Abstract
Background
Neuroimaging studies of emotion in schizophrenia have reported abnormalities in amygdala and other regions, although divergent results and heterogeneous paradigms complicate conclusions from single experiments. To identify more consistent patterns of dysfunction, a meta-analysis of functional imaging studies of emotion was undertaken.
Methods
Searching Medline and PsycINFO databases up through January of 2011, 88 potential articles were identified, of which 26 met inclusion criteria, comprising 450 patients with schizophrenia and 422 healthy comparison subjects. Contrasts were selected to include emotion perception and emotion experience. Foci from individual studies were subjected to a voxel-wise meta-analysis using multi-level kernel density analysis.
Results
For emotional experience, comparison subjects showed greater activation in the left occipital pole. For emotional perception, schizophrenia subjects showed reduced activation in bilateral amygdala, visual processing areas, anterior cingulate cortex (ACC), dorsolateral frontal cortex, medial frontal cortex and subcortical structures. Schizophrenia subjects showed greater activation in the cuneus, parietal lobule, precentral gyrus and superior temporal gyrus. Combining across studies and eliminating studies that did not balance on effort and stimulus complexity eliminated most differences in visual processing regions as well as most areas where schizophrenia subjects showed a greater signal. Reduced reactivity of the amygdala appeared primarily in implicit studies of emotion, whereas deficits in ACC activity appeared throughout all contrasts.
Conclusions
Processing emotional stimuli, schizophrenia patients show reduced activation in areas engaged by emotional stimuli, although in some conditions, schizophrenia patients exhibit increased activation in areas outside those traditionally associated with emotion, possibly representing compensatory processing.
Keywords: amygdala, medial frontal cortex, functional magnetic resonance imaging, anterior cingulate cortex, positron emission tomography, occipital cortex
Emotional disturbances in schizophrenia affect a diverse set of processes, including altered emotional expressivity (1), decreased anticipation of hedonic events (1), increased trait negative affect (2), impaired perception of socio-emotional signals (3, 4), and increased experience of negative emotions (5, 6). Other emotional functions, such as the ability to appraise the valence of emotional stimuli, appear relatively intact in schizophrenia (7). Understanding the neural mechanism of affective pathology is an important step towards improving treatment because socio-emotional perceptual deficits (8, 9), and negative emotions (2) have been associated with poor outcome, independent of positive symptoms and neurocognitive impairment.
Neural structures involved in emotion processing, particularly the amygdala, have been an active subject of neuroimaging research in schizophrenia. Findings from different paradigms and different laboratories have reported a variety of results, from reduced (10–14) to increased reactivity (15–17) to emotional stimuli. To make sense of these disparate findings, meta-analyses have recently appeared. Li and colleagues (18) used activation likelihood estimation (ALE) to examine 15 studies of face emotion processing in schizophrenia. ALE is a voxel-based meta-analytic technique for examining the spatial distribution of activation foci reported across studies, comparing that distribution to a random distribution, and determining where clusters of foci exceed a chance distribution (19). Li and colleagues found that patients with schizophrenia underactivated the amygdala. In a meta-analysis focused only on amgydala effect sizes in 35 neuroimaging studies, Anticevic and colleagues (20) found reduced reactivity in contrasts between negative and neutral stimuli (including more than just face stimuli), although of small effect size (0.20 SD). However, they found no difference between patients and controls in a direct comparison of amygdala activity for a negative condition. Thus, questions about amygdala dysfunction in emotion processing in schizophrenia remain.
Voxel-based meta-analytic studies, such as ALE, have the advantage of revealing consistent patterns across studies, to confirm tentative conclusions from single studies and generate new hypotheses from unexpected commonalities. Thus, the voxel-based approach can identify regions outside the amygdala that may be relevant for emotion dysfunction in schizophrenia. For example, visual cortex is modulated by emotional visual stimuli (21, 22). Other regions implicated in neuroimaging studies of emotion include the superior temporal sulcus, the supramarginal gyrus, dorsomedial prefrontal, anterior cingulate cortex, orbitofronal cortex, insula and subcortical areas (23). While the study by Li and colleagues identified reduced activity in the fusiform gyrus, right superior frontal gyrus, and lentiform nucleus of schizophrenia patients, 4 studies (24–27) out of the 15 included in their meta-analysis used restricted search regions, focused on the amygdala. The inclusion of coordinates from such studies biases a meta-analysis away from the excluded regions and in favor of the included regions. The ALE-type analysis is based on testing the null hypothesis that foci are randomly distributed throughout the brain. Thus, an unbiased meta-analysis is critical to addressing the question of which brain regions are implicated in emotion processing deficits in schizophrenia. Furthermore, in light of the very modest effect size observed by Anticevic and colleagues for the amygdala (20), the findings reported by Li and colleagues (18) may not persist in an unbiased analysis.
In the present study, we sought to mine the growing literature on emotion in schizophrenia to generate maps of regions throughout the brain, both in and outside the amygdala. Because emotional processing deficits are many, and not all emotional constructs are equally well-probed, we confined our analysis to two related processes – emotional experience and emotion perception (28), both of which fall within the construct identified by Ochsner as ‘recognizing/responding to socio-emotional stimuli’ (29). Combining studies within this construct maximized the power to identify common areas of difference. We predicted reduced activation in the amygdala, as well as reduced modulation of visual cortex during emotion perception tasks. In addition to specific predictions, this whole-brain meta-analysis had an exploratory aim to identify other regions of difference. For example, previous meta-analyses of emotion processing have not observed enhanced activity in schizophrenia patients (18, 20), which would constitute an important finding that neural responses to emotional stimuli in schizophrenia are not all characterized by hypoactivity.
Methods
Study Selection
Medline and PsycINFO databases were searched through January of 2011. Search terms included “emotion,” “affect,” “affective,” “emotional,” “social perception [MeSH term],” crossed with schizophrenia (as well as variants, including schizoaffective, psychosis) and neuroimaging stems (MRI, magnetic resonance imaging, PET, positron emission tomography). In addition, bibliographies of published studies were also scrutinized. Studies were reviewed and classified by one of the co-authors (IFT, IB, AH), and then reviewed again by the first author (SFT). Studies of pain were excluded, unless they focused on the anticipation of pain. Studies of reward were not included. Eigthy-eight articles were identified that could possibly meet initial inclusion criteria, which were: 1) published in English; 2) included control and patient groups, with statistical comparisons between groups; 3) used PET or fMRI; 4) used standardized coordinates to locate differential activation via subtraction between tasks (‘contrasts’); and 5) conducted unbiased, whole brain search. Of these 88, 3 did not include stereotactic coordinates, 19 did not include a healthy comparison group, 17 did not use whole brain coordinates, and 6 were excluded for various reasons (re-analysis of previously published data, analytic technique not comparable in a general linear model framework). Of the 43 remaining articles, we identified 26 (see Table 1) that contained specific contrasts, which isolated either 1) emotion perception, 2) emotion experience, or 3) a related category of cognitive tasks using emotional stimuli and isolating valence-specific processing. Together, the 26 studies involved 450 patients with schizophrenia and 422 healthy comparison subjects. Except for one (30), all studies included medicated patients, or mixed samples of medicated and unmedicated patients. All studies appeared unique, although two by Williams and colleagues (14, 31) used the same set of patients in both, studied in separate sessions. Since the comparison subjects were different, we treated these patient-control contrasts as independent of each other.
Table 1.
Studies included in meta-analysis
| Author | Schizophrenia subjects | Healthy comparison subjects | |||||
|---|---|---|---|---|---|---|---|
| Males | Females | Age | Patient Type | Males | Females | Age | |
| Crespo-Facorro et al. (76) | 16 | 2 | 30 | Chronic | 7 | 9 | 29.5 |
| Gur et al. (11) | 10 | 4 | 28.8 | Chronic | 10 | 4 | 27.4 |
| Hempel et al. (77) | 4 | 5 | 26 | Chronic | 6 | 4 | 28 |
| Williams et al. (14) | 17 | 10 | 27.3 | Chronic | 14 | 8 | 27.2 |
| Takahashi et al. (78) | 10 | 5 | 29 | Chronic | 9 | 6 | 29 |
| Johnston et al. (12) | 8 | 2 | 30.6 | Chronic | 8 | 2 | 31.2 |
| Taylor et al. (17) | 11 | 7 | 32 | Chronic/First Episode | 6 | 4 | 27 |
| Surguladze et al. (16) | 15 | 0 | 43.1 | Chronic | 11 | 0 | 36.8 |
| Williams et al. (31) | 17 | 10 | 27.3 | Chronic | 8 | 5 | 25.1 |
| Gur et al. (66) | 12 | 4 | 30.1 | Chronic | 12 | 5 | 25 |
| Schneider et al. (43) | 13 | 0 | 32.8 | Chronic | 26 | 0 | 33.4 |
| Pauly et al. (30) | 12 | 0 | 17 | First Episode | 12 | 0 | 17 |
| Michalopoulou et al. (79) | 9 | 2 | 35 | Chronic | 5 | 4 | 32 |
| Hall et al. (51) | 12 | 7 | 37.7 | Chronic | 16 | 8 | 35.1 |
| Fakra et al. (10) | 9 | 5 | 37.29 | Chronic | 9 | 5 | 34.65 |
| Seiferth et al. (68) | 12 | 0 | 17.8 | First Episode | 12 | 0 | 17.9 |
| Reske et al. (80) | 10 | 8 | 31.94 | First Episode | 10 | 8 | 31.94 |
| Kang et al. (13) | 14 | 14 | 29.95 | Chronic | 14 | 14 | 29.9 |
| Kumari et al. (32) | 13 | 0 | 34.5 | Chronic | 14 | 0 | 33.1 |
| Becerril et al. (34) | 25 | 13 | 37 | Chronic | 21 | 11 | 36 |
| Habel et al. (35) | 14 | 0 | 37.14 | Chronic | 14 | 0 | 35.5 |
| Salgado-Pineda et al. (81) | 9 | 5 | 37.3 | Chronic | 9 | 5 | 34.6 |
| Habel et al. (67) | 15 | 0 | 34.4 | Chronic | 17 | 0 | 34.2 |
| Mier et al. (50) | 11 | 5 | 34.25 | Chronic | 11 | 5 | 37 |
| Surguladze et al. (33) | 17 | 13 | 43 | Chronic | 8 | 8 | 40 |
| Holt et al. (82) | 11 | 3 | 43 | Chronic | 14 | 4 | 44 |
| Totals/Averages | 326 | 124 | 32.5 | 303 | 119 | 31.2 | |
Contrast selection
A wide variety of contrasts appear in studies of emotion. To minimize heterogeneity between studies, we focused on emotional experience and emotion perception. We conducted 4 principal analyses to interrogate the data, exploiting different approaches to studying emotion. Six studies were identified as emotional experience, i.e., isolating the feeling elicited by a stimulus, and they included visual (words, pictures) and olfactory modalities (see Table 2 for descriptions of contrasts). For emotional experience, we focused only on negative emotions, since there were inadequate numbers of contrasts using positive emotions, reasoning that the subtraction of valenced stimuli could isolate basic valence circuits common between modalities. Seventeen studies were found using contrasts of emotion perception. In one study (11), subjects rated the valence of a face, but because multiple face emotions were presented and analyzed, we classified this as a perception task. Of these studies, only 2 (32, 33) contrasted valenced stimuli (positive and negative); thus, both were included. Many studies of emotion perception did not match for effort and stimulus complexity; however, because unmatched contrasts used salient emotional stimuli, e. g., emotional faces contrasted with shapes, we elected to include these unmatched contrasts in the emotion perception analysis.
Table 2.
Contrasts analyzed
| Author | Affective Stimuli | Task | Type | Face Processing | Contrast: Not Matched | Contrast: Matched |
|---|---|---|---|---|---|---|
| Crespo-Facorro et al. (76) | Odors | Passive | Experience | Negative > Positive | ||
| Gur et al. (11) | Faces | Emotion ID | Perception | Explicit | Rate valence > Age determination | |
| Hempel et al. (77) | Faces | Label emotion Match emotion | Perception | Explicit | Emotional > Inverted | |
| Williams et al. (14) | Faces | Gender ID | Perception | Implicit | Fear > Neutral | |
| Takahashi et al. (78) | IAPS | Rate valence | Experience | Negative > Neutral | ||
| Johnston et al. (12) | Faces | Emotion ID Gender ID | Perception | Faces > Shapes | Fear/Angry > Neutral | |
| Taylor et al. (17) | IAPS | Rate valence | Experience | Negative > Neutral Positive > Neutral | ||
| Surguladze et al. (16) | Faces | Gender ID | Perception | Implicit | Neutral > Fear | |
| Williams et al. (31) | Faces | Gender ID | Perception | Implicit | Anger/Fear/Disgust > Neutral | |
| Gur et al. (66) | Faces | Emotion ID | Perception | Explicit | Face > Scrambled Face | |
| Schneider et al. (43) | Odors | Passive | Experience | Negative > Neutral, Positive > Neutral | ||
| Pauly et al. (30) | Odors | N-back | Cognitive | Negative > Neutral | ||
| Michalopoulou et al. (79) | Faces | Gender ID | Perception | Implicit | Fear > Neutral | |
| Hall et al. (51) | Faces | Gender ID | Perception | Implicit | Fear > Neutral | |
| Fakra et al. (10) | Faces | Match emotion | Perception | Explicit | Match Fear/Angry > Match Shapes | |
| Seiferth et al. (68) | Faces | Label emotion | Perception | Explicit | Sad/Angry/Fear/Happ y > Baseline | |
| Reske et al. (80) | Faces | Label emotion | Perception | Explicit | Sad > Neutral/Happy | |
| Kang et al. (13) | Speech | Gender ID | Perception | Laugh > Neutral Cry > Neutral | ||
| Kumari et al. (32) | Words | Anticipate shock | Experience | Shock warning > Safety signal | ||
| Becerril et al. (34) | Faces | N-back | Cognitive | Implicit | Negative > Neutral/Positive Neutral/Positive > Negative | |
| Habel et al. (35) | Odors | N-back | Cognitive | Negative > Neutral | ||
| Salgado-Pineda et al. (81) | Faces | Match emotion | Perception | Explicit | Angry/Fear > Neutral | |
| Habel et al. (67) | Faces | Label emotion | Perception | Explicit | Sad/Angry/Fear/Happ y > Baseline | |
| Mier et al. (50) | Faces | Label emotion | Perception | Explicit | Emotion ID > Baseline | |
| Surguladze et al. (33) | Faces | Gender ID | Perception | Implicit | Fear/Happy > Neutral | |
| Holt et al. (82) | Words | Rate valence | Experience | Negative > Neutral Negative > Positive Positive > Neutral |
We also conducted a third analysis to identify group differences in more generally defined emotion-related areas, not specifically tied to mode of presentation or nature of stimuli. This general emotion analysis combined studies from experience and perception, but eliminated studies with emotion perception contrasts that did not match on stimulus complexity and overall effort. To expand across stimulus domains, we included a third category of cognitive tasks, which used emotional stimuli without directing attention to emotional aspects of the stimuli. The cognitive studies happened to be ‘n-back’ tasks (in which subjects determine whether or not a token in a series matches a token presented 1, 2 or ‘n’ tokens back) that either used emotional faces as tokens (34) or presented odor (30, 35) during the performance of the cognitive task. The rationale for including these cognitive tasks is that, formally, an n-back task with faces is identical to a gender identification task, in which subjects do not pay attention to the emotional expression of the face. While odorants were not the attended stimuli in the n-back, they elicit negative experiences, which have been shown to elicit some of the same brain regions as other emotional stimuli (28). The combined analysis, which included 20 studies, also permitted separate analyses for negative and positive valences.
Tasks of emotional processing have also been classified as implicit or explicit, depending upon whether or not attention is directed at the emotional expression, or some other characteristic of the face. For example, identifying the gender of emotional faces constitutes implicit processing, whereas labeling the emotion of a face constitutes explicit processing. It has been suggested that the act of labeling face emotion reduces amygdala activity, compared to passive viewing (36), although a recent meta-analysis suggests that explicit processing of emotional faces is generally associated with greater signal in the amygdala and the fusiform gyrus (37). Thus, to examine how the implicit/explicit distinction might affect differential activation, we classified all of the tasks using face processing as either implicit (7 studies) or explicit (9 studies), and report meta-analytic results.
Meta-analytic procedure
The meta-analysis was conducted using multilevel kernel density analysis (MKDA; 23, 28, 38). The analysis focused only on group contrasts conducted within each study for schizophrenia > healthy control subjects (SCZ>HC), or healthy control > schizophrenia subjects (HC>SCZ). Foci from contrasts of interests from each study were mapped onto the Montreal Neurological Institute (MNI) brain template (39) in 2 mm isotropic voxels. Coordinates reported in Talairach space (40) were transformed into MNI space, using SPM (tal2mni.m; 39, 41). Foci within each study were convolved with a spherical kernel of radius 10mm (42), and voxel-wise significance was obtained via a permutation test (Monte Carlo simulation; n = 30,000), corrected for the multiple comparisons across all gray-matter voxels to derive a family-wise error rate of 0.05 (23). As an additional safeguard against Type I errors, we report only meta-analytic foci with 10 or more voxels (80 mm3). In MKDA, the unit of analysis is the contrast, not the activation foci, so that multiple activation foci from one study count the same as a single focus from another study, which partially corrects for differences in thresholding of activation foci across studies. In the results, we list both the foci contributed by the analyzed contrasts, as well as the number of independent contrasts. For additional details of the MKDA analysis, please see Supplemental Materials.
Results
Emotional Experience
In the contrasts of emotional experience, there were only 7 independent contrasts for SCZ>HC, yielding no significant meta-foci, and 9 independent contrasts for HC>SCZ, yielding a single meta-focus in the left occipital pole (see Figure 1; coordinates: −34, −70, 16; 34 voxels, MKDA density = 0.349, p = 0.0029). The single significant focus was generated by 2 studies, one using aversive visual stimuli (17) and the other using aversive olfactory stimuli (43).
Figure 1.
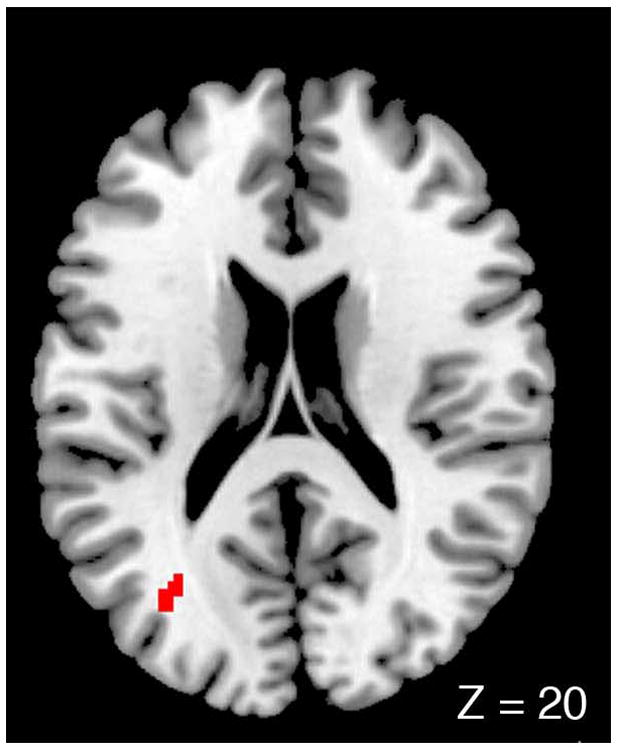
Meta-focus for healthy subjects showing greater activity than schizophrenia patients during emotion experience studies.
Emotion Perception
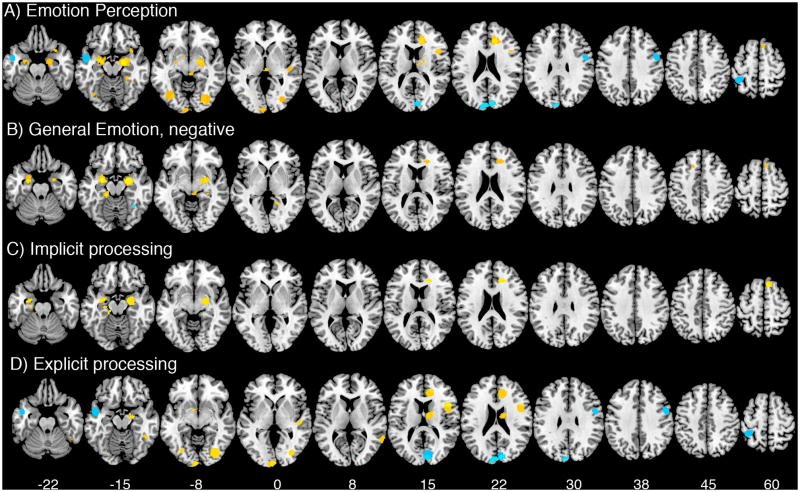
For emotion perception, there were 16 contrasts for SCZ>HC and 35 for HC>SCZ, yielding 4 significant meta-foci for SCZ>HC and 17 for HC>SCZ (Figure 2A and Table 3). The highest density of contrasts occurred in the left amygdala/hippocampal region for HC>SCZ, which was paralleled by a similar meta-focus on the right, though less dense. Control subjects also showed greater signal in early visual processing regions, along with anterior cingulate cortex, dorsolateral frontal cortex, medial frontal cortex, and subcortical structures (thalamus, caudate, midbrain). Schizophrenia patients exhibited greater signals in cuneus, parietal lobulue, precentral gyrus, and superior temporal gyrus.
Figure 2.
Results of meta-analysis for with meta-foci overlaid on a Montreal Neurological Institute template brain. Yellow indicates HC>SCZ and light blue indicates SCZ>HC. Numbers on the bottom row indicate Z-coordinates in MNI space.
Table 3.
Emotion Perception
| Meta-foci | (x, y, z) | Voxels | MKDA Value | P Value |
|---|---|---|---|---|
| Schiz > Controls (Foci = 58, Contrasts = 16) | ||||
| L superior temporal gyrus (BA21) | −44, 4, −18 | 266 | 0.247 | 0.0011 |
| R precentral_G (BA6) | 50, 0, 28 | 215 | 0.195 | 0.0076 |
| L parietal lobule (BA2) | −36, −44, 54 | 204 | 0.182 | 0.0497 |
| L cuneus (BA19) | −4, −88, 32 | 439 | 0.326 | <0.0001 |
| Controls > Schiz (Foci = 165, Contrasts = 35) | ||||
| R anterior cingulate (BA24/32)* | 16, 32, 14 | 347 | 0.192 | <0.0001 |
| R medial frontal (BA8) | 4, 22, 52 | 122 | 0.141 | 0.0013 |
| R dorsolateral frontal (BA44) | 48, 12, 12 | 118 | 0.15 | 0.0004 |
| R anterior temporal pole (BA38) | 34, 12, −24 | 59 | 0.113 | 0.0264 |
| R caudate body | 16, −4, 12 | 18 | 0.12 | 0.0125 |
| L amygdala | −22, −6, −20 | 305 | 0.185 | <0.0001 |
| Thalamus | 10, −10, 12 | 21 | 0.129 | 0.0027 |
| L amygdala/hippocampus | 24, −12, −18 | 515 | 0.233 | <0.0001 |
| R superior temporal g (BA22) | 50, −20, 4 | 89 | 0.121 | 0.0106 |
| Midbrain | 0, −22, −6 | 51 | 0.121 | 0.0106 |
| Midbrain | 4, −28, −12 | 62 | 0.124 | 0.0069 |
| R temporal fusiform gyrus (BA37) | 28, −36, −18 | 32 | 0.144 | 0.0009 |
| R temporal fusiform gyrus (BA37) | 38, −44, −18 | 12 | 0.12 | 0.0125 |
| L occipital fusiform g (BA19) | −34, −76, −12 | 340 | 0.137 | 0.0015 |
| R occipital pole (BA19) | 32, −86, −10 | 413 | 0.165 | 0.0001 |
| L occipital pole (BA17) | −10, −100, −6 | 146 | 0.153 | 0.0002 |
| L cerebellum | −16, −32, −22 | 11 | 0.119 | 0.0144 |
Focus of peak in adjacent white matter
General emotion
In the general emotion analysis, only negative contrasts generated significant group differences. There were 16 contrasts for SCZ>HC and 28 contrasts from HC>SCZ (Table 4). No significant meta-foci appeared for positive stimuli, which had only 4 contrasts for SCZ>HC and 7 for HC>SCZ. As Figure 2B shows, some meta-foci from the emotion perception contrast remained, whereas others disappeared. Meta-foci in the bilateral amygdala for HC>SCZ remained, and a new meta-focus appeared in the right hippocampus. Meta-foci in the anterior cingulate cortex and medial frontal cortex remained. However, meta-foci in early visual processing regions did not appear. For the SCZ>HC contrast, a single meta-focus appeared in the right fusiform gyrus.
Table 4.
General emotion, negative
| Meta-foci | (x, y, z) | Voxels | MKDA Value | P Value |
|---|---|---|---|---|
| Schiz > Controls (Foci = 71, Contrasts = 16) | ||||
| R fusiform gyrus (BA37) | 34, −54, −20 | 37 | 0.202 | 0.0387 |
| Controls > Schiz (Foci = 121, Contrasts = 28) | ||||
| R anterior cingulate (BA24)* | 22, 30, 14 | 149 | 0.121 | 0.0279 |
| R dorsomedial frontal (BA32) | 10, 26, 46 | 29 | 0.132 | 0.0136 |
| R medial frontal (BA6) | 8, 20, 52 | 67 | 0.132 | 0.0136 |
| L anterior cingulate (BA32) | −12, 20, 40 | 22 | 0.12 | 0.0353 |
| L amygdala | −22, −6, −20 | 304 | 0.228 | <0.0001 |
| R amygdala | 26, −8, −20 | 363 | 0.221 | <0.0001 |
| R hippocampus | 30, −32, −6 | 14 | 0.18 | 0.0002 |
| Midbrain | 2, −30, −10 | 62 | 0.154 | 0.0027 |
| R lingual_G (BA19) | 18, −48, −2 | 34 | 0.151 | 0.0045 |
| L dorsal cerebellum | −18, −34, −20 | 76 | 0.183 | 0.0001 |
Focus of peak in adjacent white matter
Implicit and explicit face emotional processing
Examining the effects of implicit versus explicit face processing shed some light on the meta-foci from the emotion perception contrast (Figure 2C & 2D and Tables 5 and 6). Implicit face processing carried the signal from the amygdala contrast for HC>SCZ, although a small meta-focus appeared in the comparable contrast for explicit face processing in the right amygdala. The right anterior cingulate focus appeared in both implicit and explicit conditions. By contrast, group differences for HC>SCZ in the occipital cortex during emotion perception only appeared during explicit processing, as did the cuneus signal seen for SCZ>HC. Similarly, the left parietal and right precentral gyrus meta-foci for SCZ>HC during emotion perception only appeared during explicit emotion processing. The right medial frontal meta-focus for HC>SCZ only appeared during implicit processing. There were no meta-foci for SCZ>HC during implicit processing.
Table 5.
Implicit face emotion processing
| Meta-foci | (x, y, z) | Voxels | MKDA Value | P Value |
|---|---|---|---|---|
| Schiz > Controls (Foci = 30, Contrasts = 5) | ||||
| No foci above threshold | ||||
| Controls > Schiz (Foci = 49, Contrasts = 17) | ||||
| R anterior cingulate (BA24)* | 22, 30, 14 | 149 | 0.202 | 0.0035 |
| R medial frontal (BA6) | 8, 20, 52 | 314 | 0.22 | 0.0014 |
| R amygdala | 28, 0, −16 | 309 | 0.241 | 0.0003 |
| L amygdala/hippocampus | −26, −10, −24 | 138 | 0.184 | 0.0183 |
| L hippocampus | −24, −20, −20 | 41 | 0.22 | 0.0014 |
| L cerebellum | −16, −32, −22 | 34 | 0.184 | 0.0183 |
Focus of peak in adjacent white matter
Table 6.
Explicit face emotion processing
| Meta-foci | (x, y, z) | Voxels | MKDA Value P Value | |
|---|---|---|---|---|
| Schiz > Controls (Foci = 52, Contrasts = 13) | ||||
| L superior temporal gyrus (BA21) | −44, 4, −18 | 266 | 0.305 | 0.0007 |
| R precentral_G (BA6) | 50, 0, 28 | 215 | 0.24 | 0.007 |
| L parietal lobule (BA2) | −36, −44, 54 | 204 | 0.225 | 0.0331 |
| L cuneus (BA19) | −4, −88, 32 | 650 | 0.402 | <0.0001 |
| Controls > Schiz (Foci = 105, Contrasts = 17) | ||||
| R anterior cingulate (BA32)* | 12, 36, 12 | 336 | 0.25 | 0.0007 |
| R dorsolateral frontal (BA44) | 48, 12, 12 | 393 | 0.309 | <0.0001 |
| L ventral pallidum | −6, 2, −10 | 26 | 0.244 | 0.0015 |
| R caudate body | 16, −4, 12 | 213 | 0.247 | 0.0009 |
| R amygdala/hippocampus | 12, −6, −18 | 37 | 0.244 | 0.0015 |
| R superior temporal gyrus (BA22) | 48, −24, −6 | 82 | 0.227 | 0.0076 |
| Middle_Temporal_gyrus (BA21) | 60, −48, 10 | 110 | 0.246 | 0.0012 |
| R temporal fusiform gyrus (BA20/37) | 46, −52, −22 | 74 | 0.188 | 0.0285 |
| L occipital fusiform g (BA19) | −30, −74, −16 | 82 | 0.216 | 0.0084 |
| L occipital fusiform g (BA19) | 32, −78, −14 | 365 | 0.225 | 0.0076 |
| L occipital pole (BA18) | −8, −102, −10 | 197 | 0.25 | 0.0007 |
Focus of peak in adjacent white matter
Discussion
This meta-analysis of emotion perception and experience in schizophrenia identified several salient differences in functional anatomic networks. Findings of reduced amygdala activation (18, 20) were confirmed for emotion perception, but not for emotional experience. In studies using contrasts of negative stimuli, matched on stimulus complexity and task effort, bilateral amygdala meta-foci showed the densest and largest region of greater activation in healthy subjects, demonstrating the relative robustness of this finding, even with the exclusion of studies using biased, a priori search regions. Results also confirmed reduced modulation of visual processing areas in schizophrenia, as well as reduced activation of the right medial frontal gyrus. Emotion processing also recruits subcortical structures besides the amygdala, in the midbrain, diencephalon, hippocampus, striatum, and thalamus (23), which were showed reduced activation in schizophrenia. While the meta-analysis could not precisely evaluate the role of performance deficits on activation, the findings for implicit face processing, in which emotional valence was not the focus of attention, suggest that reduced activity in some regions, such as the medial frontal wall and bilateral amygdala, were not driven by a failure to carry out the overt task. Lastly, the demonstration of overactivity -- in the cuneus, superior temporal gyrus, parietal lobule, and precentral gyrus -- in patients provides new information on the extent of emotion processing dysfunction in schizophrenia.
Emotional experience
The study of emotion in schizophrenia has revealed paradoxes, such as preserved appraisals of emotional stimuli in the presence of reduced affective expressiveness (7). Published results vary, but a recent meta-analysis concluded that schizophrenia patients do not differ in their subjective ratings of negative stimuli from control subjects, although they do report more negative experience of neutral and positive stimuli (5). The current meta-analysis attempted to systematically address whether or not neural substrates associated with emotional experience were, indeed, abnormal. Tasks that isolate emotional experience focus on a specific feeling induced by an emotional stimulus, whereas emotion perception involves rating or judging normative aspects of a stimulus, e. g. labeling the emotion of a face. A meta-focus of reduced activation in schizophrenia appeared in the left occipital region, with foci contributed by a visual task (17) and an olfactory task (43). The occipital meta-focus falls outside the regions commonly recruited by emotion tasks (28), and it may reflect aberrant connectivity in networks processing emotional stimuli, which has been reported in the occipital pole of schizophrenia patients processing emotional faces (44). The number of studies contributing foci to this analysis was small, but the existence of a common meta-focus implies that more studies may reveal more meta-foci. Thus, even though subjective ratings of emotional experience may not differ between patients and controls, neural activity to negative stimuli may look different.
Amygdala
Our results demonstrate that when contrasting valenced with neutral stimuli, schizophrenia subjects do not show the same specific reactivity as comparison subjects. The amygdala, comprised of several nuclei with different functional roles, is sensitive to stimuli indicating threat, such as fearful faces (45, 46), and more generally serves as a salience detector (47, 48). The meta-analytic finding was most concentrated for negatively-valenced stimuli, which were mostly fearful faces. Amygdala reactivity differences were largely driven by implicit contrasts, e.g., identification of face gender, and much less so by explicit emotion tasks, such as labeling a facial emotion, possibly related to findings that labeling emotion reduces the neural signal (36, 45, 49). Importantly, as we did not test implicit versus explicit processing, we cannot conclude that implicit processing shows a greater difference in the amgydala than explicit processing, and as more studies are added to the literature, a strong amygdala signal may yet emerge for explicit processing. Although we found no differences in amygdala activity for emotional experience, experience-based tasks, in general, are poor elicitors of amygdala activity (28), and the small number of studies in the emotional experience contrast makes any conclusions about a negative finding very dubious. While the positive findings in the other contrasts might reflect the fact that patients with schizophrenia have more difficulty perceiving facial emotion (3), there was insufficient data amongst the reported studies to relate performance to neural activity.
It is important to emphasize that the meta-analytic finding showed reduced, specific reactivity of the amygdala, and not overall reactivity. As mentioned above, Anticevic and colleagues found no differences between patients and control subjects when contrasts were made between schizophrenia patients and controls that did not involve an interaction between valenced and neutral stimuli (20). Increased activity to neutral faces has been noted in the amygdala (50, 51) and adjacent parahippocampal gyrus (16, 25). It was not possible to evaluate the response to neutral faces in this meta-analysis, but a plausible interpretation of the results is that patients may have an overactive response of the amygdala to neutral stimuli, meaning that contrasts, e.g., between fearful and neutral faces, would appear to show ‘underactivation.’ Given that fMRI BOLD studies can only show relative change between conditions, it is instructive to note that of the 4 positron emission tomography studies in Anticevic et al, which can measure absolute regional blood flow, two reported significantly greater amygdala activity for all conditions (15, 17), one reported non-significantly greater activity for all conditions (52) and one reported less amygdala activity (53). Taken together, findings in the literature raise the issue that reduced specific reactivity, or underactivity in an emotion–neutral contrast, might reflect a general overactivity of the amygdala, an interpretation that fits with conceptualizations of schizophrenia as responding to non-salient stimuli, as if they were salient (54). Other possibilities need to be considered, e. g. that schizophrenia causes a reduced responsiveness to salient socio-emotional stimuli in general (less activity to both neutral and emotional faces). Data measuring baseline activity, e.g., with cerebral perfusion, as well as examination of contrasts between neutral faces and baseline, will be necessary to determine the effect driving the observed data.
Anterior cingulate and medial frontal cortex
One meta-focus of reduced activation in schizophrenia that appeared across all analyzed contrasts was in the anterior cingulate cortex (ACC). Another meta-focus in the dorsal medial fontal cortex (dmPFC) appeared in all contrasts but explicit face processing. Both regions are consistently recruited by emotion tasks, and the ACC is preferably activated during emotion perception tasks (23). The ACC is thought to play a key integrative role in emotion, performance monitoring, motivation, and arousal (55, 56). Structural (57, 58), post-mortem (59), and functional studies (60) have implicated this structure in schizophrenia. The dmPFC processes complex social stimuli, including social inference and self-relatedness (61, 62). Also appearing was the dorsolateral prefrontal cortex for explicit emotion perception, a region less implicated by emotion tasks than medial frontal cortex, but still involved in higher-level emotional appraisals and re-appraisals (63), and also well-known to exhibit aberrant function in schizophrenia (60, 64). However, the important finding in the medial wall, particularly the ACC, reinforces the role of these areas in the pathophysiology of schizophrenia, which can now be seen to include basic emotion processing.
Posterior areas
Reduced activation of posterior, visual processing regions during emotion perception was expected, and it replicated and extended prior meta-analytic work (18). Bilateral fusiform gyri and occipital poles are modulated by salient, emotional visual stimuli (23), possibly via back projections from the amygdala (65), and the majority of the studies in the meta-analysis used visual stimuli. The signal in visual processing areas only appeared in one small meta-focus in right lingual gyrus in the general emotion analysis, which excluded contrasts not matched on visual complexity, such as judging faces relative to shapes (10, 12), to scrambled faces (66), or to baseline (50, 67, 68). Thus, it is possible that majority of these occipital meta-foci represent less the effect of valence-specific emotion, and more the effect of processing complex socio-emotional stimuli, such as faces. Reduced activation of the fusiform face area (FFA) has been reported (69, 70), (although see (71), along with abnormal frontal connectivity (44, 72) and reduced early event-related potentials (73–75) while processing faces. With this important distinction in mind, in so far as face stimuli beyond to a broad class of socio-emotional stimuli (29), the meta-analytic results implicate posterior, occipital regions as showing reduced reactivity while processing salient stimuli.
Greater activation for schizophrenia subjects
Greater activation for schizophrenia patients during emotion processing was found, something not revealed in prior work. During emotion perception, the patients showed a larger signal in the cuneus, left parietal lobule, right precentral gyrus and left temporal lobe –- all regions (except the left temporal lobe) not commonly activated in emotion tasks (23). In the general emotion analysis, which excluded studies not matched on stimulus and response complexity, these foci of greater activity disappeared, a fact that might explain why regions outside typically recruited emotion-processing areas were found in the emotion perception contrast. In the general emotion contrast, patients showed increased activity in the right fusiform gyrus. Examination of this result revealed two studies that contributed foci – one using an n-back with faces (34), and the other using words to indicate a potential shock (32), neither of which would be expected to recruit fusiform gyrus. One may speculate that schizophrenia patients recruited non-emotional regions as a compensatory process, e. g. greater cognitive effort to encode emotional stimuli, more ambivalent interpretation, etc. Whatever the reason(s) for the greater activation signal, it is important to note that schizophrenia patients do not simply show reduced activation when processing emotional stimuli.
Limitations and conclusion
This meta-analysis represents an ‘interim report’ from the field, and with the following caveats. In spite of the attempt to limit the analysis to relatively homogeneous contrasts, differences between studies and non-standardized experimental parameters (stimulus duration, trial design, timing of response, etc.) introduces an unavoidable heterogeneity. In general, this fact reduces the ability to generate common findings, but it also raises the possibility that common areas of activation are functionally different and cannot be lumped together. While it may seem surprising that only 2 studies can generate significant foci, this reflects the relatively small numbers of contrasts for some of the analyses, and significant foci indicate the low probability of a chance occurrence of foci in the same vicinity. With 30,000 Monte Carlo simulations and a relatively conservative cluster size (> 10 voxels), we feel that our positive findings are solid and should endure as more studies are added to the literature. However, the absence of findings, e.g., in the insula, may reflect poor experimental power with relatively few contrasts; consequently, no conclusions should be made about areas that do not show up in the meta-analysis. It is very likely that as more published data become available, more regions of the brain will be implicated, along with information relating symptoms and functioning to specific networks. Lastly, all of the samples in the included studies, save 1, used medicated patients, raising the question of whether medication effects contributed to the results. Despite these limitations, the data begin to demonstrate the networks implicated in this important phenomenological dimension of the illness.
Supplementary Material
Acknowledgments
This project was supported by MH086701 to S.F.T.
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006;32:238–245. doi: 10.1093/schbul/sbj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanchard WPJJ, Clark LA, Green MF. Affective traits in schizophrenia and schizotypy. Schizophr Bull. 2008;34:856–874. doi: 10.1093/schbul/sbn083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull. 2010;36:1009–1019. doi: 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoekert M, Kahn RS, Pijnenborg M, Aleman A. Impaired recognition and expression of emotional prosody in schizophrenia: review and meta-analysis. Schizophr Res. 2007;96:135–145. doi: 10.1016/j.schres.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Cohen AS, Minor KS. Emotional Experience in Patients With Schizophrenia Revisited: Meta-analysis of Laboratory Studies. Schizophr Bull. 2010;36:143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myin-Germeys I, Delespaul PA, deVries MW. Schizophrenia patients are more emotionally active than is assumed based on their behavior. Schizophr Bull. 2000;26:847–854. doi: 10.1093/oxfordjournals.schbul.a033499. [DOI] [PubMed] [Google Scholar]
- 7.Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophr Bull. 2008;34:819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horan WP, Green MF, Degroot M, Fiske A, Hellemann G, Kee K, et al. Social Cognition in Schizophrenia, Part 2: 12-Month Stability and Prediction of Functional Outcome in First-Episode Patients. Schizophr Bull. 2011 doi: 10.1093/schbul/sbr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32(Suppl 1):S44–63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fakra E, Salgado-Pineda P, Delaveau P, Hariri AR, Blin O. Neural bases of different cognitive strategies for facial affect processing in schizophrenia. Schizophr Res. 2008;100:191–205. doi: 10.1016/j.schres.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 11.Gur RE, McGrath C, Chan RM, Schroeder L, Turner T, Turetsky BI, et al. An fMRI study of facial emotion processing in patients with schizophrenia. Am J Psychiatry. 2002;159:1992–1999. doi: 10.1176/appi.ajp.159.12.1992. [DOI] [PubMed] [Google Scholar]
- 12.Johnston PJ, Stojanov W, Devir H, Schall U. Functional MRI of facial emotion recognition deficits in schizophrenia and their electrophysiological correlates. Eur J Neurosci. 2005;22:1221–1232. doi: 10.1111/j.1460-9568.2005.04294.x. [DOI] [PubMed] [Google Scholar]
- 13.Kang JI, Kim JJ, Seok JH, Chun JW, Lee SK, Park HJ. Abnormal brain response during the auditory emotional processing in schizophrenic patients with chronic auditory hallucinations. Schizophrenia Research. 2009;107:83–91. doi: 10.1016/j.schres.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Williams LM, Das P, Harris AW, Liddell BB, Brammer MJ, Olivieri G, et al. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. American Journal of Psychiatry. 2004;161:480–489. doi: 10.1176/appi.ajp.161.3.480. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Egea E, Parellada E, Lomena F, Falcon C, Pavia J, Mane A, et al. 18FDG PET study of amygdalar activity during facial emotion recognition in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2010;260:69–76. doi: 10.1007/s00406-009-0020-6. [DOI] [PubMed] [Google Scholar]
- 16.Surguladze S, Russell T, Kucharska-Pietura K, Travis MJ, Giampietro V, David AS, et al. A Reversal of the Normal Pattern of Parahippocampal Response to Neutral and Fearful Faces Is Associated with Reality Distortion in Schizophrenia. Biol Psychiatry. 2006;60:423–31. doi: 10.1016/j.biopsych.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Taylor SF, Phan KL, Britton JC, Liberzon I. Neural response to emotional salience in schizophrenia. Neuropsychopharmacology. 2005;30:984–995. doi: 10.1038/sj.npp.1300679. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Chan RC, McAlonan GM, Gong QY. Facial emotion processing in schizophrenia: a meta-analysis of functional neuroimaging data. Schizophr Bull. 2010;36:1029–1039. doi: 10.1093/schbul/sbn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anticevic A, Van Snellenberg JX, Cohen RE, Repovs G, Dowd EC, Barch DM. Amygdala Recruitment in Schizophrenia in Response to Aversive Emotional Material: A Meta-analysis of Neuroimaging Studies. Schizophr Bull. 2010 doi: 10.1093/schbul/sbq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pizzagalli DA, Lehmann D, Hendrick AM, Regard M, Pascual-Marqui RD, Davidson RJ. Affective judgments of faces modulate early activity (approximately 160 ms) within the fusiform gyri. Neuroimage. 2002;16:663–677. doi: 10.1006/nimg.2002.1126. [DOI] [PubMed] [Google Scholar]
- 22.Taylor SF, Liberzon I, Koeppe RA. The effect of graded aversive stimuli on limbic and visual activation. Neuropsychologia. 2000;38:1415–1425. doi: 10.1016/s0028-3932(00)00032-4. [DOI] [PubMed] [Google Scholar]
- 23.Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Habel U, Klein M, Shah NJ, Toni I, Zilles K, Falkai P, et al. Genetic load on amygdala hypofunction during sadness in nonaffected brothers of schizophrenia patients. American Journal of Psychiatry. 2004;161:1806–1813. doi: 10.1176/ajp.161.10.1806. [DOI] [PubMed] [Google Scholar]
- 25.Holt DJ, Kunkel L, Weiss AP, Goff DC, Wright CI, Shin LM, et al. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophr Res. 2006;82:153–162. doi: 10.1016/j.schres.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 26.Holt DJ, Weiss AP, Rauch SL, Wright CI, Zalesak M, Goff DC, et al. Sustained activation of the hippocampus in response to fearful faces in schizophrenia. Biol Psychiatry. 2005;57:1011–1019. doi: 10.1016/j.biopsych.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 27.Kosaka H, Omori M, Murata T, Iidaka T, Yamada H, Okada T, et al. Differential amygdala response during facial recognition in patients with schizophrenia: an fMRI study. Schizophr Res. 2002;57:87–95. doi: 10.1016/s0920-9964(01)00324-3. [DOI] [PubMed] [Google Scholar]
- 28.Wager T, Barrett LF, Bliss-Moreau E, Lindquist K, Duncan S, Kober H, et al. The neuroimaging of emotion. In: Lewis MH, Haviland-Jones JM, Barrett LF, editors. Handbook of Emotion. 3. New York: Guilford Press; 2008. pp. 249–271. [Google Scholar]
- 29.Ochsner KN. The social-emotional processing stream: five core constructs and their translational potential for schizophrenia and beyond. Biol Psychiatry. 2008;64:48–61. doi: 10.1016/j.biopsych.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pauly K, Seiferth NY, Kellermann T, Backes V, Vloet TD, Shah NJ, et al. Cerebral dysfunctions of emotion-cognition interactions in adolescent-onset schizophrenia. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:1299–1310. doi: 10.1097/CHI.0b013e318184ff16. [DOI] [PubMed] [Google Scholar]
- 31.Williams LM, Das P, Liddell BJ, Olivieri G, Peduto AS, David AS, et al. Fronto-limbic and autonomic disjunctions to negative emotion distinguish schizophrenia subtypes. Psychiatry Research. 2007;155:29–44. doi: 10.1016/j.pscychresns.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 32.Kumari V, Das M, Taylor PJ, Barkataki I, Andrew C, Sumich A, et al. Neural and behavioural responses to threat in men with a history of serious violence and schizophrenia or antisocial personality disorder. Schizophr Res. 2009;110:47–58. doi: 10.1016/j.schres.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Surguladze SA, Chu EM, Marshall N, Evans A, Anilkumar AP, Timehin C, et al. Emotion processing in schizophrenia: fMRI study of patients treated with risperidone long-acting injections or conventional depot medication. J Psychopharmacol. 2011;25:722–733. doi: 10.1177/0269881110363316. [DOI] [PubMed] [Google Scholar]
- 34.Becerril K, Barch D. Influence of Emotional Processing on Working Memory in Schizophrenia. Schizophr Bull. 2011;37:0127–38. doi: 10.1093/schbul/sbq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Habel U, Pauly K, Koch K, Kellermann T, Reske M, Backes V, et al. Emotion-cognition interactions in schizophrenia. World J Biol Psychiatry. 2010;11:934–944. doi: 10.3109/15622975.2010.501820. [DOI] [PubMed] [Google Scholar]
- 36.Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 37.Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- 38.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philos Trans R Soc Lond B Biol Sci. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Talairach J, Tournoux P. A co-planar stereotaxic atlas of a human brain. Stuttgart: Thieme-Verlag; 1988. [Google Scholar]
- 41.Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 43.Schneider F, Habel U, Reske M, Toni I, Falkai P, Shah NJ. Neural substrates of olfactory processing in schizophrenia patients and their healthy relatives. Psychiatry Research. 2007;155:103–112. doi: 10.1016/j.pscychresns.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Taylor SF, Chen AC, Tso IF, Liberzon I, Welsh RC. Social appraisal in chronic psychosis: Role of medial frontal and occipital networks. J Psychiatr Res. 2011;45:526–538. doi: 10.1016/j.jpsychires.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Research Reviews. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7:1271–1278. doi: 10.1038/nn1341. [see comment] [DOI] [PubMed] [Google Scholar]
- 47.Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 48.Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Reviews in the Neurosciences. 2003;14:303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- 49.Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage. 2003;18:650–659. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- 50.Mier D, Sauer C, Lis S, Esslinger C, Wilhelm J, Gallhofer B, et al. Neuronal correlates of affective theory of mind in schizophrenia out-patients: evidence for a baseline deficit. Psychol Med. 2010;40:1607–1617. doi: 10.1017/S0033291709992133. [DOI] [PubMed] [Google Scholar]
- 51.Hall J, Whalley HC, McKirdy JW, Romaniuk L, McGonigle D, McIntosh AM, et al. Overactivation of fear systems to neutral faces in schizophrenia. Biological Psychiatry. 2008;64:70–73. doi: 10.1016/j.biopsych.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 52.Taylor SF, Liberzon I, Decker LR, Koeppe RA. A functional anatomic study of emotion in schizophrenia. Schizophr Res. 2002;58:159–172. doi: 10.1016/s0920-9964(01)00403-0. [DOI] [PubMed] [Google Scholar]
- 53.Paradiso S, Andreasen NC, Crespo-Facorro B, O’Leary DS, Watkins GL, Boles Ponto LL, et al. Emotions in unmedicated patients with schizophrenia during evaluation with positron emission tomography. Am J Psychiatry. 2003;160:1775–1783. doi: 10.1176/appi.ajp.160.10.1775. [DOI] [PubMed] [Google Scholar]
- 54.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatr. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 55.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 56.Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- 57.Fornito A, Yucel M, Patti J, Wood SJ, Pantelis C. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr Res. 2009;108:104–113. doi: 10.1016/j.schres.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 58.Baiano M, David A, Versace A, Churchill R, Balestrieri M, Brambilla P. Anterior cingulate volumes in schizophrenia: a systematic review and a meta-analysis of MRI studies. Schizophrenia Research. 2007;93:1–12. doi: 10.1016/j.schres.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 59.Benes FM. Model generation and testing to probe neural circuitry in the cingulate cortex of postmortem schizophrenic brain. Schizophr Bull. 1998;24:219–230. doi: 10.1093/oxfordjournals.schbul.a033322. [DOI] [PubMed] [Google Scholar]
- 60.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Archives of General Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- 62.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 63.Kalisch R, Wiech K, Herrmann K, Dolan RJ. Neural correlates of self-distraction from anxiety and a process model of cognitive emotion regulation. J Cogn Neurosci. 2006;18:1266–1276. doi: 10.1162/jocn.2006.18.8.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [see comments] [DOI] [PubMed] [Google Scholar]
- 65.Sabatinelli D, Lang PJ, Bradley MM, Costa VD, Keil A. The timing of emotional discrimination in human amygdala and ventral visual cortex. Journal of Neuroscience. 2009;29:14864–14868. doi: 10.1523/JNEUROSCI.3278-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gur RE, Loughead J, Kohler CG, Elliott MA, Lesko K, Ruparel K, et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Archives of General Psychiatry. 2007;64:1356–1366. doi: 10.1001/archpsyc.64.12.1356. [DOI] [PubMed] [Google Scholar]
- 67.Habel U, Chechko N, Pauly K, Koch K, Backes V, Seiferth N, et al. Neural correlates of emotion recognition in schizophrenia. Schizophr Res. 2010;122:113–123. doi: 10.1016/j.schres.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 68.Seiferth NY, Pauly K, Kellermann T, Shah NJ, Ott G, Herpertz_Dahlmann B, et al. Neuronal correlates of facial emotion discrimination in early onset schizophrenia. Neuropsychopharmacology. 2009;34:477–487. doi: 10.1038/npp.2008.93. [DOI] [PubMed] [Google Scholar]
- 69.Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res. 2008;99:164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walther S, Federspiel A, Horn H, Bianchi P, Wiest R, Wirth M, et al. Encoding deficit during face processing within the right fusiform face area in schizophrenia. Psychiatry Res. 2009;172:184–191. doi: 10.1016/j.pscychresns.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 71.Yoon JH, D’Esposito M, Carter CS. Preserved function of the fusiform face area in schizophrenia as revealed by fMRI. Psychiatry Res. 2006;148:205–216. doi: 10.1016/j.pscychresns.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 72.Dima D, Roiser JP, Dietrich DE, Bonnemann C, Lanfermann H, Emrich HM, et al. Understanding why patients with schizophrenia do not perceive the hollow-mask illusion using dynamic causal modelling. Neuroimage. 2009;46:1180–1186. doi: 10.1016/j.neuroimage.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 73.Bediou B, Henaff MA, Bertrand O, Brunelin J, d’Amato T, Saoud M, et al. Impaired fronto-temporal processing of emotion in schizophrenia. Neurophysiol Clin. 2007;37:77–87. doi: 10.1016/j.neucli.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 74.Yeap S, Kelly SP, Sehatpour P, Magno E, Javitt DC, Garavan H, et al. Early visual sensory deficits as endophenotypes for schizophrenia: high-density electrical mapping in clinically unaffected first-degree relatives. Arch Gen Psychiatry. 2006;63:1180–1188. doi: 10.1001/archpsyc.63.11.1180. [DOI] [PubMed] [Google Scholar]
- 75.Turetsky BI, Kohler CG, Indersmitten T, Bhati MT, Charbonnier D, Gur RC. Facial emotion recognition in schizophrenia: when and why does it go awry? Schizophr Res. 2007;94:253–263. doi: 10.1016/j.schres.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Crespo-Facorro B, Paradiso S, Andreasen NC, O’Leary DS, Watkins GL, Ponto LL, et al. Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. Jama. 2001;286:427–435. doi: 10.1001/jama.286.4.427. [DOI] [PubMed] [Google Scholar]
- 77.Hempel A, Hempel E, Schonknecht P, Stippich C, Schroder J. Impairment in basal limbic function in schizophrenia during affect recognition. Psychiatry Res. 2003;122:115–124. doi: 10.1016/s0925-4927(02)00126-9. [DOI] [PubMed] [Google Scholar]
- 78.Takahashi H, Koeda M, Oda K, Matsuda T, Matsushima E, Matsuura M, et al. An fMRI study of differential neural response to affective pictures in schizophrenia. Neuroimage. 2004;22:1247–1254. doi: 10.1016/j.neuroimage.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 79.Michalopoulou PG, Surguladze S, Morley LA, Giampietro VP, Murray RM, Shergill SS. Facial fear processing and psychotic symptoms in schizophrenia: functional magnetic resonance imaging study. British Journal of Psychiatry. 2008;192:191–196. doi: 10.1192/bjp.bp.106.032649. [DOI] [PubMed] [Google Scholar]
- 80.Reske M, Habel U, Kellermann T, Backes V, Jon Shah N, von Wilmsdorff M, et al. Differential brain activation during facial emotion discrimination in first_episode schizophrenia. Journal of Psychiatric Research. 2009;43:592–599. doi: 10.1016/j.jpsychires.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 81.Salgado-Pineda P, Fakra E, Delaveau P, Hariri AR, Blin O. Differential patterns of initial and sustained responses in amygdala and cortical regions to emotional stimuli in schizophrenia patients and healthy participants. J Psychiatry Neurosci. 2010;35:41–48. doi: 10.1503/jpn.090017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Holt DJ, Lakshmanan B, Freudenreich O, Goff DC, Rauch SL, Kuperberg GR. Dysfunction of a Cortical Midline Network During Emotional Appraisals in Schizophrenia. Schizophr Bull. 2010;37:164–176. doi: 10.1093/schbul/sbp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.