Abstract
The ion selectivity of pumps and channels is central to their ability to perform a multitude of functions. Here we investigate the mechanism of the extraordinary selectivity of the human voltage gated proton channel1, hHV1. This selectivity is essential to its ability to regulate reactive oxygen species production by leukocytes2–4, histamine secretion by basophils5, sperm capacitation6, and airway pH7. The most selective ion channel known, HV1 shows no detectable permeability to other ions1. Opposing classes of selectivity mechanisms postulate that (a) a titratable amino acid residue in the permeation pathway imparts proton selectivity1, 8–11, or (b) water molecules “frozen” in a narrow pore conduct protons while excluding other ions12. Here we identify Aspartate112 as a crucial component of the selectivity filter of hHV1. When a neutral amino acid replaced Asp112, the mutant channel lost proton specificity and became anion selective or did not conduct. Only the glutamate mutant remained proton specific. Mutation of the nearby Asp185 did not impair proton selectivity, suggesting that Asp112 plays a unique role. Although histidine shuttles protons in other proteins, when histidine or lysine replaced Asp112, the mutant channel was still anion permeable. Evidently, the proton specificity of hHV1 requires an acidic group at the selectivity filter.
Voltage gated proton channels are considered specific (perfectly selective) for protons, because no evidence exists for permeation of anything but H+. Specificity, combined with a large deuterium isotope effect9 and extraordinarily strong temperature dependence of conduction10 suggests a permeation pathway more complex than a simple water wire, as exists in gramicidin13. All proton conduction appears consistent with a hydrogen-bonded chain (HBC) mechanism14; a HBC including a titratable group could explain several unique properties of HV11, especially proton selectivity14. Yet in a recent study, mutation of each titratable amino acid in all four transmembrane (TM) helices of hHV1 failed to abolish conduction12. Thus, the mechanism producing proton selectivity remained unknown.
We noticed that a human gene, C15orf27 (of unknown function), contains a predicted voltage sensor domain (VSD) that shares 25% sequence identity and 52% similarity [http://www.ebi.ac.uk/Tools/emboss/align/] with the VSD of hHV1, and includes three Arg residues in the S4 TM helix that are conserved among all known HV1 homologues. Phylogenetic analysis of VSD sequences (Fig. S1) reveals that a group comprising HV1, C15orf27, and voltage sensitive phosphatase (VSP) sequences separated early from the two phylogenetically distinct groups of depolarization activated VSDs described previously (KV channels and NaV/CaV channels), supporting the modular evolution of VSD-containing proteins15. Furthermore, HV1 VSDs occupy a discrete lineage, distinct from those of VSP and C15orf27 orthologs.
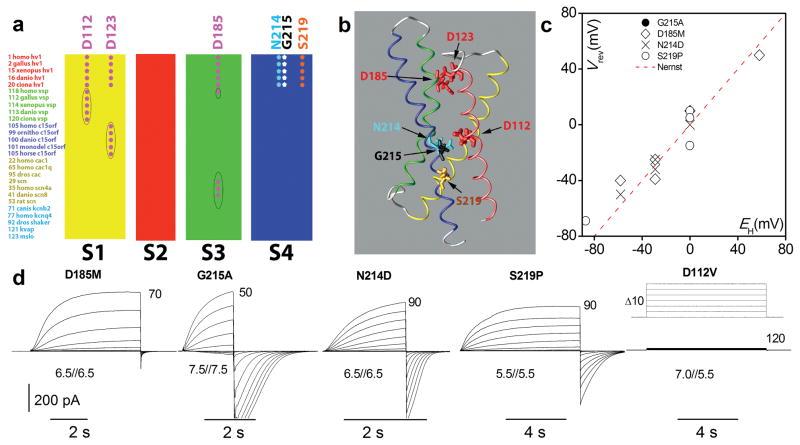
When we cloned the C15orf27 gene and expressed the product in HEK-293 or COS-7 cells, the GFP-tagged protein localised at the plasma membrane (Fig. S2), but we detected no currents beyond those in non-transfected cells. We reasoned that substitutions based on sequence elements that differ between hHV1 and C15orf27 should be structurally tolerated while revealing residues responsible for proton conduction. We therefore mutated residues that are perfectly conserved in 21 HV1 family members and differ between C15orf27 and HV1. We replaced five candidate residues in hHV1 (D112, D185, N214, G215, and S219) (Fig. 1a & 1b) with the corresponding residue in C15orf27. Four mutants exhibited large currents under whole-cell voltage clamp (Fig. 1d). The reversal (zero current) potential, Vrev, measured at several pHo and pHi, was close to the Nernst potential for protons, EH (Fig. 1c), demonstrating proton selectivity. D112V mutants localised to the plasma membrane (Fig. S3), but displayed no convincing current (Fig. 1d). Some D112V transfected HEK-293 or COS-7 cells (and non-transfected cells) exhibited small native proton currents. His140A/H193A double mutants16, 17, in which the two Zn2+ binding His residues are neutralized, resemble WT, with similar ΔpH dependent gating12, and Vrev near EH (Fig. S4). We expressed mutants in this Zn2+ insensitive background (D112x/A/A) to distinguish their currents from native currents that are abolished by 100 μM Zn2+ at pHo 7.0. We tentatively concluded that Asp112 is crucial to proton conduction.
Figure 1. Identification of five key amino acids that differ in hHV1 and C15orf27, and the currents generated in a heterologous expression system by hHV1 mutants in which hHV1 residues were replaced by the corresponding amino acid in the non-conducting C15orf27.
a, Representative subset of multiple sequence alignment of 122 VSDs; only TM helices are shown. Gene families include: HV1; voltage sensitive phosphatases; C15orf27; Ca2+ and Na+ channels; and K+ channels (cf. Fig. S1). b, Location of the key amino acids in the open hHV1 channel VSD viewed from the side (membrane), based on a homology model17. c, Vrev in the four conducting mutants is near EH (dashed line), indicating proton selectivity. Vrev was measured using tail currents; in G215A Vrev was positive to threshold and was observed directly. d, Voltage-clamp current families in cells expressing hHV1 mutants. Depolarizing pulses were applied in 10-mV increments from a holding voltage, Vhold = −40 mV (D185M, D112V), −60 mV (G215A, S219P), or −90 mV (N214D), with the most positive pulse labelled. After membrane repolarisation, an inward “tail current” is seen as channels close (see inset for D185M); pH is given as pHo//pHi. D112V exhibited no clear current.
The absence of detectable currents in D112V led us to make other D112x substitutions. These mutants (Fig 2a) exhibited slowly activating outward currents upon depolarization that resembled hHV1 currents. As reported previously12, Asp112 mutation had little effect on the ΔpH dependence of gating. The proton conductance-voltage, gH-V, relationship of all D112x mutants shifted roughly −60 mV when pHo increased from 5.5 to 7.0 (Fig. S5), as in WT channels1, 8, 18. Mutation of Asp112 did influence channel opening and closing kinetics (Table S2).
Figure 2. Currents in Asp112 mutants resemble proton currents, but are not.
a, Currents generated by WT, D112E, D112H, D112K/A/A, D112N/A/A, D112S, D112A/A/A, and D112F/A/A in COS-7 cells (pHi 5.5) at pHo 5.5 (column 1) or 7.0 (column 2), during families of pulses in 10 mV increments up to indicated voltages. Tail currents at pHo 7.0 (column 3) reveal that Vrev deviates from EH, indicating loss of proton selectivity. At pHo 5.5 Vhold was −40 mV (−60 mV for WT). At pHo 7.0 Vhold was −40 mV (K, N, S), −50 mV (F), −60 mV (H, A), −80 mV (E), or −90 mV (WT); Vpre was −65 mV (WT), −40 mV (E), −10 mV (H), +50 mV (K), +40 mV (N, S), or +20 mV (A, F). Vrev (arrows) was determined from the amplitude and direction of tail current decay. For D112N, Vrev was above Vthreshold and was evident during pulse families. b, Shift in Vrev when the TMACH3SO3 bath solution was changed from pH 5.5 to 7.0. There is no difference between WT and D112E, but the shift in all other mutants is smaller than WT (p<0.001, by one-way ANOVA followed by Tukey’s test, n = 7, 4, 9, 8, 6, 7, 9, and 4. Dashed line shows EH.
Measurements of Vrev in Asp112 mutants revealed a marked departure from WT hHV1 properties. At symmetrical pH 5.5, Vrev was near 0 mV (not shown). At pHo 7.0, pHi 5.5 (Fig. 2a, column 3), WT channels reversed near EH (−87 mV), indicating proton selectivity. But for all mutants except D112E, Vrev was substantially positive to EH (Fig. 2b), ranging from −58 mV (D112H) to −13 mV (D112N). Substitutions at Asp112 eliminated the proton specificity that distinguishes HV1 from all other ion channels1. A previous study described currents in D112A and D112N mutants12 but did not report Vrev.
We expected that loss of proton selectivity would result in nonselective permeation of cations. Surprisingly, Vrev did not change detectably when Na+, K+, N-methyl-D-glucamine+, or TEA+ replaced TMA+ (Table S4). To test anion vs. cation selectivity, we adopted the classical tactic of replacing a fraction of the bath solution with isotonic sucrose19. The Nernst equation predicts that dilution of all extracellular ions except H+ and OH− (leaving internal ion concentrations unchanged) will shift Vrev negatively for a cation selective channel, but positively for an anion selective one. Despite the 10-fold reduction of buffer concentration, direct measurement confirmed that pH remained constant.
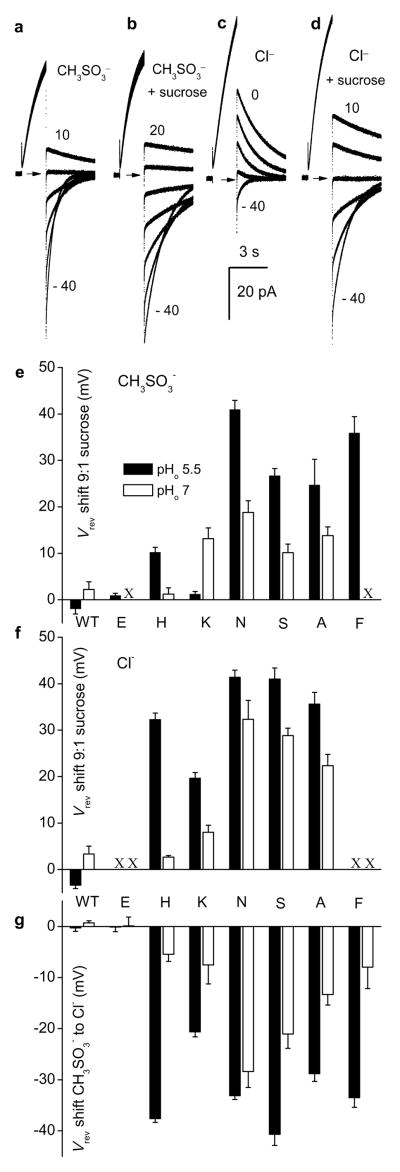
Fig. 3 illustrates determination of Vrev from tail currents in a D112H transfected cell at pH 5.5//5.5 in CH3SO3− (Fig. 3a) or Cl− solution (Fig. 3c), and after 90% reduction of external ionic strength (Figs. 3b & 3d). Astonishingly, for all Asp112 mutants except D112K/A/A, sucrose shifted Vrev positively (Fig. S6), indicating anion selectivity both in CH3SO3− (Fig. 3e) and Cl− solutions (Fig. 3f). For hHV1 and D112E, Vrev did not change, reaffirming their proton specificity. Neutralization of a single Asp residue converts a proton channel into a predominantly anion selective channel. Thus, Asp112 mediates charge selectivity as well as proton selectivity.
Figure 3. Dilution of ionic strength by 90% with isotonic sucrose shifted Vrev positively, indicating that most Asp112 mutants are anion selective.
a Measurement of Vrev by tail currents in a cell transfected with D112H at pH 5.5//5.5 and (b) after sucrose. c, Vrev in the same cell in pH 5.5 Cl− solution, and (d) after sucrose. Arrows indicate zero current. Vhold = −40 mV, Vpre = +60 mV. e, Mean shifts of Vrev with decreasing ionic strength in CH3SO3− solutions or (f) in Cl− solutions. Each value was determined in 3–6 cells. X = not done. g, Shifts of Vrev when CH3SO3− was replaced by Cl−. Values for WT and D112E do not differ significantly from 0 mV. For all anion selective mutants except D112N, the difference between shifts at pH 5.5 and 7.0 was significant (p<0.001, one-way ANOVA followed by Tukey’s test; n = 3–8). Error bars in e–g are s.e.
To confirm anion permeability of Asp112 mutants, we replaced the main external anion, methanesulfonate− (CH3SO3−), with Cl−. Consistent with previous studies1, Vrev in hHV1 was unchanged. As shown in D112H (Fig. 3a vs. 3c), Vrev shifted negatively in Cl solutions in all mutants (except D112E), indicating that Cl− is more permeant than the larger CH3SO3− anion (Fig. 3g). That all conducting non-acidic mutants exhibited Cl-permeability indicates that Asp112 mediates not only proton selectivity, but also charge selectivity. Currents were smaller than WT in cells expressing some mutants (Fig. S7), suggesting a smaller unitary conductance. Evidently, these channels conduct anions, but not very well.
Although the mutant channels have diminished selectivity, Vrev did shift negatively when pHo increased from 5.5 to 7.0 (Fig. 2b). Because these solutions differ mainly in buffer species and concentrations of H+ and OH−, Asp112 mutants must have significant permeability to H+ and/or OH−. The Goldman-Hodgkin-Katz equation shows how Vrev depends on ion concentrations:
| (1) |
Ions with greater permeability dominate Vrev. Permeation of H+ and OH− are difficult to distinguish because they have the same Nernst potential1. The data can be interpreted assuming permeation of either (Table S3), but the anion selectivity of Asp112 mutants and the pH dependence of sucrose effects (Figs. 3e & 3f) support OH− permeation. The relative permeability of conducting Asp112 mutants was OH− (or H+) > Cl− > CH3SO3−.
Although Asp112 is essential to selectivity, other acidic groups might participate. We mutated Asp185, located in the presumed conduction pore (Fig. 1b)12, 17. However, like D185M (Fig. 1b), D185V, D185A, and D185N remained proton selective (Fig. S8). Speaking against additive effects, the double mutant, D112N/D185M did not differ from D112N (Fig. S8).
Consistent with earlier predictions that a titratable amino acid provides the selectivity filter of HV11, 8–11, only channels with acidic residues (Glu or Asp) at position 112 manifested proton specificity. Asp112 lies at the constriction of the presumed pore (Fig. 1c), a logical location for a selectivity filter, and just external to the postulated gating charge transfer centre20. Our original prediction envisioned selectivity arising from protonation/deprotonation of a residue during conduction, but other mechanisms are possible. For example, proton selectivity of the influenza A M2 viral proton channel has been explained by (a) immobilized water21, (b) successive proton transfer and release by His37 (refs.22, 23), or (c) delocalization of the proton among His37 and nearby waters24.
The Cl− permeability of D112H was completely unexpected given strong precedents for His imparting proton selectivity to channels. Histidine shuttles protons in K+ or Na+ channel VSDs with Arg→His mutations25–27, in carbonic anhydrase28, and in M2 channels22, 23. However, these molecules are not proton specific27, 29. Evidently, His shuttles protons, but does not guarantee proton selectivity. In hHV1, Asp112 (or Glu112 in D112E) excludes anions, resulting in proton specific conduction. His may fail to exclude anions because it is cationic when protonated, whereas Glu and Asp are neutral.
The anion selectivity of neutral Asp112 mutants suggests that electrostatic forces due to the charge distribution in the rest of the channel deter cation permeation, and that the cation selectivity of the WT channel is due to the anionic charge of Asp112. Asp185 does not participate directly in selectivity (Fig. S8). VSP family members possess the equivalent of Asp112 (Fig. 1a), yet conduct no current30, illustrating that Asp112 requires a specific microenvironment to achieve selectivity. Although permeation of Cl− and CH3SO3− suggests a wide pore in D112x mutants, local geometry might differ in WT channels due to the presence of anionic Asp112.
Regulation of voltage gating by ΔpH is distinct from permeation. Pathognomonic of HV1 is a strict correlation between the gH-V relationship and Vrev, in which Vthreshold shifts 40 mV/Unit change in ΔpH8. The ΔpH dependence persisted in mutants exhibiting shifted gH-V relationships 12. Here we show uncoupling of Vrev and voltage gating. Asp112 mutants retained normal ΔpH dependence (Fig. S5), despite the dissociation of Vrev from ΔpH (Figs. 2 & S9). This uncoupling of pH control of gating from permeation speaks against any mechanism that invokes regulation by local proton concentration in the vicinity of S4 Arg residues12.
In summary, Asp112 is a critical component of the selectivity filter of hHV1, crucial to both proton selectivity and charge selectivity. That D112E was proton selective, but D112H conducted anions indicates that this proton channel requires an acid at the selectivity filter. That neutralization of nearby Asp185 did not affect selectivity suggests that Asp112 plays a unique role.
‘Methods Summary’
The pipette solution (also used externally) contained (mM) 130 TMACH3SO3, 2 MgCl2, 2 EGTA, 80 MES, titrated to pH 5.5 with ~20 TMAOH. In the pH 5.5 TMACl solution, TMACl replaced TMACH3SO3. Bath solutions at pH 7.0 had (mM) 90 TMACH3SO3 or TMACl, 3 CaCl2, 1 EGTA, 100 BES, and 36–40 TMAOH. For experiments with Zn2+, solutions contained PIPES without EGTA. Experiments were done at 20–25°C. Currents are shown without leak correction. Vrev data were corrected for liquid junctions potentials measured in each solution19.
‘Methods’
Exhaustive searches to identify HV1 homologs were performed using protein BLAST and PSI-BLAST. A sample of VSDs from K+, Na+, and Ca2+ channels (that open with depolarization like HV1, and in addition one that opens with hyperpolarization), along with putative HV1, VSP, and C15orf27 homologs were chosen. For cation channels, we sampled from the range of subfamilies, from the VSD repeats within Na+ and Ca2+ channels, and from the range of species. VSD sequences, including crystallized K+ channels (PDB ids 1ORS, 2R9R, and 2A79), were aligned using PromalS3D31, which incorporates structural information, allowing high confidence identification of VSD boundaries. Sequences were trimmed to the VSD, realigned with PromalS3D, and the resulting alignment was analyzed with PhyML (maximum likelihood)32 and Protpars (maximum parsimony)33 at the Mobyle portal34. Trees were visualized with TreeDyn35 and iTOL36. Parsimony (not shown) and maximum likelihood trees had similar topology, including HV1 and C15orf27 families separating into discrete branches. A homology model of the VSD of hHV1 was constructed as described previously17.
The C15orf27 clone was PCR amplified from human cerebellum and subcloned into pcDNA3.1(+) expression vector (Invitrogen, Carlsbad, CA). The coding sequence of human HV1 (HVCN1) was cloned into either pcDNA3.1(−) or pQBI25-fC3 (to make GFP-HV1) vectors as described previously16. Site directed mutants were created using the Stratagene Quikchange (Agilent, Santa Clara, CA) procedure according to the manufacturer’s instructions. All the positive clones were sequenced to confirm the presence of the introduced mutation. HEK-293 or, more often COS-7 cells were grown to ~80% confluency in 35 mm cultures dishes, usually by seeding cells 1 d ahead of transfection. Cells were transfected with 0.4–0.5 μg of the appropriate cDNA using Lipofectamine 2000 (Invitrogen). After 6 h at 37oC in 5% CO2, the cells were trypsinized and re-plated onto glass cover slips at low density for patch clamp recording the following day. We selected green cells under fluorescence for recording. Patch clamp methods were described previously18.
The main pipette solution (also used externally) contained (in mM) 130 TMACH3SO3, 2 MgCl2, 2 EGTA, 80 MES, titrated to pH 5.5 with ~20 TMAOH. In the pH 5.5 TMACl solution, TMACl replaced TMACH3SO3. Bath solutions at pH 7.0 had (mM) 90 TMACH3SO3 or TMACl, 3 CaCl2, 1 EGTA, 100 BES, and 36–40 TMAOH. For experiments with Zn2+, solutions contained PIPES buffer37 without EGTA. Experiments were done at 20–25°C. Currents are shown without leak correction. Vrev data were corrected for liquid junctions potentials measured in each solution.
Supplementary Material
Acknowledgments
We thank Peter H. Barry, Dirk Gillespie, Vladislav S. Markin, John F. Nagle, Régis Pomés, David Silverman, and Valerij Sokolov for discussions or comments on the manuscript. Supported by NSF grant MCB-0943362 (SS & TD) and NIH grant GM087507 (TD). The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institute of General Medical Sciences or the National Institutes of Health.
Footnotes
Supplementary Information accompanies the paper on www.nature.com/nature.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
AUTHORS’ CONTRIBUTIONS
S.R. identified the similarity of C15orf27 to hHV1 and cloned the C15orf27 gene; S.S. conceived the strategic approach based on molecular model, sequence, and phylogenetic analysis; S.R. and S.S. created mutants; T.D., B.M., and V.C. designed experiments; B.M., D.M. and V.C. recorded, analyzed, and interpreted data; T.D. wrote the manuscript; all authors read and approved the manuscript.
References
- 1.DeCoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]
- 2.Capasso M, et al. HVCN1 modulates BCR signal strength via regulation of BCR-dependent generation of reactive oxygen species. Nat Immunol. 2010;11:265–272. doi: 10.1038/ni.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeCoursey TE, Morgan D, Cherny VV. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature. 2003;422:531–534. doi: 10.1038/nature01523. [DOI] [PubMed] [Google Scholar]
- 4.Henderson LM, Chappell JB, Jones OTG. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem J. 1987;246:325–329. doi: 10.1042/bj2460325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musset B, et al. A pH-stabilizing role of voltage-gated proton channels in IgE-mediated activation of human basophils. Proc Natl Acad Sci USA. 2008;105:11020–11025. doi: 10.1073/pnas.0800886105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lishko PV, Botchkina IL, Fedorenko A, Kirichok Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell. 2010;140:327–337. doi: 10.1016/j.cell.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 7.Iovannisci D, Illek B, Fischer H. Function of the HVCN1 proton channel in airway epithelia and a naturally occurring mutation, M91T. J Gen Physiol. 2010;136:35–46. doi: 10.1085/jgp.200910379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherny VV, Markin VS, DeCoursey TE. The voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient. J Gen Physiol. 1995;105:861–896. doi: 10.1085/jgp.105.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeCoursey TE, Cherny VV. Deuterium isotope effects on permeation and gating of proton channels in rat alveolar epithelium. J Gen Physiol. 1997;109:415–434. doi: 10.1085/jgp.109.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeCoursey TE, Cherny VV. Temperature dependence of voltage-gated H+ currents in human neutrophils, rat alveolar epithelial cells, and mammalian phagocytes. J Gen Physiol. 1998;112:503–522. doi: 10.1085/jgp.112.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeCoursey TE, Cherny VV. Voltage-activated hydrogen ion currents. J Membr Biol. 1994;141:203–223. doi: 10.1007/BF00235130. [DOI] [PubMed] [Google Scholar]
- 12.Ramsey IS, et al. An aqueous H+ permeation pathway in the voltage-gated proton channel Hv1. Nat Struct Mol Biol. 2010;17:869–875. doi: 10.1038/nsmb.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levitt DG, Elias SR, Hautman JM. Number of water molecules coupled to the transport of sodium, potassium and hydrogen ions via gramicidin, nonactin or valinomycin. Biochim Biophys Acta. 1978;512:436–451. doi: 10.1016/0005-2736(78)90266-3. [DOI] [PubMed] [Google Scholar]
- 14.Nagle JF, Morowitz HJ. Molecular mechanisms for proton transport in membranes. Proc Natl Acad Sci USA. 1978;75:298–302. doi: 10.1073/pnas.75.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson RD, Kuan G, Saier MH, Jr, Montal M. Modular assembly of voltage-gated channel proteins: a sequence analysis and phylogenetic study. J Mol Microbiol Biotechnol. 1999;1:281–287. [PubMed] [Google Scholar]
- 16.Ramsey IS, Moran MM, Chong JA, Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musset B, et al. Zinc inhibition of monomeric and dimeric proton channels suggests cooperative gating. J Physiol. 2010;588:1435–1449. doi: 10.1113/jphysiol.2010.188318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musset B, et al. Detailed comparison of expressed and native voltage-gated proton channel currents. J Physiol. 2008;586:2477–2486. doi: 10.1113/jphysiol.2007.149427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barry PH. The reliability of relative anion-cation permeabilities deduced from reversal (dilution) potential measurements in ion channel studies. Cell Biochem Biophys. 2006;46:143–154. doi: 10.1385/CBB:46:2:143. [DOI] [PubMed] [Google Scholar]
- 20.Tao X, Lee A, Limapichat W, Dougherty DA, MacKinnon R. A gating charge transfer center in voltage sensors. Science. 2010;328:67–73. doi: 10.1126/science.1185954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sansom MSP, Kerr ID, Smith GR, Son HS. The influenza A virus M2 channel: a molecular modeling and simulation study. Virology. 1997;233:163–173. doi: 10.1006/viro.1997.8578. [DOI] [PubMed] [Google Scholar]
- 22.Hu F, Luo W, Hong M. Mechanisms of proton conduction and gating in influenza M2 proton channels from solid-state NMR. Science. 2010;330:505–508. doi: 10.1126/science.1191714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venkataraman P, Lamb RA, Pinto LH. Chemical rescue of histidine selectivity filter mutants of the M2 ion channel of influenza A virus. J Biol Chem. 2005;280:21463–21472. doi: 10.1074/jbc.M412406200. [DOI] [PubMed] [Google Scholar]
- 24.Acharya R, et al. Structure and mechanism of proton transport through the transmembrane tetrameric M2 protein bundle of the influenza A virus. Proc Natl Acad Sci USA. 2010;107:15075–15080. doi: 10.1073/pnas.1007071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starace DM, Bezanilla F. Histidine scanning mutagenesis of basic residues of the S4 segment of the shaker K+ channel. J Gen Physiol. 2001;117:469–490. doi: 10.1085/jgp.117.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Starace DM, Bezanilla F. A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature. 2004;427:548–553. doi: 10.1038/nature02270. [DOI] [PubMed] [Google Scholar]
- 27.Sokolov S, Scheuer T, Catterall WA. Ion permeation and block of the gating pore in the voltage sensor of NaV1.4 channels with hypokalemic periodic paralysis mutations. J Gen Physiol. 2010;136:225–236. doi: 10.1085/jgp.201010414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tu CK, Silverman DN, Forsman C, Jonsson BH, Lindskog S. Role of histidine 64 in the catalytic mechanism of human carbonic anhydrase II studied with a site-specific mutant. Biochemistry. 1989;28:7913–7918. doi: 10.1021/bi00445a054. [DOI] [PubMed] [Google Scholar]
- 29.Leiding T, Wang J, Martinsson J, DeGrado WF, Årsköld SP. Proton and cation transport activity of the M2 proton channel from influenza A virus. Proc Natl Acad Sci USA. 2010;107:15409–15414. doi: 10.1073/pnas.1009997107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- 31.Pei J, Kim BH, Grishin NV. PROMALS3D: a tool for multiple protein sequence and structure alignments. Nucleic Acids Res. 2008;36:2295–2300. doi: 10.1093/nar/gkn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 33.Felsenstein J. Distributed by the author. Seattle: 1993. [Google Scholar]
- 34.Néron B, et al. Mobyle: a new full web bioinformatics framework. Bioinformatics. 2009;25:3005–3011. doi: 10.1093/bioinformatics/btp493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chevenet F, Brun C, Bauñls AL, Jacq B, Christen R. TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics. 2006;7:439. doi: 10.1186/1471-2105-7-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Letunic I, Bork P. Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics. 2007;23:127–128. doi: 10.1093/bioinformatics/btl529. [DOI] [PubMed] [Google Scholar]
- 37.Cherny VV, DeCoursey TE. pH-dependent inhibition of voltage-gated H+ currents in rat alveolar epithelial cells by Zn2+ and other divalent cations. J Gen Physiol. 1999;114:819–838. doi: 10.1085/jgp.114.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.