Abstract
Sphingosine 1-phosphate (S1P) is a lipid mediator produced from sphingomyelin by the sequential enzymatic actions of sphingomyelinase, ceramidase, and sphingosine kinase. Five subtypes of cell surface G-protein-coupled receptors, S1P1–5, mediate the actions of S1P in various organs systems, most notably cardiovascular, immune, and central nervous systems. S1P is enriched in blood and lymph but is present at much lower concentrations in interstitial fluids of tissues. This vascular S1P gradient is important for the regulation of trafficking of various immune cells. FTY720, which was recently approved for the treatment of relapsing-remitting multiple sclerosis, potently sequesters lymphocytes into lymph nodes by functionally antagonizing the activity of the S1P1 receptor. S1P also plays critical roles in the vascular barrier integrity, thereby regulating inflammation, tumor metastasis, angiogenesis, and atherosclerosis. Recent studies have also revealed the involvement of S1P signaling in coagulation and in tumor necrosis factor α-mediated signaling. This review highlights the importance of S1P signaling in these inflammatory processes as well as the contribution of each receptor subtype, which exhibits both cooperative and redundant functions.
Keywords: Sphingosine 1-phosphate, G-protein-coupled receptor, Vascular barrier integrity, Immune cell trafficking, Coagulation, Inflammation
Introduction
Sphingosine 1-phosphate (S1P) is a pleiotropic lipid mediator involved in regulation of proliferation, migration, rearrangement of cytoskeleton, adhesion, and inflammation in various cell types, including those of the cardiovascular system, both innate- and adaptive-immune systems, and the central nervous system. Fingolimod (FTY720/Gilenya™; Novartis), a structural analog of sphingosine, was recently approved as the first-line oral treatment for the relapsing–remitting multiple sclerosis by the US Food and Drug Administration. In this review, we outline the metabolic pathways of S1P, S1P receptors, and physiological S1P functions revealed by analyses of receptor deficient mice. We also mention its roles in immune cell trafficking that provide a basic understanding of FTY720 action as a potent immunomodulator. Finally, we highlight recently uncovered roles of S1P in coagulation and inflammation.
Metabolic pathways of S1P
Sphingolipids
Sphingosine (2-amino-4-octadecene-1,3-diol) is a C18 amino alcohol that contains one amino group and two hydroxy groups and serves as a backbone for all sphingolipids (Fig. 1a). Ceramides are produced from sphingosine when the C2 amino group is acylated with one of several fatty acid chains. Ceramides are further metabolized when the C1 hydroxy group is esterified by phosphocholine to sphingomyelin, the most abundant sphingolipid in plasma membrane. Ceramides are also metabolized when the C1 hydroxy group is glycosylated by a sugar chain to glycosphingolipids like cerobrosides and gangliosides. On the other hand, when sphingosine is phosphorylated at the C1 hydoxy group, S1P is produced.
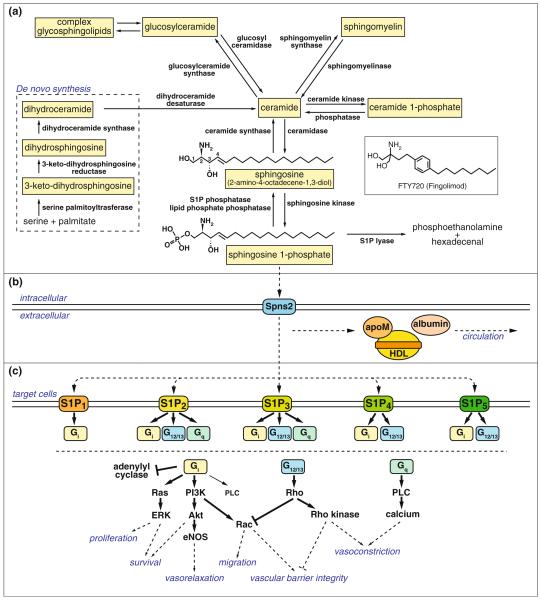
Fig. 1.
Metabolic pathways, transport, and receptors of S1P. a De novo sphingolipid metabolism starts at the endoplasmic reticulum where serine palmitoyltransferase condesates serine and palmitate into 3-keto-dihydrosphingosine, followed by sequential conversion to dihydrosphingosine, dihydroceramide, and ceramide by the action of 3-keto-dihydrosphingosine reductase, dihydroceramide synthases, and dihydroceramide desaturase, respectively. Ceramide, generated either from the de novo pathway or from degradation of complex sphingolipids such as sphingomyelin and glycosphingolipids, is deacylated by ceramidase to sphingosine, followed by phosphorylation by sphingosine kinase to produce S1P. S1P is dephosphorylated to sphingosine by S1P phosphatase or by lipid phosphate phosphatases, or is irreversibly degraded to phosphoethanolamine and hexadecenal by S1P lyase. b S1P produced intracellularly is transported by specific transporters such as Spns2 and carried by carrier proteins such as HDL-associated apoM and albumin. c S1P activates S1P receptors, S1P1–5, which transmit diverse intracellular signals depending on the coupled Gα subunit of heterotrimeric G protein and the expression pattern of each receptors in a given cell type
The metabolism of each sphingolipid class is highly interconnected and orchestrated by specific enzymes at each step. Although the family name “sphingolipid” is derived from the common sphingosine backbone, sphingosine molecule itself is not directly generated by de novo synthesis. De novo synthesis of sphingolipids starts at the cytosolic leaflet of the endoplasmic reticulum where 3-keto-dihydrosphingosine is generated from serine and palmitate by the action of serine palmitoyltransferase (Fig. 1a). Then, 3-keto-dihydrosphingosine is sequentially converted to dihydrosphingosine, dihydroceramide, and ceramide by the action of 3-keto-dihydrosphingosine reductase, dihydroceramide synthases, and dihydroceramide desaturase, respectively. Ceramide can be considered as a central player in sphingolipid metabolism, from which either complex sphingolipids such as sphingomyelin and glycosphingolipid are synthesized, or signaling molecules such as ceramide 1-phosphate and S1P are produced.
Although sphingolipids have been regarded as merely building blocks of cellular membranes for a long time, recent studies revealed their diverse functions both as intercellular and intracellular signaling mediators. Sphingomyelin is not only a major constituent of myelin sheath but also plays important roles in lipid raft formation on plasma membranes [1]. Ceramides play roles not only in moisture retention function of epidermis but also in many cell-stress responses, including the regulation of apoptosis [2] and cell senescence [3]. Among all sphingolipids, S1P is the most potent intercellular signaling molecules. Structurally, S1P is classified as a lysophospholipid—for example, lysophosphatidic acid and platelet activating factor, with a polar head group and hydrophobic acyl tail. This amphipathic character allows lysophospholipids to leave plasma membrane and work as intercellular mediators. Lysophospholipids signal via specific G-protein-coupled receptors (GPCR) and exert various physiological functions.
S1P production and degradation
S1P is enriched in blood and lymph in the submicromolar range, whereas it is much lower in interstitial fluids of tissues, creating a steep S1P gradient [4]. This vascular S1P gradient is utilized to regulate trafficking of immune cells such as lymphocytes, hematopoietic progenitor cells, and dendritic cells as discussed later. Since half-life of S1P when bound to albumin is <15 min in plasma [5], it is likely that S1P is continuously produced to maintain the high concentration in blood and lymph. Platelets store S1P abundantly and have been regarded as a main source of plasma S1P [6]. However, contribution of platelets to plasma S1P levels has been challenged by the observation that plasma from NF-E2-deficient mice and antibody-induced thrombocytopenic mice have normal S1P concentrations despite having virtually no circulating platelets [5, 7]. Recent studies have shown that erythrocytes play important roles in the maintenance of S1P concentration in plasma [7, 8]. Platelets probably contribute to local exaggerated synthesis of S1P during thrombotic episodes. The results from our group have shown that vascular endothelial cells also contribute to plasma S1P levels and that S1P production is stimulated by fluid shear stress in endothelial cells [5].
The main pathway for S1P production is from sphingomyelin hydrolysis. Sphingomyelin is sequentially converted to ceramide, sphingosine, and S1P by the action of sphingomyelinase, ceramidase, and sphingosine kinase, respectively (Fig. 1a). Two types of sphingosine kinases (SphK) have been identified, SphK1 and SphK2 [9]. Although SphK1 and SphK2 have slight differences in substrate specificities and subcellular localization, they catalyze the same reaction, that is, phosphorylation of sphingosine to produce S1P, and have redundant functions since neither SphK1 nor SphK2 knockout mice display any obvious developmental abnormalities alone, but double knockout mice show complete loss of sphingosine kinase activity and embryonic lethality due to improper neural and vascular development [10].
Importantly, enzymatic activity of SphK1 and SphK2 are regulated by extracellular stimulus. The first insight into this came from the observation that SphK1 translocated from the cytoplasm to the plasma membrane in response to phorbol ester treatment [11]. This translocation is regulated by extracellular signal-regulated kinase (ERK) 1/2-dependent phosphorylation [12] and leads to increased S1P production [11, 13]. Many kinds of growth factors have been shown to induce translocation and activation of SphK1 to exert their proliferative effects. These include platelet-derived growth factor (PDGF) [14], vascular endothelial growth factor (VEGF) [15], nerve growth factor [16], epidermal growth factor [17], insulin [18], and insulin-like growth factor-1 [19]. SphK1 is also activated by tumor necrosis factor-α (TNF-α) stimulation in a mechanism dependent on TNF receptor-associated factor 2 (TRAF2) [20], and knockdown studies have implicated that SphK1 mediates the effects of TNF-α on induction of cyclooxygenase-2 (COX-2) and adhesion molecules, production of prostaglandins, and activation of endothelial nitric oxide synthase (eNOS) (discussed later) [21–23]. It seems that many extracellular stimulus including growth factors and pro-inflammatory mediators converge on SphK1 as an intracellular target and exert their effects via subsequent S1P production. However, it is important to note that there is significant basal activity of SphK in cells, which may be related to normal metabolic functions of the enzyme in sphingolipid turnover. In addition, many growth factors and cytokines appear to work well even under conditions of SphK isoenzyme knockout, suggesting that the activation of this pathway may be dispensable under various pathophysiological conditions.
S1P is dephosphorylated to sphingosine either by a family of lipid phosphate phosphatases, LPP1-3, at the cell surface, or by S1P specific phosphatases, SPP1 and SPP2, at the endoplasmic reticulum. LPPs have broad substrate specificity, dephosphorylating S1P, ceramide 1-phosphate, lysophosphatidic acid, etc. and is thought to be the primary mechanism by which extracellular S1P signaling is attenuated [24]. Overexpression of specific LPPs reduces S1P-dependent signaling events in HEK293 cells [25, 26]. On the other hand, SPPs are S1P-specific phosphatases and are mainly localized to the endoplasmic reticulum. SPPs are thought to play roles in regulating cytoplasmic S1P level and reintroduction of sphingoid bases into ceramide species [27].
An alternative pathway to regulate S1P levels is irreversible degradation of S1P to phosphoethanol amine and hexadecenal by S1P lyase (SPL), which serves as the final degradation of sphingolipids species. SPL is exclusively localized to the endoplasmic reticulum [28]. SPL has a wide tissue distribution with its highest expression in the thymus and intestines, followed by spleen, liver, and testis [28]. In contrast, SPL activity cannot be detected in platelets and erythrocytes in which S1P is actively produced [28]. Expression of SPL in lymphoid organs seems to be essential for proper lymphocyte trafficking since inhibition of SPL by the food colorant 2-acetyl-4-tetrahydroxybutylimidazole (THI) induces lymphopenia due to the disruption of S1P gradient between blood or lymph and lymphoid organs (see below) [29].
S1P transport
Since several enzymes involved in S1P production like sphingomyelinase [30], ceramidase [31], and SphK1 [32, 33] are secreted from endothelial cells, S1P can be produced in the extracellular space. However, it is generally regarded that most of S1P are produced in the intracellular space and exported after production (Fig. 1b). Several members of ATP-binding cassette (ABC) transporter super-family have been suggested to regulate S1P transport. Those include ABCC1 in mast cells [34], ABCA1 and ABCA7 in platelet [35], ABCA1 in astrocytes [36], ABCC1 in fibroblasts [37], and ABCC1 and ABCG2 in breast cancer cells [38]. However, none of ABCA1, ABCA7, or ABCC1 null mice showed significant changes in S1P levels in plasma as compared to wild-type counter parts [39]. This indicates that these transporters are not required in the maintenance of plasma S1P or that they regulate S1P transport redundantly. In addition, it is well known that ABC transporters have other functions; for example, ABCA1 is a sterol transporter important for HDL generation. Thus, the requirement for ABC transporters in the S1P export is unclear at present.
Recently, Spinster 2 (Spns2) was reported as a critical S1P transporter required for heart development [40]. Spns2 has putative 12 transmembrane domains with structural similarity to the glycerol-3-phosphate transporter. Spns2 mutants in zebrafish display cardia bifida resembling the phenotype of S1P receptor-2 (Mil) mutant [40]. When ectopically expressed in CHO cells with SphK1, only Spns2 but not ABCA1, ABCB1, ABCC1, or ABCG2 promotes the secretion of S1P [40, 41]. Since both genetic and biochemical data support the role of Spns2 as a S1P transporter, it is likely that this protein plays a physiological role in S1P transport. Its function in mammalian systems needs further elucidation.
Due to its amphipathic character, most of S1P is bound to carrier proteins in blood (Fig. 1b). In human plasma, more than half of S1P is found in HDL fraction, about 36% in albumin fraction, and 8% in LDL fraction [42]. However, it was not clear which molecule on HDL was specifically serving as a S1P carrier. Recently, we identified apolipoprotein M (apoM) as a specific carrier for S1P on the HDL particles [43]. ApoM is a 25-kDa lipoprotein-associated plasma protein mainly synthesized in the liver [44]. The retained hydrophobic NH2-terminal signal peptide anchors apoM in the phospholipids layer of lipoprotein particles [45, 46]. The determination of crystal structure of human recombinant apoM has elucidated that apoM has a typical lipocalin fold characterized by an eight-stranded β-barrel that encloses an internal binding pocket and suggested that S1P is one of the potent ligand for apoM [47]. Accordingly, the 1.7-Å structure of the S1P–apoM complex has revealed that S1P highly specifically interacts with an amphiphilic pocket in the lipocalin fold of apoM [43]. Although apoM is found only in 5% of total HDL in human plasma [48], S1P was shown to be specifically associated with apoM-containing HDL [43]. ApoM-containing HDL induced S1P receptor-dependent signaling in human umbilical vein embryonic endothelial cells (HUVEC), whereas apoM-depleted HDL did not. Moreover, lack of S1P in the HDL fraction of Apom−/− mice decreased basal endothelial barrier function in lung tissue [43]. These results strongly suggest apoM serves as a specific carrier of S1P on HDL that mediates vasoprotective actions on the endothelium.
S1P receptors and physiological functions of S1P
S1P receptors
Diverse physiological functions of S1P are mediated by specific, high-affinity GPCRs. Five receptors for S1P have been identified so far since the first S1P receptor S1P1 was reported in 1998 [49]; those are S1P1–S1P5, formerly termed endothelial differentiation gene (EDG) receptor-1, EDG-5, EDG-3, EDG-6, and EDG-8, respectively [50]. All of these receptors are activated by nanomolar doses of S1P.
The differential but overlapping expression patterns and intracellular signaling pathways of each receptor enable S1P to exert its diverse functions. S1P1–3 receptors are widely expressed, especially rich in cardiovascular and immune system. S1P4 is expressed in the lymphoid system [51, 52] and airway smooth muscle cells [53], and S1P5 is in the white matter tracts of the central nervous system [54, 55], though the expression level of S1P4 and S1P5 are relatively low compared to S1P1-3.
S1P1 exclusively couples with Gi/o alpha subunit of heterotrimeric G proteins, whereas S1P2 and S1P3 couple with Gi/o, Gq, and G12/13, and S1P4 and S1P5 with Gi/o and G12/13 (Fig. 1c). Generally, signaling through Gi/o leads to the activation of the Ras/ERK pathway to promote proliferation, the PI3K/Akt pathway to prevent apoptosis, and the PI3K/Rac pathway to promote cytoskeletal rearrangement and migration, as well as phospholipase C (PLC) activation to increase intracellular calcium concentration and inhibition of adenylyl cyclase to reduce cAMP production. Signaling through Gq primarily activates the PLC pathway, and signaling through G12/13 promotes Rho activation to inhibit Rac and migration. It seems that the balance of those signaling pathways downstream of each receptor type determines the divergent effects of S1P, depending on the expression pattern of S1P receptor subtypes in a given cell type. For example, S1P1 and S1P2 work competitively for cell migration; S1P1 promotes migration via Rac activation [56], whereas S1P2 suppresses migration by Rho-mediated inhibition of Rac [57, 58]. Our group showed that migration toward S1P was markedly attenuated by the Rho- and PTEN-dependent pathways when S1P2 was overexpressed in HUVEC, which predominantly express S1P1 [59].
Most of the S1P biological functions are regarded to be mediated by S1P receptors mentioned above, but there are also several reports showing intracellular S1P effects. Hait et al. [60] recently reported that S1P specifically bound to histone deacetylases HDAC1 and HDAC2 and inhibited their enzymatic activity. Alvarez et al. [61] showed that S1P specifically bound to TRAF2, one of the key components in NF-κB signaling triggered by TNF-α, and stimulated its E3 ubiquitin ligase activity. Physiological relevance of these findings remains to be established.
Physiological functions of S1P revealed by the analysis of receptor/enzyme-deficient mice
Genetic deletion of S1P1 causes embryonic lethality between E12.5 and E14.5 as a result of excessive hemorrhage [62]. No clear defect is observed in vasculogenesis and angiogenesis. However, vascular maturation is incomplete due to defective coverage by vascular smooth muscle cells/pericytes in the dorsal aorta [62]. Furthermore, endothelial-specific deletion of S1P1 shows the same phenotype as the global deletion [63], clearly demonstrating that S1P1 in endothelial cells play critical roles in vascular stabilization by recruiting mural cells. It was shown that S1P1 regulated N-cadherin-mediated cell adhesion between endothelial and mural cells [64]. The importance of S1P in the maintenance of vascular integrity in the adults is also clear, since the mutant mice engineered to selectively lack plasma S1P by an inducible SphK1/SphK2 double-deletion system display increased vascular leak and impaired survival after anaphylaxis [65].
Deletion of S1P1 also causes severe disturbances in neurogenesis. Embryos at E12.5 have cell loss in the forebrain, increased apoptotic cells in the neuroepithelium of the telencephalon and diencephalon, and decreased mitotic cells in the telencephalon [10]. SphK1/SphK2 double-knockout mice show very similar phenotypes [10]. SphK1 is highly expressed in the brain, especially rich in telenchphalon, while SphK2 is rich in the limb buds, eyes, and branchial arches [10]. The expression patterns overlap with those of S1P1, establishing an important role of the SphK/S1P/S1P1 axis in the neuronal development.
Single deletion of either S1P2 or S1P3 does not lead to the embryonic lethality, but simultaneous deletion of S1P2 and S1P3 results in about 50% embryonic death around E13.5 due to the hemorrhage [66–68]. Triple null mice lacking all of the S1P1–3 show the severest bleeding, leading to embryonic death between E10.5 and E11.5 [68], which is a little earlier than S1P1 single deletion. These results indicate that S1P1 plays a critical role in the vascular maturation, with S1P2 and S1P3 redundant and/or supportive roles. SphK1/SphK2 double knockout embryos have undetectable levels of S1P, which also results in the embryonic lethality around E12.5 due to the defects in vascular maturation, just like S1P1 deletion mice [10].
Although S1P2-deficient mice are born with no apparent anatomical or physiological defects, the mice develop spontaneous, sporadic, and occasionally lethal seizures between 3 and 7 weeks of age [69]. At a cellular level, a large increase in the excitability of neocortical pyramidal neurons are observed in these mice [69], suggesting that S1P2 is important for the development and/or mediation of neuronal excitability. S1P2 is also essential for the proper functioning of the auditory and vestibular systems since S1P2-deficient mice show deafness due to the impairments in the stria vascularis and cochlear hair cells [70, 71]. Recently, it was shown that the balance between S1P1- and S1P2-mediated signaling controlled the chemotactic behavior of osteoclast precursors between bone and blood [72]. S1P2 also has an inhibitory role in macrophage recruitment during inflammation [73].
Several studies using S1P2-dificient mice have elucidated critical roles of S1P2 in angiogenesis and atherogenesis. Our group showed that S1P2-driven inflammatory process was an important event in hypoxia-triggered pathological angiogenesis of the retina [74]. Du et al. [75] showed that S1P2 expressed in endothelial cells and bone-marrow-derived cells negatively regulated tumor angiogenesis and tumor growth. S1P2 also regulates macrophage retention and inflammatory cytokine secretion in the atherosclerotic plaques, thereby promoting atherosclerosis [76, 77].
S1P3 is highly expressed in heart, lung, spleen, kidney, intestine, and diaphragm, but S1P3-deficient mice show no obvious phenotype [66]. However, clear involvements of S1P3 have been demonstrated in several cardiovascular functions such as regulation of heart rate and blood pressure in rodents [78, 79], regulation of myocardial perfusion and consequent protection of cadiomyocytes in ischemia–reperfusion injury [80–82], vasorelaxation [83], and cardiac fibrosis [84]. S1P3 is also shown to regulate the endothelial cells in splenic marginal sinus organization [85] and the stimulation of endothelial progenitor cells [86]. Furthermore, pro-inflammatory roles of S1P3 have been reported. Niessen et al. [87] reported that the dendritic cell PAR1-S1P3 crosstalk played a critical role in coupling coagulation events with inflammation in sepsis syndrome (see below). Keul et al. [88]showed that S1P3 mediated the chemotactic effect of S1P in macrophages and played a causal role in atherosclerosis by promoting inflammatory monocyte/macrophage recruitment and altering smooth muscle cell behavior.
S1P4 is primary expressed in lymphoid tissues [52], and pharmacological analysis has suggested that S1P4 mediates immunosuppressive effects of S1P by inhibiting T-cell proliferation and secretion of effector cytokines [89]. Recently, S1P4-deficient mice were reported, showing that S1P4 played a role in shaping the terminal differentiation of megakaryocytes and proplatelet development [90].
S1P5 is mainly expressed in oligodendrocytes in the brain, and immature oligodendrocytes from S1P5-dificient mice shows diminished responses in S1P-induced process retraction, though S1P5-deficient mice have no deficits in myelination [91]. S1P5 is also expressed in natural killer cells and regulates their trafficking by a FTY720-resistant mechanism [92, 93].
Taken together, S1P exerts its effects through specific receptors in many organ systems, most notably cardiovascular, immune, and central nervous systems. Multiple receptor system enables S1P to have pleiotropic effects, where each receptor has differential but partially overlapping tissue distributions and intracellular signaling pathways, and works sometimes cooperatively and sometimes competitively each other.
S1P as a regulator of lymphocyte trafficking
Recent studies have elucidated a critical role of S1P–S1P1 signaling in the regulation of lymphocytes egress from the thymus and secondary lymphoid organs. S1P receptors are the targets of FTY720, which is a newly developed immunomodulatory drug and was recently approved as the first oral treatment for the relapsing–remitting multiple sclerosis by the US Food and Drug Administration [94, 95]. In this section, we summarize the functions of S1P signaling in lymphocyte trafficking and the effects of FTY720. For a detailed description, the reader is referred to recent comprehensive reviews [96, 97].
FTY720 was synthesized as a potent immunosuppressor with low cytotoxicity using myriocin as a lead compound [98]. Myriocin is one of the active constituents of Isaclaria sinclarii, a traditional herb for Eastern medicine [99]. Different from calcinuerin inhibitors like cyclosporine, FTY720 does not affect proliferation and activation of lymphocytes, but it induces marked decrease of circulating lymphocytes (lymphopenia), especially of mature T lymphocytes [100, 101]. Structural similarity between FTY720 and sphingosine (Fig. 1a) led to the elucidation that S1P signaling plays a critical role in lymphocyte trafficking and that FTY720 modulates S1P signaling as detailed below.
FTY720 is phosphorylated to FTY720-P by SphK2 [102–104], and FTY720-P acts as a potent agonist on all of the S1P receptors except S1P2 in the nanomolar range [105, 106]. After agonist stimulation, S1P1 is desensitized by phosphorylation of its C-terminal tail and subsequent internalization by a β-arrestin-mediated clathrin-coated vesicle mechanism [107, 108]. In the case of S1P stimulation, most of the internalized S1P1 receptors recycle back to the plasma membrane. In contrast, FTY720-P induces sustained internalization, followed by WWP2 (ubiquitin E3 ligase)-dependent polyubiquitinylation and degradation of S1P1, resulting in the massive decrease of S1P1 expression level [108, 109]. Lymphocytes from S1P1 mutant-knockin mice in which C-terminal phosphorylation sites are mutated show delayed S1P1 internalization and defective desensitization after agonist stimulation, and these mutant mice exhibit significantly delayed lymphopenia after FTY720 administration [110], supporting the idea that FTY720 exerts its effects through modulating the cell surface residence and activity of S1P1 and/or other S1P receptors. Administration of S1P or S1P1-selective agonist also causes lymphopenia [78, 79, 106], and histological analysis revealed that lymph node medullary sinuses of FTY720-treated mice were emptied of lymphocytes, which suggests that lymphocytes cannot access the egress structure [106].
In conditional knockout mice whose hematopoietic cells lack S1P1, there is no clear defect in lymphocyte maturation, but almost no mature lymphocytes is found in circulating blood and lymph due to the defect in egress from thymus or peripheral lymphoid organs [111]. When S1P1-deficient mature lymphocytes are adoptively transferred intravenously into wild-type mice, they enter but cannot exit secondary lymphoid organs [111]. Furthermore, S1P1-dependent chemotactic responsiveness is strongly upregulated in T-cell development before exit from the thymus, whereas S1P1 is downregulated during peripheral lymphocyte activation [111]. These observations clearly show that S1P1 is essential for the regulation of lymphocyte egress from thymus and peripheral lymphoid organs and suggest that lymphocytes utilize an S1P gradient between lymphoid organs and circulation as a cue of the egress process.
As mentioned above, S1P concentration is maintained high in blood and lymph and low in tissues. Although exact numbers vary, the S1P concentration in plasma generally has been found to be in the low micromolar range and that, in lymph, is approximately one sixth of the concentration in plasma [7, 29, 39]. S1P1 on thymocytes gets internalized almost completely after incubation with 1 nM S1P for 20 min ex vivo [29], indicating that lymphocytes are very sensitive to S1P. Although S1P1 is undetectable on the surfaces of circulating T cells, the receptor is readily detectable on the surfaces of T cells in the spleen and lymph nodes [29]. These results suggest that S1P concentration in the interstitial fluids of lymphoid organs is much lower than that in circulation, creating a steep S1P gradient.
S1P lyase inhibition either by the food colorant THI, by treatment with the vitamin B6 antagonist deoxypyridoxine, or by short hairpin RNA-mediated knockdown in hematopoietic cells leads to the increase of S1P concentration in lymphoid organs and consequently induces lymphopenia [29], which suggests that S1P lyase is critical for maintaining the low concentration of S1P in lymphoid organs. Similarly, conditional deletion of both SphK1 and SphK2 leads to the destruction of the S1P gradient and results in lymphopenia [7].
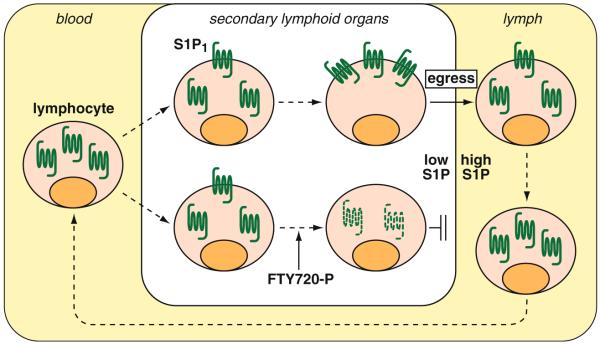
The current model for the regulation of lymphocyte egress by S1P signaling is as follows (Fig. 2); lymphocytes in circulation have their S1P1 mostly internalized due to the high S1P concentration, and upon entrance in lymphoid organs where S1P concentration is low, they gradually recover the surface expression of S1P1 and regain ability to migrate out from lymphoid organs toward S1P in blood or lymph. FTY720 induces the degradation of S1P1 and thereby inhibits the lymphocyte egress from lymphoid organs. S1P signaling is also utilized for the regulation of trafficking of dendritic cells [112], natural killer cells [92, 93], and hematopoietic stem cells [113] based on the almost same mechanism as in lymphocytes.
Fig. 2.
Regulation of lymphocyte trafficking by S1P. Lymphocytes in circulation have their S1P1 mostly internalized due to the high S1P concentration in plasma. Upon entrance in secondary lymphoid organs where S1P concentration is low, they gradually recover the surface expression of S1P1 and regain ability to egress from the lymphoid organs toward S1P in circulation. FTY720 is phosphorylated by sphingosine kinase 2 and induces the internalization and degradation of S1P1 and thereby inhibits the lymphocyte egress
S1P in coagulation and inflammation
As mentioned above, S1P is maintained rich in blood and lymph and low in tissues, creating a vascular S1P gradient [4]. This marks a sharp contrast with most of pro-inflammatory mediators such as eicosanoids and cytokines that are acutely produced upon stimulation. The vascular S1P gradient is utilized for regulation of vascular integrity (see below) and immune cell trafficking. Thus, S1P is unlikely to be a pro-inflammatory mediator acting on endothelium and circulating cells under homeostatic conditions. However, disturbances of the vascular S1P gradient by local exaggerated production of S1P or by ectopic S1P increase in tissues make S1P exert both pro- and anti-inflammatory actions in various types of cell, including endothelial cells, smooth muscle cells, fibroblasts, monocyte/macrophages, mast cells, lymphocytes, and so on. Expression pattern of S1P receptor subtypes in a given cell type and local S1P concentration seem to determine the consequences of S1P actions. In this section, we mainly discuss roles of S1P signaling in vascular barrier integrity and coagulation processes. We also mention pro-inflammatory actions of S1P, particularly in mast cells and in TNF-α-mediated signaling. Finally, we discuss involvement of S1P signaling in a chronic inflammatory disease, atherosclerosis. S1P has a wide spectrum of effects on diverse cell types and is involved in various diseases, such as cancer, asthma, multiple sclerosis, and rheumatoid arthritis. The reader interested is referred to excellent reviews by other authors [97, 114–121].
S1P in platelet
Platelets contain high concentrations of S1P because platelets have constitutive SphK activity, while they lack S1P lyase activity [28, 122, 123]. The concentrations of S1P in serum are usually higher than that in plasma [6, 124, 125], indicating that S1P is released from platelets during the coagulation processes. In fact, several reports showed that platelets secreted S1P upon stimulation, such as thrombin, collagen, and phorbol ester [122, 123, 126]. Therefore, platelets were initially thought to be a source of plasma S1P. However, it has been shown that transcriptional factor NF-E2-deficent mice and acute experimental thrombocytopenic mice have normal plasma S1P concentrations despite having virtually no circulating platelets [5, 7, 8, 127]. Nonetheless, local exaggerated production of S1P upon platelet activation might be important for wound-healing processes and vascular barrier integrity as discussed below. Very high concentration of S1P (>10 μM) was shown to induce platelet activation such as intracellular calcium increase, shape change, and aggregation [122], but physiological range of S1P does not activate platelets.
Although S1P is not a major activator of platelet, S1P signaling seems to play a role in the platelet production. Recently, Golfier et al. [90] reported that S1P4 was specifically up-regulated during the development of human megakaryocytes from hematopoietic progenitor cells and that megakaryocytes generated from S1P4-deficient murine bone marrow showed atypical and reduced formation of proplatelets in vitro. The recovery of platelet numbers after experimental thrombocytopenia was significantly delayed in S1P4-deficient mice. These observations indicate that S1P4 plays a role in shaping the terminal differentiation of megakaryocytes and proplatelet development.
S1P in vascular barrier integrity
Dysfunction of endothelial barrier integrity results in marked increases in vascular permeability that is one of the central features of inflammation, tumor metastasis, angiogenesis, and atherosclerosis. Many reports have demonstrated the critical roles of S1P signaling in the maintenance of vascular barrier integrity. Camerer et al. [65] showed that mutant mice, which selectively lacked S1P in plasma by an inducible SphK1/SphK2 double-deletion system, displayed basal vascular leak and impaired survival after anaphylaxis challenges by platelet-activating factor or histamine. They also showed that increased leak was associated with increased inter-endothelial cell gaps in venules and was reversed by transfusion with wild-type erythrocytes (which restored plasma S1P levels) and by acute treatment with an agonist for S1P1. These results strongly suggest that plasma S1P maintains basal vascular integrity under homeostatic conditions via S1P1 and prevents lethal responses to leak-inducing mediators.
Important events to maintain vascular integrity are rearrangements of cytoskeleton and assembly of adherens junctions in endothelial cells. Lee et al. [128] showed that S1P induced reorganization of actin cytoskeleton and localization of VE-cadherin and α-, β-, and γ-catenin to the sites of cell–cell contact in conjunction with adherens junction assembly in HUVEC. Furthermore, Rac and an associated guanine nucleotide exchange factor Tiam1 also accumulated to the sites of cell–cell contact after S1P stimulation, and adherens junction assembly by S1P was attenuated by dominant-negative Rac, by inhibition of Rho with C3 endotoxin and by suppression of S1P1 or S1P3 expression. Garcia et al. [129] also showed that S1P preferentially induced the activation of Rac and subsequent p21-associated kinase activation in a pertussis toxin-sensitive manner in bovine endothelial cells, which led to the prominent cortical actomyosin ring formation, whereas thrombin induced dissolution of the cortical cytoskeleton, prominent stress fiber formation, and increase in intercellular gaps. They further showed that S1P induced rapid, sustained, and dose-dependent increases in transmonolayer electrical resistance (TER) across both human and bovine pulmonary artery and lung microvascular endothelial cells. S1P also reversed barrier dysfunction elicited by thrombin. The S1P-elicited TER increase was mediated mainly by S1P1 and partly by S1P3 via activation of Rac and actin filament rearrangement in a pertussis toxin-sensitive manner.
The role of S1P3 in endothelial barrier integrity is complex and may be context dependent. Recent studies showed that silencing of S1P3 receptor attenuated increases of vascular permeability by edemagenic stimulus [130, 131]. Activation of S1P3 leads to Rho activation through coupling with G12/13. Although coordinated activation of Rac and Rho is required for the barrier integrity [128], dominant activation of Rho leads to stress fiber formation and disruption of adherens junction [132]. Similarly, activation of S1P2 promotes Rho-dependent stress fiber formation and disruption of adherens junction that increases vascular permeability [133]. Thus, the concerted actions of S1P1, S1P2, and S1P3 regulate vascular permeability, with S1P1 primarily barrier protective and S1P2/S1P3 promoting vascular leakage, albeit in a context dependent manner (Fig. 3a). This concept is supported by several in vivo experiments. Administration of S1P or S1P1 selective agonist shows protective effects in rodent models of lipopolysaccharide (LPS)-induced lung injury and vascular permeability [134, 135], VEGF-induced vascular leakage [102], and anaphylaxis challenges [65], while administration of S1P1 antagonists induces a loss of capillary integrity and vascular leakage in lung and skin [136]and FTY720-mediated S1P1 degradation leads to vascular leakage in lung [109]. Efficacy of S1P administration in LPS-induced vascular permeability is dependent on the S1P concentration, with a low S1P dose barrier protective whereas a high S1P dose barrier disruptive [135]. This is probably due to the activation of S1P2 and S1P3 by ahighdoseof S1P. Indeed, S1p2−/− mice as well as mice with reduced S1P3 expression are protected against LPS-induced barrier disruption compared with control mice [135].
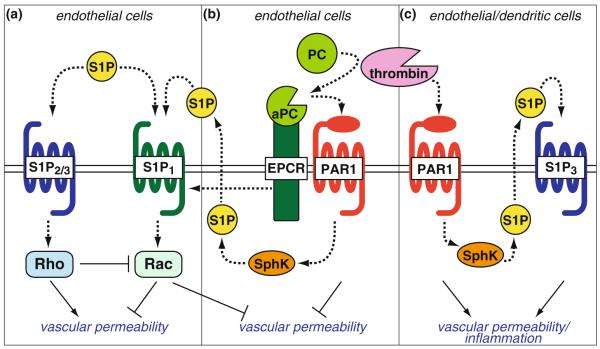
Fig. 3.
Regulation of vascular permeability by S1P and crosstalk with PAR1 signaling. a In endothelial cells, S1P1 induces Rac-mediated formation of cortical actin and adherens junctions and decreases vascular permeability, whereas S1P2 and S1P3 induce Rho-mediated increases in vascular permeability. b PAR1, when activated by the complex of endothelial protein C receptor (EPCR) and activated protein C (aPC), potently inhibits thrombin-induced vascular permeability. This barrier-protective activity of aPC is partly dependent on cross-activation of S1P1 either by SphK1-mediated S1P production or by EPCR-mediated trans-activation of S1P1. c Thrombin-activated PAR1 increases vascular permeability partly via trans-activation of S1P3. PAR1-S1P3 coupling in dendritic cells leads to the systemic inflammation in the late stage of severe sepsis
S1P production by SphK1 in endothelial cells after edemagenic stress is shown to be important for S1P1 to exert the barrier protective function. Tauseef et al. [137] showed that SphK1 activity and S1P concentration was significantly increased in the lung after a thrombin-mimetic peptide administration and that Sphk1−/− mice showed markedly enhanced pulmonary edema formation in response to LPS and thrombin compared to wild-type mice. They further showed in human pulmonary arterial endothelial cells that S1P produced by SphK1 activated S1P1 in an autocrine/paracrine manner and restored endothelial barrier integrity after thrombin stimulation. It is also shown that bone-marrow-derived endothelial progenitor cells contribute as another source of S1P to restore endothelial barrier integrity after LPS challenges [138].
S1P in angiogenesis
In addition to the role in the vascular barrier integrity, S1P is a potent pro-angiogenic factor that stimulates migration, proliferation, and morphogenesis of endothelial cells. S1P1 and S1P3 promote chemotaxis of endothelial cells toward S1P through mechanisms involving Rho and PI3K/Akt/Rac pathways [56, 58, 139–143], whereas S1P2 mediates Rho- and PTEN-dependent inhibition of migration [59]. S1P also stimulates proliferation of endothelial cells [140, 144] and morphogenesis of endothelial cells into capillary-like networks [128]. Furthermore, S1P synergistically potentiates fibroblast growth factor (FGF)-2- and VEGF-induced angiogenesis in vivo [128, 144]. Thus, it is assumed that locally produced S1P from activated platelets during coagulation processes at the sites of trauma and wound healing is one of the major constituents to induce angiogenesis, including migration, proliferation, and morphogenesis of endothelial cells in concert with several angiogenic growth factors such as FGF, VEGF, and PDGF [145, 146].
In vivo studies have also revealed a role of S1P signaling in pathological angiogenesis. RNA interference-mediated S1P1 silencing inhibited tumor angiogenesis and growth in an animal model of subcutaneous tumor implantation [147], whereas S1P2 expressed in endothelial cells and bone-marrow-derived cells negatively regulates tumor angiogenesis and growth [75]. Skoura et al. [74] showed using the hypoxia-triggered pathological angiogenesis model of mouse retina that activation of S1P2 on corneal endothelial cells was essential for COX-2 induction and subsequent inflammation and drove the neovascularization in the vitreous chamber. In S1P2-deficient mice, this abnormal angiogenesis was significantly inhibited, which was correlated with decreased COX-2 expression and increased eNOS expression.
S1P in coagulation
Recent studies have revealed that S1P signaling is actively coupled with coagulation processes. Takeya et al. [148] reported a synergistic effect of S1P on thrombin-induced tissue factor expression in HUVEC. Although S1P itself did not induce tissue factor expression, S1P strongly potentiated thrombin-induced tissue factor expression through the activation of transcriptional factors NF-κB and early growth response-1 (Egr-1) in an ERK1/2-dependent manner. In addition, co-stimulation of thrombin and S1P rapidly up-regulated the expression of S1P1 and S1P3. Matsushita et al. [149] showed that S1P activated exocytosis of the Weibel–Palade body that contains several thrombotic factors such as P-selectin, von Willebrand factor, and tissue plasminogen activator in human aortic endothelia cells. Interestingly, they reported two opposing effects of S1P in regulating endothelial exocytosis: S1P activated the exocytosis through phospholipase C-γ activation and intracellular calcium increase, and on the other hand, S1P suppressed the exocytosis through production of NO by activating the PI3K/Akt/eNOS pathway. This is probably due to the differential activation of S1P1 and S1P3. S1P might modulate the tone of coagulation processes depending on the presence of other coagulants or on the receptor subtypes activated.
Furthermore, S1P signaling has crosstalk with protease-activated receptor (PAR) 1-mediated signaling pathways. PAR1 is a GPCR that serves as a major thrombin receptor in endothelial cells [150]. PAR1 is cleaved by thrombin or by tissue factor coagulation initiation complex [151] and mediates their barrier-disruptive effects, leading to the increase in vascular permeability [150]. On the other hand, PAR1 is also cleaved by activated protein C (aPC), an anticoagulant serine protease that is produced by thrombin/thrombomodulin complex from the zymogen protein C [152]. Endothelial protein C receptor (EPCR) binds to protein C and facilitates the activation of protein C [153]. Activation of PAR1 by EPCR/aPC complex induces distinct signaling pathways from thrombin in endothelial cells and potently inhibits thrombin-induced vascular permeability [154–158]. This barrier-protective activities of aPC have been shown to be partly dependent on cross-activation of S1P1 [154, 155].
Feistritzer et al. [154] showed that aPC reduced thrombin-elicited hyperpermeability of the confluent HUVEC monolayer in a dual-chamber system using Evans blue-labeled bovine serum albumin and that the effects of aPC were dependent on binding to EPCR, activation of PAR1, and activity of SphK. Targeted silencing of S1P1 or SphK1 expression using small interfering RNA (siRNA) attenuated this protective signaling by aPC, whereas that of S1P3 did not. Finigan et al. [155] also showed that aPC attenuated thrombin-elicited reductions in TER across human pulmonary artery endothelial cells, which was dependent on Rac activation and subsequent cortical actin assembly and myosin light chain phosphorylation. They further showed that aPC induced S1P1 phosphorylation on threonine residues in an EPCR- and PI3K-dependent manner and that S1P1 co-immunoprecipitated with EPCR upon aPC treatment. Targeted silencing of S1P1 expression using siRNA significantly reduced aPC-mediated barrier protection against thrombin. One of the threonine residues phosphorylated upon aPC treatment is likely to be Thr236 since Akt-mediated phosphorylation of this residue is shown to be indispensable for Rac activation and cortical actin assembly upon S1P stimulation in HUVEC [56]. In contrast, RhoA-mediated trans-activation of S1P3 is implicated as a cause of thrombin-elicited endothelial barrier disruption [131]. These in vitro studies have suggested that S1P1 plays a role in aPC-mediated endothelial barrier protection, either by SphK1-mediated S1P production or by EPCR-mediated direct trans-activation of S1P1 (Fig. 3b), whereas a differential coupling of thrombin-activated PAR1 with S1P3 leads to endothelial barrier disruption (Fig. 3c). This concept is supported by a recent careful in vivo study using a combination of genetic mouse models (Par1−/−, S1p3−/−, Sphk1−/−, EPCRlow (low EPCR expression) [159], and TMpro (lack of thrombomodulin-dependent activation of protein C) [160]) and several pharmacological modulators in LPS-induced sepsis model [161]. Thus, the activating proteases (thrombin vs. aPC/EPCR) and coupling to S1P receptor subtypes (S1P1 vs. S1P3) determine PAR1 signaling specificity in endothelial cells in the regulation of vascular permeability.
In addition to the crosstalk in endothelial cells, PAR1-S1P3 coupling in dendritic cells has been shown to amplify inflammatory responses in the LPS-induced sepsis model. Niessen et al. [87] found that the levels of dendritic-cell- and T-cell-derived inflammatory cytokines were markedly reduced in Par1−/− mice at 18 h after challenge with a high dose of LPS, although initial inflammatory responses were indistinguishable between Par1−/− and wild-type mice. Adoptive transfer of immature, bone marrow-derived wild-type dendritic cell populations into Par1−/− mice restored wild-type levels of all inflammatory parameters, indicating that dendritic cells orchestrate the inflammatory responses in the late stages of the sepsis model. In Par1−/− mice, late stage inflammatory exacerbation was restored with a nonselective agonist for S1P receptors, but not with a selective agonist for S1P1. Furthermore, S1p3−/− or Sphk1−/− mice showed similar reduced late stage inflammation as Par1−/− mice and were markedly protected from LPS challenge and bacterial sepsis. Adoptive transfer of wild-type dendritic cells, but not Par1−/− dendritic cells, into S1p3−/− mice recovered the severe late stage inflammation, indicating that expression of both PAR1 and S1P3 in dendritic cells is critical for the late stage inflammatory exacerbation. S1p3−/− mice also showed markedly reduced levels of circulating thrombin–antithrombin at the late stage, which was restored by adoptive transfer of wild-type dendritic cells, indicating that PAR1-S1P3 coupling in dendritic cell compartment is important in both disseminated intravascular coagulation and inflammation in late stage sepsis. Mechanistically, the loss of PAR1-S1P3 signaling sequestered dendritic cells and inflammation into draining lymph nodes after LPS challenges due to reduced dendritic cell mobility and attenuated dissemination of interleukin-1β to the lungs that is directly downstream of the lymphatic draining of the thoracic duct into the central circulation. Thus, PAR1-S1P3 signaling in dendritic cells couples coagulation and inflammation in severe sepsis conditions (Fig. 3c).
S1P in mast cell
Mast cells are tissue-resident cells that are important for both innate and acquired immunity [162]. Mast cells play a critical role especially in allergy and anaphylaxis. Antigen engagement of the high-affinity Fc receptor for IgE (FcεRI) on mast cells results in activation of SphK1 and SphK2 and subsequent production of S1P [163–165]. SphK2 is predominantly responsible for S1P production in mast cells dependent on activation of non-receptor tyrosine kinase, Fyn [166, 167]. Olivera et al. [167] demonstrated that mast cells from Sphk2−/− mice showed decreased S1P production upon FcεRI stimulation and resulted in a decrease in the extent of degranulation and in the production of various cytokines and eicosanoids, which are key mediators of allergic responses.
Intracellularly produced S1P seems to activate S1P1 and S1P2 that are expressed in mast cells in an autocrine and/or paracrine manner during mast cell activation [168]. Genetic deletion of S1P2 or antisense nucleotide/siRNA-mediated knockdown of S1P1 or S1P2 indicated that S1P1 was involved in migration toward antigen, while S1P2 was indispensable for degranulation [168, 169]. Especially, S1P2 was shown to be essential for IgE-triggered anaphylactic responses, including elevation of circulating histamine and associated pulmonary edema in mice [169]. Thus, S1P1/2 and/or SphK1/2 might be beneficial targets for allergy suppression and anaphylaxis attenuation.
S1P in TNF-α signaling
It has been implicated that S1P and SphK1 are involved in the actions of TNF-α, a pleiotropic cytokine crucial for systemic inflammation and a wide range of autoimmune disorders such as rheumatoid arthritis, inflammatory bowel disease, and asthma. TNF-α induces ERK1/2-mediated phosphorylation of SphK1 and subsequent translocation to the plasma membrane to catalyze the production of S1P in 293T cells [12]. It was suggested that TRAF2, an essential signaling intermediate in TNF-α signaling, bound directly to SphK1, which in turn was required for TRAF2-mediated activation of NF-κB[20].
In L929 fibroblasts and A549 lung carcinoma cells, TNF-α-induced up-regulation of COX-2 expression and subsequent prostaglandin E2 (a potent pro-inflammatory eicosanoid) production is dependent on activation of SphK1 and S1P production [21, 22]. Furthermore, TNF-induced transcription of pro-inflammatory cytokines, chemokines, and adhesion molecules (IL-6, RANTES, MCP-1, and VCAM-1) was found to require SphK1 activation [22]. Since S1P stimulation of the cells also induces the COX-2 expression [21], TNF-α-induced effects are likely to be mediated by S1P receptors in an autocrine manner. S1P also activates cytosolic phospholipase A2 via an S1P3-mediated intracellular calcium increase in A549 cells and releases arachidonic acid that serves as a substrate for COX-2 [170]. In macrophages, SphK1 is also required for complement component C5a-triggered intracellular calcium increase, degranulation, cytokine generation (TNF-α, IL-6, and IL-8), and chemotaxis [171], and for TNF-α-triggered prostaglandin E2 production [172].
These in vitro findings are supported by recent in vivo studies using Sphk1−/− mice or siRNA-mediated SphK1 knockdown in the models of dextran sulfate sodium-induced colitis [173], collagen- or TNF-α-induced arthritis [174, 175], and LPS-induced sepsis [176]. Furthermore, increased expression of SphK1 is observed in the colon epithelial and stromal cells from patients with ulcerative colitis [173] and in peritoneal macrophages from patients with severe sepsis [176]. Thus, SphK1-S1P axis regulates both local and systemic pro-inflammatory responses in concert with TNF-α, particularly in epithelial cells and macrophages.
On the other hand, in endothelial cells, S1P prevents TNF-α-mediated monocyte adhesion both in vitro and in vivo [177, 178]. TNF-α induces SphK1 activation and S1P production, which in turn activates S1P1 and S1P3 in an autocrine manner to increase NO production by eNOS [23]. NO is known to attenuate expression of adhesion molecules and leukocyte adhesion [179, 180]. The difference of pro- and anti-inflammatory actions of SphK1-S1P axis might be explained by differences in the cell types and the expression pattern of S1P receptor subtypes, or by mode of S1P actions, that is, receptor-dependent and -independent actions of S1P. Recently, Alvarez et al. [61] showed that S1P produced by SphK1 upon TNF-α stimulation of HEK293 cells specifically bound to TRAF2 and stimulated its E3 ubiquitin ligase activity, which was necessary for lysine-63-linked polyubiquitinylation of receptor interacting protein 1 that then served as a platform for recruitment and stimulation of IκB kinase and subsequent activation of NF-κB. They further showed that these responses were mediated by intracellular S1P independently of cell surface S1P receptors. Although highly intriguing, this model does not take into account why endogenous S1P under homeostatic conditions fail to activate the NF-κB pathway. Perhaps, a localized production of S1P is required for TRAF2 activation. The relevance of intracellular signaling of S1P under physiological conditions remains to be established.
S1P in atherosclerosis
Atherosclerosis is a chronic inflammatory condition that involves complex interactions among lipids, vascular endothelial cells, and immune cells as well as smooth muscle cells. It is well established that the plasma level of oxidized LDL is closely correlated, whereas that of HDL is inversely correlated, with the risk of atherosclerosis and associated cardiovascular diseases [181, 182]. HDL removes excess cholesterols from arterial and non-liver cells, transport them to liver, and excrete them as bile [181, 182]. In addition to this so-called reverse cholesterol transport, HDL exerts a wide range of actions such as inhibition of LDL oxidation and platelet aggregation. Importantly, HDL also exerts beneficial effects on endothelium, including stimulation of proliferation, cell survival, migration, and NO synthesis, and inhibition of adhesion molecule expression [183]. Recent studies have revealed that those anti-atherogenic HDL actions on endothelial cells are partly mediated by S1P that is bound to HDL-associated apoM [43].
In vitro dissection of signaling pathways using siRNA-mediated knockdown of S1P1, S1P3, and scavenger receptor BI (apolipoprotein A-I receptor) in HUVEC by Kimura et al. [184–187] has revealed that HDL-bound S1P mediates survival, migration, and NO production as well as inhibition of TNF-α-induced adhesion molecule expression via S1P1 and S1P3 in concert with the scavenger receptor-BI-mediated signaling. Furthermore, HDL-induced endothelial barrier enhancement is mediated by S1P1 through Akt activation in HUVEC [188], and HDL-induced vasorelaxation is mediated by S1P3 through NO production in mouse artery [83].
Since S1P1-deficient mice are embryonic lethal, S1P1 involvement has not been explored in the murine model of atherosclerosis using apolipoprotein E (apoE)-deficient mice or LDL receptor (LDLR)-deficient mice fed a cholesterol-rich diet. Recently, roles of S1P2 and S1P3 in these disease models were reported. Apoe−/−S1p2−/− mice show markedly attenuated development of the atherosclerotic plaques compared with Apoe−/− mice, with reduced accumulation of macrophages, lipid, and collagen and reduced expression of pro-inflammatory cytokines and adhesion molecules [76, 77]. Bone marrow transplant experiments indicate that S1P2 functions in the hematopoietic compartment are critical for the atherogenesis [76, 77]. In fact, macrophages from Apoe−/−S1p2−/− mice show reduced cytokine expression and uptake of LDL and enhanced cholesterol efflux, associated with decreased expression of scavenger receptor and increased expression of cholesterol efflux transporter, probably due to diminished Rho/Rho kinase/NF-κB activity [76]. Furthermore, LPS-induced inflammatory cytokine productions (IL-1β and IL-18) are significantly reduced in the serum of S1p2−/− mice [77]. Thus, S1P2 regulates macrophage functions and promotes vascular inflammation and atherosclerosis.
Although Apoe−/−S1p3−/− mice do not show a significant difference in the atherosclerotic lesion sizes after 25 or 45 weeks of normal chow diet, monocyte/macrophage content in the lesions is markedly reduced, whereas smooth muscle cell content is increased compared with Apoe−/− mice [88]. Bone marrow transplant experiment indicates that S1P3 in both hematopoietic and nonhematopoietic cells contributes to monocyte/macrophage accumulation in atherosclerotic lesions. Elicited peritoneal macrophages during thioglycollate-induced peritonitis are reduced in S1P3-deficient mice and express lower levels of TNF-α and monocyte chemoattractant protein-1 [88]. These results suggest that S1P3 may play a causal role in atherosclerosis by altering inflammatory monocyte/macrophage recruitment and smooth muscle cell behavior.
Administration of S1P analogue FTY720 has been shown to dramatically attenuate the development of atherosclerosis in both Apoe−/− [189] and Ldlr−/− mice [190]. FTY720 administration reduces blood lymphocyte count and significantly interferes with lymphocyte function as evidenced by reduced splenocyte proliferation and interferon-γ levels in plasma. Plasma concentrations of pro-inflammatory cytokines such as TNF-α and IL-6 are also reduced by FTY720. Moreover, FTY720 modulates macrophage activation, favoring an anti-inflammatory M2-type macrophage activation profile [190]. In isolated aortic segments and cultured vascular smooth muscle cell, FTY720 potently inhibits thrombin-induced release of monocyte chemoattractant protein-1 via S1P3 [189]. Taken together, FTY720 inhibits atherosclerosis by suppressing lymphocyte functions (as discussed above) as well as modulating macrophage and smooth muscle functions.
Concluding remarks
Multiple receptor system enables S1P to have pleiotropic effects, where each receptor has a different but partially overlapping intracellular signaling pathway and works cooperatively or competitively each other. Depending on the expression pattern of S1P subtypes in a given cell type, S1P has both pro- and anti-inflammatory effects. Especially, regulation of vascular barrier integrity is one of the highlights of S1P functions in both homeostatic and inflammatory conditions, in which S1P1 basically protects the vasculature from leakage with counteracting actions of S1P2/3. In addition, S1P signaling regulates the trafficking of various types of immune cells, including lymphocytes, dendritic cells, mast cells, monocyte/macrophages, and neutrophils [191], and affects functions of these cells such as degranulation and production of pro-inflammatory mediators, again depending on the receptor subtypes. Thus, therapeutic intervention targeting S1P signaling has beneficial effects as well as adverse side effects. It would be of great use to further clarify the regulation and contribution of each S1P subtypes in a specific pathological context and develop a way for tissue- and receptor subtype-specific interventions of S1P signaling.
Acknowledgments
This work was supported by NIH grants HL-67330 and HL-89934.
References
- 1.Westerlund B, Slotte JP. How the molecular features of glycosphingolipids affect domain formation in fluid membranes. Biochim Biophys Acta. 2009;1788:194–201. doi: 10.1016/j.bbamem.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 3.Venable ME, Lee JY, Smyth MJ, Bielawska A, Obeid LM. Role of ceramide in cellular senescence. J Biol Chem. 1995;270:30701–30708. doi: 10.1074/jbc.270.51.30701. [DOI] [PubMed] [Google Scholar]
- 4.Hla T, Venkataraman K, Michaud J. The vascular S1P gradient-cellular sources and biological significance. Biochim Biophys Acta. 2008;1781:477–482. doi: 10.1016/j.bbalip.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venkataraman K, Lee Y, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi R, Asazuma N, Satoh K, Ozaki Y, Kume S. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem (Tokyo) 1997;121:969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- 7.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 8.Hänel P, Andréani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 9.Spiegel S, Milstien S. Functions of the multifaceted family of sphingosine kinases and some close relatives. J Biol Chem. 2007;282:2125–2129. doi: 10.1074/jbc.R600028200. [DOI] [PubMed] [Google Scholar]
- 10.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson KR, Becker KP, Facchinetti MM, Hannun YA, Obeid LM. PKC-dependent activation of sphingosine kinase 1 and translocation to the plasma membrane. Extracellular release of sphingosine-1-phosphate induced by phorbol 12-myristate 13-acetate (PMA) J Biol Chem. 2002;277:35257–35262. doi: 10.1074/jbc.M203033200. [DOI] [PubMed] [Google Scholar]
- 12.Pitson SM, Moretti PAB, Zebol JR, Lynn HE, Xia P, Vadas MA, Wattenberg BW. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22:5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitson SM, Xia P, Leclercq TM, Moretti PAB, Zebol JR, Lynn HE, Wattenberg BW, Vadas MA. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J Exp Med. 2005;201:49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olivera A, Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- 15.Shu X, Wu W, Mosteller RD, Broek D. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol Cell Biol. 2002;22:7758–7768. doi: 10.1128/MCB.22.22.7758-7768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edsall LC, Pirianov GG, Spiegel S. Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation. J Neurosci. 1997;17:6952–6960. doi: 10.1523/JNEUROSCI.17-18-06952.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarkar S, Maceyka M, Hait NC, Paugh SW, Sankala H, Milstien S, Spiegel S. Sphingosine kinase 1 is required for migration, proliferation and survival of MCF-7 human breast cancer cells. FEBS Lett. 2005;579:5313–5317. doi: 10.1016/j.febslet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 18.Ma MM, Chen JL, Wang GG, Wang H, Lu Y, Li JF, Yi J, Yuan YJ, Zhang QW, Mi J, Wang LS, Duan HF, Wu CT. Sphingosine kinase 1 participates in insulin signalling and regulates glucose metabolism and homeostasis in KK/Ay diabetic mice. Diabetologia. 2007;50:891–900. doi: 10.1007/s00125-006-0589-5. [DOI] [PubMed] [Google Scholar]
- 19.El-Shewy HM, Johnson KR, Lee M, Jaffa AA, Obeid LM, Luttrell LM. Insulin-like growth factors mediate heterotrimeric G protein-dependent ERK1/2 activation by transactivating sphingosine 1-phosphate receptors. J Biol Chem. 2006;281:31399–31407. doi: 10.1074/jbc.M605339200. [DOI] [PubMed] [Google Scholar]
- 20.Xia P, Wang L, Moretti PAB, Albanese N, Chai F, Pitson SM, D’Andrea RJ, Gamble JR, Vadas MA. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-alpha signaling. J Biol Chem. 2002;277:7996–8003. doi: 10.1074/jbc.M111423200. [DOI] [PubMed] [Google Scholar]
- 21.Pettus BJ, Bielawski J, Porcelli AM, Reames DL, Johnson KR, Morrow J, Chalfant CE, Obeid LM, Hannun YA. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-α. FASEB J. 2003;17:1411–1421. doi: 10.1096/fj.02-1038com. [DOI] [PubMed] [Google Scholar]
- 22.Billich A, Bornancin F, Mechtcheriakova D, Natt F, Huesken D, Baumruker T. Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: function in cell survival and IL-1β and TNF-α induced production of inflammatory mediators. Cell Signal. 2005;17:1203–1217. doi: 10.1016/j.cellsig.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 23.De Palma C, Meacci E, Perrotta C, Bruni P, Clementi E. Endothelial nitric oxide synthase activation by tumor necrosis factor alpha through neutral sphingomyelinase 2, sphingosine kinase 1, and sphingosine 1 phosphate receptors: a novel pathway relevant to the pathophysiology of endothelium. Arterioscler Thromb Vasc Biol. 2006;26:99–105. doi: 10.1161/01.ATV.0000194074.59584.42. [DOI] [PubMed] [Google Scholar]
- 24.Pyne S, Long JS, Ktistakis NT, Pyne NJ. Lipid phosphate phosphatases and lipid phosphate signalling. Biochem Soc Trans. 2005;33:1370–1374. doi: 10.1042/BST0331370. [DOI] [PubMed] [Google Scholar]
- 25.Alderton F, Darroch P, Sambi B, McKie A, Ahmed IS, Pyne N, Pyne S. G-protein-coupled receptor stimulation of the p42/p44 mitogen-activated protein kinase pathway is attenuated by lipid phosphate phosphatases 1, 1a, and 2 in human embryonic kidney 293 cells. J Biol Chem. 2001;276:13452–13460. doi: 10.1074/jbc.M006582200. [DOI] [PubMed] [Google Scholar]
- 26.Long J, Darroch P, Wan KF, Kong KC, Ktistakis N, Pyne NJ, Pyne S. Regulation of cell survival by lipid phosphate phosphatases involves the modulation of intracellular phosphatidic acid and sphingosine 1-phosphate pools. Biochem J. 2005;391:25–32. doi: 10.1042/BJ20050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Stunff H, Galve-Roperh I, Peterson C, Milstien S, Spiegel S. Sphingosine-1-phosphate phosphohydrolase in regulation of sphingolipid metabolism and apoptosis. J Cell Biol. 2002;158:1039–1049. doi: 10.1083/jcb.200203123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikeda M, Kihara A, Igarashi Y. Sphingosine-1-phosphate lyase SPL is an endoplasmic reticulum-resident, integral membrane protein with the pyridoxal 5′-phosphate binding domain exposed to the cytosol. Biochem Biophys Res Commun. 2004;325:338–343. doi: 10.1016/j.bbrc.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 29.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 30.Marathe S, Schissel SL, Yellin MJ, Beatini N, Mintzer R, Williams KJ, Tabas I. Human vascular endothelial cells are a rich and regulatable source of secretory sphingomyelinase. Implications for early atherogenesis and ceramide-mediated cell signaling. J Biol Chem. 1998;273:4081–4088. doi: 10.1074/jbc.273.7.4081. [DOI] [PubMed] [Google Scholar]
- 31.Romiti E, Meacci E, Tani M, Nuti F, Farnararo M, Ito M, Bruni P. Neutral/alkaline and acid ceramidase activities are actively released by murine endothelial cells. Biochem Biophys Res Commun. 2000;275:746–751. doi: 10.1006/bbrc.2000.3370. [DOI] [PubMed] [Google Scholar]
- 32.Ancellin N, Colmont C, Su J, Li Q, Mittereder N, Chae S, Stefansson S, Liau G, Hla T. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem. 2002;277:6667–6675. doi: 10.1074/jbc.M102841200. [DOI] [PubMed] [Google Scholar]
- 33.Venkataraman K, Thangada S, Michaud J, Oo ML, Ai Y, Lee YM, Wu M, Parikh NS, Khan F, Proia RL, Hla T. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem J. 2006;397:461–471. doi: 10.1042/BJ20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci USA. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi N, Nishi T, Hirata T, Kihara A, Sano T, Igarashi Y, Yamaguchi A. Sphingosine 1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. J Lipid Res. 2006;47:614–621. doi: 10.1194/jlr.M500468-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Sato K, Malchinkhuu E, Horiuchi Y, Mogi C, Tomura H, Tosaka M, Yoshimoto Y, Kuwabara A, Okajima F. Critical role of ABCA1 transporter in sphingosine 1-phosphate release from astrocytes. J Neurochem. 2007;103:2610–2619. doi: 10.1111/j.1471-4159.2007.04958.x. [DOI] [PubMed] [Google Scholar]
- 37.Nieuwenhuis B, Lüth A, Chun J, Huwiler A, Pfeilschifter J, Schäfer-Korting M, Kleuser B. Involvement of the ABC-transporter ABCC1 and the sphingosine 1-phosphate receptor subtype S1P3 in the cytoprotection of human fibroblasts by the glucocorticoid dexamethasone. J Mol Med. 2009;87:645–657. doi: 10.1007/s00109-009-0468-x. [DOI] [PubMed] [Google Scholar]
- 38.Takabe K, Kim RH, Allegood JC, Mitra P, Ramachandran S, Nagahashi M, Harikumar KB, Hait NC, Milstien S, Spiegel S. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J Biol Chem. 2010;285:10477–10486. doi: 10.1074/jbc.M109.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee Y, Venkataraman K, Hwang S, Han D, Hla T. A novel method to quantify sphingosine 1-phosphate by immobilized metal affinity chromatography (IMAC) Prostaglandins Other Lipid Mediat. 2007;84:154–162. doi: 10.1016/j.prostaglandins.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–527. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- 41.Hisano Y, Kobayashi N, Kawahara A, Yamaguchi A, Nishi T. The sphingosine 1-phosphate transporter, SPNS2, functions as a transporter of the phosphorylated form of the immunomodulating agent FTY720. J Biol Chem. 2011;286:1758–1766. doi: 10.1074/jbc.M110.171116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murata N, Sato K, Kon J, Tomura H, Yanagita M, Kuwabara A, Ui M, Okajima F. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem J. 2000;352:809–815. [PMC free article] [PubMed] [Google Scholar]
- 43.Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnström J, Sevvana M, Egerer-Sieber C, Muller YA, Hla T, Nielsen LB, Dahlbäck B. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc Natl Acad Sci USA. 2011;108:9613–9618. doi: 10.1073/pnas.1103187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu N, Dahlbäck B. A novel human apolipoprotein (apoM) J Biol Chem. 1999;274:31286–31290. doi: 10.1074/jbc.274.44.31286. [DOI] [PubMed] [Google Scholar]
- 45.Christoffersen C, Ahnström J, Axler O, Christensen EI, Dahlbäck B, Nielsen LB. The signal peptide anchors apolipoprotein M in plasma lipoproteins and prevents rapid clearance of apolipoprotein M from plasma. J Biol Chem. 2008;283:18765–18772. doi: 10.1074/jbc.M800695200. [DOI] [PubMed] [Google Scholar]
- 46.Axler O, Ahnström J, Dahlbäck B. Apolipoprotein M associates to lipoproteins through its retained signal peptide. FEBS Lett. 2008;582:826–828. doi: 10.1016/j.febslet.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Sevvana M, Ahnström J, Egerer-Sieber C, Lange HA, Dahlbäck B, Muller YA. Serendipitous fatty acid binding reveals the structural determinants for ligand recognition in apolipoprotein M. J Mol Biol. 2009;393:920–936. doi: 10.1016/j.jmb.2009.08.071. [DOI] [PubMed] [Google Scholar]
- 48.Christoffersen C, Nielsen LB, Axler O, Andersson A, Johnsen AH, Dahlbäck B. Isolation and characterization of human apolipoprotein M-containing lipoproteins. J Lipid Res. 2006;47:1833–1843. doi: 10.1194/jlr.M600055-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.Lee M, Van Brocklyn J, Thangada S, Liu C, Hand A, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG 1. Science. 1998;279:1552. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 50.Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, Pyne S, Tigyi G. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- 51.Gräler MH, Bernhardt G, Lipp M. EDG6, a novel G-protein-coupled receptor related to receptors for bioactive lysophospholipids, is specifically expressed in lymphoid tissue. Genomics. 1998;53:164–169. doi: 10.1006/geno.1998.5491. [DOI] [PubMed] [Google Scholar]
- 52.Graeler M, Goetzl EJ. Activation-regulated expression and chemotactic function of sphingosine 1-phosphate receptors in mouse splenic T cells. FASEB J. 2002;16:1874–1878. doi: 10.1096/fj.02-0548com. [DOI] [PubMed] [Google Scholar]
- 53.Jolly PS, Rosenfeldt HM, Milstien S, Spiegel S. The roles of sphingosine-1-phosphate in asthma. Mol Immunol. 2002;38:1239–1245. doi: 10.1016/s0161-5890(02)00070-6. [DOI] [PubMed] [Google Scholar]
- 54.Im DS, Heise CE, Ancellin N, O’Dowd BF, Shei GJ, Heavens RP, Rigby MR, Hla T, Mandala S, McAllister G, George SR, Lynch KR. Characterization of a novel sphingosine 1-phosphate receptor, Edg 8. J Biol Chem. 2000;275:14281–14286. doi: 10.1074/jbc.275.19.14281. [DOI] [PubMed] [Google Scholar]
- 55.Terai K, Soga T, Takahashi M, Kamohara M, Ohno K, Yatsugi S, Okada M, Yamaguchi T. Edg-8 receptors are preferentially expressed in oligodendrocyte lineage cells of the rat CNS. Neuroscience. 2003;116:1053–1062. doi: 10.1016/s0306-4522(02)00791-1. [DOI] [PubMed] [Google Scholar]
- 56.Lee MJ, Thangada S, Paik JH, Sapkota GP, Ancellin N, Chae SS, Wu M, Morales-Ruiz M, Sessa WC, Alessi DR, Hla T. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol Cell. 2001;8:693–704. doi: 10.1016/s1097-2765(01)00324-0. [DOI] [PubMed] [Google Scholar]
- 57.Ryu Y, Takuwa N, Sugimoto N, Sakurada S, Usui S, Okamoto H, Matsui O, Takuwa Y. Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circ Res. 2002;90:325–332. doi: 10.1161/hh0302.104455. [DOI] [PubMed] [Google Scholar]
- 58.Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol Cell Biol. 2003;23:1534–1545. doi: 10.1128/MCB.23.5.1534-1545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanchez T, Thangada S, Wu M, Kontos CD, Wu D, Wu H, Hla T. PTEN as an effector in the signaling of antimigratory G protein-coupled receptor. Proc Natl Acad Sci USA. 2005;102:4312–4317. doi: 10.1073/pnas.0409784102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T, Milstien S, Spiegel S. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Allende ML, Yamashita T, Proia RL. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood. 2003;102:3665–3667. doi: 10.1182/blood-2003-02-0460. [DOI] [PubMed] [Google Scholar]
- 64.Paik J, Skoura A, Chae S, Cowan AE, Han DK, Proia RL, Hla T. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev. 2004;18:2392–2403. doi: 10.1101/gad.1227804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, Pham TH, Wong JS, Pappu R, Coughlin SR. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest. 2009;119:1871–1879. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ishii I, Friedman B, Ye X, Kawamura S, McGiffert C, Contos JJ, Kingsbury MA, Zhang G, Brown JH, Chun J. Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its G protein-coupled receptor, LPB3/EDG 3. J Biol Chem. 2001;276:33697–33704. doi: 10.1074/jbc.M104441200. [DOI] [PubMed] [Google Scholar]
- 67.Ishii I, Ye X, Friedman B, Kawamura S, Contos JJ, Kingsbury MA, Yang AH, Zhang G, Brown JH, Chun J. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P2/LPB2/EDG-5 and S1P3/LPB3/EDG 3. J Biol Chem. 2002;277:25152–25159. doi: 10.1074/jbc.M200137200. [DOI] [PubMed] [Google Scholar]
- 68.Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu Y, Yamashita T, Proia RL. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem. 2004;279:29367–29373. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- 69.MacLennan AJ, Carney PR, Zhu WJ, Chaves AH, Garcia J, Grimes JR, Anderson KJ, Roper SN, Lee N. An essential role for the H218/AGR16/Edg-5/LPB2 sphingosine 1-phosphate receptor in neuronal excitability. Eur J Neurosci. 2001;14:203–209. doi: 10.1046/j.0953-816x.2001.01634.x. [DOI] [PubMed] [Google Scholar]
- 70.Herr DR, Grillet N, Schwander M, Rivera R, Müller U, Chun J. Sphingosine 1-phosphate (S1P) signaling is required for maintenance of hair cells mainly via activation of S1P2. J Neurosci. 2007;27:1474–1478. doi: 10.1523/JNEUROSCI.4245-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kono M, Belyantseva IA, Skoura A, Frolenkov GI, Starost MF, Dreier JL, Lidington D, Bolz SS, Friedman TB, Hla T, Proia RL. Deafness and stria vascularis defects in S1P2 receptor-null mice. J Biol Chem. 2007;282:10690–10696. doi: 10.1074/jbc.M700370200. [DOI] [PubMed] [Google Scholar]
- 72.Ishii M, Kikuta J, Shimazu Y, Meier-Schellersheim M, Germain RN. Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J Exp Med. 2010;207:2793–2798. doi: 10.1084/jem.20101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Michaud J, Im D, Hla T. Inhibitory role of sphingosine 1-phosphate receptor 2 in macrophage recruitment during inflammation. J Immunol. 2010;184:1475–1483. doi: 10.4049/jimmunol.0901586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest. 2007;117:2506–2516. doi: 10.1172/JCI31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Du W, Takuwa N, Yoshioka K, Okamoto Y, Gonda K, Sugihara K, Fukamizu A, Asano M, Takuwa Y. S1P2, the G protein-coupled receptor for sphingosine-1-phosphate, negatively regulates tumor angiogenesis and tumor growth in vivo in mice. Cancer Res. 2010;70:772–781. doi: 10.1158/0008-5472.CAN-09-2722. [DOI] [PubMed] [Google Scholar]
- 76.Wang F, Okamoto Y, Inoki I, Yoshioka K, Du W, Qi X, Takuwa N, Gonda K, Yamamoto Y, Ohkawa R, Nishiuchi T, Sugimoto N, Yatomi Y, Mitsumori K, Asano M, Kinoshita M, Takuwa Y. Sphingosine-1-phosphate receptor-2 deficiency leads to inhibition of macrophage proinflammatory activities and atherosclerosis in apoE-deficient mice. J Clin Invest. 2010;120:3979–3995. doi: 10.1172/JCI42315. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Skoura A, Michaud J, Im D, Thangada S, Xiong Y, Smith JD, Hla T. Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:81–85. doi: 10.1161/ATVBAHA.110.213496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, Rosen H. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- 79.Forrest M, Sun SY, Hajdu R, Bergstrom J, Card D, Doherty G, Hale J, Keohane C, Meyers C, Milligan J, Mills S, Nomura N, Rosen H, Rosenbach M, Shei GJ, Singer II, Tian M, West S, White V, Xie J, Proia RL, Mandala S. Immune cell regulation and cardiovascular effects of sphingosine 1-phosphate receptor agonists in rodents are mediated via distinct receptor subtypes. J Pharmacol Exp Ther. 2004;309:758–768. doi: 10.1124/jpet.103.062828. [DOI] [PubMed] [Google Scholar]
- 80.Levkau B, Hermann S, Theilmeier G, van der Giet M, Chun J, Schober O, Schäfers M. High-density lipoprotein stimulates myocardial perfusion in vivo. Circulation. 2004;110:3355–3359. doi: 10.1161/01.CIR.0000147827.43912.AE. [DOI] [PubMed] [Google Scholar]
- 81.Theilmeier G, Schmidt C, Herrmann J, Keul P, Schäfers M, Herrgott I, Mersmann J, Larmann J, Hermann S, Stypmann J, Schober O, Hildebrand R, Schulz R, Heusch G, Haude M, von Wnuck Lipinski K, Herzog C, Schmitz M, Erbel R, Chun J, Levkau B. Sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006;114:1403–1409. doi: 10.1161/CIRCULATIONAHA.105.607135. [DOI] [PubMed] [Google Scholar]
- 82.Means CK, Xiao C, Li Z, Zhang T, Omens JH, Ishii I, Chun J, Brown JH. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H2944–H2951. doi: 10.1152/ajpheart.01331.2006. [DOI] [PubMed] [Google Scholar]
- 83.Nofer J, Giet MVD, Tölle M, Wolinska I, von Wnuck Lipinski K, Baba HA, Tietge UJ, Gödecke A, Ishii I, Kleuser B, Schäfers M, Fobker M, Zidek W, Assmann G, Chun J, Levkau B. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest. 2004;113:569–581. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takuwa N, Ohkura S, Takashima S, Ohtani K, Okamoto Y, Tanaka T, Hirano K, Usui S, Wang F, Du W, Yoshioka K, Banno Y, Sasaki M, Ichi I, Okamura M, Sugimoto N, Mizugishi K, Nakanuma Y, Ishii I, Takamura M, Kaneko S, Kojo S, Satouchi K, Mitumori K, Chun J, Takuwa Y. S1P3-mediated cardiac fibrosis in sphingosine kinase 1 transgenic mice involves reactive oxygen species. Cardiovasc Res. 2010;85:484–493. doi: 10.1093/cvr/cvp312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Girkontaite I, Sakk V, Wagner M, Borggrefe T, Tedford K, Chun J, Fischer K. The sphingosine-1-phosphate (S1P) lysophospholipid receptor S1P3 regulates MAdCAM-1+ endothelial cells in splenic marginal sinus organization. J Exp Med. 2004;200:1491–1501. doi: 10.1084/jem.20041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walter DH, Rochwalsky U, Reinhold J, Seeger F, Aicher A, Urbich C, Spyridopoulos I, Chun J, Brinkmann V, Keul P, Levkau B, Zeiher AM, Dimmeler S, Haendeler J. Sphingosine-1-phosphate stimulates the functional capacity of progenitor cells by activation of the CXCR4-dependent signaling pathway via the S1P3 receptor. Arterioscler Thromb Vasc Biol. 2007;27:275–282. doi: 10.1161/01.ATV.0000254669.12675.70. [DOI] [PubMed] [Google Scholar]
- 87.Niessen F, Schaffner F, Furlan-Freguia C, Pawlinski R, Bhattacharjee G, Chun J, Derian CK, Andrade-Gordon P, Rosen H, Ruf W. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature. 2008;452:654–658. doi: 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- 88.Keul P, Lucke S, von Wnuck Lipinski K, Bode C, Gräler M, Heusch G, Levkau B. Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ Res. 2011;108:314–323. doi: 10.1161/CIRCRESAHA.110.235028. [DOI] [PubMed] [Google Scholar]
- 89.Wang W, Graeler MH, Goetzl EJ. Type 4 sphingosine 1-phosphate G protein-coupled receptor (S1P4) transduces S1P effects on T cell proliferation and cytokine secretion without signaling migration. FASEB J. 2005;19:1731–1733. doi: 10.1096/fj.05-3730fje. [DOI] [PubMed] [Google Scholar]
- 90.Golfier S, Kondo S, Schulze T, Takeuchi T, Vassileva G, Achtman AH, Graler MH, Abbondanzo SJ, Wiekowski M, Kremmer E, Endo Y, Lira SA, Bacon KB, Lipp M. Shaping of terminal megakaryocyte differentiation and proplatelet development by sphingosine-1-phosphate receptor S1P4. FASEB J. 2010;24:4701–4710. doi: 10.1096/fj.09-141473. [DOI] [PubMed] [Google Scholar]
- 91.Jaillard C, Harrison S, Stankoff B, Aigrot MS, Calver AR, Duddy G, Walsh FS, Pangalos MN, Arimura N, Kaibuchi K, Zalc B, Lubetzki C. Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. J Neurosci. 2005;25:1459–1469. doi: 10.1523/JNEUROSCI.4645-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Walzer T, Chiossone L, Chaix J, Calver A, Carozzo C, Garrigue-Antar L, Jacques Y, Baratin M, Tomasello E, Vivier E. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat Immunol. 2007;8:1337–1344. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- 93.Jenne CN, Enders A, Rivera R, Watson SR, Bankovich AJ, Pereira JP, Xu Y, Roots CM, Beilke JN, Banerjee A, Reiner SL, Miller SA, Weinmann AS, Goodnow CC, Lanier LL, Cyster JG, Chun J. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med. 2009;206:2469–2481. doi: 10.1084/jem.20090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, Vera AD, Jin J, Stites T, Wu S, Aradhye S, Kappos L. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 95.Kappos L, Radue EW, O’connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P. A placebo-controlled trial of oral Fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 96.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 97.Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 98.Adachi K, Kohara T, Nakao N, Arita M, Chiba K, Mishina T, Sasaki S, Fujita T. Design, synthesis, and structure-activity relationships of 2-substituted-2-amino-1,3-propanediols: discovery of a novel immunosuppressant, FTY720. Bioorg Med Chem Lett. 1995;5:853–856. [Google Scholar]
- 99.Fujita T, Inoue K, Yamamoto S, Ikumoto T, Sasaki S, Toyama R, Chiba K, Hoshino Y, Okumoto T. Fungal metabolites. Part 11. A potent immunosuppressive activity found in Isaria sinclairii metabolite. J Antibiot. 1994;47:208–215. doi: 10.7164/antibiotics.47.208. [DOI] [PubMed] [Google Scholar]
- 100.Chiba K, Yanagawa Y, Masubuchi Y, Kataoka H, Kawaguchi T, Ohtsuki M, Hoshino Y. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J Immunol. 1998;160:5037–5044. [PubMed] [Google Scholar]
- 101.Yanagawa Y, Sugahara K, Kataoka H, Kawaguchi T, Masubuchi Y, Chiba K. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. II. FTY720 prolongs skin allograft survival by decreasing T cell infiltration into grafts but not cytokine production in vivo. J Immunol. 1998;160:5493–5499. [PubMed] [Google Scholar]
- 102.Sanchez T, Estrada-Hernandez T, Paik J, Wu MT, Venkataraman K, Brinkmann V, Claffey K, Hla T. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J Biol Chem. 2003;278:47281–47290. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- 103.Kharel Y, Lee S, Snyder AH, Sheasley-O’neill SL, Morris MA, Setiady Y, Zhu R, Zigler MA, Burcin TL, Ley K, Tung KS, Engelhard VH, Macdonald TL, Pearson-White S, Lynch KR. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J Biol Chem. 2005;280:36865–36872. doi: 10.1074/jbc.M506293200. [DOI] [PubMed] [Google Scholar]
- 104.Zemann B, Kinzel B, Müller M, Reuschel R, Mechtcheriakova D, Urtz N, Bornancin F, Baumruker T, Billich A. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood. 2006;107:1454–1458. doi: 10.1182/blood-2005-07-2628. [DOI] [PubMed] [Google Scholar]
- 105.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 106.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 107.Liu CH, Thangada S, Lee MJ, Van Brocklyn JR, Spiegel S, Hla T. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG 1. Mol Biol Cell. 1999;10:1179–1190. doi: 10.1091/mbc.10.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oo ML, Thangada S, Wu M, Liu CH, Macdonald TL, Lynch KR, Lin C, Hla T. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitiny-lation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 109.Oo ML, Chang S, Thangada S, Wu MT, Rezaul K, Blaho V, Hwang S, Han DK, Hla T. Engagement of S1P1-degradative mechanisms leads to vascular leak in mice. J Clin Invest. 2011;121:2290–2300. doi: 10.1172/JCI45403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thangada S, Khanna KM, Blaho VA, Oo ML, Im D, Guo C, Lefrancois L, Hla T. Cell-surface residence of sphingosine 1-phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J Exp Med. 2010;207:1475–1483. doi: 10.1084/jem.20091343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 112.Czeloth N, Bernhardt G, Hofmann F, Genth H, Förster R. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J Immunol. 2005;175:2960–2967. doi: 10.4049/jimmunol.175.5.2960. [DOI] [PubMed] [Google Scholar]
- 113.Massberg S, Schaerli P, Knezevic-Maramica I, Köllnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, von Andrian UH. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 115.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nixon GF. Sphingolipids in inflammation: pathological implications and potential therapeutic targets. Br J Pharmacol. 2009;158:982–993. doi: 10.1111/j.1476-5381.2009.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Okajima F, Sato K, Kimura T. Anti-atherogenic actions of high-density lipoprotein through sphingosine 1-phosphate receptors and scavenger receptor class B type I. Endocr J. 2009;56:317–334. doi: 10.1507/endocrj.k08e-228. [DOI] [PubMed] [Google Scholar]
- 118.Ruf W, Furlan-Freguia C, Niessen F. Vascular and dendritic cell coagulation signaling in sepsis progression. J Thromb Haemost. 2009;7(Suppl 1):118–121. doi: 10.1111/j.1538-7836.2009.03374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lucke S, Levkau B. Endothelial functions of sphingosine-1-phosphate. Cell Physiol Biochem. 2010;26:87–96. doi: 10.1159/000315109. [DOI] [PubMed] [Google Scholar]
- 120.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 121.Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yatomi Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86:193–202. [PubMed] [Google Scholar]
- 123.Yatomi Y, Yamamura S, Ruan F, Igarashi Y. Sphingosine 1-phosphate induces platelet activation through an extracellular action and shares a platelet surface receptor with lysophosphatidic acid. J Biol Chem. 1997;272:5291–5297. doi: 10.1074/jbc.272.8.5291. [DOI] [PubMed] [Google Scholar]
- 124.Caligan TB, Peters K, Ou J, Wang E, Saba J, Merrill AH. A high-performance liquid chromatographic method to measure sphingosine 1-phosphate and related compounds from sphingosine kinase assays and other biological samples. Anal Biochem. 2000;281:36–44. doi: 10.1006/abio.2000.4555. [DOI] [PubMed] [Google Scholar]
- 125.Ruwisch L, Schäfer-Korting M, Kleuser B. An improved high-performance liquid chromatographic method for the determination of sphingosine-1-phosphate in complex biological materials. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:358–363. doi: 10.1007/s002100000365. [DOI] [PubMed] [Google Scholar]
- 126.Yatomi Y, Ruan F, Ohta J, Welch RJ, Hakomori S, Igarashi Y. Quantitative measurement of sphingosine 1-phosphate in biological samples by acylation with radioactive acetic anhydride. Anal Biochem. 1995;230:315–320. doi: 10.1006/abio.1995.1480. [DOI] [PubMed] [Google Scholar]
- 127.Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R, Coughlin SR, McDonald DM, Schwab SR, Cyster JG. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med. 2010;207:17–27. doi: 10.1084/jem.20091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha’afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 129.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Singleton PA, Dudek SM, Ma S, Garcia JG. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family. J Biol Chem. 2006;281:34381–34393. doi: 10.1074/jbc.M603680200. [DOI] [PubMed] [Google Scholar]
- 131.Singleton PA, Moreno-Vinasco L, Sammani S, Wanderling SL, Moss J, Garcia JG. Attenuation of vascular permeability by methylnaltrexone: role of mOP-R and S1P3 transactivation. Am J Respir Cell Mol Biol. 2007;37:222–231. doi: 10.1165/rcmb.2006-0327OC. [DOI] [PubMed] [Google Scholar]
- 132.Spindler V, Schlegel N, Waschke J. Role of GTPases in control of microvascular permeability. Cardiovasc Res. 2010;87:243–253. doi: 10.1093/cvr/cvq086. [DOI] [PubMed] [Google Scholar]
- 133.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol. 2007;27:1312–1318. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- 134.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 135.Sammani S, Moreno-Vinasco L, Mirzapoiazova T, Singleton PA, Chiang ET, Evenoski CL, Wang T, Mathew B, Husain A, Moitra J, Sun X, Nunez L, Jacobson JR, Dudek SM, Natarajan V, Garcia JG. Differential effects of sphingosine 1-phosphate receptors on airway and vascular barrier function in the murine lung. Am J Respir Cell Mol Biol. 2010;43:394–402. doi: 10.1165/rcmb.2009-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, Cahalan MD, Wong CH, Rosen H. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol. 2006;2:434–441. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- 137.Tauseef M, Kini V, Knezevic N, Brannan M, Ramchandaran R, Fyrst H, Saba J, Vogel SM, Malik AB, Mehta D. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ Res. 2008;103:1164–1172. doi: 10.1161/01.RES.0000338501.84810.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhao YD, Ohkawara H, Rehman J, Wary KK, Vogel SM, Minshall RD, Zhao YY, Malik AB. Bone marrow progenitor cells induce endothelial adherens junction integrity by sphingosine-1-phosphate-mediated Rac1 and Cdc42 signaling. Circ Res. 2009;105:696–704. doi: 10.1161/CIRCRESAHA.109.199778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang F, Van Brocklyn JR, Hobson JP, Movafagh S, Zukowska-Grojec Z, Milstien S, Spiegel S. Sphingosine 1-phosphate stimulates cell migration through a Gi-coupled cell surface receptor. Potential involvement in angiogenesis. J Biol Chem. 1999;274:35343–35350. doi: 10.1074/jbc.274.50.35343. [DOI] [PubMed] [Google Scholar]
- 140.Kimura T, Watanabe T, Sato K, Kon J, Tomura H, Tamama K, Kuwabara A, Kanda T, Kobayashi I, Ohta H, Ui M, Okajima F. Sphingosine 1-phosphate stimulates proliferation and migration of human endothelial cells possibly through the lipid receptors, Edg-1 and Edg 3. Biochem J. 2000;348(Pt 1):71–76. [PMC free article] [PubMed] [Google Scholar]
- 141.Morales-Ruiz M, Lee MJ, Zöllner S, Gratton JP, Scotland R, Shiojima I, Walsh K, Hla T, Sessa WC. Sphingosine 1-phosphate activates Akt, nitric oxide production, and chemotaxis through a Gi protein/phosphoinositide 3-kinase pathway in endothelial cells. J Biol Chem. 2001;276:19672–19677. doi: 10.1074/jbc.M009993200. [DOI] [PubMed] [Google Scholar]
- 142.Paik JH, Chae SS, Lee MJ, Thangada S, Hla T. Sphingosine 1-phosphate-induced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of αvβ3- and β1-containing integrins. J Biol Chem. 2001;276:11830–11837. doi: 10.1074/jbc.M009422200. [DOI] [PubMed] [Google Scholar]
- 143.Liu F, Verin AD, Wang P, Day R, Wersto RP, Chrest FJ, English DK, Garcia JG. Differential regulation of sphingosine-1-phosphate- and VEGF-induced endothelial cell chemotaxis. Involvement of Giα2-linked Rho kinase activity. Am J Respir Cell Mol Biol. 2001;24:711–719. doi: 10.1165/ajrcmb.24.6.4323. [DOI] [PubMed] [Google Scholar]
- 144.English D, Welch Z, Kovala AT, Harvey K, Volpert OV, Brindley DN, Garcia JG. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB J. 2000;14:2255–2265. doi: 10.1096/fj.00-0134com. [DOI] [PubMed] [Google Scholar]
- 145.Yatomi Y, Ohmori T, Rile G, Kazama F, Okamoto H, Sano T, Satoh K, Kume S, Tigyi G, Igarashi Y, Ozaki Y. Sphingosine 1-phosphate as a major bioactive lysophospholipid that is released from platelets and interacts with endothelial cells. Blood. 2000;96:3431–3438. [PubMed] [Google Scholar]
- 146.English D, Garcia JG, Brindley DN. Platelet-released phospholipids link haemostasis and angiogenesis. Cardiovasc Res. 2001;49:588–599. doi: 10.1016/s0008-6363(00)00230-3. [DOI] [PubMed] [Google Scholar]
- 147.Chae S, Paik J, Furneaux H, Hla T. Requirement for sphingosine 1-phosphate receptor-1 in tumor angiogenesis demonstrated by in vivo RNA interference. J Clin Invest. 2004;114:1082–1089. doi: 10.1172/JCI22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Takeya H, Gabazza EC, Aoki S, Ueno H, Suzuki K. Synergistic effect of sphingosine 1-phosphate on thrombin-induced tissue factor expression in endothelial cells. Blood. 2003;102:1693–1700. doi: 10.1182/blood-2002-11-3607. [DOI] [PubMed] [Google Scholar]
- 149.Matsushita K, Morrell CN, Lowenstein CJ. Sphingosine 1-phosphate activates Weibel–Palade body exocytosis. Proc Natl Acad Sci USA. 2004;101:11483–11487. doi: 10.1073/pnas.0400185101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 151.Riewald M, Ruf W. Mechanistic coupling of protease signaling and initiation of coagulation by tissue factor. Proc Natl Acad Sci USA. 2001;98:7742–7747. doi: 10.1073/pnas.141126698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 153.Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT. The endothelial cell protein C receptor augments protein C activation by the thrombin–thrombomodulin complex. Proc Natl Acad Sci USA. 1996;93:10212–10216. doi: 10.1073/pnas.93.19.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105:3178–3184. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 155.Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JG. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem. 2005;280:17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 156.Feistritzer C, Schuepbach RA, Mosnier LO, Bush LA, Di Cera E, Griffin JH, Riewald M. Protective signaling by activated protein C is mechanistically linked to protein C activation on endothelial cells. J Biol Chem. 2006;281:20077–20084. doi: 10.1074/jbc.M600506200. [DOI] [PubMed] [Google Scholar]
- 157.Bae J, Yang L, Manithody C, Rezaie AR. The ligand occupancy of endothelial protein C receptor switches the protease-activated receptor 1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood. 2007;110:3909–3916. doi: 10.1182/blood-2007-06-096651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bae J, Yang L, Rezaie AR. Lipid raft localization regulates the cleavage specificity of protease activated receptor 1 in endothelial cells. J Thromb Haemost. 2008;6:954–961. doi: 10.1111/j.1538-7836.2008.02924.x. [DOI] [PubMed] [Google Scholar]
- 159.Castellino FJ, Liang Z, Volkir SP, Haalboom E, Martin JA, Sandoval-Cooper MJ, Rosen ED. Mice with a severe deficiency of the endothelial protein C receptor gene develop, survive, and reproduce normally, and do not present with enhanced arterial thrombosis after challenge. Thromb Haemost. 2002;88:462–472. [PubMed] [Google Scholar]
- 160.Weiler H, Lindner V, Kerlin B, Isermann BH, Hendrickson SB, Cooley BC, Meh DA, Mosesson MW, Shworak NW, Post MJ, Conway EM, Ulfman LH, von Andrian UH, Weitz JI. Characterization of a mouse model for thrombomodulin deficiency. Arterioscler Thromb Vasc Biol. 2001;21:1531–1537. doi: 10.1161/hq0901.094496. [DOI] [PubMed] [Google Scholar]
- 161.Niessen F, Furlan-Freguia C, Fernández JA, Mosnier LO, Castellino FJ, Weiler H, Rosen H, Griffin JH, Ruf W. Endogenous EPCR/aPC-PAR1 signaling prevents inflammation-induced vascular leakage and lethality. Blood. 2009;113:2859–2866. doi: 10.1182/blood-2008-12-192385. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 162.Dawicki W, Marshall JS. New and emerging roles for mast cells in host defence. Curr Opin Immunol. 2007;19:31–38. doi: 10.1016/j.coi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 163.Choi OH, Kim JH, Kinet JP. Calcium mobilization via sphingosine kinase in signalling by the Fc epsilon RI antigen receptor. Nature. 1996;380:634–636. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- 164.Prieschl EE, Csonga R, Novotny V, Kikuchi GE, Baumruker T. The balance between sphingosine and sphingosine-1-phosphate is decisive for mast cell activation after Fc epsilon receptor I triggering. J Exp Med. 1999;190:1–8. doi: 10.1084/jem.190.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Melendez AJ, Khaw AK. Dichotomy of Ca2+ signals triggered by different phospholipid pathways in antigen stimulation of human mast cells. J Biol Chem. 2002;277:17255–17262. doi: 10.1074/jbc.M110944200. [DOI] [PubMed] [Google Scholar]
- 166.Olivera A, Urtz N, Mizugishi K, Yamashita Y, Gilfillan AM, Furumoto Y, Gu H, Proia RL, Baumruker T, Rivera J. IgE-dependent activation of sphingosine kinases 1 and 2 and secretion of sphingosine 1-phosphate requires Fyn kinase and contributes to mast cell responses. J Biol Chem. 2006;281:2515–2525. doi: 10.1074/jbc.M508931200. [DOI] [PubMed] [Google Scholar]
- 167.Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, Proia RL, Rivera J. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–297. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 168.Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, Milstien S, Spiegel S. Transactivation of sphingosine-1-phosphate receptors by FcεRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199:959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Oskeritzian CA, Price MM, Hait NC, Kapitonov D, Falanga YT, Morales JK, Ryan JJ, Milstien S, Spiegel S. Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema. J Exp Med. 2010;207:465–474. doi: 10.1084/jem.20091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen L, Woszczek G, Nagineni S, Logun C, Shelhamer JH. Cytosolic phospholipase A2α activation induced by S1P is mediated by the S1P3 receptor in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;295:L326–L335. doi: 10.1152/ajplung.00393.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Melendez AJ, Ibrahim FB. Antisense knockdown of sphingosine kinase 1 in human macrophages inhibits C5a receptor-dependent signal transduction, Ca2+ signals, enzyme release, cytokine production, and chemotaxis. J Immunol. 2004;173:1596–1603. doi: 10.4049/jimmunol.173.3.1596. [DOI] [PubMed] [Google Scholar]
- 172.Hammad SM, Crellin HG, Wu BX, Melton J, Anelli V, Obeid LM. Dual and distinct roles for sphingosine kinase 1 and sphingosine 1 phosphate in the response to inflammatory stimuli in RAW macrophages. Prostaglandins Other Lipid Mediat. 2008;85:107–114. doi: 10.1016/j.prostaglandins.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Snider AJ, Kawamori T, Bradshaw SG, Orr KA, Gilkeson GS, Hannun YA, Obeid LM. A role for sphingosine kinase 1 in dextran sulfate sodium-induced colitis. FASEB J. 2009;23:143–152. doi: 10.1096/fj.08-118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lai W, Irwan AW, Goh HH, Melendez AJ, McInnes IB, Leung BP. Distinct roles of sphingosine kinase 1 and 2 in murine collagen-induced arthritis. J Immunol. 2009;183:2097–2103. doi: 10.4049/jimmunol.0804376. [DOI] [PubMed] [Google Scholar]
- 175.Baker DA, Barth J, Chang R, Obeid LM, Gilkeson GS. Genetic sphingosine kinase 1 deficiency significantly decreases synovial inflammation and joint erosions in murine TNF-α-induced arthritis. J Immunol. 2010;185:2570–2579. doi: 10.4049/jimmunol.1000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Puneet P, Yap CT, Wong L, Yulin L, Koh DR, Moochhala S, Pfeilschifter J, Huwiler A, Melendez AJ. SphK1 regulates proinflammatory responses associated with endotoxin and polymicrobial sepsis. Science. 2010;328:1290–1294. doi: 10.1126/science.1188635. [DOI] [PubMed] [Google Scholar]
- 177.Bolick DT, Srinivasan S, Kim KW, Hatley ME, Clemens JJ, Whetzel A, Ferger N, Macdonald TL, Davis MD, Tsao PS, Lynch KR, Hedrick CC. Sphingosine-1-phosphate prevents tumor necrosis factor-α-mediated monocyte adhesion to aortic endothelium in mice. Arterioscler Thromb Vasc Biol. 2005;25:976–981. doi: 10.1161/01.ATV.0000162171.30089.f6. [DOI] [PubMed] [Google Scholar]
- 178.Kimura T, Tomura H, Mogi C, Kuwabara A, Ishiwara M, Shibasawa K, Sato K, Ohwada S, Im DS, Kurose H, Ishizuka T, Murakami M, Okajima F. Sphingosine 1-phosphate receptors mediate stimulatory and inhibitory signalings for expression of adhesion molecules in endothelial cells. Cell Signal. 2006;18:841–850. doi: 10.1016/j.cellsig.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 179.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Khan BV, Harrison DG, Olbrych MT, Alexander RW, Medford RM. Nitric oxide regulates vascular cell adhesion molecule 1 gene expression and redox-sensitive transcriptional events in human vascular endothelial cells. Proc Natl Acad Sci USA. 1996;93:9114–9119. doi: 10.1073/pnas.93.17.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Assmann G, Gotto AM. HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004;109:III8–III14. doi: 10.1161/01.CIR.0000131512.50667.46. [DOI] [PubMed] [Google Scholar]
- 182.Choi BG, Vilahur G, Yadegar D, Viles-Gonzalez JF, Badimon JJ. The role of high-density lipoprotein cholesterol in the prevention and possible treatment of cardiovascular diseases. Curr Mol Med. 2006;6:571–587. doi: 10.2174/156652406778018590. [DOI] [PubMed] [Google Scholar]
- 183.Rohrer L, Hersberger M, Eckardstein VA. High density lipoproteins in the intersection of diabetes mellitus, inflammation and cardiovascular disease. Curr Opin Lipidol. 2004;15:269–278. doi: 10.1097/00041433-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 184.Kimura T, Sato K, Kuwabara A, Tomura H, Ishiwara M, Kobayashi I, Ui M, Okajima F. Sphingosine 1-phosphate may be a major component of plasma lipoproteins responsible for the cytoprotective actions in human umbilical vein endothelial cells. J Biol Chem. 2001;276:31780–31785. doi: 10.1074/jbc.M104353200. [DOI] [PubMed] [Google Scholar]
- 185.Kimura T, Sato K, Malchinkhuu E, Tomura H, Tamama K, Kuwabara A, Murakami M, Okajima F. High-density lipoprotein stimulates endothelial cell migration and survival through sphingosine 1-phosphate and its receptors. Arterioscler Thromb Vasc Biol. 2003;23:1283–1288. doi: 10.1161/01.ATV.0000079011.67194.5A. [DOI] [PubMed] [Google Scholar]
- 186.Kimura T, Tomura H, Mogi C, Kuwabara A, Damirin A, Ishizuka T, Sekiguchi A, Ishiwara M, Im DS, Sato K, Murakami M, Okajima F. Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells. J Biol Chem. 2006;281:37457–37467. doi: 10.1074/jbc.M605823200. [DOI] [PubMed] [Google Scholar]
- 187.Kimura T, Tomura H, Sato K, Ito M, Matsuoka I, Im DS, Kuwabara A, Mogi C, Itoh H, Kurose H, Murakami M, Okajima F. Mechanism and role of high density lipoprotein-induced activation of AMP-activated protein kinase in endothelial cells. J Biol Chem. 2010;285:4387–4397. doi: 10.1074/jbc.M109.043869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Argraves KM, Gazzolo PJ, Groh EM, Wilkerson BA, Matsuura BS, Twal WO, Hammad SM, Argraves WS. High density lipoprotein-associated sphingosine 1-phosphate promotes endothelial barrier function. J Biol Chem. 2008;283:25074–25081. doi: 10.1074/jbc.M801214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Keul P, Tölle M, Lucke S, von Wnuck Lipinski K, Heusch G, Schuchardt M, der Giet VM, Levkau B. The sphingosine-1-phosphate analogue FTY720 reduces atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:607–613. doi: 10.1161/01.ATV.0000254679.42583.88. [DOI] [PubMed] [Google Scholar]
- 190.Nofer J, Bot M, Brodde M, Taylor PJ, Salm P, Brinkmann V, Van Berkel T, Assmann G, Biessen EA. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;115:501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- 191.Allende ML, Bektas M, Lee BG, Bonifacino E, Kang J, Tuymetova G, Chen W, Saba JD, Proia RL. Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J Biol Chem. 2011;286:7348–7358. doi: 10.1074/jbc.M110.171819. [DOI] [PMC free article] [PubMed] [Google Scholar]