maroteaux et al., 1988). Subsequent studies have shown that α-Syn proteins are abundantly expressed and widely distributed in mammalian central and peripheral nervous systems (Bayer et al., 1999; Mori et al., 2002). In neurons, α-Syn proteins are enriched at synapses and may play a regulatory role in presynaptic vesicle cycling and neurotransmitter release (Abeliovich et al., 2000; Chandra et al., 2004; Totterdell and Meredith, 2005; Lee et al., 2008; Watson et al., 2009). However, the nuclear localization of α-Syn remains controversial, as conflicting results have been obtained on the existence of endogenous α-Syn proteins in nuclei of mammalian brain neurons (Li et al., 2002; Yu et al., 2007; Zhang et al., 2008; Vivacqua et al., 2009; Zhong et al., 2010; Vivacqua et al., 2011). Based on published reports, this discrepancy is possibly attributable to different α-Syn antibodies used in various studies, but the underlying cause is still unknown. On the other hand, experiments conducted in cultured primary neurons and transfected mammalian cells have consistently demonstrated the localization of α-Syn proteins in the nucleus where they may act to inhibit histone acetylation and promote neurotoxicity (McLean et al., 2000; Goers et al., 2003; Kontopoulos et al., 2006). In addition, nuclear localization of α-Syn has been observed in transgenic mice expressing a mutant form of α-Syn (A53T). Interestingly, these studies noted nuclear accumulation of phosphorylated α-Syn (Pser129) in specific brain regions of the transgenic mice, suggesting a potential role of nuclear α-Syn in neuropathology (Wakamatsu et al., 2007; Schell et al., 2009).
In this study, we examined the subcellular localization of endogenous α-Syn in mouse brains using a panel of antibodies. Our data suggested that nuclear staining by some of the α-Syn antibodies may result from their non-specific cross-reactivity to other antigen(s) with epitope(s) similar to that of α-Syn. We also determined that endogenous α-Syn was minimally expressed in neuronal cell bodies, while enriched in neuropil throughout the mouse brain. Our findings should help resolve the ongoing debate on nuclear localization of α-Syn and contribute to our understanding of α-Syn’s role in the brain.
EXPERIMENTAL PROCEDURES
Antibodies and cDNAs
Polyclonal anti-α-synuclein antibodies were generated by immunizing rabbits with a GST fusion protein containing the C-terminal region (residues 94–140) of human α-synuclein, and purified using an affinity column containing the same fusion protein. In addition, the following antibodies were used in this study: monoclonal anti-α-synuclein antibody 3D5 (Yu et al., 2007), polyclonal anti-α-synuclein antibody C-20 (Santa Cruz Biotechnology, Inc.), polyclonal anti-α-synuclein antibody AB5038P (Millipore), polyclonal anti-α-synuclein antibody (Cell Signaling Technology, Inc.), monoclonal anti-α-synuclein (phosphorylated Ser129) antibody (Wako Chemicals USA, Inc.), monoclonal anti-α-synuclein (nitrated Tyr123/133) antibody (Novus Biologicals) and monoclonal anti-Tyrosine 3-Hydroxylase antibody (Epitomics, Inc.). The flag-tagged and EGFP-fused α-synuclein cDNAs were constructed by either cloning the human α-synuclein coding sequence into a mammalian expression vector pcDNA3, or fusing EGFP to the carboxyl terminus of α-synuclein coding region in the pEGFP-N1 vector.
Animals
α-synuclein knock-out mice were purchased from the Jackson Laboratory and maintained in the FSU animal facility. Generation and characterization of the homozygous null mice have been described previously (Abeliovich et al., 2000). Both the wild type and α-synuclein knock-out mice used in this study were on the C57BL/6J background.
Brain sections
All procedures were performed in accordance with the FSU Animal Care and Use Committee and NIH guidelines. Adult mice, weighed 25–30g, were anesthetized and transcardially perfused with 4% formaldehyde in 0.1 M phosphate buffer, pH 7.4 (PBS). After an overnight postfixation in the same fixative at 4°C, the brains were cut into 40 µm coronal sections on a vibratome (Leica Microsystems).
Immunofluorescence imaging
Mouse brain sections were permeabilized and blocked in a PBS solution containing 0.4% Triton (PBST) and 5% normal goat serum for 1 hour at room temperature. They were then incubated in the same solution containing appropriate primary antibodies at 4°C overnight. The next day, sections were washed with PBST 3 × 5 minutes, followed by incubation with Alexa Fluor® 647 donkey anti-rabbit or anti-mouse secondary antibodies (Invitrogen) for 2 hours at room temperature. After several washes with PBST and PBS, the sections were counterstained with 5 µg/mL 4′,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich, St. Louis, MO) in PBS for 5 minutes, washed in PBS for 5 minutes, and mounted with Vectashield to retard fluorescence fading. Tissue sections were imaged on a Leica TCS SP2 SE laser scanning confocal microscope (Leica Microsystems, Bannockburn, IL) using a 63× objective at 1024 × 1024 resolution. Serial stack images were taken at 0.5 µm steps, and all images were acquired by sequential scanning to avoid bleed-through.
In α-Syn presbsorption experiments, the AB5038P, 3D5 or Ab-CS antibody was preincubated with different amount of recombinant human α-Syn proteins (GenWay Biotench, Inc.) at 4°C for 4 hours before it was applied to wild type and α-Syn knock-out mouse brain sections. To preabsorb antibodies with brain slices, the Ab-CS antibody was preincubated at 4°C for 4 hours with one section of the wild type or α-Syn knock-out mouse brains that were first permeabilized in PBS solution containing 0.4% Triton and 5% normal goat serum.
To image α-Syn proteins in heterologous cells, tsA 201 cells were grown on poly-L-lysine-treated cover glasses and transfected with α-Syn-pEGFP-N1 or Flag-α-Syn-pcDNA3 cDNAs. After 48 hours, cells were fixed and permeabilized with 0.2% Triton, and then incubated with anti-α-Syn antibodies and Alexa Fluor® 647 donkey anti-rabbit or anti-mouse secondary antibodies in the presence of 10% goat serum.
Western blot analyses
Cytoplasmic and nuclear fractions of mouse brains were isolated using the Nuclear Complex Co-IP kit (Active Motif) based on the manufacture’s protocol. Briefly, mouse brains were homogenized in ice-cold hypotonic buffer supplemented with DTT and detergent. After a low speed centrifugation at 850 Xg for 10 minutes, the cell pellets were homogenized again and centrifuged at 14,000 Xg for 30 seconds. The supernatant was taken as the cytoplasmic fraction, and the nuclear pellet was digested with the enzymatic shearing cocktail for 10 minutes at 37°C and centrifuged at 14,000 Xg for 10 minutes to yield the supernatant containing nuclear proteins.
After determining total protein concentrations in cytoplasmic and nuclear fractions using the Bio-Rad Protein Assay kit, α-Syn proteins in these fractions were analyzed by immunoblot using α-Syn antibodies as described previously (Li et al., 2006). To verify the efficacy of fractionations, sample of the nuclear fraction was probed with a Histone H3 antibody (Millipore).
RESULTS
Distinct nuclear immunoreactivity of different α-Syn antibodies
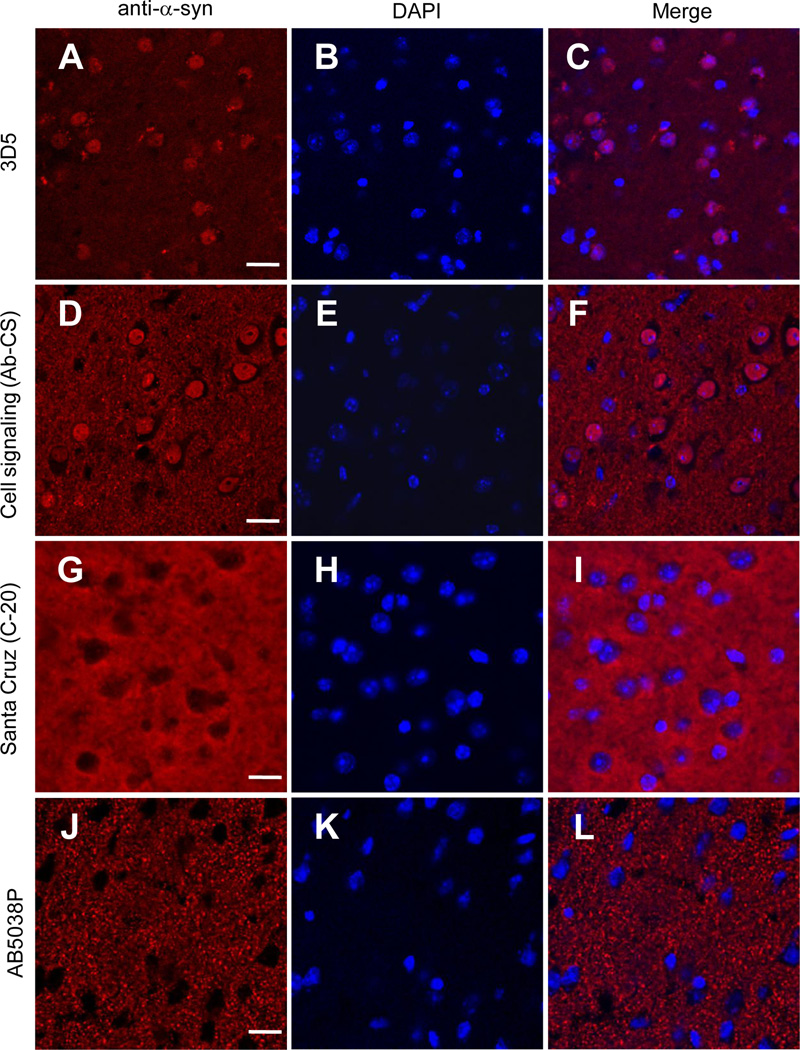
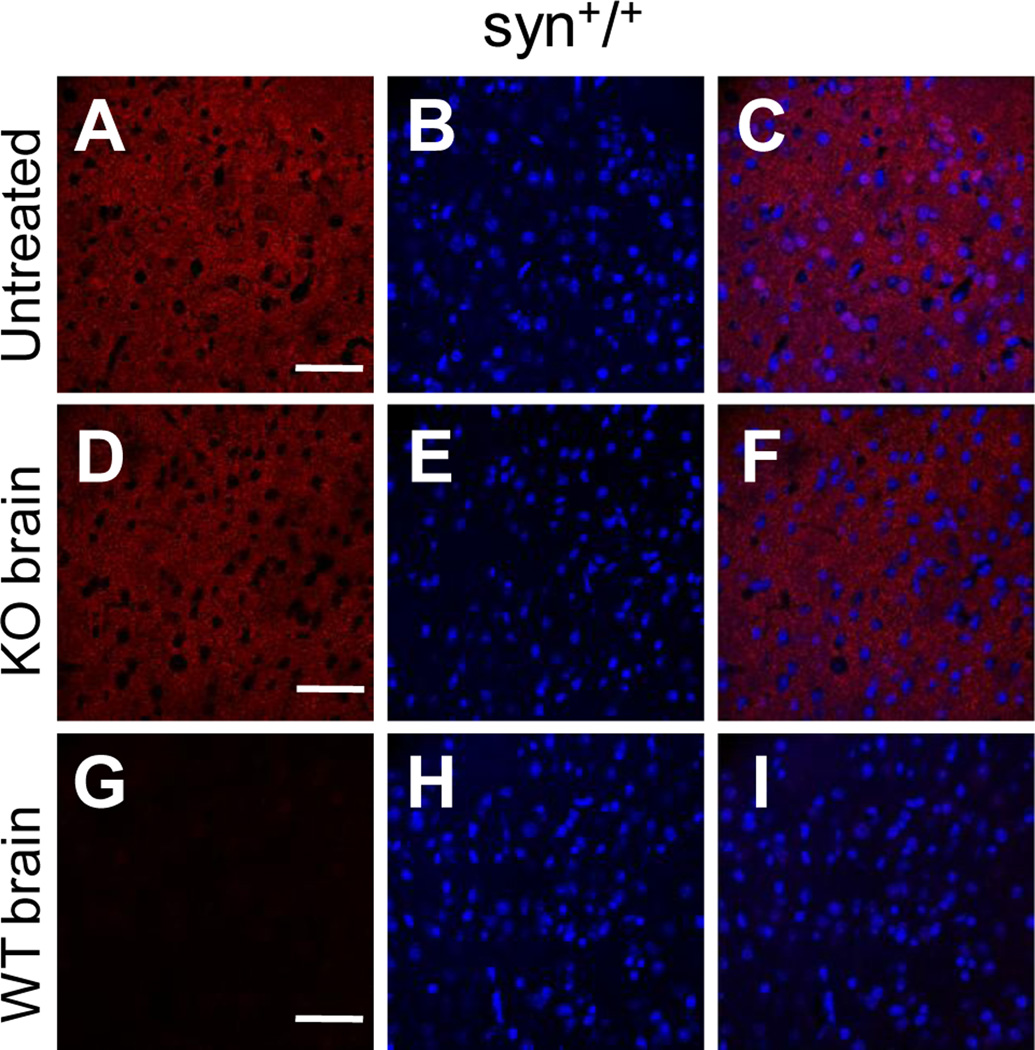
To determine the subcellular localization of α-Syn proteins in mouse brain neurons, we first immunostained brain slices of wild type mice using recently characterized 3D5 antibody that generates intensive nuclear signals in both central and peripheral neurons (Yu et al., 2007; Vivacqua et al., 2011). Consistent with previous reports, immunoreactivity of the 3D5 antibody was present in the neuronal processes and concentrated in the nuclei of neurons throughout the mouse brain. Figs. 1A–1C depicts such staining pattern by 3D5 in the cerebral cortex. We also observed similar neuritic and nuclear staining with a commercial α-Syn antibody from Cell Signaling (herein referred as Ab-CS) (Figs. 1D–1F). In contrast, nuclear localization was not found with staining from the other three α-Syn antibodies examined in this study. These included the C-20 antibody from Santa Cruz (Figs. 1G–1I), a polyclonal α-Syn antibody generated by us (data not shown), and the AB5038P antibody from Millipore (Figs. 1J–1L). Together, these results confirmed the variability of different α-Syn antibodies in nuclear staining of mouse brain neurons.
Figure 1. α-Syn antibodies exhibited different nuclear immunoreactivity in mouse brain neurons.
Confocal immunofluorescence images of mouse cerebral cortex stained with four different α-Syn antibodies (A, D, G, J), and counterstained with DAPI to depict the nuclei (B, E, H, K). Intensive nuclear staining was revealed in brain slices probed with both the 3D5 (A, C) and Cell Signaling (D, F) antibodies, but absent in that stained with either the C-20 (G, I) or the AB5038P antibody (J, L). Representative images from one of five independent experiments are shown. Scale bar = 20 µm.
Assessment of specific α-Syn signals using the knock-out mice
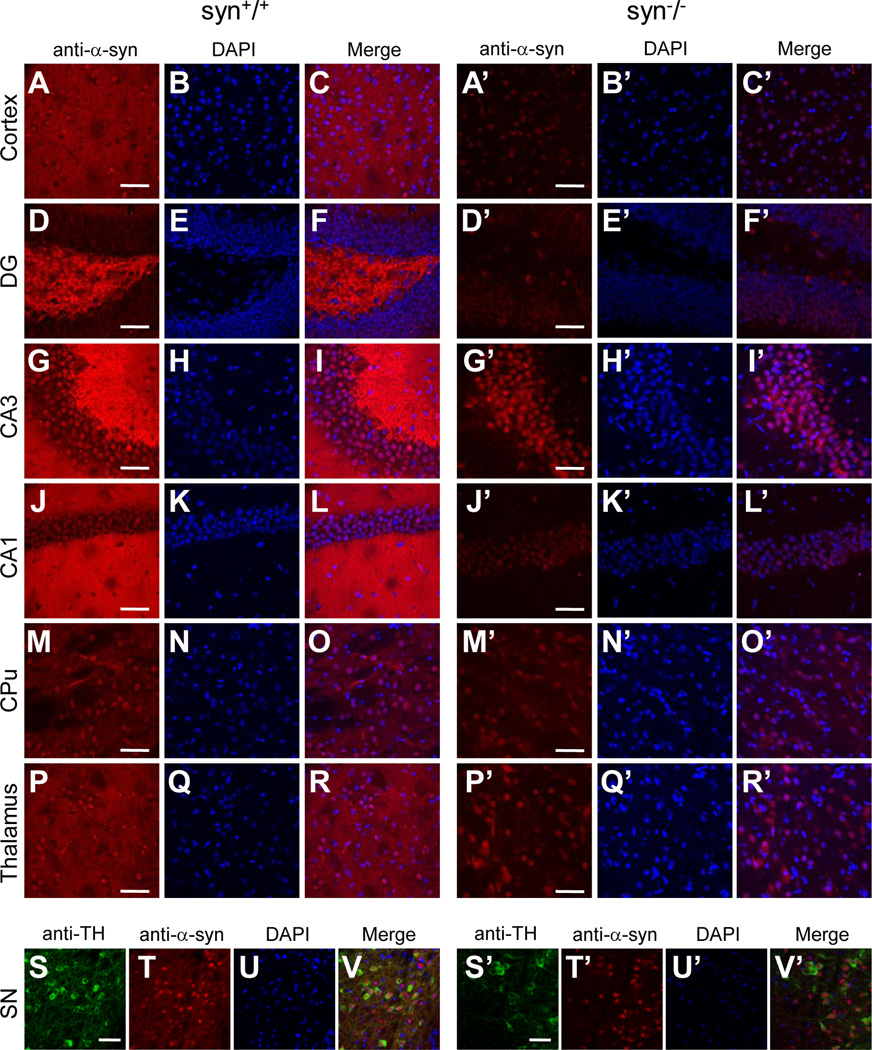
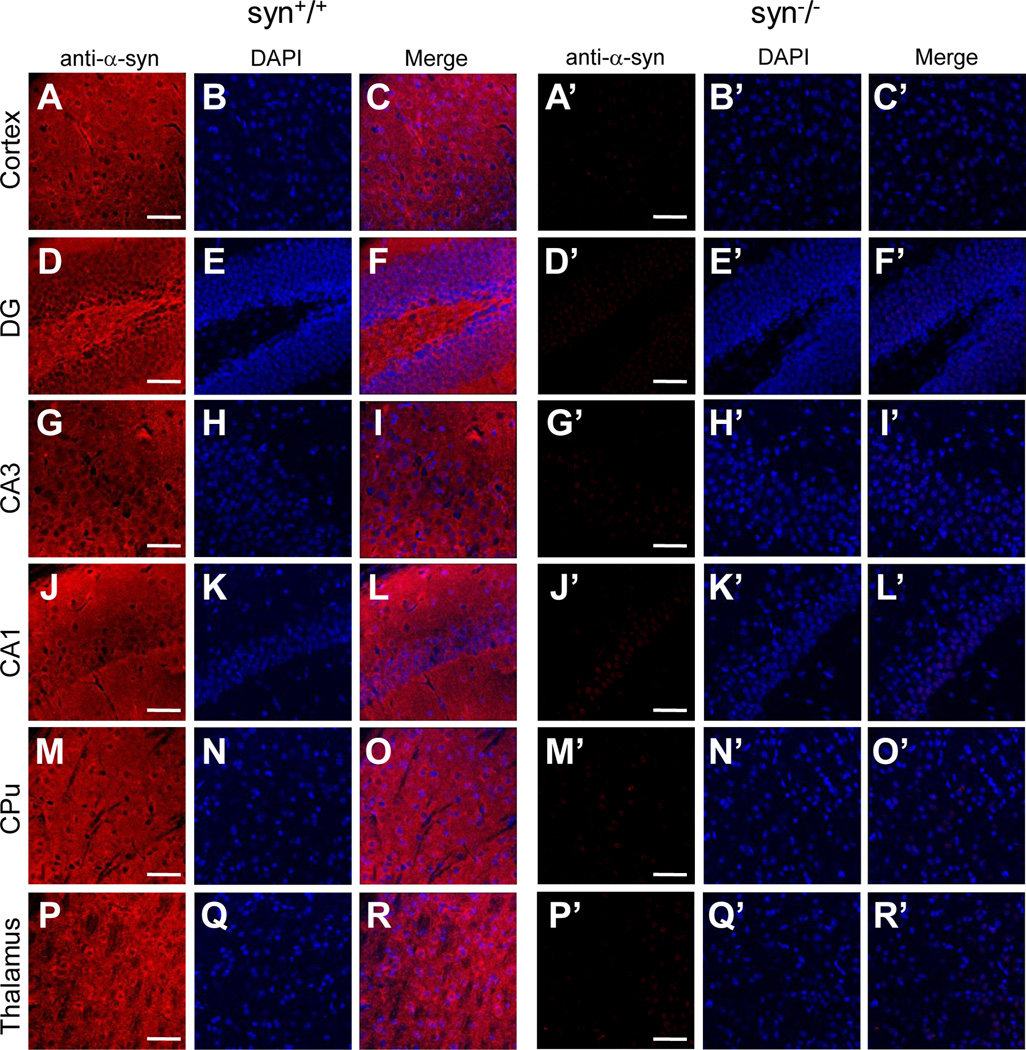
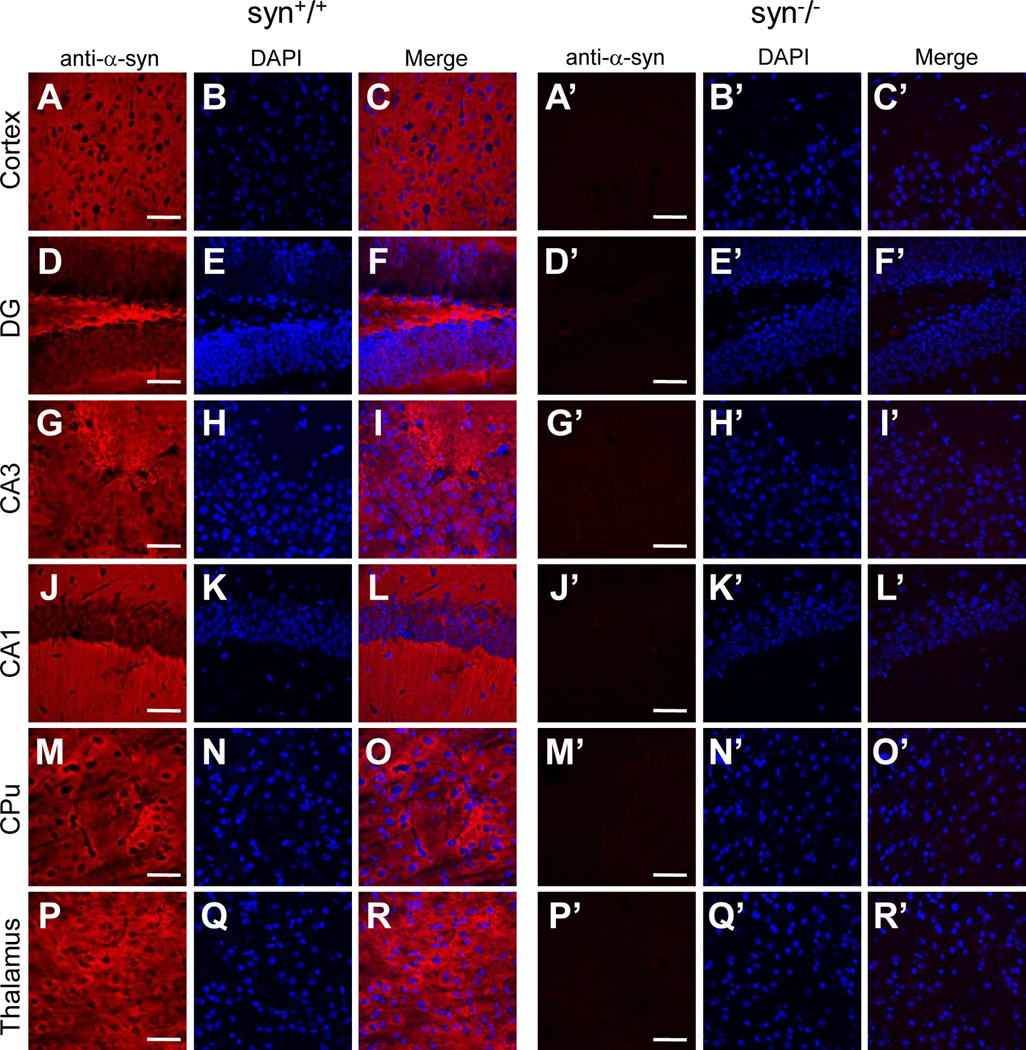
One of the possible causes for the discrepancy in nuclear staining is non-specific immunoreactivity of some of the antibodies to α-Syn-unrelated antigens in the nuclei. To confirm this, we evaluated the specificity of these antibodies to α-Syn proteins by comparing their staining patterns in the brain of the wild type with that of the α-synuclein knock-out mice which are devoid of α-Syn proteins (Abeliovich et al., 2000). To minimize experimental variability, we immunostained brain slices of the age-matched wild type and α-synuclein knock-out mice using the same dilutions of primary and secondary antibodies, and imaged these slices on confocal microscope with identical laser intensity and photomultiplier (PMT) settings. In wild type mice, all four α-Syn antibodies (3D5, Ab-CS, C-20 and AB5038P) further evaluated here produced extensive staining in neuronal processes throughout the brain (Figs. 2–5). Given that the neuritic staining by these antibodies were comparable with each other and completely absent in the α-Syn knock-out (syn−/−) mice (Figs. 2–5), it reflected the distribution of endogenous α-Syn proteins in the neuronal processes and terminals of brain neurons. On the other hand, these antibodies exhibited a significant difference in nuclear immunoreactivity. While C-20 and AB5038P did not generate any nuclear staining (Figs. 4 & 5), 3D5 and Ab-CS antibodies stained nuclei in various regions of mouse brains, including cerebral cortex, hippocampus, basal ganglia, thalamus, midbrain, etc (Figs. 2 & 3). The nuclear staining pattern of these two antibodies (3D5 and Ab-CS) in the syn−/− mouse brains had intensity and regional distribution similar to that of the wild type mice (Figs. 2 & 3). Since α-Syn proteins are not expressed in the syn−/− mice, the nuclear staining of these two antibodies can not result from their specific immunoreactivities to endogenous α-Syn proteins.
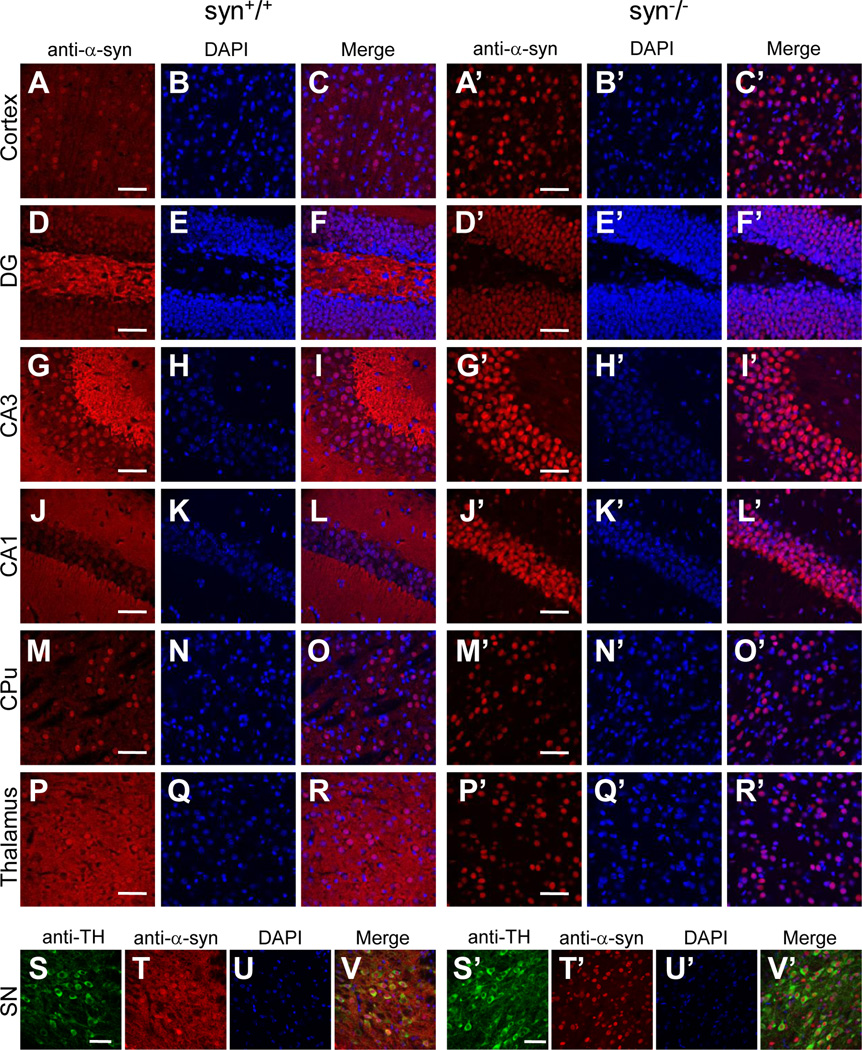
Figure 2. Subcellular localization of α-Syn proteins in various mouse brain regions revealed by the 3D5 antibody.
Confocal immunofluorescence images showing staining patterns of the 3D5 antibody in cerebral cortex (A, A’), DG, CA3 and CA1 of hippocampus (D, G, J, D’, G’, J’), striatum (CPu) (M, M’), thalamus (P, P’) and substantia nigra (SN) (T, T’) of wild type and α-Syn knock-out mice. In wild type mouse brains, the 3D5 antibody generated intensive staining in both neuronal fibers and a majority of nuclei in these regions (C, F, I, L, O, R, V). In the knock-out mice, there was no visible neuritic staining, but the nuclear staining was present and similar to that of wild type mice in these regions (C’, F’, I’, L’, O’, R’, V’). Sections of SN were stained with Tyrosine 3-Hydroxylase antibody to visualize dopaminergic neurons (S, S’). Representative images from one of five independent experiments are shown. Scale bar = 50 µm.
Figure 5. Subcellular localization of α-Syn proteins in various mouse brain regions revealed by the AB5038P antibody.
Confocal immunofluorescence images showing staining patterns of the AB5038P antibody in cerebral cortex (A, A’), DG, CA3 and CA1 of hippocampus (D, G, J, D’, G’, J’), striatum (CPu) (M, M’) and thalamus (P, P’) of wild type and α-Syn knock-out mice. In wild type mouse brains, the AB5038P antibody only stained neuronal processes and terminals, but not nuclei in these regions (C, F, I, L, O, R). The immunostaining was completely absent in all brain regions of the α-Syn knock-out mice (C’, F’, I’, L’, O’, R’). Representative images from one of three independent experiments are shown. Scale bar = 50 µm.
Figure 4. Subcellular localization of α-Syn proteins in various mouse brain regions revealed by the C-20 antibody.
Confocal immunofluorescence images showing staining patterns of the C-20 antibody in cerebral cortex (A, A’), DG, CA3 and CA1 of hippocampus (D, G, J, D’, G’, J’), striatum (CPu) (M, M’) and thalamus (P, P’) of wild type and α-Syn knock-out mice. In wild type mouse brains, the C-20 antibody only stained neuronal processes and terminals, but not nuclei in these regions (C, F, I, L, O, R). The immunostaining was completely absent in all brain regions of the α-Syn knock-out mice (C’, F’, I’, L’, O’, R’). Representative images from one of four independent experiments are shown. Scale bar = 50 µm.
Figure 3. Subcellular localization of α-Syn proteins in various mouse brain regions revealed by the Ab-CS antibody.
Confocal immunofluorescence images showing staining patterns of the Ab-CS antibody in cerebral cortex (A, A’), DG, CA3 and CA1 of hippocampus (D, G, J, D’, G’, J’), striatum (CPu) (M, M’), thalamus (P, P’) and substantia nigra (SN) (T, T’) of wild type and α-Syn knock-out mice. In wild type mouse brains, the Ab-CS antibody generated intensive staining in both neuronal fibers and a majority of nuclei in these regions (C, F, I, L, O, R, V). In the knock-out mice, there was no visible neuritic staining, but the nuclear staining was present and similar to that of wild type mice in these regions (C’, F’, I’, L’, O’, R’, V’). Sections of SN were stained with anti-Tyrosine 3-Hydroxylase antibody to visualize dopaminergic neurons (S, S’). Representative images from one of four independent experiments are shown. Scale bar = 50 µm.
To further examine the specificity of these antibodies, we conducted preabsorption experiments in which some of the α-Syn antibodies were preincubated with recombinant human α-Syn proteins before they were applied to brain sections. As expected, neuritic signal was diminished by preabsorption of the AB5038P, 3D5 or Ab-CS antibody with soluble α-Syn proteins in a dose-dependent manner (Figs. 6, 7 & 8). Importantly, nuclear staining generated by both 3D5 and Ab-CS antibodies was also eliminated by preincubation of them with recombinant α-Syn proteins (Figs. 7 & 8), indicating that the nuclear staining of 3D5 and Ab-CS antibodies was conferred by their cross-reactivity to some nuclear antigen(s) that may contain epitope(s) similar to that of the α-Syn protein. For both 3D5 and Ab-CS antibodies, a significantly less α-Syn proteins was required to abolish the nuclear staining than what was needed to abolish the neuritic staining (Figs. 7 & 8). Since 3D5 is a homogenous monoclonal antibody, this observation could reflect a higher affinity of this antibody for α-Syn in neuronal processes than the cross-reacted nuclear antigens. On the other hand, the polyclonal Ab-CS antibody consist of a mixture of antibodies which may recognize different epitopes on the target proteins and thus could lead to different dose-responses to preabsorption with α-Syn proteins. To test this possibility, we preincubated the Ab-CS antibody with permeabilized brain tissue of the α-Syn knock-out mice, and then used the preaborbed antibody to immunostain brain sections of wild type mice. As shown in Fig. 9A–9F, this preabsorption treatment did not affect α-Syn derived neuritic signal, but eradicated the nonspecific nuclear staining. In contrast, staining in both processes and nuclei was completely lost after preabsorption of Ab-CS antibodies with brain slices of the wild-type mouse (Figs. 9G–9I). Taken together, these results provided additional support for our hypothesis that the nuclear staining of the two α-Syn antibodies is caused by their cross-reactivity to some nuclear antigens that are not α-Syn proteins.
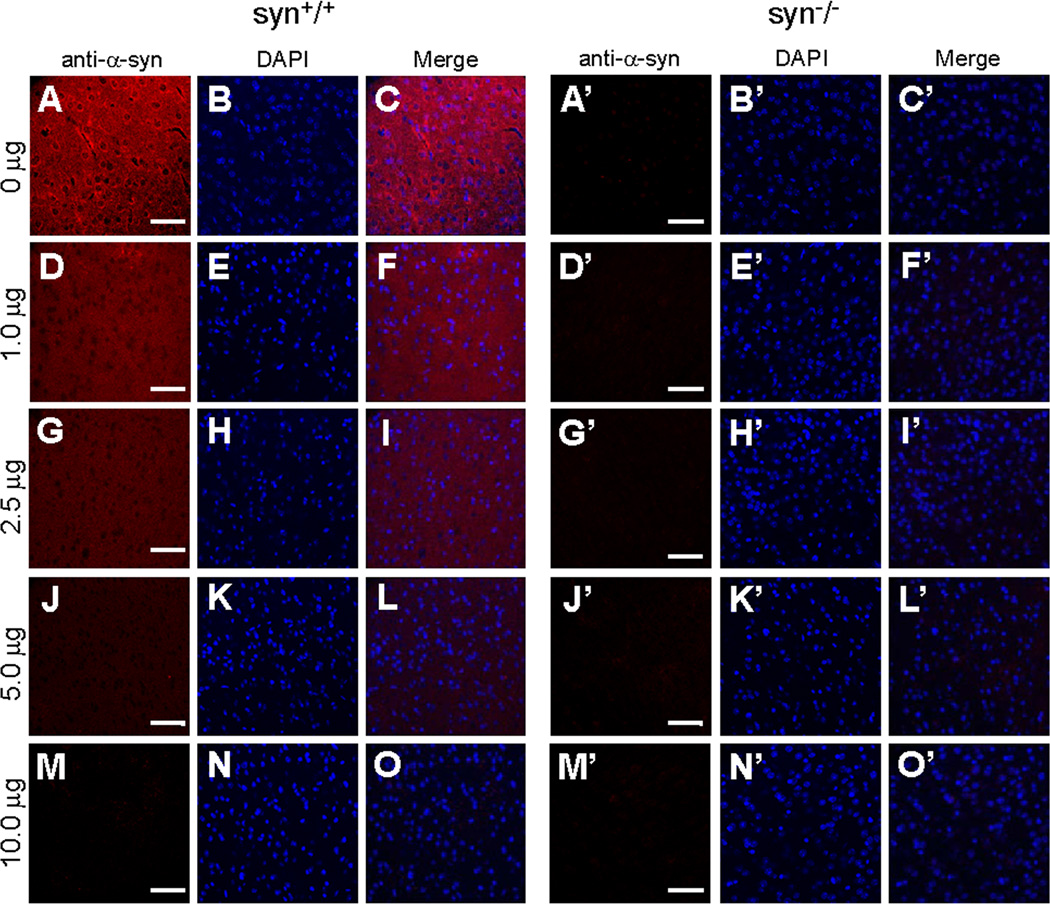
Figure 6. Specificity of AB5038P antibody immunoreactivity assessed by preabsorption assays.
Confocal immunofluorescence images of mouse cerebral cortex stained with the AB5038P antibody preabsorbed with different amount of recombinant human α-Syn proteins. In wild type mouse brains, α-Syn signals in neurites (A–C) were reduced by preabsorption of AB5038P with 1.0 or 2.5 µg α-Syn proteins (D–F, G–I). No visible α-Syn staining was left when preabsorption with α-Syn was increased to 5.0 or 10.0 µg of recombinant α-Syn (J–L, M–O). The immunostaining was completely absent in all groups of the α-Syn knock-out mice (A’–O’). Representative images from one of three independent experiments are shown. Scale bar = 50 µm.
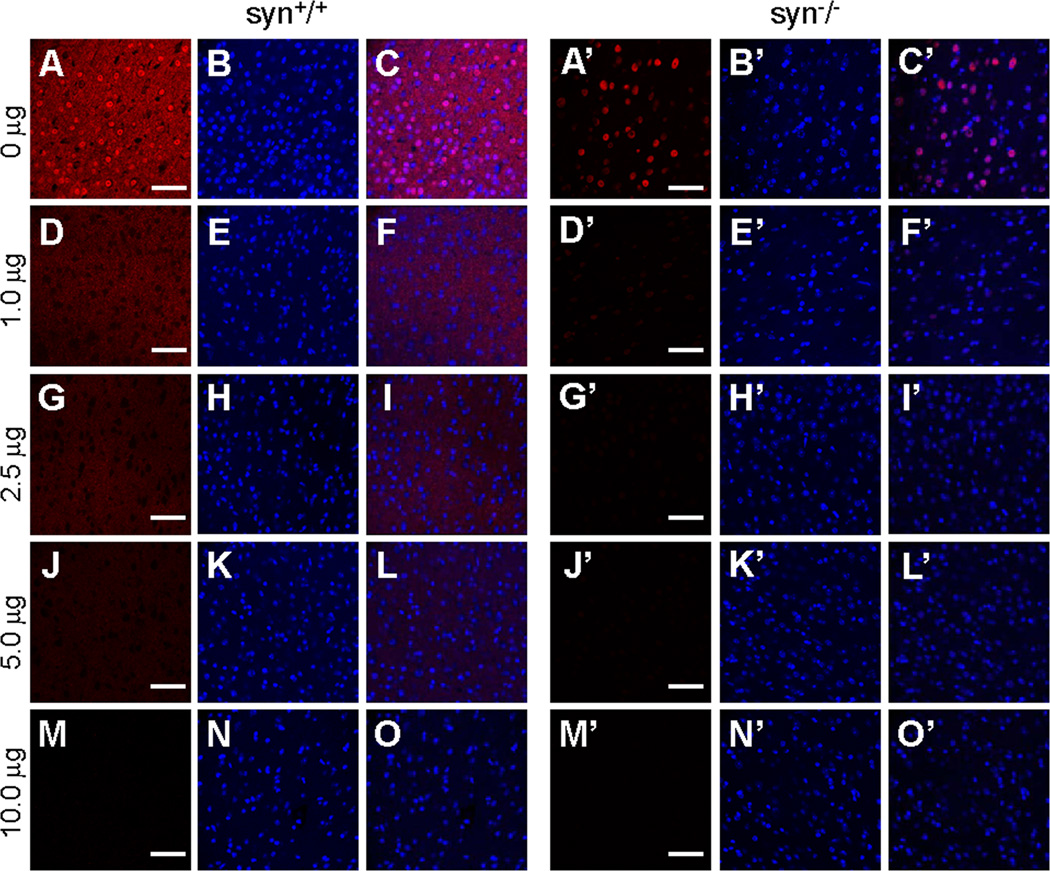
Figure 7. Specificity of 3D5 antibody immunoreactivity assessed by preabsorption assays.
Confocal immunofluorescence images of mouse cerebral cortex stained with the 3D5 antibody preabsorbed with different amount of recombinant human α-Syn proteins. Neuritic signals in wild type mouse brains (A–C) were reduced by preabsorption of 3D5 with 1.0, 2.5 or 5.0 µg α-Syn proteins (D–F, G–I, J–L), and abolished when α-Syn was increased to 10.0 µg (M–O). In both wild type and α-Syn knock-out mouse brains, nuclear staining of 3D5 was substantially reduced by preincubation of the 3D5 antibody with 1.0 µg α-Syn proteins (D–F, D’–F’), and completely abolished when α-Syn proteins were increased to 2.5, 5.0 or 10.0 µg (G–O, G’–O’). Representative images from one of three independent experiments are shown. Scale bar = 50 µm.
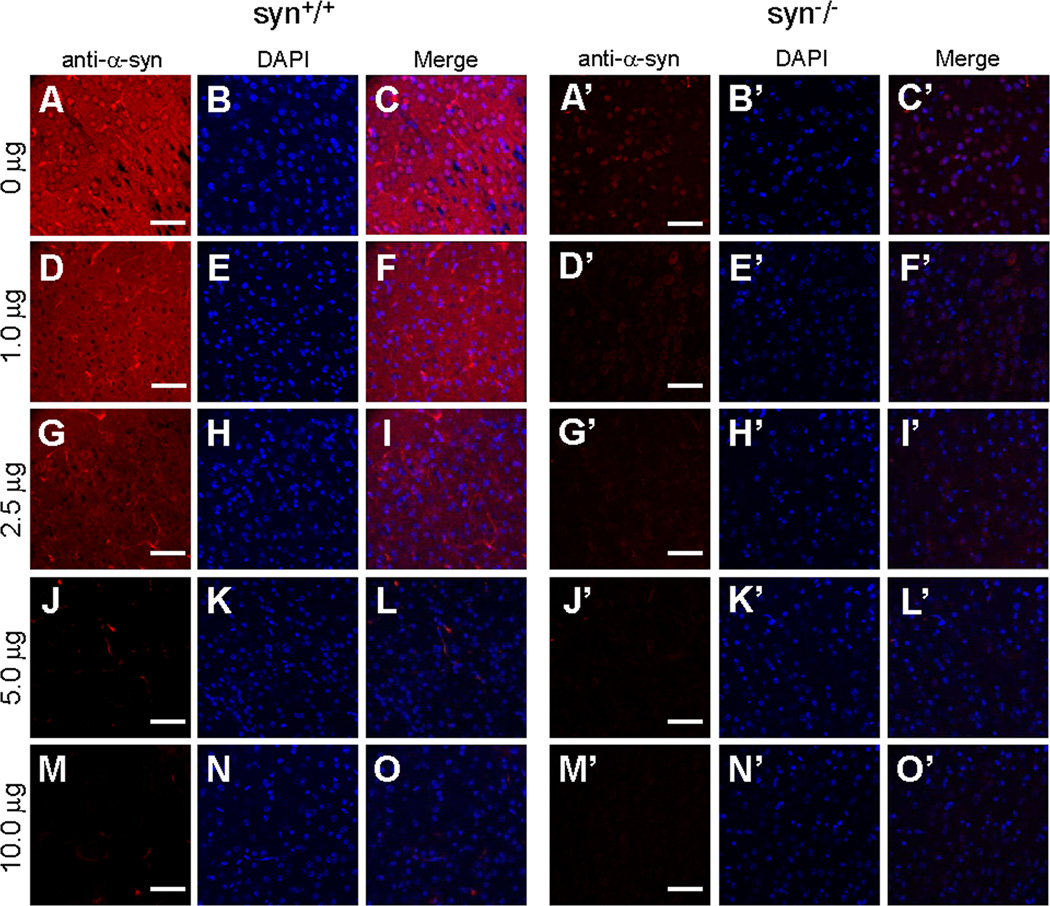
Figure 8. Specificity of Ab-CS antibody immunoreactivity assessed by preabsorption assays.
Confocal immunofluorescence images of mouse cerebral cortex stained with the Ab-CS antibody preabsorbed with different amount of recombinant human α-Syn proteins. Neuritic signals in wild type mouse brains (A–C) were gradually reduced by preabsorption of Ab-CS antibodies with 1.0, 2.5 or 5.0 µg α-Syn proteins (D–F, G–I, J–L), and abolished when α-Syn was increased to 10.0 µg (M–O). In both wild type and α-Syn knock-out mouse brains, nuclear staining of Ab-CS antibodies was virtually eliminated by preincubation of the antibody with 1.0 µg α-Syn proteins (D–F, D’–F’), and completely absent when α-Syn proteins were increased to 2.5, 5.0 or 10.0 µg (G–O, G’–O’). Representative images from one of two independent experiments are shown. Scale bar = 50 µm.
Figure 9. Elimination of Ab-CS’s nuclear immunoreactivity by preincubation of the antibody with brain tissues of α-Syn knock-out mice.
Confocal immunofluorescence images of wild type mouse cerebral cortex stained with Ab-CS antibodies that were either untreated (top panels, A–C), or preincubated with α-Syn knock-out (middle panels, D–F) or wild type (bottom panels, G–I) mouse brain slices. Preincubation of Ab-CS antibodies with brain slices of α-Syn knock-out mice completely abolished their nuclear staining, but had little effect on the neuritic signals (D–F). Neither nuclear nor neuritic staining was left after preincubation of Ab-CS antibodies with brain slices of wild-type mice (G–I). Representative images from one of two independent experiments are shown. Scale bar = 50 µm. Note that a lower dilution (1:300 vs. 1:100 in Figs 1, 3 & 7)) of the Ab-CS antibody was used in these experiments.
Biochemical analyses of α-Syn antibodies
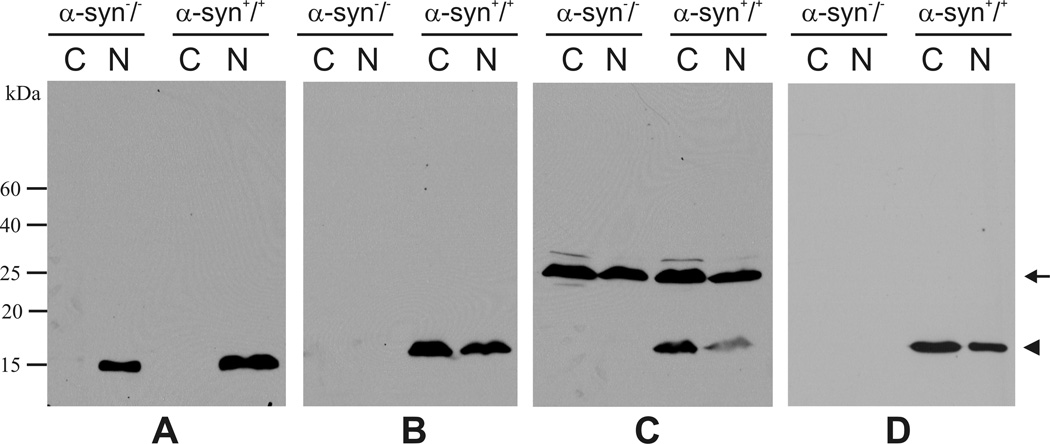
In previous reports, the presence of α-Syn immunoreactivity in the nuclei was also indicated by western blot analyses (Yu et al., 2007). We thus carried out similar studies by probing the cytoplasmic and nuclear fractions of mouse brains with these α-Syn antibodies. As a control for our fractionation procedures, proteins from the two subcellular compartments were probed with a Histone H3 antibody to mark the nuclear fraction (Fig. 10A). Among the three antibodies tested here, the C-20 antibody recognized a single band in both cytoplasmic and nuclear fractions of the wild type mouse brains (Fig. 10B, right panels). These bands represented mouse α-Syn proteins, as they migrated at a position corresponding to the molecular mass of mouse α-Syn and were absent in neither subcellular faction derived from the α-Syn knock-out mice (Fig. 10B, left panels). The Ab-CS antibody revealed the α-Syn band, but also recognized another higher molecular weight band (~27 kd) in these two subcellular fractions (Fig. 10C, right panels). As the higher molecular weight band was present in both wild type and α-Syn knock-out mice (Fig. 10C), it should not be related to α-Syn proteins. However, this non-specific band is unlikely the source of intense nuclear staining by this antibody since it appeared in both the cytoplasmic and nuclear fractions on western blots (Fig. 10C). Moreover, we found that the 3D5 antibody specifically recognized α-Syn in both cytoplasmic and nuclear fractions without producing any apparent non-specific bands on western blots (Fig. 10D). This is consistent with the previous report by Yu et al. and indicates that the cause of nuclear staining by some of the α-Syn antibodies may not be easily elucidated by conventional biochemical approaches.
Figure 10. Western blot analyses of mouse brains.
α-Syn proteins (arrow head) were detected in both cytoplasmic (C) and nuclear (N) fractions of the wild type, but not the knock-out mice by C-20 (B), Ab-CS (C), and 3D5 (D) antibodies. Note the presence of a higher molecular band (arrow) in western blots probed by the Ab-CS antibody. The blots were also probed with a Histone H3 antibody to mark the nuclear fraction (A).
Subcellular localization of recombinant α-Syn in heterologous cells
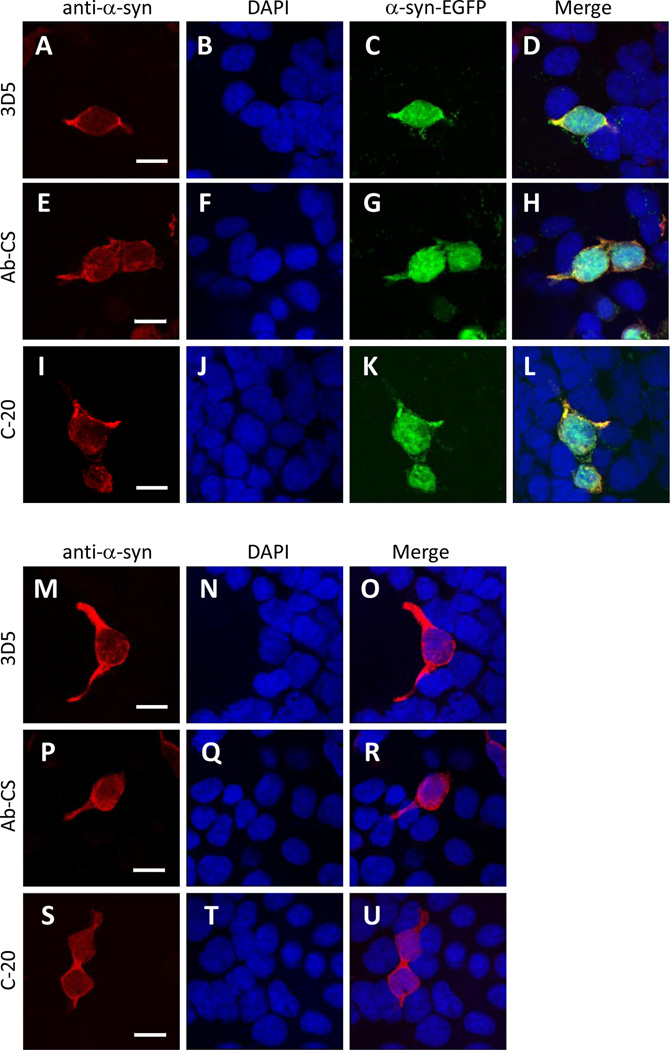
Despite the controversy on the nuclear localization of endogenous α-Syn in the brain, a number of previous studies have consistently reported the existence of recombinant α-Syn in the nuclei of transfected cells (Specht et al., 2005). Likewise, we found that the exogenously expressed α-Syn-EGFP fusion proteins were localized to both cytoplasmic and nuclear compartments as judged by GFP signals under confocal microscopy (Figs. 11C, 11G & 11K). However, there was no significant difference in subcellular distribution of immunofluorescence generated by staining the cells with the three different α-Syn antibodies (Figs. 11A, 11E & 11I), suggesting that all of them were capable of accessing cytoplasmic and nuclear α-Syn proteins in transfected cells. This was further confirmed by our parallel experiments in which we observed a generally uniform distribution of α-Syn proteins in both cytoplasmic and nuclear compartments of cells transfected with a flag-tagged α-Syn cDNA and immunostained with these α-Syn antibodies (Figs. 11M–11U). Interestingly, in non-transfected tsA 201 cells, we did not observe any cytoplasmic or nuclear staining by these antibodies. This observation thus indicated that the non-specific nuclear antigen(s) recognized by 3D5 and Ab-CS antibodies may be specific to neurons, but not to cell lines derived from some other tissues.
Figure 11. Subcellular localization of exogenous α-Syn in heterologous cells.
Confocal images of tsA 201 cells transfected with either α-Syn-EGFP or Flag-α-Syn cDNAs. Cells were immunostained with one of the three α-Syn antibodies (A, E, I, M, P, S) and counterstained with DAPI to depict the nuclei (B, F, J, N, Q, T). For α-Syn-EGFP-transfected cells, GFP fluorescence was also visualized in a green channel (C, G, K). All three antibodies immunostained only the transfected cells in which α-Syn proteins were localized to both the cytosol and nucleus (D, H, L, O, R, U). Representative images from one of four independent experiments are shown. Scale bar = 15 µm. Note that no signals were produced by either antibody in none-transfected cells.
Specificity of other α-Syn-related antibodies
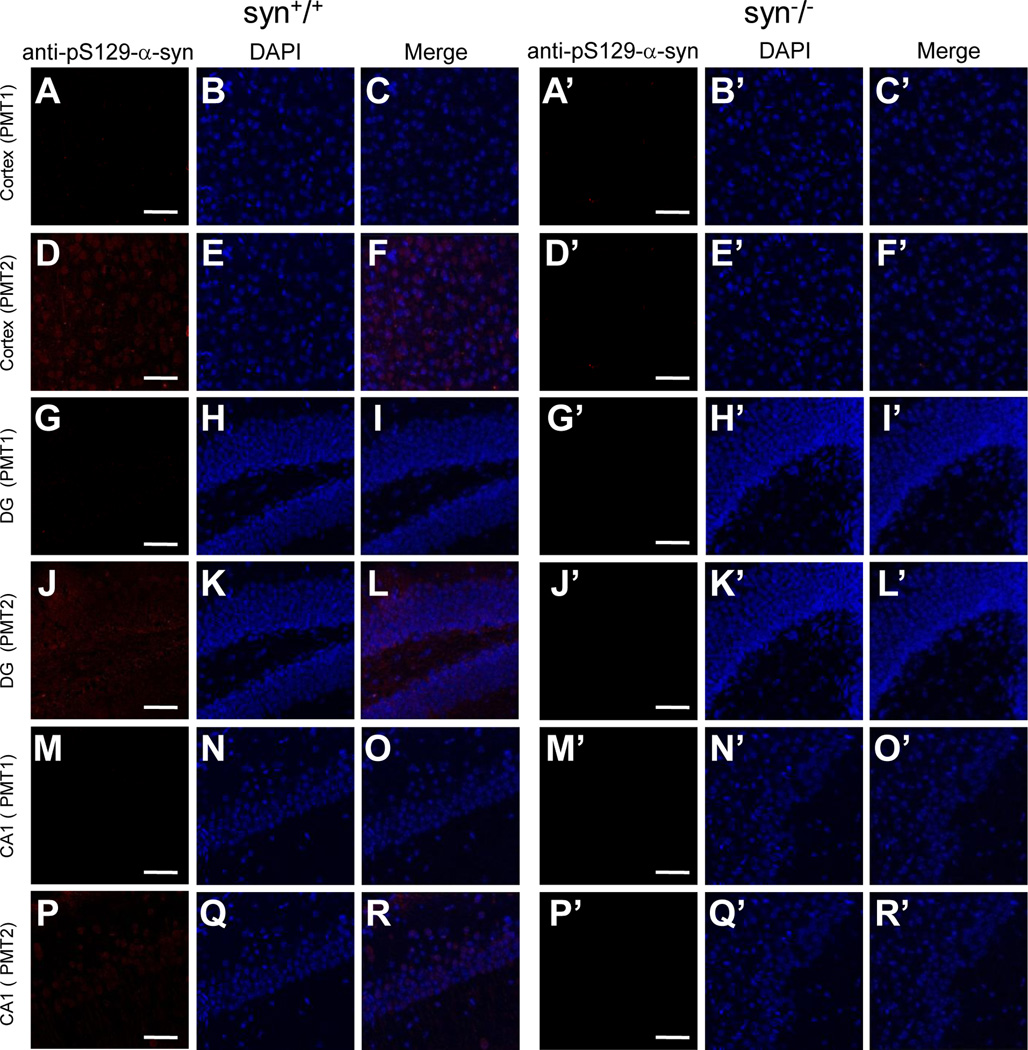
Having found cross-reactivity for some of the α-Syn antibodies, we further examined other antibodies specific for certain types of modifications in α-Syn proteins. One of them is a commercial monoclonal antibody that recognizes Ser129 phosphorylated α-Syn (anti-pS129-α-Syn, Wako Chemicals). No immunoreactivity was detected in mouse brains with normal confocal settings (Fig. 12). However, when immunofluorescence images were intensified by using a higher photomultiplier setting (PMT 2), the pS129-α-Syn antibody generated some weak signals in several brain regions of wild type, but not the α-Syn knock-out mice (Fig. 12). Thus, these data are consistent with previous report showing a low level of S129 phosphorylation under physiological conditions (Schell et al., 2009), as well as indicated a high specificity of this antibody.
Figure 12. Immunoreactivity of anti-phosphorylated-α-Syn antibody in wild type and α-Syn knock-out mouse brains.
Confocal immunofluorescence images showing staining patterns of an anti-phosphorylated-α-Syn antibody (anti-pS129-α-Syn) in cerebral cortex, DG and CA1 of hippocampus of wild type and α-Syn knock-out mice. In these regions of wild type mouse brains, the anti-pS129-α-Syn antibody generated little signals at normal photomultiplier setting (PMT 1) (A–C, G–I, M–O), while some faint staining was visible at a higher photomultiplier setting (PMT 2) (D–F, J–L, P–R). The immunostaining was completely absent in all groups of the α-Syn knock-out mice (A’–R’). Representative images from one of two independent experiments are shown. Scale bar = 50 µm.
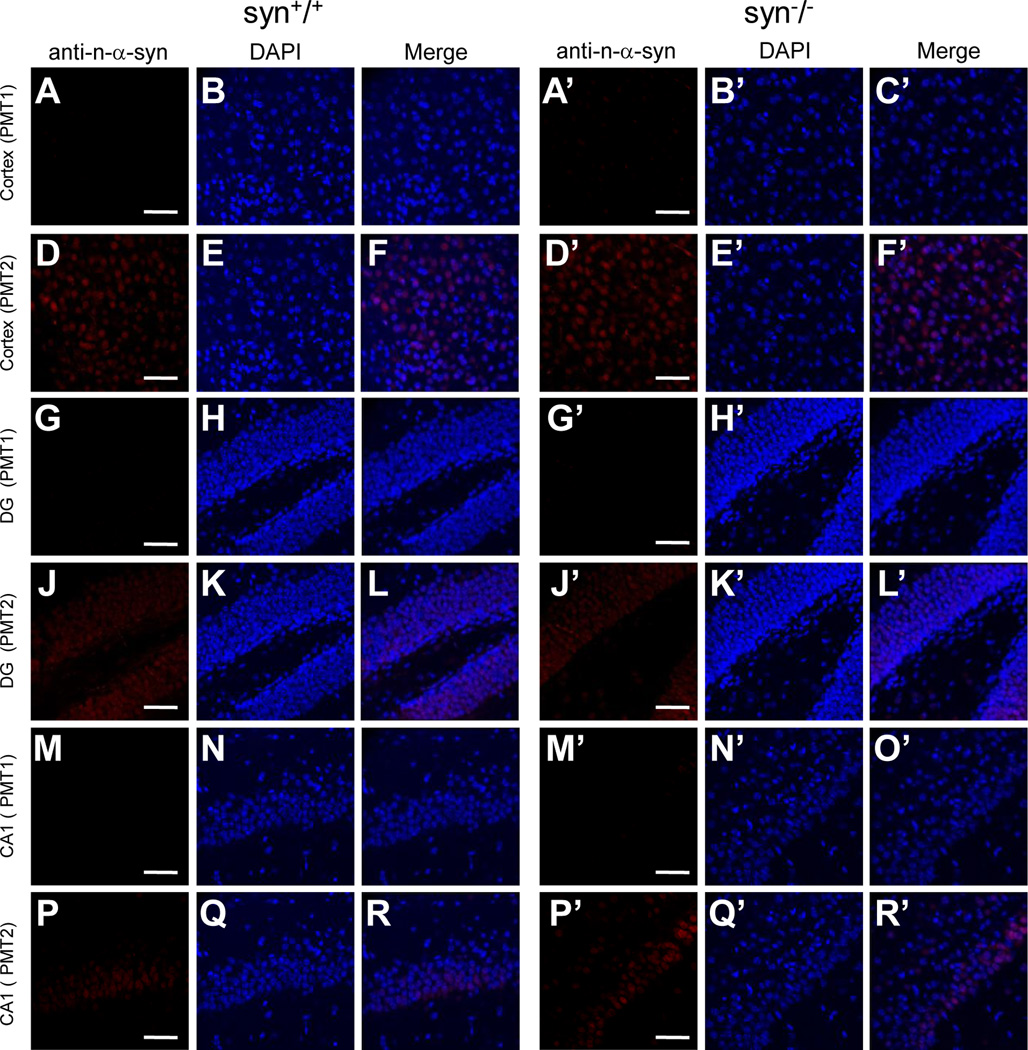
In addition, we immunostained mouse brain sections with another commercial monoclonal antibody that detects nitration of α-Syn at residues Tyr123/133, a type of modification occurring after oxidative injury (anti-n-α-Syn, Novus Biologicals) (Giasson et al., 2000a). As shown in Figure 13, the anti-n-α-Syn produced little signals when brain sections were imaged with normal confocal settings, but some weak staining was revealed in several regions of mouse brains at a higher photomultiplier setting (Fig. 13). Since these signals are similarly present in both wild type and α-Syn knock-out mice, they are not likely be derived from nitrated α-Syn, but rather possibly reflect some degree of nonspecific cross-reactivity of this antibody.
Figure 13. Immunoreactivity of anti- nitrated-α-Syn antibody in wild type and α-Syn knock-out mouse brains.
Confocal immunofluorescence images showing staining patterns of an anti-nitrated-α-Syn antibody (anti-n-α-Syn) in cerebral cortex, DG and CA1 of hippocampus of wild type and α-Syn knock-out mice. In these regions of wild type mouse brains, the anti-n-α-Syn antibody generated little signals at normal photomultiplier setting (PMT 1) (A–C, G–I, M–O), while weak nuclear staining was visible at a higher photomultiplier setting (PMT 2) (D–F, J–L, P–R). A similar nuclear staining at PMT 2 was present in these regions of the knock-out mice (D’–F’, J’–L’, P’–R’). Representative images from one of two independent experiments are shown. Scale bar = 50 µm.
DISCUSSION
α-Syn was originally named based on its subcellular localization in presynaptic terminals and nuclei of electric organ of Torpedo (Maroteaux et al., 1988). The nuclear localization of α-Syn in the vertebrate brain neurons has been a subject of debate (Galvin et al., 2001; Li et al., 2002; Yu et al., 2007; Lin et al., 2009; Vivacqua et al., 2011). Here we have addressed this controversial issue by comparing staining patterns of several α-Syn antibodies in the brain of wild type and the α-Syn knock-out mice. Similar to previous reports, we observed inconsistent nuclear staining by five different α-Syn antibodies tested in this study. Three antibodies produced little nuclear signal; however, the other two (3D5 and Ab-CS) exhibited extensive nuclear immunoreactivities throughout the mouse brain. Moreover, nuclear staining by these two antibodies persisted in the brain of the α-Syn knock-out mice, i.e., mice which have no expression of α-Syn proteins. These results thus demonstrate that nuclear staining of these two antibodies does not result from their specific immunoreactivities to α-Syn proteins. As the nuclear staining was abolished by preincubation of these two antibodies with recombinant α-Syn proteins, it is likely that their nuclear immunoreactivities were directed toward some nuclear antigen(s) that may contain epitope(s) similar to that of the α-Syn protein. For the polyclonal Ab-CS antibody, we further showed that incubation of this antibody with brain tissues of the α-Syn knock-out mice could preabsorb a portion of the polyclonal antibodies preferentially responding to the nuclear antigens, and thus selectively eliminate its nuclear staining. Taken together, our studies provide strong evidence that nuclear staining by some of α-Syn antibodies is caused by their nonspecific cross-reactivity to α-Syn unrelated antigen(s) in neuronal nuclei.
What might the nonspecific nuclear antigen(s) recognized by some of the α-Syn antibodies be? One clue is that they are probably uniquely expressed in the neuronal cells, as little nuclear staining was revealed by these antibodies in non-neuronal cells in which no exogenous α-Syn was expressed. Interestingly, all of the antibodies examined here were generated against carboxyl regions of the α-Syn protein (Table 1). It is therefore unclear why only some of them reacted with the nonspecific antigens in the nucleus. Previously, Yu et al. has determined the epitope for the 3D5 antibody using phage display and found no apparent nonspecific band on western blots in either cytoplasmic or nuclear fraction prepared from rat brains (Yu et al., 2007). Our western blot analyses of 3D5 were consistent with these findings and detected mainly α-Syn proteins from mouse brains. On the other hand, the Ab-CS antibody yielded an unknown band on western blot that had a higher molecular weight than α-Syn and was present in both wild type and α-Syn knock-out mice. However, this unknown band is unlikely the source of the nuclear immunoreactivity to this antibody, because it was evenly distributed in the cytoplasmic and nuclear fractions of mouse brains, and also existed in some non-neuronal cell lines in which the Ab-CS antibody produced little nuclear staining. Hence, it appears that conventional biochemical approaches may not be sufficient to elucidate the molecular identity of the α-Syn unrelated nuclear antigens.
Table 1.
Summary of α-Syn antibodies used in this study
| Antibodies | Host species | Types | Epitopes | Nuclear staining |
|---|---|---|---|---|
| 3D5 | Mouse | Monoclonal | Amino acids 103–130 | Yes |
| Cell Signaling (Ab-CS) | Rabbit | Polyclonal | Carboxyl terminus* | Yes |
| Santa Cruz (C-20) | Rabbit | Polyclonal | Carboxyl terminus* | No |
| Millipore (AB5038P) | Rabbit | Polyclonal | Amino acids 111–131 | No |
Amino acid sequences information is not available
Regardless of their difference in nuclear immunoreactivity, all of the α-Syn antibodies we tested produced extensive staining in the neuronal processes and nerve terminals. As the neuropil staining among different antibodies was comparable in the wild type mouse brain and absent in the α-Syn knock-out mice, it is clear that endogenous α-Syn proteins are primarily distributed in the processes and terminals of brain neurons. Interestingly, exogenous α-Syn proteins are distributed in both cytoplasmic and nuclear compartments when transfected into heterologous cells or primary neurons. This raises a possibility that α-Syn proteins may be preferentially localized to neuropil due to their specific interactions with certain structural or molecular components uniquely existed in the neuronal processes and nerve terminals. It is worth note that, under some pathological conditions, α-Syn proteins are known to accumulate in cell bodies, as well as form protein aggregates in the cytoplasm or nuclei of brain neurons (Takeda et al., 1998; Giasson et al., 2000b; Ma et al., 2003; Lin et al., 2004; Lin et al., 2009). This may reflect a disease related change in α-Syn tethering mechanism at neuronal processes and bear some relevance to Parkinson’s disease and other synucleinopathies.
As the two nuclear reactive antibodies (3D5 and Ab-CS) recognize epitopes at carboxyl regions of the α-Syn protein, we extended our analysis on two other commercial antibodies that have been utilized to monitor certain types of modifications in the carboxyl portion of α-Syn proteins. These include an antibody that recognizes phosphorylation of α-Syn at residue Ser129 (anti-pS129-α-Syn), and an antibody that detects nitration of α-Syn at residues Tyr123/133. By comparing their immuno-staining patterns in wild type mouse brains with that of the α-Syn knock-out mice, we determined that both antibodies produced little nonspecific signals under basal conditions. Such information should be beneficial for future applications of these antibodies in characterizing post-translational modification of α-Syn proteins in pathological conditions.
CONCLUSION
In summary, we have determined that the nuclear staining revealed by some of the α-Syn antibodies is caused by their immunoreactivity to some unknown antigen(s) that is present in neuronal nuclei but is not α-Syn. In mouse brains, α-Syn proteins are primarily distributed in the neuronal processes and nerve terminals, while minimally localized to the somata. These findings help define the subcellular localization of α-Syn proteins in the brain and may set a stage for further investigation of the role of α-Syn in physiological and pathological processes.
Highlights.
-
>
Nuclear localization of endogenous α-Syn in the brain is controversial.
-
>
Specificity of a panel of α-Syn antibodies was assessed by using the α-Syn knock-out mice.
-
>
Nuclear staining by some of α-Syn antibodies results from the nonspecific cross-reactivity.
-
>
Exogenously expressed α-Syn is distributed in both cytoplasmic and nuclear compartments.
ACKNOWLEDGEMENTS
This work was supported by NIH grant NS50355 (to Y.Z.). We would like to thank Drs. Shun Yu and Piu Chan for providing us the 3D5 antibody, and Dr. Jianhua Zhang for the α-Syn cDNA constructs. We also thank Ruth Didier of the Confocal Facility in the FSU College of Medicine for her help with imaging, and Dr. Richard Nowakowski for proofreading this manuscript.
Abbreviations
- α-Syn
alpha-Synuclein
- PD
Parkinson’s disease
- LB
Lewy bodies
- DAPI
4′,6-diamidino-2-phenylindole
- PMT
photomultiplier
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 2.Bayer TA, Jakala P, Hartmann T, Egensperger R, Buslei R, Falkai P, Beyreuther K. Neural expression profile of alpha-synuclein in developing human cortex. Neuroreport. 1999;10:2799–2803. doi: 10.1097/00001756-199909090-00019. [DOI] [PubMed] [Google Scholar]
- 3.Cantuti-Castelvetri I, Klucken J, Ingelsson M, Ramasamy K, McLean PJ, Frosch MP, Hyman BT, Standaert DG. Alpha-synuclein and chaperones in dementia with Lewy bodies. J Neuropathol Exp Neurol. 2005;64:1058–1066. doi: 10.1097/01.jnen.0000190063.90440.69. [DOI] [PubMed] [Google Scholar]
- 4.Chandra S, Fornai F, Kwon HB, Yazdani U, Atasoy D, Liu X, Hammer RE, Battaglia G, German DC, Castillo PE, Sudhof TC. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc Natl Acad Sci U S A. 2004;101:14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galvin JE, Schuck TM, Lee VM, Trojanowski JQ. Differential expression and distribution of alpha-, beta-, and gamma-synuclein in the developing human substantia nigra. Exp Neurol. 2001;168:347–355. doi: 10.1006/exnr.2000.7615. [DOI] [PubMed] [Google Scholar]
- 6.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000a;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 7.Giasson BI, Jakes R, Goedert M, Duda JE, Leight S, Trojanowski JQ, Lee VM. A panel of epitope-specific antibodies detects protein domains distributed throughout human alpha-synuclein in Lewy bodies of Parkinson's disease. J Neurosci Res. 2000b;59:528–533. doi: 10.1002/(SICI)1097-4547(20000215)59:4<528::AID-JNR8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Goers J, Manning-Bog AB, McCormack AL, Millett IS, Doniach S, Di Monte DA, Uversky VN, Fink AL. Nuclear localization of alpha-synuclein and its interaction with histones. Biochemistry. 2003;42:8465–8471. doi: 10.1021/bi0341152. [DOI] [PubMed] [Google Scholar]
- 9.Kontopoulos E, Parvin JD, Feany MB. Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum Mol Genet. 2006;15:3012–3023. doi: 10.1093/hmg/ddl243. [DOI] [PubMed] [Google Scholar]
- 10.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Jeon H, Kandror KV. Alpha-synuclein is localized in a subpopulation of rat brain synaptic vesicles. Acta Neurobiol Exp (Wars) 2008;68:509–515. doi: 10.55782/ane-2008-1717. [DOI] [PubMed] [Google Scholar]
- 12.Li JY, Henning JP, Dahlstrom A. Differential localization of alpha-, beta- and gamma-synucleins in the rat CNS. Neuroscience. 2002;113:463–478. doi: 10.1016/s0306-4522(02)00143-4. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Wu Y, Zhou Y. Modulation of inactivation properties of CaV2.2 channels by 14-3-3 proteins. Neuron. 2006;51:755–771. doi: 10.1016/j.neuron.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Lin WL, DeLucia MW, Dickson DW. Alpha-synuclein immunoreactivity in neuronal nuclear inclusions and neurites in multiple system atrophy. Neurosci Lett. 2004;354:99–102. doi: 10.1016/j.neulet.2003.09.075. [DOI] [PubMed] [Google Scholar]
- 15.Lin X, Parisiadou L, Gu XL, Wang L, Shim H, Sun L, Xie C, Long CX, Yang WJ, Ding J, Chen ZZ, Gallant PE, Tao-Cheng JH, Rudow G, Troncoso JC, Liu Z, Li Z, Cai H. Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson's-disease-related mutant alpha-synuclein. Neuron. 2009;64:807–827. doi: 10.1016/j.neuron.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma QL, Chan P, Yoshii M, Ueda K. Alpha-synuclein aggregation and neurodegenerative diseases. J Alzheimers Dis. 2003;5:139–148. doi: 10.3233/jad-2003-5208. [DOI] [PubMed] [Google Scholar]
- 17.Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLean PJ, Ribich S, Hyman BT. Subcellular localization of alpha-synuclein in primary neuronal cultures: effect of missense mutations. J Neural Transm. 2000 Suppl:53–63. doi: 10.1007/978-3-7091-6284-2_5. [DOI] [PubMed] [Google Scholar]
- 19.Mori F, Tanji K, Yoshimoto M, Takahashi H, Wakabayashi K. Immunohistochemical comparison of alpha- and beta-synuclein in adult rat central nervous system. Brain Res. 2002;941:118–126. doi: 10.1016/s0006-8993(02)02643-4. [DOI] [PubMed] [Google Scholar]
- 20.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 21.Rampello L, Cerasa S, Alvano A, Butta V, Raffaele R, Vecchio I, Cavallaro T, Cimino E, Incognito T, Nicoletti F. Dementia with Lewy bodies: a review. Arch Gerontol Geriatr. 2004;39:1–14. doi: 10.1016/j.archger.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Recchia A, Debetto P, Negro A, Guidolin D, Skaper SD, Giusti P. Alpha-synuclein and Parkinson's disease. FASEB J. 2004;18:617–626. doi: 10.1096/fj.03-0338rev. [DOI] [PubMed] [Google Scholar]
- 23.Schell H, Hasegawa T, Neumann M, Kahle PJ. Nuclear and neuritic distribution of serine-129 phosphorylated alpha-synuclein in transgenic mice. Neuroscience. 2009;160:796–804. doi: 10.1016/j.neuroscience.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Specht CG, Tigaret CM, Rast GF, Thalhammer A, Rudhard Y, Schoepfer R. Subcellular localisation of recombinant alpha- and gamma-synuclein. Mol Cell Neurosci. 2005;28:326–334. doi: 10.1016/j.mcn.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 26.Spira PJ, Sharpe DM, Halliday G, Cavanagh J, Nicholson GA. Clinical and pathological features of a Parkinsonian syndrome in a family with an Ala53Thr alpha-synuclein mutation. Ann Neurol. 2001;49:313–319. [PubMed] [Google Scholar]
- 27.Takeda A, Mallory M, Sundsmo M, Honer W, Hansen L, Masliah E. Abnormal accumulation of NACP/alpha-synuclein in neurodegenerative disorders. Am J Pathol. 1998;152:367–372. [PMC free article] [PubMed] [Google Scholar]
- 28.Totterdell S, Meredith GE. Localization of alpha-synuclein to identified fibers and synapses in the normal mouse brain. Neuroscience. 2005;135:907–913. doi: 10.1016/j.neuroscience.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 29.Venda LL, Cragg SJ, Buchman VL, Wade-Martins R. alpha-Synuclein and dopamine at the crossroads of Parkinson's disease. Trends Neurosci. 2010;33:559–568. doi: 10.1016/j.tins.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vivacqua G, Casini A, Vaccaro R, Fornai F, Yu S, D'Este L. Different sub-cellular localization of alpha-synuclein in the C57BL\6J mouse's central nervous system by two novel monoclonal antibodies. J Chem Neuroanat. 2011;41:97–110. doi: 10.1016/j.jchemneu.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Vivacqua G, Yin JJ, Casini A, Li X, Li YH, D'Este L, Chan P, Renda TG, Yu S. Immunolocalization of alpha-synuclein in the rat spinal cord by two novel monoclonal antibodies. Neuroscience. 2009;158:1478–1487. doi: 10.1016/j.neuroscience.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Wakamatsu M, Ishii A, Ukai Y, Sakagami J, Iwata S, Ono M, Matsumoto K, Nakamura A, Tada N, Kobayashi K, Iwatsubo T, Yoshimoto M. Accumulation of phosphorylated alpha-synuclein in dopaminergic neurons of transgenic mice that express human alpha-synuclein. J Neurosci Res. 2007;85:1819–1825. doi: 10.1002/jnr.21310. [DOI] [PubMed] [Google Scholar]
- 33.Watson JB, Hatami A, David H, Masliah E, Roberts K, Evans CE, Levine MS. Alterations in corticostriatal synaptic plasticity in mice overexpressing human alpha-synuclein. Neuroscience. 2009;159:501–513. doi: 10.1016/j.neuroscience.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wenning GK, Jellinger KA. The role of alpha-synuclein in the pathogenesis of multiple system atrophy. Acta Neuropathol. 2005;109:129–140. doi: 10.1007/s00401-004-0935-y. [DOI] [PubMed] [Google Scholar]
- 35.Yu S, Li X, Liu G, Han J, Zhang C, Li Y, Xu S, Liu C, Gao Y, Yang H, Ueda K, Chan P. Extensive nuclear localization of alpha-synuclein in normal rat brain neurons revealed by a novel monoclonal antibody. Neuroscience. 2007;145:539–555. doi: 10.1016/j.neuroscience.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 36.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez TE, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, Zhang C, Zhu Y, Cai Q, Chan P, Ueda K, Yu S, Yang H. Semi-quantitative analysis of alpha-synuclein in subcellular pools of rat brain neurons: an immunogold electron microscopic study using a C-terminal specific monoclonal antibody. Brain Res. 2008;1244:40–52. doi: 10.1016/j.brainres.2008.08.067. [DOI] [PubMed] [Google Scholar]
- 38.Zhong SC, Luo X, Chen XS, Cai QY, Liu J, Chen XH, Yao ZX. Expression and subcellular location of alpha-synuclein during mouse-embryonic development. Cell Mol Neurobiol. 2010;30:469–482. doi: 10.1007/s10571-009-9473-4. [DOI] [PMC free article] [PubMed] [Google Scholar]