Abstract
Hepatocellular carcinoma (HCC) results from the accumulation of deregulated tumor suppressor genes and/or oncogenes in hepatocytes. Inactivation of TP53 and inhibition of transforming growth factor-beta (TGF-β) signaling are among the most common molecular events in human liver cancers. Thus, we assessed whether inactivation of TGF-β signaling, by deletion of the TGF-β receptor, type II (Tgfbr2), cooperates with Trp53 loss to drive HCC formation. Albumincre transgenic mice were crossed with floxed Trp53 and/or floxed Tgfbr2 mice to generate mice lacking p53 and/or Tgfbr2 in the liver. Deletion of Trp53 alone (Trp53KO) resulted in liver tumors in approximately 41% of mice by 10 months of age, while inactivation of Tgfbr2 alone (Tgfbr2KO) did not induce liver tumors. Surprisingly, deletion of Tgfbr2 in the setting of p53 loss (Trp53KO;Tgfbr2KO) decreased the frequency of mice with liver tumors to around 17% and delayed the age of tumor onset. Interestingly, Trp53KO and Trp53KO;Tgfbr2KO mice develop both HCC and cholangiocarcinomas, suggesting that loss of p53, independent of TGF-β, may affect liver tumor formation through effects on a common liver stem cell population. Assessment of potential mechanisms through which TGF-β signaling may promote liver tumor formation in the setting of p53 loss revealed a subset of Trp53KO tumors that express increased levels of alpha-fetoprotein. Furthermore, tumors from Trp53KO mice express increased TGF-β1 levels compared to tumors from Trp53KO;Tgfbr2KO mice. Increased phosphorylated Smad3 and ERK1/2 expression was also detected in the tumors from Trp53KO mice and correlated with increased expression of the TGF-β responsive genes, Pai1 and Ctgf.
Conclusion
TGF-β signaling paradoxically promotes the formation of liver tumors that arise in the setting of p53 inactivation.
Keywords: HCC, CC, AFP, Smad3, ERK1/2
Hepatocellular carcinoma (HCC) is one of the deadliest forms of cancer worldwide, with a five-year survival rate of less than 5 percent (1). The high death rate is due in part to the fact that liver cancer is often detected at advanced stages, usually after metastatic spread of the primary tumor has already occurred (2). This is particularly problematic because, apart from surgical resection or ablation of the primary tumors, no curative treatment options are available (3). Therefore, the need for understanding the molecular mechanisms involved in the initiation and progression of the disease is critical in order to develop more effective medical therapies for this form of cancer.
Hepatocarcinogenesis is the result of progressive genetic and epigenetic changes that accumulate in liver epithelial cells and lead to the deregulation of fundamental behaviors of the cells, such as proliferation, apoptosis, etc. (4, 5). Chronic viral infection by hepatitis B virus or hepatitis C virus and concurrent cirrhosis are among the known factors that predispose the liver to HCC (6). The viral proteins HBx and NS5 have been shown to bind and inhibit the tumor suppressor p53 (7). The inactivation of p53 by these viral proteins is believed to be a major contributing event in the formation of HCCs (8). Furthermore, somatic mutations or deletion of TP53 are also common molecular events in human liver cancer (9).
In addition to TP53 mutations, alterations in the transforming growth factor-beta (TGF-β) signaling pathway are commonly observed in HCC. TGF-β is a secreted cytokine that initiates downstream signals through binding to a heteromeric cell-surface receptor complex that consists of two transmembrane serine-threonine kinases, TGF-β receptor, type I (TGFBR1) and type II (TGFBR2). This activated receptor complex induces both Smad-dependent and Smad-independent signaling pathways (10). TGF-β has been found to be overexpressed in 40% of HCCs (11), while Tgfbr2 has been shown to be downregulated in 37-70% of tumors (12, 13). In the liver, TGF-β has been shown to play both tumor suppressive and tumor promoting roles (14, 15). This paradoxical role of TGF-β in cancer is believed to be a consequence of the context dependence of the TGF-β signaling pathway on tumor cells. Among other factors, the concurrent gene alterations present in a tumor cell can influence whether TGF-β signaling has primarily an oncogenic or tumor suppressive role. Thus, it is important to determine cooperative effects of specific gene mutations on the TGF-β signaling pathway in order to determine what effect therapies directed at the TGF-β pathway may have on cancers carrying specific mutations that affect the pathway output (16).
Studies from in vitro systems have revealed that p53 and TGF-β can cooperate to regulate a number of cellular responses (17). p53 physically interacts with Smad2 and Smad3 in a TGF-β dependent manner (18). In mouse embryonic fibroblasts, p53 is required for TGF-β mediated growth arrest, and in Xenopus, defective embryonic development results from impaired TGF-β/Activin/Nodal signaling caused by the loss of p53 (18). Although p53 and Smads function as transcription factors that bind distinct promoter sequences, they have been shown to coordinately regulate a number of target genes. For example, at the Mix.2 promoter, p53 binding is required for expression and is believed to help stabilize a larger complex consisting of Smad2, Smad4, and FAST1 (18). Additionally, the repression of alpha-fetoprotein (AFP), a clinical marker of HCC, depends on the interaction with Smads, p53, and the corepressors, SnoN and mSin3A (19, 20). Therefore, the importance of the relationship between the p53/TGF-β signaling pathways in regulating the transcriptional response of cells to various stimuli has been established, but the relevance to in vivo HCC formation remains to be determined. Thus, we developed a mouse model system to investigate if p53 and Tgfbr2 cooperate in vivo to affect HCC formation.
Materials and Methods
Generation and characterization of Alb-Cre;Trp53flx/flx;Tgfbr2flx/flx mice
The generation of Albumin-Cre (Alb-Cre), Trp53F2-10/F2-10 (Trp53flx/flx) and Tgfbr2flx/flx mice has been described previously (21-23). Tgfbr2flx/flx mice were crossed with Alb-Cre transgenic mice and Trp53flx/flx mice to generate the following genotypes: Alb-Cre;Trp53flx/flx;Tgfbr2wt/wt (Trp53KO), Alb-Cre;Trp53flx/flx;Tgfbr2flx/flx (Trp53KO;Tgfbr2KO), Alb-Cre;Trp53wt/wt;Tgfbr2flx/flx (Tgfbr2KO), and Trp53flx/flx;Tgfbr2flx/flx (Control). Mice were backcrossed in order to obtain a strain background that was on average C57BL6 (87.5%) / FVB (12.5%). Both male and female mice were used for this study. Tissues from non-breeders were used for qRT-PCR, ELISA and Western Blot assays. Genotypes were determined by PCR following published protocols (21, 24). All mice were maintained and cared for using protocols approved by the institutional IACUC. Mice that became moribund or reached approximately 15 months of age were sacrificed and necropsied. Total body weight and liver weight were measured.
Mouse tissue processing
Mouse tissues were either snap-frozen in liquid nitrogen and used for RNA and protein preparations; or fixed in 10% neutral buffered formalin phosphate (Fisher Scientific, Pittsburgh, PA), embedded in paraffin, and cut into 4 μm sections for H&E staining or immunohistochemistry (See Supporting Information).
Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Gene expression studies were performed as described in Supporting Information. The results of the qRT-PCR assays were normalized to β-glucuronidase. Statistical analysis was performed using the GraphPad Prism version 4.00 software. The Mann-Whitney test was used for comparisons of quantitative results from the ELISA and qRT-PCR assays. A P value of <0.05 was regarded as significant.
Protein lysate preparation
Total protein lysates were prepared from frozen tumor or non-tumor liver tissue. Samples were homogenized on ice with a Dounce Tissue Grinder (Wheaton Science Products, Millville, NJ) in Triton X-100 Lysis Buffer (See Supporting Information).
TGF-β1 ELISA
Mouse TGF-β1 was assessed in protein lysates (21ug per sample) obtained from selected paired frozen tumor and non-tumor tissues, as well as from grossly normal appearing livers. The samples were activated and quantified according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN).
Western blot analysis
Protein lysates (20 ug per lane) were resolved by 10% SDS-PAGE and transferred to PVDF membranes (Thermo Scientific, Rockford, IL). Antibodies used are described in the Supporting Information. Densitometric quantification of immunoblots was performed using the ImageJ 1.43 software.
Results
Targeted deletion of Trp53 in the liver results in tumor formation
Since inactivation of the TGF-β signaling pathway and mutation of TP53 are common molecular events observed in human HCC, we assessed whether deletion of Tgfbr2 and Trp53 cooperate in the mouse liver to affect tumor formation. To this end, we crossed Alb-Cre transgenic mice with mice conditionally null for either Tgfbr2 and/or Trp53 to generate mice with liver-specific deletion of these genes (21-23). No liver tumors were observed in the control mice lacking Alb-Cre (Control) (Table 1). Likewise, deletion of Tgfbr2 alone (Tgfbr2KO) did not induce liver tumors by 15 months of age. The Tgfbr2KO mice had a normal liver to body weight ratio of 0.050, which is not statistically different from the Control mice (Table 1). In contrast, deletion of Trp53, in the context of intact Tgfbr2 (Trp53KO), resulted in a significant number of mice developing tumors (P = 0.0034) as compared to the Control mice. The median lifespan for the entire Trp53KO cohort was 46.6 weeks, while the median lifespan for the subset of mice with tumors was 22.7 weeks. Survival curves illustrate that 52% of Trp53KO mice died by 50 weeks of age (Fig. 1). Additionally, the liver to body weight ratio was increased nearly 2X (P = 0.0002) in the Trp53KO cohort, presumably secondary to the tumor load present in the Trp53KO mice (Table 1). Histological analysis of the primary tumors from the Trp53KO livers revealed the tumors to be both HCC and cholangiocarcinoma (CC) (Fig. 2). The tumors consisted of a variety of histologic subtypes, ranging from trabecular HCC with necrosis to CC with necrosis and fibrosis. Biliary hyperplasia, cholangiohepatitis, multifocal coagulative necrosis, oval cell hyperplasia, and arterial thrombosis were also noted in the adjacent liver tissue. Of the 12 liver tumor bearing mice, 2 also had multiple lung metastases that likely arose from large primary CCs.
Table 1.
Liver Tumors in Trp53KO and Trp53KO;Tgfbr2KO Mice
| Genotype | Total # of Mice |
Median Survival of Entire Cohort (weeks) |
Median Survival of Mice with Tumors (weeks) |
# of Mice with Liver Tumors |
% of Mice with Liver Tumors |
Liver/Body Weight Ratio of Entire Cohort |
Liver/Body Weight Ratio of Mice with Tumors |
|---|---|---|---|---|---|---|---|
| Trp53KO | 29 | 46.6 | 22.7€ | 12§ | 41 | 0.096† | 0.132‡ |
| Trp53KO;Tgfbr2KO | 36 | 64.0 | 71.6€ | 6§ | 17 | 0.054† | 0.063‡ |
| Tgfbr2KO | 19 | 65.3 | N/A | 0 | 0 | 0.050 | N/A |
| Control | 14 | 65.0 | N/A | 0 | 0 | 0.050 | N/A |
P = 0.0017, Trp53KO vs. Trp53KO;Tgfbr2KO mice, Mann-Whitney test
P = 0.0265, Trp53KO (12/29) vs. Trp53KO;Tgfbr2KO (6/36) mice, Fisher’s exact test
P = 0.0002, Trp53KO vs. Trp53KO;Tgfbr2KO mice, Mann-Whitney test
P = 0.0149, Trp53KO vs. Trp53KO;Tgfbr2KO mice, Mann-Whitney test
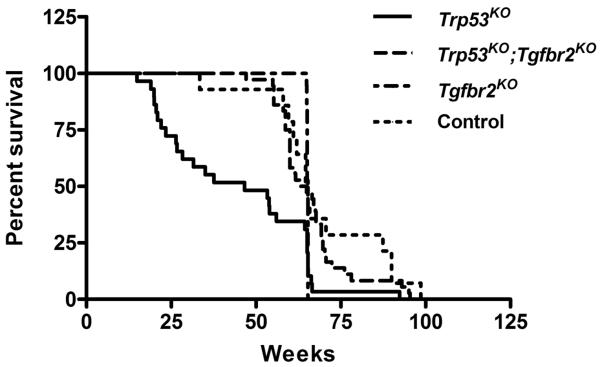
Figure 1. Survival curves of Trp53KO, Trp53KO;Tgfbr2KO, Tgfbr2KO and Control mice.
52% of Trp53KO mice (N=29) died by 50 weeks whereas 3% of Trp53KO;Tgfbr2KO mice (N=36) died by 50 weeks and no Tgfbr2KO mice (N=19) died by 50 weeks. Seven percent of Control mice (N=14) died by 50 weeks of age. A significant decrease in the survival of Trp53KO mice as compared to Trp53KO;Tgfbr2KO, Tgfbr2KO or Control mice was observed (P = 0.0001, 0.0213, 0.0082, respectively). There was no significant difference in survival between the Trp53KO;Tgfbr2KO, Tgfbr2KO or Control mice (P = 0.4590, Log rank Test).
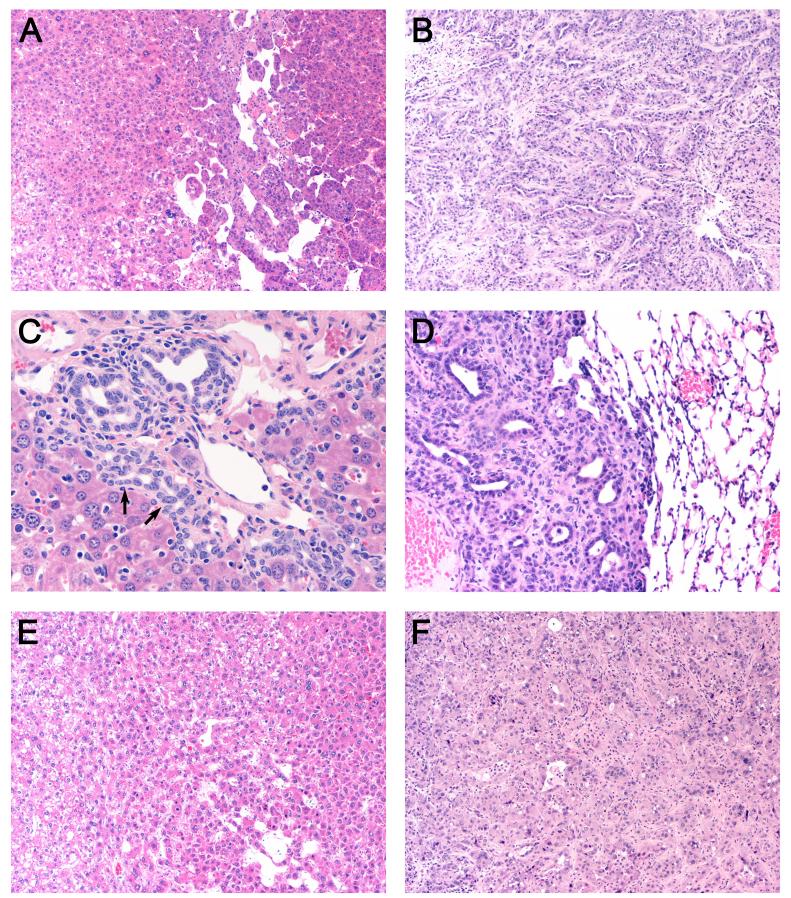
Figure 2. H&E stained sections of representative liver tumors and lung metastasis.
(A) HCC, 100x magnification (B) CC, 100x (C) Oval cell (arrows) and biliary hyperplasia with mononuclear infiltration, 400x and (D) Metastatic lung lesion derived from a CC from Trp53KO mice, 200x. (E) HCC, 100x (F) CC from Trp53KO;Tgfbr2KO mice, 100x.
Deletion of Tgfbr2 in p53 null livers decreases tumor formation
In light of the known common occurrence of TGF-β signaling inactivation and TP53 mutation in human HCC and the development of HCCs and CCs in the Trp53KO mice, we assessed the effect of Tgfbr2 deletion on liver tumor formation in these mice. Livers from mice with both inactive p53 and Tgfbr2 (Trp53KO;Tgfbr2KO) were analyzed. Interestingly, the double knock-out mice displayed a survival curve similar to the Control and Tgfbr2KO mice (Fig. 1). Additionally, fewer mice developed liver tumors by 15 months (Table 1, P = 0.0265) compared to the Trp53KO mice. The median lifespan for the mice with tumors was 71.6 weeks. Furthermore, the liver to body weight ratio of the tumor-bearing Trp53KO;Tgfbr2KO mice was significantly lower than the ratio of tumor-bearing Trp53KO mice (P = 0.0149). The double knock-out mice displayed a tumor spectrum similar to the Trp53KO mice in that they also developed both HCC and CC (Fig. 2). However, no lung metastases were observed in the tumor bearing Trp53KO;Tgfbr2KO mice. Assessment of the gene status of the normal liver and tumors of the various genotypes confirmed the tissue specific recombination and deletion status as predicted (Supporting Fig. 1).
A subset of Trp53KO tumors express high Afp mRNA levels
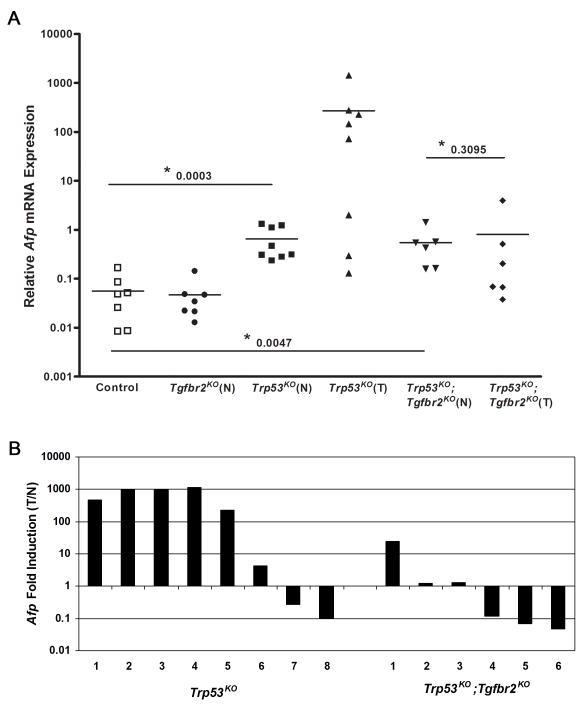
Based on our analysis of the Trp53KO vs. Trp53KO;Tgfbr2KO mice, it was clear that mice lacking p53 and with intact Tgfbr2 developed tumors at a younger age, had increased liver to body weight ratios, and displayed overall worse survival rates compared to the mice lacking both p53 and Tgfbr2. Subsequently, we conducted a series of studies assessing candidate mechanisms that may be responsible for the pro-tumorigenic effects of TGF-β in the setting of loss of p53 in the liver. We initially focused on AFP, a gene frequently overexpressed in human liver cancer that may promote HCC formation (25). AFP has been shown to be regulated by both p53 and TGF-β (19, 20) and is thought to play a pathogenic role in liver cancer by acting as a growth factor and immunosuppressor (26, 27). Afp mRNA levels were analyzed in tumor and non-tumor tissue isolated from mice of various genotypes (Fig. 3A). Afp mRNA was expressed at very low levels in normal liver tissue harvested from Control mice, consistent with previous reports (28, 29). There was no significant difference in the median level of Afp mRNA detected in the normal livers of Tgfbr2KO mice, compared to Control mice (P = 0.7104). A significant increase in Afp mRNA levels was observed in normal tissue from Trp53KO mice and normal tissue from Trp53KO;Tgfbr2KO mice (Trp53KO and Trp53KO;Tgfbr2KO vs. Control, P = 0.0003 and 0.0047, respectively). This moderate increase over basal levels in normal liver is consistent with the role of p53 in Afp repression. Analysis of the levels of Afp mRNA in Trp53KO tumors revealed two distinct subsets of tumors – a high Afp expressing group and a moderate/low Afp expressing group (Fig. 3A). This was in contrast to tumors from Trp53KO;Tgfbr2KO mice which all had moderate/low Afp expression. The ratio of Afp mRNA expression (tumor:normal liver, T/N) was also calculated for Trp53KO and Trp53KO;Tgfbr2KO mice (Fig. 3B). In the Trp53KO mice, the ratio of Afp mRNA expression in tumors vs. normal liver in a subset of tumors was higher than in tumors arising in the Trp53KO;Tgfbr2KO mice (P = 0.0426).
Figure 3. Afp mRNA expression in normal and liver tumor tissues.
(A) qRT-PCR analysis reveals that Afp mRNA levels are increased in the normal (N) tissue from Trp53KO and Trp53KO;Tgfbr2KO mice compared to normal (Control) tissue (P = 0.0003 and 0.0047, respectively). There is no significant difference between Afp levels in Trp53KO;Tgfbr2KO tumors (T) and normal (N) tissue (P = 0.3095). However, analysis of Afp levels in tumors from Trp53KO mice revealed that the tumors separate into two distinct groups, those expressing high Afp mRNA levels and those with moderate to low levels of Afp mRNA. (B) Ratio of Afp mRNA expression levels (T/N) in Trp53KO and Trp53KO;Tgfbr2KO mice. Five out of eight tumors from the Trp53KO mice exhibited over a hundred fold increase in Afp mRNA levels as compared to normal tissue. In contrast, only one Trp53KO;Tgfbr2KO tumor exhibited a 24 fold increase, while the other tumors remained unchanged or slightly decreased in comparison to their corresponding normal tissue. The average age of the mice used for analysis in each group is: Control, 72.3 weeks; Tgfbr2KO, 65.2 weeks; Trp53KO, 20.0 weeks; Trp53KO;Tgfbr2KO, 72.3 weeks.
Increased TGF-β1 production in Trp53KO tumors
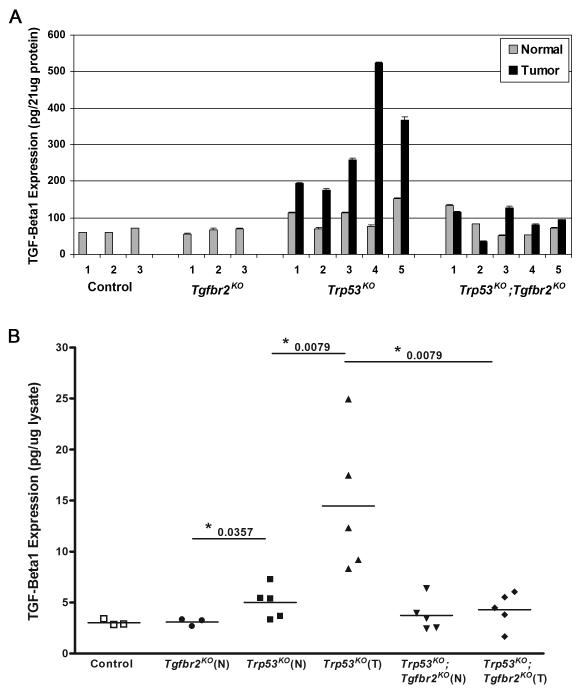
Although we observed increased Afp in a subset of Trp53KO tumors, it is clear that elevated Afp levels is not the sole mechanism responsible for increased liver tumor formation in the Trp53KO mice. Therefore we determined if there were other concurrent mechanisms that may help explain how Tgfbr2 cooperates with loss of p53 to promote liver tumor formation. One possible mechanism could be through regulation of the TGF-β signaling pathway itself. TGF-β1 has been shown to be upregulated in a number of tumors, including HCC (30, 31). There is evidence in many tumor types that early in tumor development, TGF-β functions as a tumor suppressor, but as tumors progress, TGF-β can work as a tumor promoter, acting in an autocrine and/or paracrine fashion to drive tumor invasion, metastasis, and angiogenesis (15). Therefore, we determined the TGF-β1 levels in the tumors of the different genotypes. A TGF-β1 ELISA was performed on lysates prepared from tumors and normal liver tissue (Fig. 4A, B). Low levels of TGF-β1 were detected in the normal Control and Tgfbr2KO livers. Assessment of TGF-β1 levels in normal Trp53KO liver tissue demonstrated a small, but significant increase over normal liver from the Tgfbr2KO mice (P = 0.0357). TGF-β1 levels were further increased in Trp53KO tumor tissue compared to normal Trp53KO liver (P = 0.0079). Comparison of TGF-β1 levels in Trp53KO tumors vs. Trp53KO;Tgfbr2KO tumors revealed that Trp53KO tumors have higher levels of TGF-β1 than Trp53KO;Tgfbr2KO tumors (P = 0.0079). These findings suggest that TGF-β signaling in the setting of p53 deletion may help promote tumor formation by inducing TGF-β1 expression.
Figure 4. TGF-β1 expression in normal and liver tumor tissues.
(A) TGF-β1 was detected in cell lysates by an ELISA based assay and is increased in the tumors of Trp53KO mice. (B) Comparison of TGF-β1 levels revealed a significant increase in the tumors (T) from Trp53KO mice as compared to the normal (N) Trp53KO liver tissue (P = 0.0079) and tumors from Trp53KO;Tgrbr2KO mice (P = 0.0079). The levels of TGF-β1 in the normal liver tissue from Trp53KO mice was also increased slightly over the levels in normal liver tissue from Tgfbr2KO mice (P = 0.0357), but not in normal liver from Trp53KO;Tgrbr2KO or Control mice (P = 0.3095 and 0.0714, respectively).
Loss of p53 and Tgfbr2 Reduce Smad3 and Erk1/2 Phosphorylation
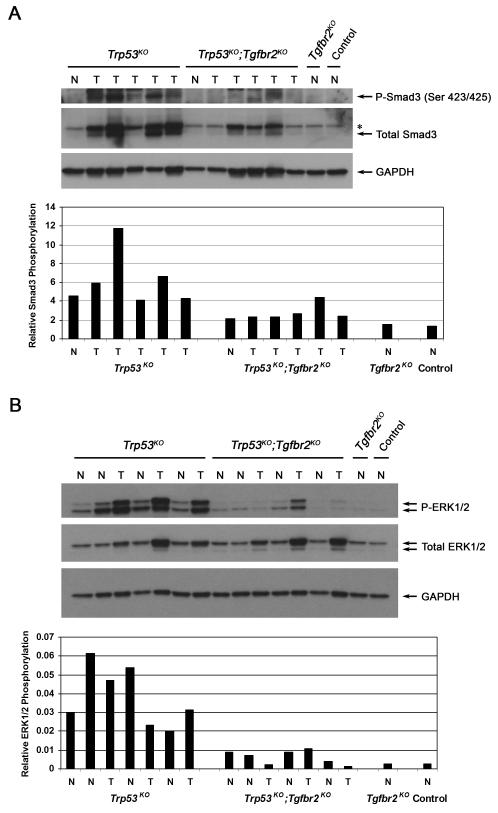
Since TGF-β1 levels were increased in Trp53KO tumors, we assessed the activation status of TGF-β signaling pathways in these tumors, including both Smad-dependent and Smad-independent pathways (Fig. 5). Immunoblot and immunohistochemistry analysis of liver tissue from both Trp53KO and Trp53KO;Tgfbr2KO mice detected the expression of phospho-Smad2 in both tumor genotypes (Supporting Fig. 2), thus indicating that the Smad2-dependent pathway is activated regardless of Tgfbr2 status, perhaps through activin signaling in the Trp53KO;Tgfbr2KO mice. The status of Smad3 was also assessed in the tumor samples (Fig. 5A). In contrast to Smad2, increased total Smad3 protein was observed in the majority of tumors from Trp53KO mice as compared to tumors from Trp53KO;Tgfbr2KO mice. This increase in total Smad3 levels corresponded to an overall increase in phospho-Smad3 levels in the Trp53KO tumors and suggests that regulation of total Smad3 levels and subsequent Smad3-dependent signaling may promote the tumors of the Trp53KO mice. Next, we analyzed the activation status of the MAPK pathway, another signaling cascade that can be induced by TGF-β1 stimulation (Fig. 5B). Interestingly, we found that the MAPK pathway, as measured by phospho-ERK1/2, is highly activated in the Trp53KO tumors compared to tumors lacking both p53 and Tgfbr2. Furthermore, increased ERK1/2 phosphorylation is also observed in the normal liver tissue in the Trp53KO mice as compared to the normal tissue from the Trp53KO;Tgfbr2KO mice. This increase in phosphorylated ERK1/2 in Trp53KO tumors was also observed by immunohistochemistry (Fig. 6 and Supporting Fig. 3).
Figure 5. Smad3 and ERK1/2 phosphorylation levels in normal and liver tumor tissues.
(A) Upper panel, Analysis of Smad3 expression by Western blot demonstrates an increase in phospho-Smad3 and total Smad3 in Trp53KO tumors (T), but not in lysates from tumors harvested from Trp53KO;Tgfbr2KO mice or normal (N) control tissues. (Asterisk indicates total Smad2 band.) Lower panel, A significant increase in relative Smad3 phosphorylation levels in Trp53KO liver samples as determined by densitometry, normalized to GAPDH (P = 0.0159). We also normalized phospho-Smad3 to total Smad3 and found no significant difference in the ratios between the different genotypes (data not shown). This is likely due to the fact that there is an overall increase in total Smad3 levels in Trp53KO tumors, which correlates with an overall increase in phospho-Smad3. (B) Upper panel, Analysis of ERK1/2 expression demonstrates an increase in phospho-ERK1/2 in Trp53KO normal tissue (N) and tumor tissues (T). Lower levels of phospho-ERK1/2 were detected in lysates from normal and tumor tissues harvested from Trp53KO;Tgfbr2KO mice or normal control tissues. The total ERK1/2 antibody preferentially detected p44 ERK1. Lower panel, There is a significant increase in relative ERK1/2 phosphorylation levels in Trp53KO liver samples, as determined by densitometry, P-ERK1/2 normalized to total ERK1/2 divided by GAPDH (P = 0.0022).
Figure 6. Phospho-ERK1/2 expression.
Phospho-ERK1/2 expression was determined by immunohistochemical analysis. Representative photomicrographs are shown. (A) HCC, 40x (B) CC, 40x and (C) Metastatic lung lesion from Trp53KO mice, 100x. (D) HCC, 40x and (E) CC from Trp53KO;Tgfbr2KO mice, 40x. (F) Normal liver from Control mouse, 40x.
Decreased expression of TGF-β responsive genes in Trp53KO;Tgfbr2KO tumors
TGF-β induces the expression of a number of downstream target genes that regulate various cellular processes including proliferation, angiogenesis and tissue remodeling. Plasminogen activator inhibitor 1 (PAI1) is both a TGF-β and p53 target gene and contains Smad-responsive elements and activator protein 1 (AP-1) sites in its proximal promoter (32). Increased PAI1 levels have been associated with HCC invasion, metastasis and recurrence (33). Therefore, we assessed the levels of Pai1 mRNA by qRT-PCR in normal and tumor tissue (Fig. 7A). Low levels of Pai1 mRNA were detected in normal liver from Control and Tgfbr2KO mice. Pai1 levels were significantly increased in the Trp53KO tumor samples compared to Control liver tissue (P = 0.0095). However, comparison of Pai1 levels in tumors from Trp53KO and Trp53KO;Tgfbr2KO mice revealed a significant decrease in Pai1 expression in the Trp53KO;Tgfbr2KO tumor samples (P = 0.0043).
Figure 7. Expression of TGF-β responsive genes.
(A) qRT-PCR analysis shows that Pai1 mRNA levels are increased in the tumor (T) tissue from Trp53KO mice and Trp53KO;Tgfbr2KO mice compared to normal Control liver tissue (P = 0.0095). Additionally, Pai1 mRNA levels are significantly higher in Trp53KO tumors compared to Trp53KO;Tgfbr2KO tumors (P = 0.0043). (B) qRT-PCR analysis of Pai1, Ctgf, Itgb1, Cdkn1a and Fn1 in tumors from Trp53KO mice and Trp53KO;Tgfbr2KO mice.
In addition to Pai1, we analyzed the expression of additional TGF-β responsive genes in various tumors (Fig. 7B). Significantly decreased levels of connective tissue growth factor (Ctgf) and integrin beta 1 (Itgb1) were also observed in the Trp53KO;Tgfbr2KO tumors (P = 0.0350 and 0.0082, respectively) compared to the tumors from the Trp53KO mice. Furthermore, Cdkn1a (the gene for p21) and Fn1 (the gene for fibronectin 1) expression also trended downward, however the difference was not significant (P = 0.0513 and 0.0593, respectively). Therefore, the decrease in overall Pai1, Ctgf and Itgb1 expression observed in the Trp53KO;Tgfbr2KO tumors are potential mechanisms for the delayed tumor development seen in these mice compared to the Trp53KO mice.
Discussion
We have developed a mouse model for liver cancer that has allowed us to assess the in vivo functional interaction of p53 and Tgfbr2 in hepatocarcinogenesis. Liver specific deletion of p53 results in the formation of either HCC or CC in approximately 41% of the Trp53KO mice by 10 months of age. However, unexpectedly, the loss of Tgfbr2, in the context of loss of p53 decreased the incidence of HCCs and CCs and attenuated many of the features seen in the tumors with inactive p53 alone.
Interestingly, the spectrum of tumors observed in our Trp53KO mice is similar to those reported for the RCAS-PyMT injected albumin-tv-a transgenic mice containing Alb-Cre and p53 floxed alleles (34). However, only around 10% of their p53 null mice injected with control virus developed tumors by 1 year, which is lower than what was seen in our Trp53KO mice. It is possible that different genetic backgrounds and/or housing conditions could be responsible for this difference. Nevertheless, increased ERK1/2 phosphorylation in the Trp53KO tumors is present in both models suggesting that this may be a significant event in tumor formation in the liver in the setting of p53 inactivation. Additionally, both mouse models developed HCC and CC tumor types, suggesting these tumors originate from a common liver stem cell population, although this was not formally assessed. In addition to increased tumor formation in the Trp53KO mice, we also observed oval cell hyperplasia in 33% of the tumor-bearing Trp53KO mice. Oval cells are bipotential liver stem cells, capable of differentiating into both hepatocytes and cholangiocytes. Studies have shown that oval cells are less sensitive to TGF-β inhibition and p53 null oval cell lines are capable of forming tumors when injected into nude mice (35, 36). It is possible that loss of p53 in the oval cell population could set-up a permissive state and permit the accumulation of genetic mutations due to the lack of the G1 checkpoint control. This could possibly account for the occurrence of both HCC and CC in these mice. Analysis of oval cell markers in the livers and tumors might provide further insight into this possibility.
The Trp53KO mouse model recapitulates features seen in many human liver cancers, including increased expression of TGF-β1, Afp, Pai1 and Ctgf. Increased TGF-β1 has been observed in a variety of human cancers, including HCC, gastric, prostate and breast cancer (11, 37-39). The increased TGF-β1 observed in human cancers was one of the first clues that TGF-β has a complex role in cancer behavior and may have a paradoxical role in tumors arising in organs like the liver. In this case, the elevated levels are presumed to promote tumor formation through effects on tumor stromal cells and local immune cells or potentially on tumor cells that have developed mechanisms for evading the cell autonomous tumor suppressor activities of TGF-β (40). Furthermore, studies by Piccolo’s laboratory (18) suggest that in certain cancer cell types, p53 inactivation may contribute to the lack of TGF-β antiproliferative effects.
Of particular relevance to the Trp53KO mouse, TGF-β and p53 cooperate to regulate a number of target genes, including Afp. AFP is highly expressed in the developing liver and is dramatically down-regulated after birth (41). AFP is the most widely used clinical biomarker for HCC, and elevated levels have been found in approximately 70% of HCC patients. Aberrant AFP expression is thought to promote tumor growth and contribute to tumor cell evasion of the immune system (26, 27). p53 appears to be required for TGF-β/Smad mediated transcriptional repression of AFP (19, 20). In our mouse model, we found that deletion of p53 in normal liver tissue resulted in an overall increase in basal Afp mRNA levels, which is consistent with previous observations (20). We also found that 4/4 HCCs and 1/4 CCs analyzed from Trp53KO mice exhibited even higher Afp mRNA levels than normal liver tissue. In contrast, we found that 0/2 HCCs and 0/4 CCs from the Trp53KO;Tgfbr2KO mice expressed high levels of Afp mRNA. These results suggest that tumors lacking both p53 and Tgfbr2 lack the transcription factor complex needed to induce high levels of AFP expression.
PAI1 has also been shown to be regulated by TGF-β and p53 (42, 43). PAI1 is an important component of the plasminogen activating system and regulates the urokinase-type plasminogen activators (uPA) and uPA receptor complex involved in tissue remodeling. Studies have shown that Smad3 and Smad4/DPC4 are important for mediating TGF-β induction of PAI1 in Hep3B cells (42). Additionally, TGF-β induced MAPK activity is thought to regulate AP-1 activity at the Pai1 promoter in rat mesangial cells (44). Clinically, increased levels of PAI1 have been found in patients with HCC and have been correlated with tumor invasion, metastasis and poor outcome (33). Similarly, CTGF is involved in fibrogenic remodeling of the liver and increased levels in HCC patients have been correlated with poor prognosis (45). Therefore, taken together, the increased levels of TGF-β1, Afp, Pai1 and Ctgf that likely results from the effects of intact TGF-β signaling in the setting of p53 inactivation may help explain why tumors develop faster and more frequently in the Trp53KO mice.
These studies broaden our understanding of the role of TGF-β signaling and p53 in liver cancer formation and provide insight into therapies directed at these molecular targets. The identification of potential targets for treatment of HCC is important for improving the clinical outcome of patients. Recent success with the BRAF inhibitor, sorafenib, in the treatment of advanced HCC offers hope that additional therapeutic gains can be made with other targeted agents (46). There are a number of TGF-β signaling pathway inhibitors, including small molecules and antibodies, that are under investigation for the treatment of HCC (16). The development of pre-clinical cancer models, such as the Trp53KO and Trp53KO;Tgfbr2KO mice, might be useful in identifying potential targeted agents that may be effective in human HCC. Our studies also provide further support for the potential of using the mutation status of individual tumors for creating personalized strategies for cancer treatment.
Supplementary Material
Acknowledgment
The authors thank the members of the Grady laboratory for helpful suggestions and discussions, Jean Campbell for critical reading of this manuscript, Elif Sozeman and Kelly T. Carter for technical assistance. We also wish to acknowledge support from the Experimental Histopathology Core and Animal Health Resources Core at the Fred Hutchinson Cancer Research Center.
Financial Support
This work supported in part by funding from the National Institutes of Health (RO1 DK60669-01) and by pilot project funding from P30 CA015704 (WMG).
Abbreviations
- Afp
alpha-fetoprotein
- Alb-Cre
Albumin-Cre
- CC
cholangiocarcinoma
- Cdkn1a
cyclin-dependent kinase inhibitor 1
- Ctgf
connective tissue growth factor
- ELISA
enzyme-linked immunosorbent assay
- ERK
extracellular signal-regulated kinase
- Fn1
fibronectin 1
- HCC
hepatocellular carcinoma
- Itgb1
integrin beta 1
- MAPK
mitogen-activated protein kinase
- Pai1
plasminogen activator inhibitor 1
- qRT-PCR
real-time quantitative reverse transcription polymerase chain reaction
- TGF-β
transforming growth factor-beta
- Tgfbr1
transforming growth factor-beta receptor, type I
- Tgfbr2
transforming growth factor-beta receptor, type II
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Lau JWY, Leow CK. Surgical management. In: Leong AS-Y, Liew CT, Lau JWY, Johnson PJ, editors. Hepatocellular carcinoma: diagnosis, investigation and management. Arnold; London, England: 1999. [Google Scholar]
- 3.Poon D, Anderson BO, Chen LT, Tanaka K, Lau WY, Van Cutsem E, Singh H, et al. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1111–1118. doi: 10.1016/S1470-2045(09)70241-4. [DOI] [PubMed] [Google Scholar]
- 4.Martin J, Dufour JF. Tumor suppressor and hepatocellular carcinoma. World J Gastroenterol. 2008;14:1720–1733. doi: 10.3748/wjg.14.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pang RW, Joh JW, Johnson PJ, Monden M, Pawlik TM, Poon RT. Biology of hepatocellular carcinoma. Ann Surg Oncol. 2008;15:962–971. doi: 10.1245/s10434-007-9730-z. [DOI] [PubMed] [Google Scholar]
- 6.Tsai WL, Chung RT. Viral hepatocarcinogenesis. Oncogene. 2010;29:2309–2324. doi: 10.1038/onc.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majumder M, Ghosh AK, Steele R, Ray R, Ray RB. Hepatitis C virus NS5A physically associates with p53 and regulates p21/waf1 gene expression in a p53-dependent manner. J Virol. 2001;75:1401–1407. doi: 10.1128/JVI.75.3.1401-1407.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueda H, Ullrich SJ, Gangemi JD, Kappel CA, Ngo L, Feitelson MA, Jay G. Functional inactivation but not structural mutation of p53 causes liver cancer. Nat Genet. 1995;9:41–47. doi: 10.1038/ng0195-41. [DOI] [PubMed] [Google Scholar]
- 9.Oda T, Tsuda H, Scarpa A, Sakamoto M, Hirohashi S. p53 gene mutation spectrum in hepatocellular carcinoma. Cancer Res. 1992;52:6358–6364. [PubMed] [Google Scholar]
- 10.Grady WM, Markowitz SD. TGF-β signaling pathway in tumor suppression. In: Derynck R, Miyazano K, editors. The TGF-β family. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 2008. pp. 889–938. [Google Scholar]
- 11.Abou-Shady M, Baer HU, Friess H, Berberat P, Zimmermann A, Graber H, Gold LI, et al. Transforming growth factor betas and their signaling receptors in human hepatocellular carcinoma. Am J Surg. 1999;177:209–215. doi: 10.1016/s0002-9610(99)00012-4. [DOI] [PubMed] [Google Scholar]
- 12.Sue SR, Chari RS, Kong FM, Mills JJ, Fine RL, Jirtle RL, Meyers WC. Transforming growth factor-beta receptors and mannose 6-phosphate/insulin-like growth factor-II receptor expression in human hepatocellular carcinoma. Ann Surg. 1995;222:171–178. doi: 10.1097/00000658-199508000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiss A, Wang NJ, Xie JP, Thorgeirsson SS. Analysis of transforming growth factor (TGF)-alpha/epidermal growth factor receptor, hepatocyte growth Factor/c-met,TGF-beta receptor type II, and p53 expression in human hepatocellular carcinomas. Clin Cancer Res. 1997;3:1059–1066. [PubMed] [Google Scholar]
- 14.Tian M, Schiemann WP. The TGF-beta paradox in human cancer: an update. Future Oncol. 2009;5:259–271. doi: 10.2217/14796694.5.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akhurst RJ, Derynck R. TGF-beta signaling in cancer--a double-edged sword. Trends Cell Biol. 2001;11:S44–51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 16.Giannelli G, Mazzocca A, Fransvea E, Lahn M, Antonaci S. Inhibiting TGF-beta signaling in hepatocellular carcinoma. Biochim Biophys Acta. 2011;1815:214–223. doi: 10.1016/j.bbcan.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Wu Q, Qiu P, Mirza A, McGuirk M, Kirschmeier P, Greene JR, et al. Analyses of p53 target genes in the human genome by bioinformatic and microarray approaches. J Biol Chem. 2001;276:43604–43610. doi: 10.1074/jbc.M106570200. [DOI] [PubMed] [Google Scholar]
- 18.Cordenonsi M, Dupont S, Maretto S, Insinga A, Imbriano C, Piccolo S. Links between tumor suppressors: p53 is required for TGF-beta gene responses by cooperating with Smads. Cell. 2003;113:301–314. doi: 10.1016/s0092-8674(03)00308-8. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson DS, Tsai WW, Schumacher MA, Barton MC. Chromatin-bound p53 anchors activated Smads and the mSin3A corepressor to confer transforming-growth-factor-beta-mediated transcription repression. Mol Cell Biol. 2008;28:1988–1998. doi: 10.1128/MCB.01442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkinson DS, Ogden SK, Stratton SA, Piechan JL, Nguyen TT, Smulian GA, Barton MC. A direct intersection between p53 and transforming growth factor beta pathways targets chromatin modification and transcription repression of the alpha-fetoprotein gene. Mol Cell Biol. 2005;25:1200–1212. doi: 10.1128/MCB.25.3.1200-1212.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 22.Chytil A, Magnuson MA, Wright CV, Moses HL. Conditional inactivation of the TGF-beta type II receptor using Cre:Lox. Genesis. 2002;32:73–75. doi: 10.1002/gene.10046. [DOI] [PubMed] [Google Scholar]
- 23.Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 24.Romero-Gallo J, Sozmen EG, Chytil A, Russell WE, Whitehead R, Parks WT, Holdren MS, et al. Inactivation of TGF-beta signaling in hepatocytes results in an increased proliferative response after partial hepatectomy. Oncogene. 2005;24:3028–3041. doi: 10.1038/sj.onc.1208475. [DOI] [PubMed] [Google Scholar]
- 25.Zhou L, Liu J, Luo F. Serum tumor markers for detection of hepatocellular carcinoma. World J Gastroenterol. 2006;12:1175–1181. doi: 10.3748/wjg.v12.i8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li MS, Ma QL, Chen Q, Liu XH, Li PF, Du GG, Li G. Alpha-fetoprotein triggers hepatoma cells escaping from immune surveillance through altering the expression of Fas/FasL and tumor necrosis factor related apoptosis-inducing ligand and its receptor of lymphocytes and liver cancer cells. World J Gastroenterol. 2005;11:2564–2569. doi: 10.3748/wjg.v11.i17.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavin LG, Venkatraman M, Factor VM, Kaur S, Schroeder I, Mercurio F, Beg AA, et al. Regulation of alpha-fetoprotein by nuclear factor-kappaB protects hepatocytes from tumor necrosis factor-alpha cytotoxicity during fetal liver development and hepatic oncogenesis. Cancer Res. 2004;64:7030–7038. doi: 10.1158/0008-5472.CAN-04-1647. [DOI] [PubMed] [Google Scholar]
- 28.Belayew A, Tilghman SM. Genetic analysis of alpha-fetoprotein synthesis in mice. Mol Cell Biol. 1982;2:1427–1435. doi: 10.1128/mcb.2.11.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tilghman SM, Belayew A. Transcriptional control of the murine albumin/alpha-fetoprotein locus during development. Proc Natl Acad Sci U S A. 1982;79:5254–5257. doi: 10.1073/pnas.79.17.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong ZZ, Yao DF, Yao M, Qiu LW, Zong L, Wu W, Wu XH, et al. Clinical impact of plasma TGF-beta1 and circulating TGF-beta1 mRNA in diagnosis of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2008;7:288–295. [PubMed] [Google Scholar]
- 31.Derynck R, Goeddel DV, Ullrich A, Gutterman JU, Williams RD, Bringman TS, Berger WH. Synthesis of messenger RNAs for transforming growth factors alpha and beta and the epidermal growth factor receptor by human tumors. Cancer Res. 1987;47:707–712. [PubMed] [Google Scholar]
- 32.Dimova EY, Kietzmann T. Metabolic, hormonal and environmental regulation of plasminogen activator inhibitor-1 (PAI-1) expression: lessons from the liver. Thromb Haemost. 2008;100:992–1006. [PubMed] [Google Scholar]
- 33.Zheng Q, Tang ZY, Xue Q, Shi DR, Song HY, Tang HB. Invasion and metastasis of hepatocellular carcinoma in relation to urokinase-type plasminogen activator, its receptor and inhibitor. J Cancer Res Clin Oncol. 2000;126:641–646. doi: 10.1007/s004320000146. [DOI] [PubMed] [Google Scholar]
- 34.Chen YW, Klimstra DS, Mongeau ME, Tatem JL, Boyartchuk V, Lewis BC. Loss of p53 and Ink4a/Arf cooperate in a cell autonomous fashion to induce metastasis of hepatocellular carcinoma cells. Cancer Res. 2007;67:7589–7596. doi: 10.1158/0008-5472.CAN-07-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen LN, Furuya MH, Wolfraim LA, Nguyen AP, Holdren MS, Campbell JS, Knight B, et al. Transforming growth factor-beta differentially regulates oval cell and hepatocyte proliferation. Hepatology. 2007;45:31–41. doi: 10.1002/hep.21466. [DOI] [PubMed] [Google Scholar]
- 36.Dumble ML, Croager EJ, Yeoh GC, Quail EA. Generation and characterization of p53 null transformed hepatic progenitor cells: oval cells give rise to hepatocellular carcinoma. Carcinogenesis. 2002;23:435–445. doi: 10.1093/carcin/23.3.435. [DOI] [PubMed] [Google Scholar]
- 37.Hirayama D, Fujimori T, Satonaka K, Nakamura T, Kitazawa S, Horio M, Maeda S, et al. Immunohistochemical study of epidermal growth factor and transforming growth factor-beta in the penetrating type of early gastric cancer. Hum Pathol. 1992;23:681–685. doi: 10.1016/0046-8177(92)90325-w. [DOI] [PubMed] [Google Scholar]
- 38.Thompson TC, Truong LD, Timme TL, Kadmon D, McCune BK, Flanders KC, Scardino PT, et al. Transforming growth factor beta 1 as a biomarker for prostate cancer. J Cell Biochem Suppl. 1992;16H:54–61. doi: 10.1002/jcb.240501212. [DOI] [PubMed] [Google Scholar]
- 39.Gorsch SM, Memoli VA, Stukel TA, Gold LI, Arrick BA. Immunohistochemical staining for transforming growth factor beta 1 associates with disease progression in human breast cancer. Cancer Res. 1992;52:6949–6952. [PubMed] [Google Scholar]
- 40.Bierie B, Stover DG, Abel TW, Chytil A, Gorska AE, Aakre M, Forrester E, et al. Transforming growth factor-beta regulates mammary carcinoma cell survival and interaction with the adjacent microenvironment. Cancer Res. 2008;68:1809–1819. doi: 10.1158/0008-5472.CAN-07-5597. [DOI] [PubMed] [Google Scholar]
- 41.Chen H, Egan JO, Chiu JF. Regulation and activities of alpha-fetoprotein. Crit Rev Eukaryot Gene Expr. 1997;7:11–41. doi: 10.1615/critreveukargeneexpr.v7.i1-2.20. [DOI] [PubMed] [Google Scholar]
- 42.Song CZ, Siok TE, Gelehrter TD. Smad4/DPC4 and Smad3 mediate transforming growth factor-beta (TGF-beta) signaling through direct binding to a novel TGF-beta-responsive element in the human plasminogen activator inhibitor-1 promoter. J Biol Chem. 1998;273:29287–29290. doi: 10.1074/jbc.273.45.29287. [DOI] [PubMed] [Google Scholar]
- 43.Kunz C, Pebler S, Otte J, von der Ahe D. Differential regulation of plasminogen activator and inhibitor gene transcription by the tumor suppressor p53. Nucleic Acids Res. 1995;23:3710–3717. doi: 10.1093/nar/23.18.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo B, Inoki K, Isono M, Mori H, Kanasaki K, Sugimoto T, Akiba S, et al. MAPK/AP-1-dependent regulation of PAI-1 gene expression by TGF-beta in rat mesangial cells. Kidney Int. 2005;68:972–984. doi: 10.1111/j.1523-1755.2005.00491.x. [DOI] [PubMed] [Google Scholar]
- 45.Mazzocca A, Fransvea E, Dituri F, Lupo L, Antonaci S, Giannelli G. Down-regulation of connective tissue growth factor by inhibition of transforming growth factor beta blocks the tumor-stroma cross-talk and tumor progression in hepatocellular carcinoma. Hepatology. 2010;51:523–534. doi: 10.1002/hep.23285. [DOI] [PubMed] [Google Scholar]
- 46.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.