Abstract
Four studies were performed to further clarify the contribution of rod/cone and intrinsically photoreceptive retinal ganglion cells to measures of entrainment, light-induced locomotor suppression and photosomnolence. Wildtype (WT), retinally degenerate (rd/rd) and melanopsin-less (OPN4−/−) mouse strains were compared. In Expt. 1, mice were exposed to a graded photoperiod in which approximately 0.26 μW/cm2 irradiance diminished to dark over a 6 hr interval. This method enabled “phase angle titration,” with individual animals assuming activity onsets according to their sensitivity to light. WT and OPN4−/− animals entrained with identical phase angles (effective irradiance=0.078 μW/cm2), but rd/rd mice required a more intense irradiance (0.161 μW/cm2) and entrainment occurred about 2.5 hr earlier. In Expt. 2, all three strains preferred the dark side of a divided light-dark chamber until the irradiance dropped to 0.5 μW/cm2 at which point, rd/rd mice no longer showed a preference. Expts. 3 and 4 determined that WT and rd/rd mice showed equivalent light-induced locomotor suppression, but the response was greatly impaired in OPN4−/− mice. Closer examination of open field locomotion using infrared video-based methods and Any-mazetm software revealed two opposing effects of light. Locomotor suppression was equivalent in WT and rd/rd mice. Responses by OPN4−/− mice varied from being absent (N=17) to normal (similar to WT and rd/rd mice; N=8). Light onset was associated with a significant, but brief, locomotion increase in WT and OPN4−/− mice, but not in rd/rd mice. Any-mazetm analysis supports the view that light-induced locomotor quiescence is followed by behavioral sleep (photosomnolence), a fact that was visually validated from the raw video files. The data show that a) classical photoreceptors, most likely rods, allow mice to prefer and entrain to very dim light such as found in natural twilight; b) the presence of melanopsin photopigment enables light-induced locomotor suppression and photosomnolence; c) light-induced locomotor suppression/photosomnolence is rod/cone mediated in 36% of mice lacking melanopsin, but not in 64% of the same OPN4−/− strain; and d) light-induced locomotor suppression encompasses an interval of behavioral sleep.
Keywords: Sleep, Circadian Rhythm, Light Aversion, Dark Preference, Masking, Locomotor Suppression, Phase Shift, OPN4, Melanopsin, Any-mazetm
1.1 INTRODUCTION
Both classical photoreceptors (rods/cones) and ganglion cells employing melanopsin-based photoreception contribute to multiple non-image forming visual functions. One of these is light-induced locomotor suppression (also known as “negative masking”) (Hattar et al., 2003, Panda et al., 2003). A typical test of this photic action compares the level of wheelrunning during a nocturnal 30–60 min light pulse to the level during the same interval 24 hr earlier when light was absent (Mrosovsky et al., 1999). This form of test has contributed to the view that light-induced locomotor suppression is contingent upon the continued presence of light (see (Morin and Studholme, 2009)). This has been disputed by recent studies showing that millisecond light exposure is sufficient to trigger a 30–40 min interval of locomotor suppression that persists in the absence of additional light (Morin and Studholme, 2009, Morin et al., 2010).
Given that living animals always engage in some form of behavior, suppression of one behavior necessarily requires concomitant increase in another. In the case of light-induced locomotor suppression, a candidate complementary behavior is sleep. This behavior unexpectedly increases in mice exposed to nocturnal light (Altimus et al., 2008, Lupi et al., 2008, Tsai et al., 2009). Moreover, tests of response to millisecond light flashes or a few minutes of light pulses have shown that mice (Morin and Studholme (2009); and hamsters, unpub.data) rapidly become quiescent, then show an interval of behavioral sleep (“photosomnolence”) that lasts about 20 min.
Circadian rhythm entrainment and phase shift responses to moderate intensity light signals are reasonably normal in mice lacking melanopsin or classical photoreceptors (Hattar et al., 2003, Panda et al., 2003). Photosomnolence may be regulated differently. Two reports suggest that photosomnolence depends exclusively on melanopsin-based photoreception by intrinsically photoreceptive retinal ganglion cells (ipRGCs) (Lupi et al., 2008, Tsai et al., 2009). Alternatively, photosomnolence, along with other non-image forming visual responses, may be elicited by either classical or ipRGC photoception (Altimus et al., 2008). Here, we provide additional information suggesting that light-induced locomotor suppression and photosomnolence are mediated exclusively by ipRGC photoreception in some individuals, but by either classical or ipRGC photoreception in others.
With respect to entrainment, hamsters and mice entrain to low light levels consistent with a rod-only function (Evans et al., 2007, 2009, Lall et al., 2010). We have employed a “phase angle titration” method to show that entrainment to low irradiance signals is exclusively mediated by classical photoreceptors. In addition, we have performed an analysis of photoreceptor contribution to the dark preference response which persists in animals lacking rods and cones (Mrosovsky and Hampton, 1997). The light-dark preference test shows that dark preference (light aversion), as for entrainment, is mediated by classical photoreceptors when irradiance of the lighted side is low.
2.1 METHODS
2.1.1 Animals
Mice were C57BL/6J wild type (WT), the retinally degenerate strain, rd/rd (B6.C3-Pde6brd1 Hps4le/J) and the melanopsin null strain, OPN4−/−, in which the ipRGCs are non-functional consequent to the absence of melanopsin photopigment (Panda et al., 2002, Ruby et al., 2002). OPN4−/− breeding stock were provided courtesy of Dr. Ignacio Provencio. This congenic strain was derived from ten successive back crosses of the mix strain melanopsin null mice (Panda et al., 2002) onto the C57BL/6J line (Warthen et al., 2011). WT animals were purchased from Jackson Labs (Bar Harbor, ME) and age on arrival in the laboratory was approximately 8 wks. The rd/rd mice were bred from stock purchased from Jackson Labs and were at least 12 wks old at the beginning of experimental use. Rod photoreceptors completely degenerate in this strain by 26–36 days post partum and cones, which represent only 2.8% of the normal rod/cone population, are approximately 61% gone by 65 days and 84% gone by 120 days post partum (Carter-Dawson et al., 1978).
The standard laboratory photoperiod for all mice was LD12:12. At the start of each experiment, each animal was transferred to a 45 L x 20 W x 20 H cm clear polycarbonate cage containing a 16.5 cm diameter stainless steel running wheel, sawdust bedding, with food and water continuously available. Cages were placed on a rack with 5 shelves with 5 or 4 cages per shelf, the rack abutting the wall of an animal housing room. Each wheel revolution closed a switch which was recorded by computer using WinCollectXP software written by Glenn Hudson, Stony Brook University. Data were collected in 1 min bins and plotted in standard actogram format or exported to a spreadsheet for further analysis.
2.2 Light Stimuli
2.2.1 Pulses and flashes
All stimuli were white light with unknown spectra. The “flash” stimuli were generated by a DynaLite Flash Head (DynaLite, Union, NJ; model 2040 which has a Dynalite model 0703 xenon flash, not color corrected, with a 5400K color temperature) mounted in a white-painted animal colony room and directed at the center of animal cage rack, approximately 2 m across the room. The Dynalite Flash Head was powered by a DynaLite M1000er power supply. The duration of each flash was 2 msec, as indicated by the manufacturer’s specifications. The energy of each flash was approximately 3.6 J/m2 ( 1 Joule = 1 Watt*sec ). The animals were exposed directly to the flashes from the flash head without intervening filters, in accordance with previous procedures (Vidal and Morin, 2007, Morin and Studholme, 2009). Light energy and illuminance were measured with a Gigahertz-Optik P-9710 photometer (Newburyport, MA) that has the capability of measuring millisecond light stimuli. When 5 min light “pulses” were employed, the source was a 100 watt incandescent reflector bulb (GE type 6E) providing an irradiance of about 40 μW/cm2 (78 lux) in each cage. Light levels, measured immediately in front of each cage with the photodetector facing the source, did not vary by more than 2% of the value measured in front of the cage nearest to and most directly in line with the light source. Timing and number of light stimuli were computer-controlled using custom software and delivered without regard to the behavior, position or orientation of the mice being tested. Pin photodiodes monitored the actual occurrence of each light stimulus and, when activated, sent a signal to the data collection computer.
2.2.2 Graded photoperiod
A set of 5 chambers, each capable of holding 5 cages were assembled on an stainless steel rack. Each chamber was enclosed and light-tight, with an interior lighting source consisting of 5 broad spectrum white light LED arrays (OSRAM model OS-CM01E-W), one above the wheel end of each cage. Irradiance within each chamber was controlled by an 8 bit D-A voltage controller, an LED dimmer (OSRAM OT DIM) and custom software (LightControl written by Glenn Hudson, Stony Brook University) allowing approximately continuous and nearly linear variation of LED output from zero to approximately 1 μW/cm2. The initial photoperiod was LD12:12, with light off at 1600 hr. The software enabled use of a graded LD16:8 photoperiod that achieved full darkness at 2000 hr, 4 hr after the time of light off in LD12:12. The most important feature of the graded photoperiod was the very gradual diminution of the light, beginning at 1200 hr when irradiance was about 0.2 μW/cm2 and ending 8 hr later at an irradiance of zero. This is a phase angle titration procedure during which animals advance or delay while adopting a preferred phase angle of entrainment. It is a useful psychophysical method for detecting differences in circadian visual system sensitivity to light (Goz et al., 2008).
2.2.3 Light-dark preference chamber
Mice were tested in a 30.5 x 61 cm arena partitioned into two equal halves. One side was lighted from above by a fluorescent lamp (Philips Soft White 4 watt T5, HomeDepot.com) and was separated from the dark side by wall with a centered, floor-level 5 cm2 opening. All light in the dark side arrived through this opening. Walls of the chamber were opaque, but pale and the chamber sat on absorbent white paper. Walls and floor maximized contrast with the black mice, facilitating video detection by a USB webcam mounted above the lighted side. Movement on the lighted side was measured by custom real time video analysis software (Glenn Hudson, Stony Brook University). A Microsoft LifeCam (TigerDirect.com) was used when irradiance was set to 50 μW/cm2; at the lower irradiances, video recordings were obtained under infrared illumination with an infrared USB camera (Sabrent M501-1160, TigerDirect.com). Water was accessible at similar locations in both lighted and dark sides of the chamber; a food pellet was placed in each side at the start of each test.
2.3.1 Video recordings with Any-Mazetm
Locomotion level is a sensitive and highly reliable correlate of EEG-determined sleep (Pack et al., 2007, Fisher et al., in press). Video recording was accomplished with Any-mazetm software (Stoelting Co., Wood Dale, IL) which simultaneously determined the position of each animal in its cage, yielding a data record of movement per second across the recorded interval. The critical Any-mazetm settings were 10 readings per second and a 95% sensitivity setting for determination of “immobility” with a bout of sleep was defined as an interval of at least 40 sec during which the animal was immobile according to the Any-mazetm software. These settings have previously been determined to optimally correlate with EEG indices of sleep (Fisher et al., in press). Visual validation of the relationship between sleep and Any-mazetm-determined immobility was accomplished by examining the video recordings corresponding to the immobility.
In addition to the immobility results, Any-mazetm returns an array of position coordinates for each animal across each test. These data were obtained up to 9 times per second from the frame-to-frame video analysis. These were averaged to obtain a distance (measured in pixels) per second. Distance related to minor head movement in the coordinate data was removed by excluding apparent positional change less than two pixels. Analysis showed that deeply anesthetized mice (N=6) generated no positional change over a 20 min period.
2.4.1 Assessment of Wheelrunning Suppression
In Experiments 1 and 2, mice were included in the data analysis if they satisfied three criteria on the test day. The criteria were: (1) no more than 10 min with zero wheel revolutions during the 30 min prior to light stimulation; (2) no more than 3 min with zero revolutions during the 5 min prior to stimulation; and (3) the last 2 min prior to stimulation could not both have zero revolutions.
Two previously described methods were used to assess the pattern and extent of locomotor suppression (Morin and Studholme, 2009, Morin et al., 2010). Briefly, a pictorial representation of relative locomotor activity level before, during and after various stimulus presentations was prepared, using the median wheel revolutions per minute for each 5 min of data across the test interval as the critical variable. This was expressed as a percentage of the median revolutions per minute between ZT1230 and ZT13. The median percentage response across each test group was plotted to obtain the pattern of locomotor suppression. This method provides a clear indication of both individual and group performance across time. However, the data are not easily subjected to statistical analysis because of the repeated measure variable and the need for non-parametric statistical analysis. The second method merges both the effect of the stimulus on the amount of locomotion and the temporal characteristic of this effect. Method 2 sums the number of minutes, for each animal in a particular group, during which the wheel revolutions are equal to zero (“zero minutes”). The zero minutes were summed, beginning with the onset of the stimulus and continued for 60 min.
In most instances of statistical analysis, the data violated tests for normality, equal variance or both. As a result, non-parametric statistics were generally employed, with major dependence on the Kruskall-Wallis one-way analysis of variance by ranks and Dunn’s test for post-hoc analysis. The inability to use parametric statistics precluded use of two-way analyses of variance. Statistics were calculated using the analytical capacity built into SigmaPlot v. 11.2.0.5 (Systat Software, San Jose, CA). There were 20–23 animals per group.
2.5.1 Retinal Histology
Mice were anesthetized (100mg/kg ketamine plus 10 mg/kg xylazine, intraperitoneally) and were transcardially perfused with saline followed by 4% paraformaldehyde in 0.1M phosphate buffered saline (PBS), pH 7.4, with sodium m-periodate (0.01 M) and lysine (0.075 M) added. Eyes were removed, hemisected, and immersed in the same fixative for 1 hour, rinsed with PBS for 30 min., followed by cryo-protection in 30% sucrose in 0.1 M PB overnight at 4°C. Eye cups were embedded in a gelatin-albumin mixture (3% gelatin, 30% egg albumin in distilled H2O) and frozen using liquid nitrogen-chilled isopentane. The 16 μm thick cryostat sections were picked up on gelatin/chromium-coated slides, air dried and stored in a frost-free freezer at −20°C until further processing.
Retinal sections were thawed at room temperature for 20 min., washed in PBS, pH 7.4, for 10 minutes ( x 3), then blocked in 2% normal donkey serum in PBS for 20 minutes. Tissues were incubated 24–48 hours at 4°C with primary antibodies diluted in PBS containing 0.3% Triton X-100 and 5% normal donkey serum. Primary antisera were rabbit anti-melanopsin (UF007 (Morin et al., 2003)) diluted 1:2000, guinea pig anti-vesicular glutamate transporter 1 (VGLUT1; AB5905 Chemicon International, Temecula, CA) diluted 1:2000 and rabbit anti-blue cone opsin (BCO; AB5407, Chemicon International, Temecula, CA) diluted 1:250. After washing in PBS for 45 minutes and blocking in 2% donkey serum the slides were incubated for 45 min. at 37°C in either donkey anti-rabbit, donkey anti-guinea pig or donkey anti-mouse Texas Red secondary antibodies (JacksonImmunoResearch Labs, West Grove, PA) diluted 1:250 in PBS with Triton-X 100 and 5% normal donkey serum. After a final washing in PBS, sections were coverslipped with ProlongGold with DAPI (#P36931, Introvitrogen, Eugene, OR) and stored at 4°C in the dark.
Fluorescent immunoreactivity in cells was observed with a Zeiss Axioplan microscope and digital photomicrographs were obtained using a Zeiss Axiophot camera. Images were adjusted for contrast and brightness.
2.6.1 Genetic Screening
Three millimeter tail clips from anesthetized mice were sent to Transnetyx (Cordova, TN) for genetic testing. Polymerase chain reaction (PCR) was used and confirmed in all animals. For OPN4−/− mice, a q-PCR based system was used to detect the presence or absence of a neomycin cassette insertion as well as a disruption in the Opn4 gene sequence at the insertion site. For rd/rd mice, a q-PCR based system was used to detect the presence or absence of LTR/provirus sequence as well as a disruption in the Pde6b gene sequence at the insertion site. Results were provided in a plus/minus format.
2.7 Experiments
2.7.1 Experiment 1
Mice representing each of the three strains were placed in cages with wheels and transferred to a chamber lighted with LEDs. The initial photoperiod was LD12:12. When all mice were stably entrained, the graded photoperiod was activated. All animals remained in this photoperiod until all were stably entrained. At this time, the phase angle of entrainment was measured by drawing a line through the locomotor onsets across the last 7–10 days of each wheelrunning record and measuring onset time relative to ZT12 (light off time under the preceding LD12:12 condition). This value accurately reflects the phase angle of entrainment (Goz et al., 2008) and is presented in terms of Zeitgeber time or as a change in phase from previous ZT12.
2.7.2 Experiment 2
Two mice randomly selected from two of the three mouse strains tested simultaneously in separate light-dark chambers. Half of each strain group started the test in the lighted side; the remainder on the dark side. Animal’s were tested for 24 hr beginning approximately 1–3 hr prior to lights off on a LD12:12 photoperiod. The video analysis measured percentage of the 24 hr spent in the lighted side. At the conclusion of each test, the chamber was washed thoroughly and its orientation changed by 180°. Irradiance was controlled by covering the fluorescent tube with neutral density filters which limited irradiance to 50.0, 5.0 or 0.5 μW/cm2. Irradiance measured from the dark side wall facing the opening was about 2 log units lower than in the lighted side. Bilaterally enucleated WT mice and rd/rd;OPN4−/− mice were tested under 50 μW/cm2 as controls.
2.7.3 Experiment 3
Groups of mice representing each of the three strains were simultaneously exposed to a series of 10 light “flashes” beginning at Zeitgeber Time 13 (ZT13). The tests were conducted with inter-flash interval (IFI) set at 0.5, 1, 4, 8, 16 or 30 sec; a test with a 5 min light “pulse” stimulus also occurred, as did tests in which no light stimulus was presented. There were at least 3 days between tests. Locomotor suppression was assessed as described below.
2.7.4 Experiment 4
Groups of mice representing each of the three strains were exposed to a series of 10 light flashes distributed over a 5 min interval beginning at ZT13. The home cage of each mouse was identical to a standard running wheel cage, but the wheel and wheel mount bracket were absent. At ZT10, the home cages of two mice were placed side by side forming a rectangle of 40 × 45 cm on a cage rack. The rectangle encompassed the field of view of an infrared (IR) USB camera (Sabrent M501-1160, TigerDirect.com) focused on the bottoms of the cages. Additional IR lighting was provided (Clover IR-01020 LED illuminator, TigerDirect.com) and all IR light passed through a diffusion filter to reduce glare. The paired cages were covered with a special lid fabricated from nylon screen (Small Parts, Miami Lakes, FL; part #B000FN1Q82). A timer managed by the Any-mazetm software initiated a 90 min interval of video recording at ZT1230. This interval encompasses the normal pattern of behavioral quiescence, sleep and recovery that follows exposure to the light pulse.
3.1 RESULTS
3.1.1 Histology
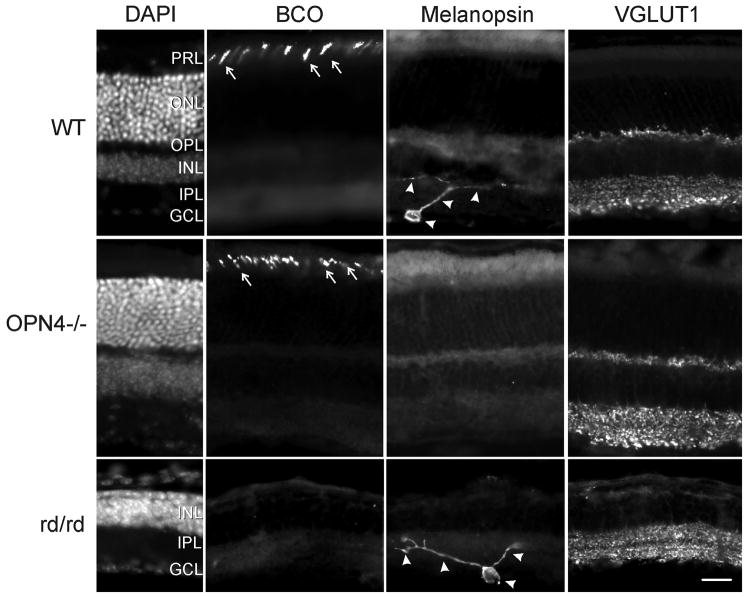
The outer nuclear layer is robustly revealed by DAPI nuclear staining in WT and OPN−/− retinas (Fig. 1). It is absent in rd/rd retina because of degeneration of rod and cone photoreceptors. BCO-IR reveals normal staining of cones in WT and OPN4−/− mice (Roberts et al., 2005), consistent with an intact classical photoreceptor layer. No BCO-IR is evident in the rd/rd retina. Although DAPI indicates the presence of the ganglion cell layer in all three strains, the melanopsin-IR is absent in ganglion cells and their processes of the OPN4−/− retina (Panda et al., 2002). The VGLUT1-IR demonstrates intact inner and outer plexiform layers (Sherry et al., 2003) in the WT and OPN4−/− mice. Because the rd/rd retina lacks a normal photoreceptor layer and outer nuclear layer, there is no VGLUT-IR staining in the outer plexiform layer of this strain. The anatomy is consistent with previous observations.
Figure 1.
Immunohistological demonstration of phenotype showing an absence of melanopsin-immunoreactive ganglion cells and processes in the OPN4−/− retina and degeneration of the photoreceptor layer with loss of rods and cones. DAPI panel - Cell bodies are identified by DAPI nuclear stain; BCO panel - blue cones are identified by an antibody against blue cone opsin (arrows); Melanopsin panel - melanopsin-IR identifies ipRGCs and processes (arrowheads); VGLUT1 panel - VGLUT1-immunoreactivity reveals the integrity of the inner nuclear layers in all three strains. Abbreviations: GCL - ganglion cell layer; INL - inner nuclear layer; IPL - inner plexiform layer; ONL - outer nuclear layer; OPL - outer plexiform layer; PRL - photoreceptor layer. Bar = 25 μm.
3.1.2 Experiment 1
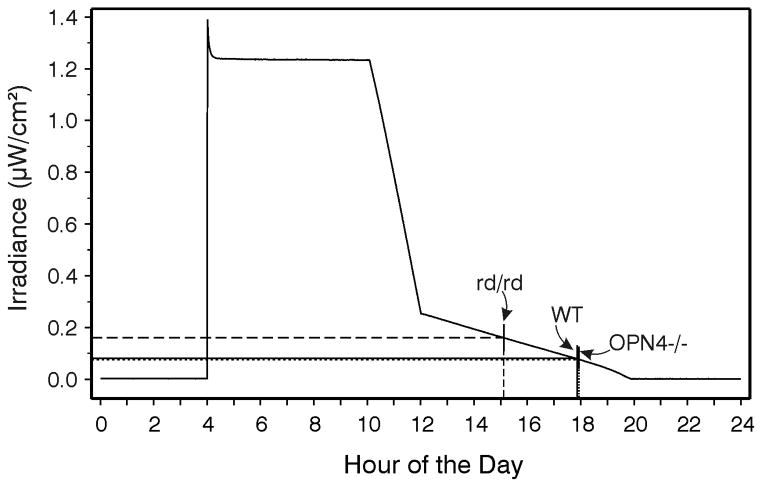
The three strains mice entrained to standard LD12:12 photoperiod as expected (Panda et al., 2002) and there were no significant differences in the phase angles of entrainment between the groups. When the animals were shifted to the graded photoperiod (Fig. 2), all WT and OPN4−/− mice achieved stable entrainment following a series of daily phase delays. In contrast, the rd/rd mice re-entrained with phase advances, eventually reaching a time of stable entrainment approximately 2.8 hr earlier than the other groups (median hours before dark and 25th/75th percentiles: WT = 1.9 (1.39/2.38); OPN4−/− = 2.2 (0.96/3.04); rd/rd = 4.76 (3.33/7.83)). There was a significant effect of strain on phase angle of entrainment (p<.001), with the rd/rd results differing significantly from those of the WT and OPN4−/− mice (which did not differ). The irradiance corresponding to the phase angle of entrainment (Fig. 2) was 0.161 μW/cm2 for the rd/rd mice and about 0.077 and 0.079 μW/cm2 for the WT and OPN4−/− mice, respectively.
Figure 2.
Measured irradiance (μW/cm2) during the daily photoperiod to which mice were exposed in Expt. 1. The vertical lines intersecting the irradiance curve and abscissa indicate the average activity onset time for each experimental group (rd/rd, dashed line; WT, solid line; OPN4−/−, dotted line ). The point at which the vertical lines intersect the irradiance curve also indicates the measured irradiance at that particular time of day, as indicated by the horizontal lines extending from the irradiance curve to the ordinate.
3.1.3 Experiment 2
Mice generally preferred the dark side of the light-dark chamber. When irradiance in the lighted side was 50 μW/cm2, all three mouse strains performed equally with an average 85.8% dark preference (Table 1). Reduction of the irradiance to 5.0 μW/cm2 resulted in greatly increased response variability by the rd/rd mice, but not by the WT or OPN4−/− mice. Dark preference by the rd/rd mice changed when irradiance was further reduced to 0.5 μW/cm2. At this level, mean dark preference remained unchanged and near 80% for WT and OPN4−/− mice (76.5 % and 85.3 %, respectively), but was only 41.9 % for rd/rd mice. The overall effect of strain was a significant (p<.001) at this irradiance, with the rd/rd group differing significantly from the other two groups (p<.05 each). Enucleated WT controls and a group of rd/rd; OPN4−/− mice had mean 42.8 and 38.8 % dark preference, respectively, during tests in which the light side was 50 μW/cm2.
Table 1.
Mean percentage (±SEM) of time spent in the dark side of a light-dark choice box varies according to which photoreceptors are present and lighted side irradiance.
| Strain/Irradiance | 50.0 μW/cm2 | 5.0 μW/cm2 | 0.5 μW/cm2 |
|---|---|---|---|
| WT | 85.5 (2.2) | 82.0 (3.4) | 77.8 (2.6) |
| rd/rd | 77.5 (6.8) | 62.4 (8.2) | a41.9 (11.3) |
| OPN4−/− | 78.4 (8.3) | 83.3 (2.1) | 85.3 (2.3) |
| rd/rd;OPN4−/− | b38.8 (9.0) | -- | -- |
| WT enucleated | c42.8 (8.4) | -- | -- |
differs significantly from WT and OPN4−/− groups under the same irradiance.
differs significantly from WT, rd/rd and OPN4−/− groups under the same irradiance.
3.1.4 Experiment 3
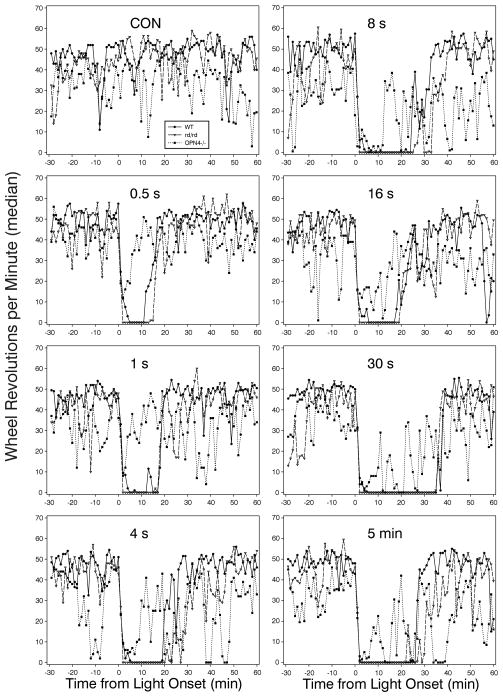
The patterns of locomotor suppression in response to light flashes with varying IFIs or to a 5 min light pulse are shown in Fig. 3. Two features are noteworthy. The first is the high degree of similarity between the rd/rd and WT groups with respect to the magnitude of locomotor suppression and the duration of the effect. The second is the erratic pattern of the OPN4−/− mice. Overall analysis of the zero count minutes indicates that only the WT responses varied significantly (became longer as the IFI increased). A between-groups comparison indicated that the OPN4−/− response to flashes with a 30 sec or 16 sec IFI were significantly less than WT and rd/rd mice, respectively. There were no other differences in this measure.
Figure 3.
Median wheel revolutions per minute in response to 10 flashes with differing inter-flash intervals (IFI; 0.5 s, 1 s, 4 s, 8 s, 16 s and 30 s correspond to the different IFIs) by WT, rd/rd and OPN4−/− mice. Animals experiencing the CON condition received no light during the test interval; those in the 5 min condition received a single 5 min light pulse.
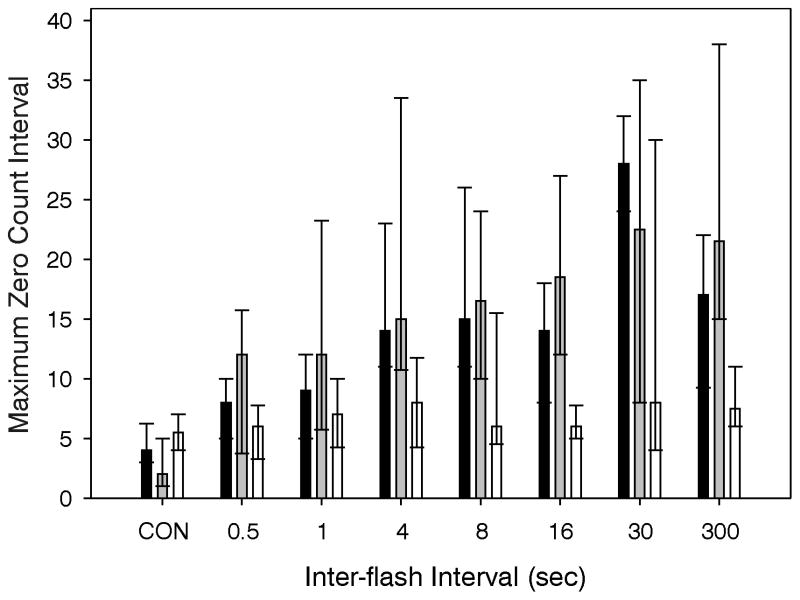
The large response variability, particularly the unpredictable responses of the OPN4−/− mice, and the resultant necessity of nonparametric statistics required a different approach to the analysis. This was achieved in two ways. First, the longest interval with zero wheel revolutions was calculated for each animal. Across light conditions, there was a significant effect of light (Fig. 4) on WT and rd/rd mice, but not on OPN4−/− mice (p<.001, .001 and 0.60, respectively; Kruskall-Wallis ANOVAs), consistent with the pictorial data in Fig. 3. However, the abrupt light-induced drop in wheel revolutions for all mouse strains seen in Fig. 3 also suggests that there is a suppressive effect of light on wheelrunning in the OPN4−/− mice. Second, the percent change in running rate (RPM during the 5 min after light onset relative to RPM prior to the stimulus) was determined. This analysis reveals that light significantly induces locomotor suppression (p<.001 for each strain). Post hoc tests showed that all light treatments of OPN4−/− mice, except the 1 sec IFI, produced significant declines in wheel revolutions relative to a no-light control result. Similar results were obtained for the WT and rd/rd groups. The RPM change (median, 25th/75th percentiles) was −81% (−86/−76), −87% (−90/−79) and −63% (−82/−38) for the WT, rd/rd and OPN4−/− groups, respectively. The CON condition was not included in these calculations. The CON group, which did not receive a light stimulus, had an approximately 9% increase in RPM during the same 5 min interval.
Figure 4.
Effect of IFI on the longest interval of consecutive minutes without a wheel revolution for WT, rd/rd or OPN4−/−. Medians and 25th/75th percentiles.
3.1.5 Experiment 4
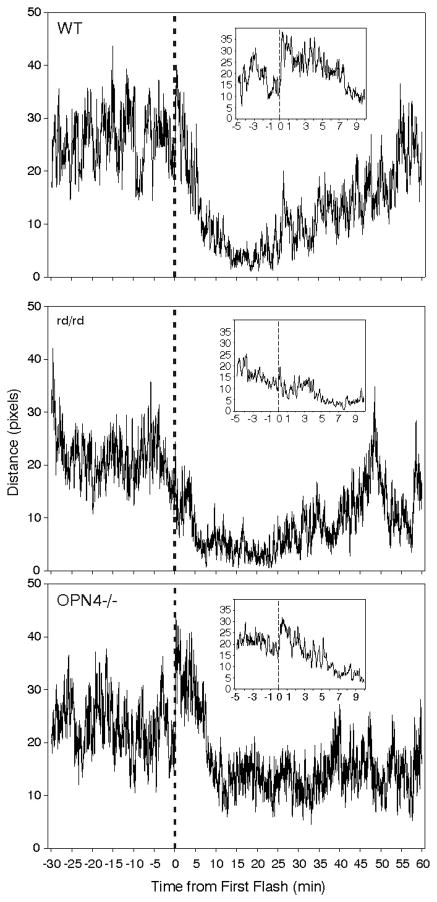
There were two opposing effects of light on a measurement of locomotion, the expected prolonged suppression (Fig. 5) and an acute increase during the initial 60 sec after the first flash(Fig. 5, insets). The acute increase in median activity varied significantly (p<.001), occurring in both WT and OPN4−/− mice (p<.05 for each comparison), but not in rd/rd mice (not significant). The more prolonged locomotion suppressing effect of light was observed in both WT and rd/rd mice which showed a robust decline over a period of 10–20 min. In contrast, the responses of OPN4−/− mice were highly variable, although the activity level of the group as a whole declined substantially during the 10 min interval after the initial flash.
Figure 5.
Effect of 10 light flashes/5 min on locomotor distance (expressed in pixel units) by all individuals in each of the three mouse strains. The large graphs show a 7 point moving average of mean distance traveled per second of the test. The first flash occurred at Time 0 and is indicated by a vertical dashed line. The inset graphs show details of a 7 point moving average distance per second during the interval beginning 5 min before the first flash (dashed vertical line at Time 0) and ending 10 min after. The increased locomotion consequent to the first flash is most evident in the insets. Statistics were performed on the change in pixel distance/sec during the minute immediately prior to the first flash relative to that during the minute immediately after the first flash.
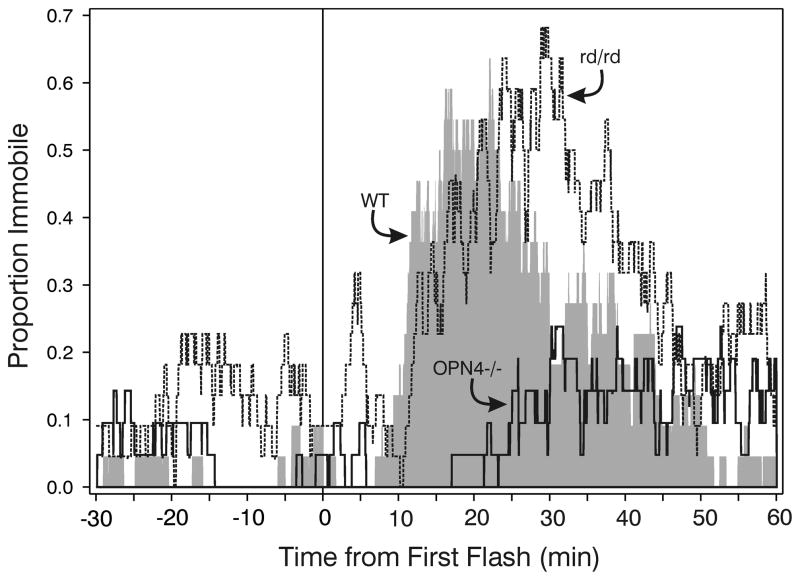
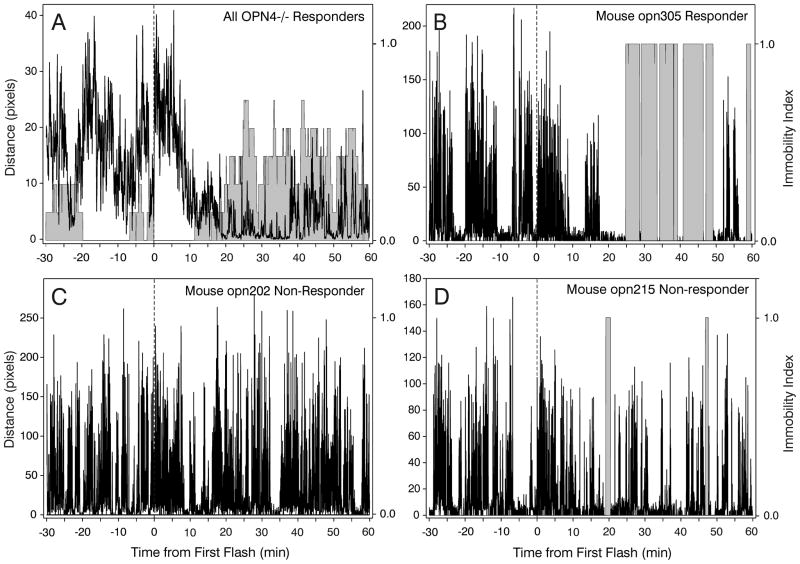
Comparison of the number of seconds mice were immobile after the first flash revealed an effect of strain (ANOVA on ranks, p<.001), with significantly greater immobility by WT and rd/rd mice than by OPN4−/− mice (Fig. 6). The effect of light on OPN4−/− mice was highly variable. It had no detected effect on immobility in 9/21 animals (43 % non-responders; e.g., Fig. 7C), but did appear to induce substantial, and more or less normal, immobility in 7 mice (Fig. 7A,B; Responders). An ANOVA on ranks comparing immobility by WT, rd/rd and the Responder OPN4−/− mice did not reveal any significant differences (median seconds immobility ± 25/75 percentiles were WT=892 ± 341/1257; rd/rd=1178 ± 564/1658; OPN4−/− Responders=1115 ± 524/2138). The remaining 5 animals had reduced locomotion with one or two fairly brief, software-detected “immobility” bouts (see below, this section) 20–40 min after the first flash (Fig. 7D), but their patterns were not in the range of those shown by WT mice. A group of WT mice (N=7) was tested for immobility responses in the absence of light and only a single brief (2.4 min) bout of immobility was observed. The apparent delay to sleep onset by the OPN4−/− mice (see Fig. 6) is the result of data from all mice in the group contributing to the plot. Inspection of Fig. 7A shows that OPN4−/− responder mice analyzed separately have a completely normal response, with sleep beginning about 10 min after stimulus onset as it does for WT mice (cf., Fig. 6, WT response).
Figure 6.
Patterns of software-detected immobility averaged across all mice in each of the three strains. The proportion of mice immobile abruptly increases 5–10 min after the first (at time 0) of 10 light flashes for the WT and rd/rd groups, but response by the OPN4−/− group is a much lower. WT, - shaded area; rd/rd - dashed line; thick solid line - OPN4−/− mice.
Figure 7.
Combined distance (black) and immobility (shaded area) plots showing (A) 7 point moving average pixels per second distance and probability of immobility across all OPN4−/− Responder mice exposed to the 10 flash stimulus; (B) a typical WT-like effect of the light stimulus by a Responder OPN4−/− mouse; (C) lack of a light stimulus effect in a typical Non-responder OPN4−/− mouse; and (D) behavioral response to light by an atypical Non-responder mouse which is quiescent, but not asleep during the two shaded immobility intervals. In panel C, no immobility is detected. In panel D, the apparent measured “immobility” occurs in an awake animal that is only moving its head. Time of first flash is indicated by the vertical dashed line.
Because mice presumably lacking melanopsin photopigment generated a wide variety of locomotion and immobility responses to the light stimulus, all OPN4−/− mice were tail clipped at the completion of the behavioral tests and their genetic background was evaluated. All mice were found to be OPN4−/−.
Visual inspection of the recorded video validated the Any-mazetm immobility measurement as a sleep index, but also demonstrated the need for such validation. The first immobility bout shown by WT and rd/rd mice was visually evaluated. Each individual was observed in the typical sleep posture, hunched with snout angled downward. The immobility bouts of each OPN4−/− mouse with at least one such bout (12 individuals) were also inspected. If the individual animal had a “normal” pattern of locomotion and immobility similar to that shown in Fig. 7B, it was also observed in the sleeping posture during the immobility bout. On the other hand, if the immobility and locomotion pattern was similar to that in Fig. 7D, the animals (N=5) were judged as being inactive (no hind or forelimb movement), but awake with the head up and moving slightly during the brief bouts of computer-determined immobility. Thus, the results revealed a “normal sleep posture” for 26/26 WT, 22/22 rd/rd and 7/21 (33%) OPN4−/− mice, with the remaining 67% of OPN4−/− mice either fully active or generally active but with one or more brief bouts of quiet wake. The visual validation procedure also revealed that the Any-mazetm software was unable to track the movements of 4 individuals (1 OPN4−/−, 1 rd/rd and 2 WT mice; their data were not included) because of improper foreground/background contrast.
4.1 DISCUSSION
The present studies employed strains of mice lacking the normal contingent of retinal photoreceptors to demonstrate separation of function between classical photoreceptors and ipRGCs with respect to several visually-mediated, non-image forming behaviors. In the first experiment, the adopted phase angle of circadian rhythm entrainment indicated greater sensitivity to light by mice bearing only classical photoreceptors (OPN4−/− mice) than seen in mice relying solely on melanopsin-based photoreception (rd/rd mice) for entrainment. Similarly, mice bearing rod/cone photoreceptors were also more sensitive to light in a test of light-dark preference (Experiment 2). In contrast, light-induced locomotor suppression, at least to a degree, was observed regardless of which photoreceptors were present, but the typical prolonged, high magnitude locomotor suppression that normally follows such stimulation was not apparent in the absence of melanopsin, while the response was normal in rd/rd mice. A fourth experiment utilized a video-based method that estimated sleep according to immobility analysis. The results demonstrated two effects of light on the amount of locomotion that were strain-dependent. A single light flash was sufficient to briefly increase locomotion in WT and OPN4−/−, but not in rd/rd mice and the longer latency effect of normal photosomnolence was observed in all WT and rd/rd mice, but generally not in mice lacking melanopsin photopigment. The OPN4−/− mice could be divided into two distinct groups according to their photosomnolence responses. Two thirds of the animals showed essentially no locomotor suppression/photosomnolence response to light and the remainder had normal responses equivalent to WT mice. Assessment of sleep using video-based tests for immobility reaffirmed the presence of sleep behavior consequent to brief nocturnal light exposure as previously determined from direct observation and EEG methods.
4.1.1 Technical Issues
Any-mazetm software is versatile and convenient, although how the data are generated is not always readily apparent. For example, Any-mazetm calculates “distance traveled” by comparing current position in the video frame with the previous position modified by the additional rule that no movement has occurred unless “distance traveled” exceeds 25% of the animal’s body length (Stoelting Co., pers.comm.). Thus, the Any-mazetm results will not explicitly match our distance data. We preferred to work directly with the raw x,y pixel coordinates and software that allows the investigator to set the limits on movement acceptability.
“Immobility” is determined according to a different form of analysis. Any-mazetm first establishes the location of the body silhouette within each video frame. Immobility is determined by measuring the percentage of the silhouette area that has not changed from frame to frame (Stoelting Co., pers.comm.). It has previously been established that the 95% sensitivity setting provides the best correlation between software-determined immobility and simultaneously recorded EEG-determined sleep (Fisher et al., in press). Hence, the use of 95% in the present study.
Pack et al. (2007) first demonstrated a simple, locomotion-based index of sleep as an alternative to the EEG. Such measures have now been validated three times with EEG and behavior observation methods (Fisher et al., in press); present data). The immobility index generated by Any-mazetm software shows a 96% correlation with simultaneously recorded EEG-based data (Fisher et al., in press). It is impossible to know whether the 4% departure from a perfect correlation lies with the EEG or the locomotion data, or is experimental error. It should be noted that while the video-based locomotor analysis is quite sensitive to movement, Pack et al. (2007) found that a much less sensitive infrared beam-breakage method also yielded locomotion data very highly correlated with EEG-determined sleep. The present data re-affirm the general utility of the Any-mazetm method of sleep detection. However, the results also demonstrate the need to validate immobility data with an alternative method if explicit statements about sleep are to be made. Here, 5/21 OPN4−/− mice had brief immobility bouts detected, with visual inspection revealing that the animals were indeed immobile, but were also obviously awake during those intervals. In addition, 4 mice were eliminated from consideration because the video review also revealed that movement tracking of those individuals had failed.
4.1.2 Rod photoreceptors are important for entrainment to dim light
Hamsters have been subjected to a variety of exotic photoperiods while being evaluated for rhythm response to dim light. Light with the intensity of a full moon lengthens the circadian period, induces phase shifts and accelerates re-entrainment (Evans et al., 2007, 2009). Such results show that, regardless of the photoreceptor involved, the rodent visual system detects and responds to irradiance below the threshold previously thought to be necessary to modify circadian rhythm timing (see Evans et al. (2007) for discussion). The authors suggest that the effects of dim illumination represent scotopic actions, presumably mediated through rod photoreceptors (Gorman et al., 2005) (but see (Naarendorp et al., 2010)). The present data support this view by showing that OPN4−/− mice entrain to a graded photoperiod with phase angles indicating the same high sensitivity of the circadian visual system to light as that shown by WT animals. This response is mediated by classical photoreceptors of which rods are overwhelmingly likely to be the most important (Lall et al., 2010).
In contrast, mice with normally functional ipRGCs, but lacking rods and cones, assume a phase angle of entrainment indicative of reduced sensitivity to low irradiance signals. These data are consistent with physiological observations indicating relative insensitivity of ipRGCs to light (Berson et al., 2002) and with data showing that mouse circadian rhythms are not ordinarily influenced by cone photoreception, but are very sensitive to rod-based photoreception (Altimus et al., 2010, Lall et al., 2010). Although melanopsin photopigment in ipRGCs has been repeatedly shown (and is shown here) to be sufficient for normal entrainment (Panda et al., 2002, Ruby et al., 2002), it is not necessary and does not appear to contribute significantly to entrainment when the photic cues are dim. Melanopsin-based ipRGC photoreception may have little or no influence on normal entrainment by mammals exposed to a natural photoperiod that includes twilight at dawn and dusk.
4.1.2 Classical photoreceptors mediate dim light-dark preference
Three strains of retinally degenerate mice show normal dark preference in a light-dark test employing a single irradiance (Mrosovsky and Hampton, 1997). Investigators studying related topics, light aversion and photophobia, have shown that two strains of retinally deficient mice, rd1 (absence of functional rods and cones) and Rpe65−/− (lacking functional cones) showed normal dark preference (Thompson et al., 2010). The authors concluded that aversion depends on non-image-forming light detection, but they did not test a low irradiance on the lighted side. In contrast, the present study evaluated responses to several irradiances and determined, first, that WT and OPN4−/− mice prefer the dark side of the test chamber even when the lighted side is very dim and, second, that preference under the dim condition is mediated by classical photoreceptors. Thus, the dark preference/light aversion results are similar to those obtained here for photoreceptor mediation of light entrainment.
4.1.3 Different photoreceptor classes mediate acute light-induced locomotor enhancement and prolonged light-induced locomotor suppression
An unexpected result from Experiment 3 was the observation of two opposing effects of light on locomotion. On the one hand, prolonged suppression of locomotion was elicited by the 10-flash stimulus. On the other, a single flash was sufficient to cause an acute, short duration increase in locomotion. Previous studies in which wheel running activity was measured lacked sufficient temporal resolution to evaluate either the rate or direction of locomotor change immediately consequent to the photic stimulus.
4.1.3.1 Locomotor enhancement
Within a few seconds of exposure to the first light flash, locomotion increased in WT animals, the increase lasting about a minute. This may have been a form of startle response to the flash, but if so, it was photoreceptor-specific because a similar response also occurred in OPN4−/−, but not in rd/rd mice. This strain-dependence supports the view that acutely enhanced locomotion is mediated by classical photoreceptors. Light-enhanced acoustic startle has been reported in mice (Salam et al., 2009), but is attenuated if animals engage in wheelrunning. There is no information regarding which photoreceptors mediate light-enhanced startle.
4.1.3.2 Locomotor suppression
Initially, rod photoreceptors were thought more likely to mediate wheelrunning suppression by flashes than cones or ipRGCs (Vidal and Morin, 2007). The rationale was that because flashes are very brief, they might be too brief to alter function of the ipRGCs which are known to respond slowly (Berson et al., 2002). In addition, the short latency pupillary light reflex, another non-image forming response, is known to be mediated by classical photoreceptors at all but high light intensities (Lucas et al., 2003). We have also demonstrated that when 10 flashes are presented with a 0.5 sec IFI, the locomotor suppression effect is diminished (present data and (Morin and Studholme, 2009)), although the total energy delivered equals that of stimuli with longer IFIs. The diminished effect of short IFIs is consistent with an expected, slightly longer rod bleaching and replenishment cycle (see Vidal and Morin (2007) for references and discussion). Nevertheless, the present data indicate that neither flash- nor 5 min light pulse-induced wheelrunning suppression depend on either rod or cone photoreception because performance by the rd/rd mice mimicked the WT response.
The alternative, that wheelrunning suppression depends on ganglion cell photoreception via melanopsin photopigment, is generally supported by the demonstration that suppression was not normal in OPN4−/− mice. As is clear (Fig. 3), OPN4−/− mice responded to a series of light flashes with a rapid decline in wheelrunning. Despite this change, the decline neither achieved the same low magnitude, nor persisted to the same extent, as shown by WT and rd/rd mice. The wheelrunning data, in general, support the conclusion that melanopsin is the photopigment used by mice to achieve maximal light-induced wheelrunning suppression, but this conclusion is tempered by the presence of very large variability in the data from the OPN4−/− mice. This variability necessitated a closer evaluation of the behavioral change (see Section 4.1.4).
Mrosovsky and Hattar (Mrosovsky and Hattar, 2003) concluded that light-induced wheelrunning suppression is mediated by both classical and ipRGC photoreceptors, an inference derived from tests with WT and OPN4−/− mice, but not from mice lacking classical photoreceptors. They showed, as did the present data, that the average OPN4−/− mouse displays substantial, but not complete, loss of wheelrunning suppression during prolonged, higher irradiance light exposure. In addition, the OPN4−/− mice showed premature, irradiance-independent recovery of wheelrunning in the presence of light (consistent with the present data). Other studies have evaluated the wheelrunning suppression response to a range of irradiances and demonstrated a contribution by both rods and cones (Thompson et al., 2008, Thompson et al., 2010).
4.1.4 Photosomnolence is mediated by ipRGCs in most, but not all, individuals
Light alters rodent sleep in at least four different ways: (1) The circadian visual system entrains the sleep-wake cycle, synchronizing it to the prevailing photoperiod (Johnson et al., 1988); (2) light-to-dark transitions increase rapid eye movement (REM) sleep, a phenomenon known as dark-induced REM (Lisk, 1966, Borbely, 1975, Borbely et al., 1975, Deboer et al., 2007); (3) Removal of light (i.e., darkness) during the daytime hours increases behavioral arousal (Altimus et al., 2008, Tsai et al., 2009); and (4) the converse effect, light exposure during the night induces sleep (Altimus et al., 2008, Lupi et al., 2008, Tsai et al., 2009). We have called this phenomenon, “photosomnolence” (Morin and Studholme, 2009). A relationship between photosomnolence and the other three effects of light on sleep has not been explored.
At the level of the retina, photic information affecting non-image-forming visual functions, including circadian rhythm phase shifts and locomotor suppression, appears to pass centrally through the ipRGCs from the classical photoreceptors (Goz et al., 2008, Guler et al., 2008, Hatori et al., 2008). With respect to ipRGC photoreception, two studies have concluded that light-induced sleep depends upon the presence of melanopsin photopigment (Lupi et al., 2008, Tsai et al., 2009), but a third (Altimus et al., 2008) has shown that rods/cones also contribute to the response. The latter is consistent with the above-discussed demonstrations (and the present data) that ganglion cell photoreceptor function only partially accounts for light-induced wheelrunning suppression. More directly, the present data show that photosomnolence was absent in 64% of OPN4−/− mice, but was apparently normal, and rod/cone regulated, in the remaining 36% of the same strain. The reason for this intra-strain variability is not known, although it could be an adaptation to the absence of melanopsin during development. Alternatively, the test conditions may not have been adequate to demonstrate the necessity of melanopsin for the induction of normal photosomnolence in all individuals.
Highlights.
Entrainment and aversion to dim light utilizes rod/cone, but not ipRGC photoreception
Light suppression of activity is greatly disrupted in the absence of melanopsin photopigment
Light acutely enhances activity by WT & OPN4−/− mice (but not rd/rd mice)
Light-induced “immobility” is actually light-induced sleep (“photosomnolence”)
Photosomnolence is normal in1/3 of OPN4−/− mice but is absent in the remaining 2/3 of those mice
Acknowledgments
Supported by NIH grant NS061804 and ARRA award NS061804-S2 to LPM. We thank Steven Mirabella and Max Grachev for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altimus CM, Guler AD, Alam NM, Arman AC, Prusky GT, Sampath AP, Hattar S. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nature Neuroscience. 2010;13:1107–1112. doi: 10.1038/nn.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altimus CM, Guler AD, Villa KL, McNeill DS, LeGates TA, Hattar S. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proceedings of the National Academy of Science of the United States of America. 2008;105:19998–20003. doi: 10.1073/pnas.0808312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Borbely AA. Circadian rhythm of vigilance in rats: modulation by short light-dark cycles. Neuroscience Letters. 1975;1:67–71. doi: 10.1016/0304-3940(75)90014-2. [DOI] [PubMed] [Google Scholar]
- Borbely AA, Huston JP, Waser PG. Control of sleep states in the rat by short light-dark cycles. Brain Research. 1975;95:89–101. doi: 10.1016/0006-8993(75)90209-7. [DOI] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM, Sidman RL. Differential effect of the rd mutation on rods and cones in the mouse retina. Invest OphthalmolVisSci. 1978;17:489–498. [PubMed] [Google Scholar]
- Deboer T, Ruijgrok G, Meijer JH. Short light-dark cycles affect sleep in mice. The European journal of neuroscience. 2007;26:3518–3523. doi: 10.1111/j.1460-9568.2007.05964.x. [DOI] [PubMed] [Google Scholar]
- Evans JA, Elliott JA, Gorman MR. Circadian effects of light no brighter than moonlight. Journal of Biological Rhythms. 2007;22:356–367. doi: 10.1177/0748730407301988. [DOI] [PubMed] [Google Scholar]
- Evans JA, Elliott JA, Gorman MR. Dim nighttime illumination accelerates adjustment to timezone travel in an animal model. Current Biology. 2009;19:R156–R157. doi: 10.1016/j.cub.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Fisher SP, Godinho SI, Hankins MW, Foster RG, Peirson SN. Rapid assessment of sleep/wake behaviour in mice. J BiolRhythms. doi: 10.1177/0748730411431550. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman MR, Kendall M, Elliott JA. Scotopic illumination enhances entrainment of circadian rhythms to lengthening light:dark cycles. J BiolRhythms. 2005;20:38–48. doi: 10.1177/0748730404271573. [DOI] [PubMed] [Google Scholar]
- Goz D, Studholme K, Lappi DA, Rollag MD, Provencio I, Morin LP. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS ONE. 2008;3:e3153. doi: 10.1371/journal.pone.0003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Le H, Vollmers C, Keding SR, Tanaka N, Schmedt C, Jegla T, Panda S. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoSONE. 2008;3:e2451. doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RF, Moore RY, Morin LP. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Research. 1988;460:297–313. doi: 10.1016/0006-8993(88)90374-5. [DOI] [PubMed] [Google Scholar]
- Lall GS, Revell VL, Momiji H, Al EJ, Altimus CM, Guler AD, Aguilar C, Cameron MA, Allender S, Hankins MW, Lucas RJ. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66:417–428. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisk RD. Increased sexual behavior in the male rat following lesions in the mammillary region. Journal of Experimental Zoology. 1966;161:129–136. doi: 10.1002/jez.1401610112. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- Lupi D, Oster H, Thompson S, Foster RG. The acute light-induction of sleep is mediated by OPN4-based photoreception. NatNeurosci. 2008;11:1068–1073. doi: 10.1038/nn.2179. [DOI] [PubMed] [Google Scholar]
- Morin LP, Blanchard JH, Provencio I. Retinal ganglion cell projections to the hamster suprachiasmatic nucleus, intergeniculate leaflet and visual midbrain: bifurcation and melanopsin immunoreactivity. Journal of Comparative Neurology. 2003;465:401–416. doi: 10.1002/cne.10881. [DOI] [PubMed] [Google Scholar]
- Morin LP, Lituma PJ, Studholme KM. Two components of nocturnal locomotor suppression by light. Journal of Biological Rhythms. 2010;25:197–207. doi: 10.1177/0748730410369890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Studholme KM. Millisecond light pulses make mice stop running, then display prolonged sleep-like behavior in the absence of light. Journal of Biological Rhythms. 2009;24:497–508. doi: 10.1177/0748730409349059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrosovsky N, Foster RG, Salmon PA. Thresholds for masking responses to light in three strains of retinally degenerate mice. Journal of Comparative PhysiologyA:Sensory, Neural and Behavioral Physiology. 1999;184:423–428. doi: 10.1007/s003590050341. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Hampton RR. Spatial responses to light in mice with severe retinal degeneration. Neurosci Lett. 1997;222:204–206. doi: 10.1016/s0304-3940(97)13374-2. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Hattar S. Impaired masking responses to light in melanopsin-knockout mice. ChronobiolInt. 2003;20:989–999. doi: 10.1081/cbi-120026043. [DOI] [PubMed] [Google Scholar]
- Naarendorp F, Esdaille TM, Banden SM, ndrews-Labenski J, Gross OP, Pugh EN., Jr Dark light, rod saturation, and the absolute and incremental sensitivity of mouse cone vision. J Neurosci. 2010;30:12495–12507. doi: 10.1523/JNEUROSCI.2186-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack AI, Galante RJ, Maislin G, Cater J, Metaxas D, Lu S, Zhang L, Von SR, Kay T, Lian J, Svenson K, Peters LL. Novel method for high-throughput phenotyping of sleep in mice. Physiol Genomics. 2007;28:232–238. doi: 10.1152/physiolgenomics.00139.2006. [DOI] [PubMed] [Google Scholar]
- Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- Roberts MR, Hendrickson A, McGuire CR, Reh TA. Retinoid X receptor (gamma) is necessary to establish the S-opsin gradient in cone photoreceptors of the developing mouse retina. Invest OphthalmolVisSci. 2005;46:2897–2904. doi: 10.1167/iovs.05-0093. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O’Hara BF. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- Salam JN, Fox JH, Detroy EM, Guignon MH, Wohl DF, Falls WA. Voluntary exercise in C57 mice is anxiolytic across several measures of anxiety. Behavioural Brain Research. 2009;197:31–40. doi: 10.1016/j.bbr.2008.07.036. [DOI] [PubMed] [Google Scholar]
- Sherry DM, Wang MM, Bates J, Frishman LJ. Expression of vesicular glutamate transporter 1 in the mouse retina reveals temporal ordering in development of rod vs. cone and ON vs. OFF circuits. JComp Neurol. 2003;465:480–498. doi: 10.1002/cne.10838. [DOI] [PubMed] [Google Scholar]
- Thompson S, Foster RG, Stone EM, Sheffield VC, Mrosovsky N. Classical and melanopsin photoreception in irradiance detection: negative masking of locomotor activity by light. Eur J Neurosci. 2008;27:1973–1979. doi: 10.1111/j.1460-9568.2008.06168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S, Recober A, Vogel TW, Kuburas A, Owens JA, Sheffield VC, Russo AF, Stone EM. Light aversion in mice depends on nonimage-forming irradiance detection. BehavNeurosci. 2010;124:821–827. doi: 10.1037/a0021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JW, Hannibal J, Hagiwara G, Colas D, Ruppert E, Ruby NF, Heller HC, Franken P, Bourgin P. Melanopsin as a sleep modulator: circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4(−/−) mice. PLoSBiol. 2009;7:e1000125. doi: 10.1371/journal.pbio.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal L, Morin LP. Absence of normal photic integration in the circadian visual system: response to millisecond light flashes. J Neurosci. 2007;27:3375–3382. doi: 10.1523/JNEUROSCI.5496-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warthen DM, Wiltgen BJ, Provencio I. Light enhances learned fear. Proceedings of the National Academy of Sciences of the United States of America. 2011 doi: 10.1073/pnas.1103214108. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]