Abstract
Therapeutic strategies following spinal cord injury must address the multiple barriers that limit regeneration. Multiple channel bridges have been developed that stabilize the injury following implantation and provide physical guidance for regenerating axons. These bridges have now been employed as a vehicle for localized delivery of lentivirus. Implantation of lentivirus loaded multiple channel bridges produced transgene expression that persisted for at least 4 weeks. Expression was maximal at the implant at the earliest time point, and decreased with increasing time of implantation, as well as rostral and caudal to the bridge. Immunohistochemical staining indicated transduction of macrophages, Schwann cells, fibroblasts, and astrocytes within the bridge and adjacent tissue. Subsequently, the delivery of lentivirus encoding the neurotrophic factors NT3 or BDNF significantly increased the extent of axonal growth into the bridge relative to empty scaffolds. In addition to promoting axon growth, the induced expression of neurotrophic factors led to myelination of axons within the channels of the bridge, where the number of myelinated axons was significantly enhanced relative to control. Combining gene delivery with biomaterials to provide physical guidance and create a permissive environment can provide a platform to enhance axonal growth and promote regeneration.
INTRODUCTION
Spinal cord injury (SCI) results in paralysis below the level of the injury. The limited regeneration observed in the spinal cord (SC) has been attributed to insufficient trophic factor support, and up-regulation of axonal growth inhibitors. SCI induces a number of processes, including neuron and oligodendrocyte cell death, demyelination, inflammation, and deposition of a glial scar. Promoting regeneration requires a combinatorial approach that can address this multitude of processes, which is exemplified by the early experiments with implantation of autologous peripheral nerve (PN) grafts [1]. Axonal elongation by the PN graft is promoted and directed by the graft architecture and cells (e.g., Schwann cells) that secrete trophic factors. PN grafts have limited clinical potential, as their source is limiting, which has motivated the development of systems and strategies to recapitulate their effects [2].
Recapitulating the effects of PN grafts is challenging, as the precise contribution of the architecture and cell-secreted factors (e.g., extracellular matrix, trophic factors) is ill defined. The structural aspects of PN grafts have been targeted with biomaterial bridges, which provide mechanical stability to the injured tissue and have channels that span the bridge to direct axonal elongation [3–5]. The bridges support cell infiltration, which helps prevent cavity formation that can occur secondary to the initial injury, and may also limit scar formation. The channels also support cell infiltration. Cells within the channels are aligned with the major axis of the bridge, which can provide a directional signal for regenerating axons.
Providing trophic factors that would normally be produced by Schwann cells within PN grafts has been explored through a range of cell and drug therapies. Direct injection and osmotic pumps have been employed to deliver trophic factors to promote neurite outgrowth [6–8]. Alternatively, transplantation of Schwann cells [9–12], stem cells [12–15], or cells genetically engineered to secrete inductive factors [16–18] are all strategies that have been reported to enhance axonal growth through the injury, however, the impact of these strategies is hampered by limited cell survival and engraftment (~15%) [19]. Gene delivery represents a versatile approach in which transduced cells can function as bioreactors for the localized production of neurotrophic factors; however, improved delivery strategies are required to localize delivery to the injury.
In this report, we investigate delivery of lentiviral vectors from multiple channel bridges as a combinatorial approach to promote regeneration in the injured SC. The bridge provides the structural support to stabilize the injury and architecture to direct axonal elongation; whereas lentivirus induced expression of neurotrophic factors can promote axon growth. Lentivirus was immobilized to nanoparticles and loaded into bridges for implantation into a rat spinal cord lateral hemisection. The transgene expression profile was characterized, as were the location and identity of transduced cells. Subsequently, lentiviral vectors encoding NT3 and BDNF were delivered to promote the growth of axons into and down the channels of the bridge. Axon growth and myelination were characterized as a function of time, treatment, and location within the bridge. The combination of soluble vectors and physical structure synergize to promote axon growth, and the vector releasing bridges provide a platform to investigate additional factors in SC regeneration.
MATERIALS AND METHODS
Virus production
Lentivirus was produced in HEK-293T cells grown in DMEM with 10% FBS at 37°C, and 5% CO2. The lentiviral packaging vectors (pMDL-GagPol, pRSV-Rev, pIVSVSV-G) were co-transfected along with plenti-CMV-GFP, plenti-CMV-BDNF, plenti-CMV-NT3, or plenti-CMV-luciferase into 293T cells using Lipofectamine 2000 (Roche Biosciences, Palo Alto, CA). After 48 h of transfection, the supernatant was collected and filtered (0.45 micron). Viruses were then concentrated using PEG-it (System Biosciences, Mountain, CA), with the precipitated lentiviruses suspended with PBS. The virus titer was determined by HIV-1 p24 Antigen ELISA Kit (ZeptoMetrix Co., Buffalo, NY).
Fabrication of multiple channel bridges
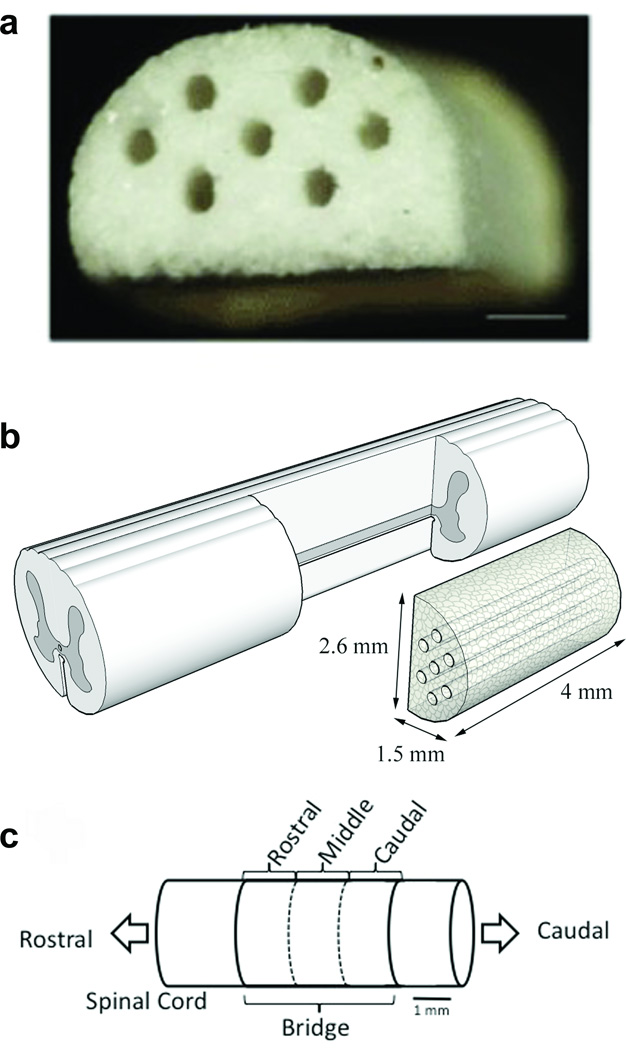
Multiple channel bridges were fabricated using a gas foaming/particulate leaching method as previously described [4, 5]. Poly(lactide-co-glycolide) (PLG) (75:25 mole ratio of D, L-lactide to glycolide, 0.76 dL/g, Lakeshore Biomaterials, Birmingham, AL) was dissolved in dichloromethane (2% w/w) and then emulsified in 1% poly(vinyl alcohol) to create microspheres. A mixture of PLG microspheres and salt particles (63–106 µm) was loaded into a custom made aluminum mold with Delrin pin guides using a layer-by-layer technique and pressurized to 200 psi using a Carver press. The bridges were then equilibrated with high pressure CO2 gas (800 psi) for 16 h in a custom-made pressure vessel. Afterwards, the pressure was released over a period of 45 min, which served to fuse adjacent microspheres creating a continuous polymer structure. The porogen was leached in water for one hour; the bridges were disinfected in ethanol for 5 minutes and dried overnight. The final bridge dimensions were 4 mm in length, 2.6 mm in width, and 1.5 mm in height, containing 7 channels 250 µm in diameter (Fig. 1b).
Figure 1. Multiple channel bridges for spinal cord regeneration.
(a) Photomicrograph of a multiple channel bridge showing 7 channels, each 250 µm in diameter. Scale bar is 500 µm. (b) Schematic of PLG bridge implantation in a spinal cord hemisection model. (c) Schematic of the regions in which the bridge was divided for analysis. Rostral analysis was done at 300 µm, middle at 2000 µm, and caudal at 3500 µm from the rostral edge of the bridge/tissue boundary.
Virus loading into multiple channel bridges
Hydroxylapatite (HA) nanoparticles (Sigma-Aldrich) were suspended in PBS and sonicated for 1 minute to dissociate aggregates. The HA (1 µL, 10 mg/mL in PBS) was then complexed with lentivirus (9 µL) encoding luciferase (LV-luc, 3×1011 LP) and incubated for 10 minutes at 4°C. HA/virus complex were then deposited into each channel of the bridge using Stripper pipet tips (Mid-Atlantic Diagnostics, Mount Laurel, NJ) and placed in dry ice until implantation.
Rat spinal cord hemisection
A rat spinal cord hemisection model (Fig. 1b) was used to analyze transgene expression and nerve regeneration. Surgery was performed as previously described [4] on female Long-Evans rats (180–200 g; Charles River) that were treated according to the Animal Care and Use Committee guidelines at Northwestern University. Animals were anesthetized using isoflurane (3% in O2), and a laminectomy was performed at T9-10. A 4-mm long spinal cord segment, lateral of the midline, was removed to create a hemisection, and bridges were implanted in the injury space and covered by Gelfoam. (Fig. 1a) The muscles were sutured together and the skin was stapled. Postoperative care consisted of the administration of Baytril (enrofloxacin 2.5 mg/kg SC, once a day for 2 weeks), buprenorphine (0.01 mg/kg SC, twice a day for 2 days), and lactate ringer solution (5 mL/100 g, once a day for 5 days). Bladders were expressed twice a day until bladder function recovered (7–14 days).
Quantification of transgene expression in a hemisection model
Bridges loaded with a lentiviral vector encoding for firefly luciferase were implanted to quantify transgene expression levels within defined spinal cord segments. One week and four weeks post implantation, rats (n = 4) were euthanized and fresh spinal segments (T4-L2) were retrieved. The injury site and five samples rostral and caudal to the injury site were collected in 5 µm intervals and frozen. The tissues were thawed and 100 µl of lysis buffer (Cell Culture Lysis Reagent 1X; Promega, Madison, WI) was added, the tissue was homogenized with microscissors and vortexed. After 10 minutes of incubation at room temperature the tissue lysate was centrifuged at 14,000 rpm for 10 minutes at 4 °C to collect the supernatant. The luciferase content in the supernatant was quantified using a luciferase assay (Promega, Madison, WI) and recorded in relative light unit (RLU) per microgram protein. The total protein amount was measured with the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL).
Transgene expression and cell type identification
Immunohistochemistry was performed on tissue sections to analyze the distribution and identity of transduced cells. Bridges loaded with a lentiviral vector encoding for EGFP were implanted into the spinal cord. After 7 days, rats (n = 4) were euthanized and the spinal cord was retrieved, frozen in isopentane (Fisher Scientific), and stored in siliconized Eppendorf tubes at −80 °C. The tissue section containing the injury site (T8-T11) was cryopreserved in optimum cutting temperature (OCT) compound and sliced longitudinally in 10-µm thick sections using a cryostat (HM 525, Thermo Scientific). Every other section was collected on glass slides, post fixed, and stained.
Sections were stained using a double-labeling immunofluorescence staining method. All collected sections were stained with polyclonal rabbit anti-EGFP (Invitrogen, Carlsbad, CA) as a primary antibody and Alexa Fluor 488 goat anti-rabbit (green) (Invitrogen, Carlsbad, CA) as a secondary antibody. Tissue sections were double stained for specific cell lineages with primary monoclonal mouse IgG1 antibodies to: (i) anti-S100 (β-subunit) for Schwann cells (Sigma); (ii) anti-rat prolyl 4-hydroxylase (rPH) for fibroblasts (Acris Antibodies, Herford, Germany); (iii) anti-rat monocytes/macrophages (ED-1) (Millipore, Billerica, MA); (iv) anti-glial fibrillary acidic protein (GFAP) for reactive astrocytes (Sigma, St. Louis, MO). Alexa Fluor 546 goat anti-mouse (red) (Invitrogen, Carlsbad, CA) was used as a secondary antibody. A Hoechst stain was used to identify the cytoarchitecture. Negative controls were performed by eliminating the primary antibodies and staining histological sections of implanted bridges with a lentivirus encoding beta-galactosidase (β-gal).
To determine the identity of transfected cells inside the bridge and the adjacent tissue, sections were double stained with antibodies for EGFP and specific cell types, and fluorescence images were captured and overlaid in Photoshop. The percentages of transduced cells identified as a specific cell type were quantified for four cell types. For each cell type, 3 rats, 9 tissue sections, and 45 images were analyzed and counted. Images were taken in three random fields of view of the bridge and two random fields of view of the adjacent tissue (200X). To count the number of transduced cells, the EGFP and Hoechst images were superimposed and the positive cells were marked. Subsequently, the location of EGFP positive cells was overlaid with red fluorescent images and all the marked transduced cells that overlapped with the red cell stain were counted as positive for each specific cell type by a blinded observer. Percentages were calculated as the ratio of the transduced cells identified as a certain cell type to the total counted number of transduced cells.
Immunohistochemistry and quantitative analysis of nerve regeneration and myelination
a. Axon growth
Immunohistochemistry was performed on tissue sections to analyze axonal growth into the bridges. Bridges were loaded with a lentiviral vector encoding for either NT3, BDNF, or no lentivirus and implanted into the SC. SCs were retrieved after 1 week (n = 3), 2 weeks (n=6), and 4 weeks (n=6) post implantation, frozen, and sliced transversally in 10-µm thick sections. Every other section was collected on glass slides. For analysis, the bridge was divided in three regions based on location; rostral, middle, and caudal (Fig. 1c). Five sections from each region were stained for neurofilament (NF200; Sigma-Aldrich) and imaged using a Leica microscope at 50X. Images were stitch together using photo stitching software (PTgui, New House Internet Services B.V., Rotterdam, The Netherlands). The total number of axons were counted per channel. Three sections located at 300 µm (rostral), 2000 µm (middle), and 3500 µm (caudal) from the rostral bridge/tissue boundary were analyzed by a blinded observer. The sections were viewed at 50X to identify the positions of the 7 channels, and axons were counted within all channels at 400X. Trends were also confirmed by analyzing the area of neurofilament per channel using ImageJ.
b. Myelination
Sections located within 30 µm from those analyzed for axon growth were stained for Myelin Basic Protein (MBP) and imaged using a Leica microscope at 400X. The total number of myelinated axons was estimated by counting the number of MBP positive rings per channel, n=6 per condition. The extent of myelination was further quantified by counting the number of channels per bridge that had stained positive for MBP. Only tissue sections from the rostral segment of the bridge were quantified as the middle and caudal segments had little to no positive staining for MBP. Results were verified by double staining adjacent tissue sections for NF200 (green) and MBP (red) and counting the number of myelinated axons.
Statistics
For multiple comparisons, pairs were compared using an ANOVA with post hoc Tukey test with a p value <0.05 defined as significant. The numbers of myelinated axons per channel were compared using a Kruskal-Wallis test. The error bars represent standard errors in all figures.
RESULTS
Bridge implantation and transgene expression from lentiviral loaded bridges
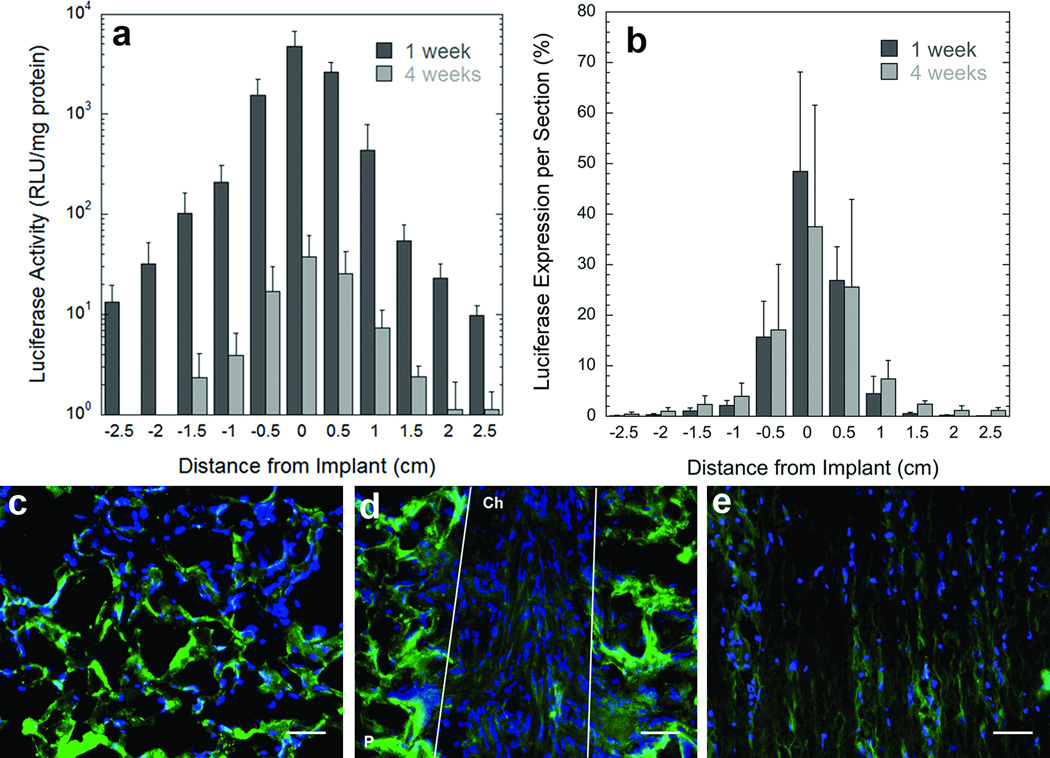
Initial studies investigated transgene expression following implantation of lentivirus loaded bridges in a rat spinal cord lateral hemisection. The bridges supported cell infiltration resulting in good tissue apposition with all edges of the bridge within a week post surgery (Fig. s1), with cells located within both the pores and channels of the bridge. Substantial transgene expression was observed within the injury at 7 days post implantation, and expression was sustained for at least 4 weeks post surgery (Fig. 2a). Transgene expression was also observed in segments adjacent to the implantation site, with expression decreasing with increasing distance from the implant. Expression at the injury site decreased 100 fold from 1 week to 4 weeks, with the greatest levels occurring at the injury site. At both time points, the greatest percentage of transgene expression was localized at the injury site, with 48% of total expression at 1 week, and 37% of total expression at 4 weeks. Approximately 90% of the transgene expression occurs within the implant and the 2 adjacent tissue segments at 1 week, with 80% of expression in the implant and adjacent segments at 4 weeks. Taken together, these studies demonstrate that delivering lentiviral vectors within the spinal cord results in localized and sustained transgene expression, transgene expression occurs in a gradient within the spinal cord, and expression is maximal at the implantation site. No significant increases in inflammation were observed when comparing macrophage infiltration within the bridges between lentivirus loaded and empty bridges (Fig. s1).
Figure 2. Transgene expression within the spinal cord.
(a) Luciferase expression in the rat spinal cord lateral hemisection model at two time points (1 week, and 4 weeks) (n=4) as a function of distance from the injury site. 0 = the bridge segment. RLU, relative light unit. (b) Percentage of total luciferase expression at each segment in the spinal cord at two time points (1 week, and 4 weeks) (n=4) as a function of distance from the injury site. 0 = the bridge segment. (c-e) Location of transduced cells after implantation of bridges loaded with a lentivirus encoding for enhanced green fluorescent protein (green). (c) Transduced cells were observed within the pores of the bridge, (d) aligned within the channels of the bridge, (e) and in the tissue adjacent to the bridge. Cell nuclei were stained with Hoechst (blue). P, polymer, Ch, channel. White lines indentify the boundaries of a channel. Scale bar is 50 µm. Error bars represent SEs.
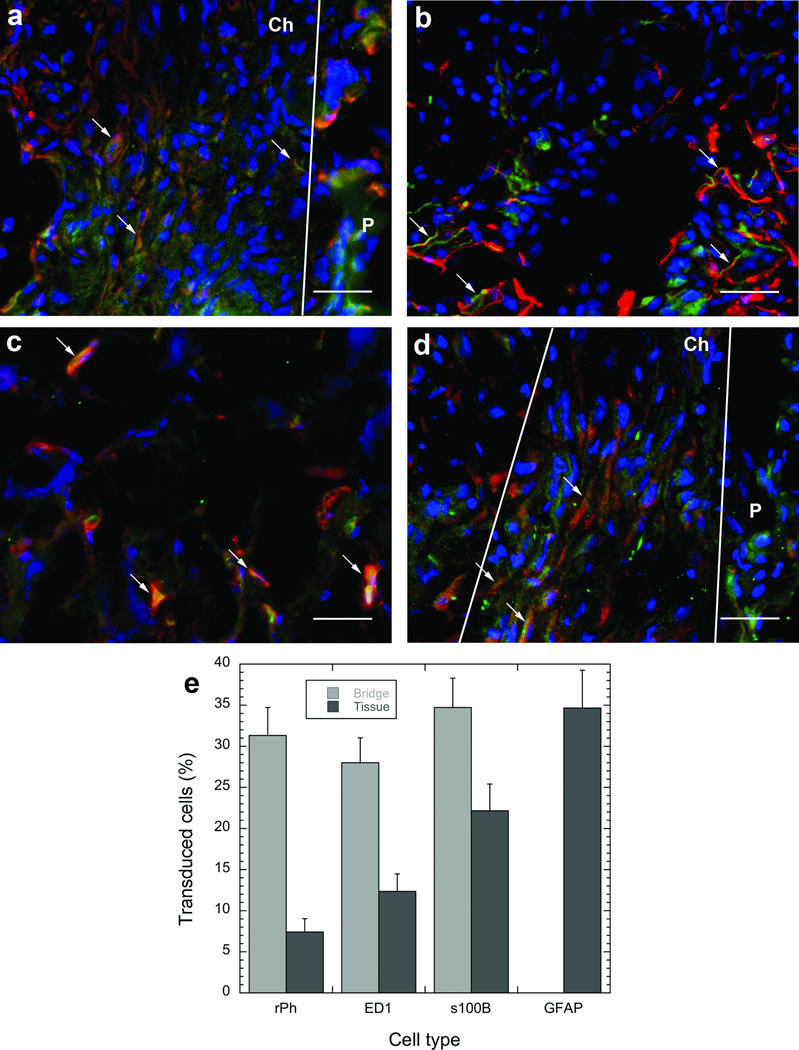
Subsequent experiments investigated the identity of transduced cells within the bridge and surrounding tissue by using EGFP transgene. At 7 days, transduced cells were localized in the channels and pores of the bridge and the adjacent tissue (Figs. 2c-e). The identity of these transduced cells was determined by double staining with antibodies for EGFP and either S-100β (includes Schwann cells and astrocytes), ED-1 (monocytes and macrophages), rPH (fibroblasts) or GFAP (reactive astrocytes). Previous studies had identified the first three cell types as the main ones that infiltrate the bridge after implantation, with these cell types and reactive astrocytes visualized in the tissue surrounding the bridge [4, 5]. Co-localization of stains for the cell-specific antigens (red) and transduced cells (green) was observed for S-100β- (Schwann cells), ED-1-(macrophages) and rPH- (fibroblasts) positive cells in the bridge and the adjacent tissue (Figs. 3a-c). The percentages of transduced cells in the bridge identified as Schwann cells, fibroblasts, and macrophages were 35%, 31%, and 28%, respectively. No transduced GFAP positive astrocytes were indentified within the bridge, consistent with previous results that astrocytes did not infiltrate the bridge [4, 5]. EGFP positive astrocytes were observed in the tissue surrounding the bridge (Fig. 3d). In this tissue, the percentages of transduced cells identified as astrocytes, Schwann cells, fibroblasts, and macrophages were 35%, 22%, 7.5%, and 12%, respectively. The percentage of unidentified (i.e. EGFP positive and red label negative) transduced cells in the bridge and adjacent tissue were 6% and 23.5%, respectively.
Figure 3. Identification of transduced cells.
Double staining of slides with antibodies to EGFP (green) and to specific cell types (red). The cell types antibodies were (a) S100β for Schwann cells, (b) GFAP for reactive astrocytes, (c) ED-1 for macrophages, or (d) rPH for fibroblasts. Cell nuclei were stained with Hoechst (blue). Arrows indicate the position of cells that stained positive for both EGFP and the specific cell antibody. P, polymer. Ch, channel. White lines indentify the boundaries of channels. Scale bar is 50 µm. Quantification of the profile of transduced cells within the bridge and the adjacent tissue (e). Error bars represent SEs.
Axon growth
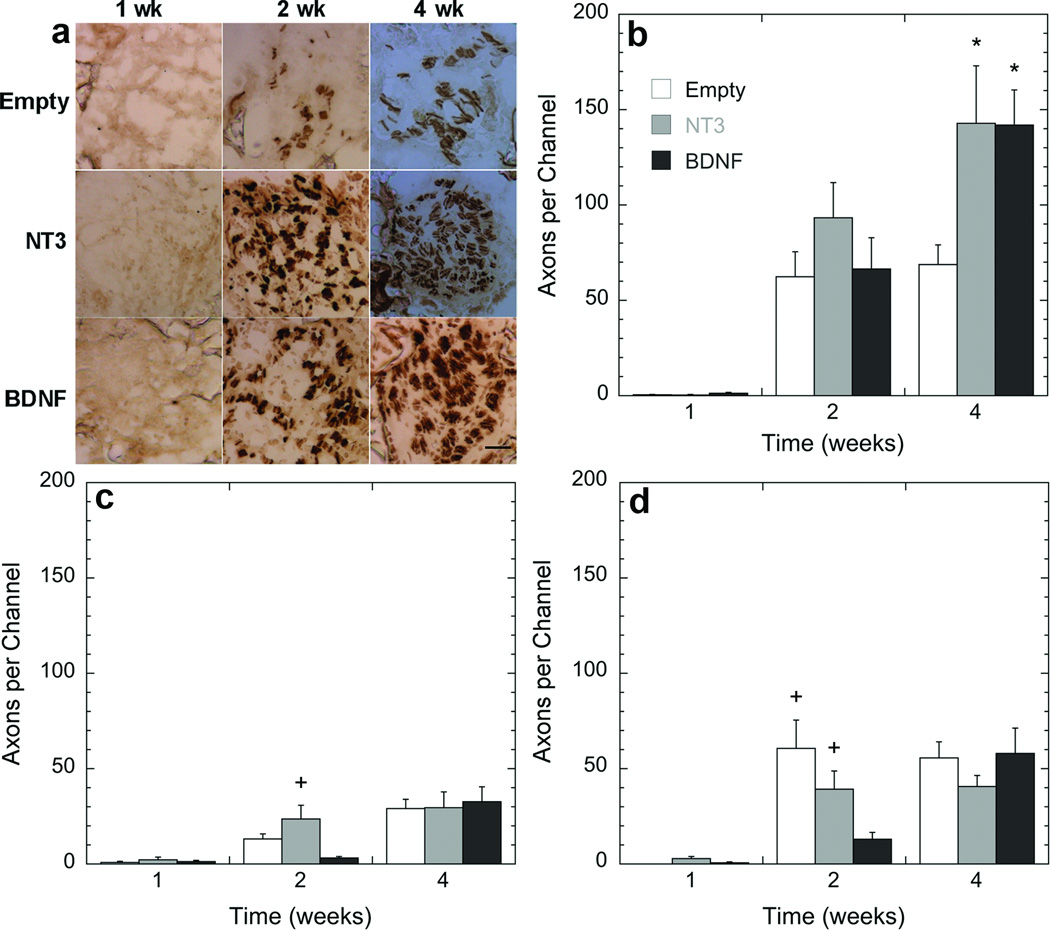
Bridges, loaded with lentivirus encoding for NT3 or BDNF, were implanted in a spinal cord hemisection to investigate the ingrowth of axons into and through the bridge at multiple time points. Bridges with no lentivirus were used as a negative control (empty). Coronal sections were grouped by dividing the bridge into 3 regions: rostral, middle and caudal (Fig. 1c). Few neurofilament positive axons were observed within the channels 1-week post surgery for any condition, but an increase in the number of axons per channel as a function of time was observed (Figs. 5b-d). At 2 weeks, substantial numbers of axons were observed within the channels, with no significant difference in neurofilament staining observed between the 7 channels (Fig. 4). Localization of positive neurofilament staining primarily to the channels suggests that that the bridge architecture is essential to promoting axonal entry and regeneration.
Figure 5. Quantification of axonal regeneration as a function of time and position within the bridges.
(a) Time course of a single channel at the rostral position. (b-d) Axons were quantified by counting the number of NF200 positive neurofilaments inside the channels. Slides analyzed were selected from the (b) rostral (300 µm), (c) middle (2000 µm), and (d) caudal (3500 µm) regions of the bridge. Statistical analysis was done by an ANOVA with Tukey post hoc test with a p<0.05 found to be significant differently. *, significant difference compared to empty at the same time point; +, significant difference compared to BDNF at the same time point). Scale bar in a is 50 µm.
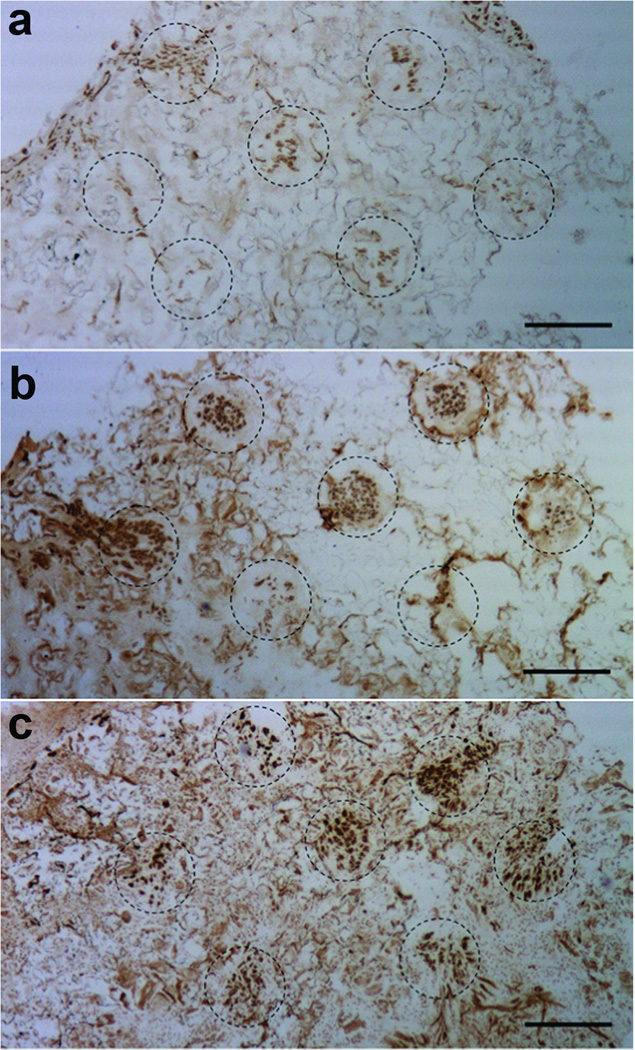
Figure 4. Axon regeneration inside the channels for different treatment conditions.
Images of an (a) empty bridge or a bridge loaded with a lentivirus encoding either (b) NT3 or (c) BDNF. Bridges were retrieved 4 weeks post implantation, and sections from the rostral position were stained with NF200 (neurofilament, brown). Channels are identified by the dash circles. Scale bar is 250 µm.
The extent of axon growth was a function of neurotrophin expression and time (overall ANOVA, F=11.8, p<0.0001). At the rostral position, delivery of lentivirus encoding either NT3 or BDNF significantly increased axon number at 4 weeks compared to the same condition at 2 weeks (p<0.005) and 1 week (p>0.0001) (Fig. 5b). However, no increase in the number of axons within the rostral location in the empty bridge was observed between 2 and 4 weeks (p=0.7087). Relative to empty bridges, delivery of lentivirus encoding either NT3 or BDNF significantly increased (p<0.005) the number of axons per channel in the rostral section at 4 weeks by approximately a factor of 2 (Fig. 5b); no significant difference was obtained between NT3 and BDNF (p=0.95) at the rostral position at 4 weeks. For NT3 and BDNF, the axon counts at 4 weeks in the rostral location were significantly greater compared to empty, NT3, and BDNF at other time points and locations (p<0.005).
The number of axons at the middle and caudal locations of the bridge were less than the numbers obtained for the rostral section. There were 4.8, 4.3, and 2.3-fold less axons for the middle segment relative to the rostral segment for NT3, BDNF, or empty loaded bridges, respectively (Figs. 5b-d). For the caudal location, the number of axons was 3.5, 2.4, and 1.2-fold less than the rostral location. No significant difference was observed between empty bridges comparing caudal and rostral locations (p=0.4269) (Figs. 5b,d). At 2 weeks in the middle section, the number of axons determined for NT3 lentivirus delivery was greater compared to the BDNF and empty conditions (p=<0.05); however the axon numbers for all conditions in the middle section reached similar levels at 4 weeks (Fig. 5c). In the caudal location, at 2 weeks both NT3 expression and empty bridges resulted in greater axon counts relative to expression of BDNF (p<0.05) (Fig. 5d). At 4 weeks all conditions resulted in similar axon counts. These results demonstrate that lentiviral delivery of neurotrophic factors, combined with the permissive environment of the bridge enhances the number of regenerating axons.
Lentivirus encoding neurotrophins, NT3 or BDNF, enhanced myelination
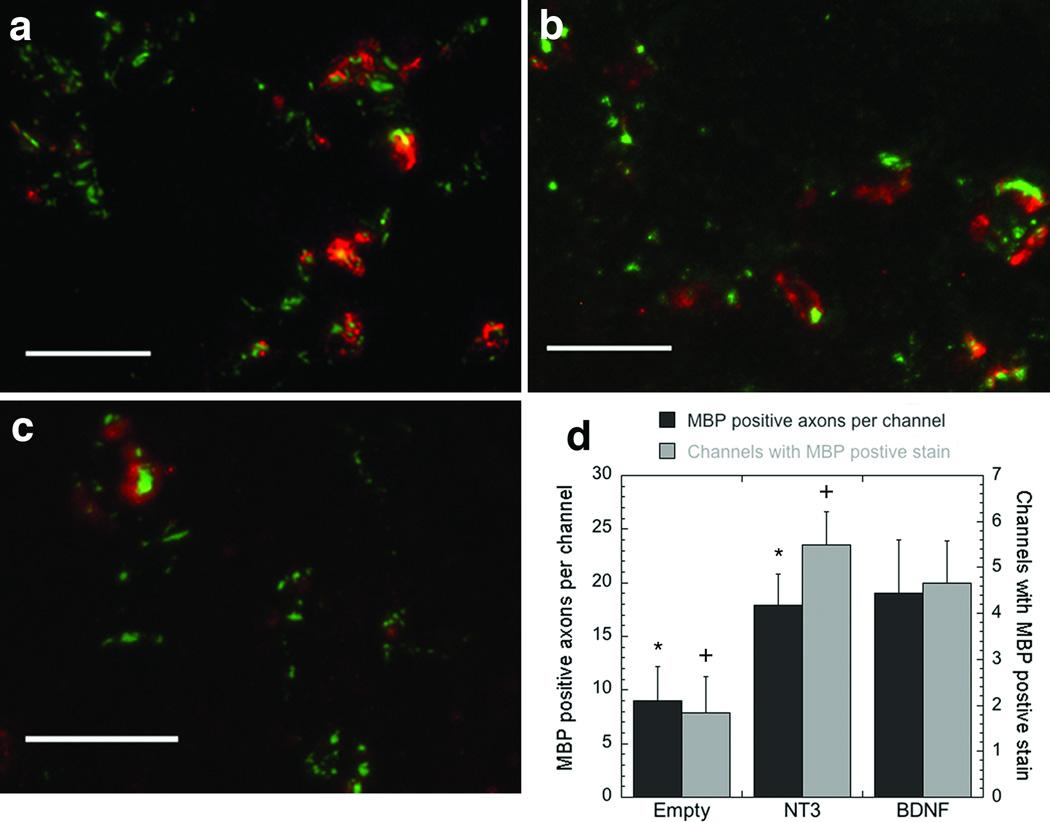
Myelination of axons within the bridge was subsequently investigated as a function of time and neurotrophic factor expression. Myelin basic protein (MBP) was observed in several of the channels in which regenerating axons were present. The middle and caudal regions had decreased levels of positive MBP staining compared to the rostral region. In the rostral location, for NT3, 84% of the channels that contained axons stained positive for MBP, compared to 68% and 32% for BDNF and empty, respectively (Figs. 6a-c). BDNF and NT3 lentiviral delivery significantly enhanced (p<0.01) the number of MBP positive axons and the number of channels in which MBP was observed, compared to empty bridges (Fig. 6d). NT3 and BDNF had approximately 18 and 19 myelinated axons per channel, compared to 9 for empty bridges. The increase in the number of myelinated axons per channel correlated with the increased number of axons per channel. For all conditions, the percentage of myelinated axons was approximately 13%.
Figure 6. Axon myelination inside the channels.
Tissue sections were stained for myelin basic protein (MBP, red) and neurofilament (NF200, green) (a-c) 4 weeks after implantation. The images are from the rostral sections of (a) a bridge loaded with a lentivirus encoding for BDNF, (b) a bridge loaded with a lentivirus encoding for NT3, and (c) an empty bridge. Scale bar is 50 µm in a-c. (d) Quantification of the MBP stain as a function of the treatment at 4 weeks post implantation and in the rostral position. Quantification was done by counting the number of myelinated axons inside the channels (■, left axis), or by counting the number of channels with positive stained myelinated axons (□, right axis). * Indicates significant difference compared to empty (p<0.01), using the Kruskal-Wallis Test. + Indicates significant difference compared to empty (p<0.01), using an ANOVA with Tukey post hoc test.
DISCUSSION
We report that delivery of lentivirus encoding the neurotrophins NT3 or BDNF from a multichannel biomaterial bridge enhanced axon regeneration and axon myelination after spinal cord injury (SCI) in a rat hemisection model. The delivery of growth factors after an injury can enhance the regenerative capacity of the body by directing or promoting cell processes that lead to regeneration. Studies delivering neurotrophic factors after SCI, including neurotrophin 3 (NT3) [6, 20–24], brain derived neurotrophin factor (BDNF) [16, 20, 25–28], nerve growth factor (NGF) [8, 21], glial-cell derived neurotrophin factor (GDNF) [29, 30], and others [31–33], have shown that these factors enhance axon regeneration by stimulating axon growth, reducing secondary injury, stimulating cell survival, and/or promoting axon myelination. These factors are commonly delivered by osmotic pumps, which can provide a sustained release. Yet, osmotic pumps can clog, require a second surgery for removal, and deliver the factors near the injury to avoid tissue damage by the catheter. Alternatively, genetically engineered cells have been transplanted within the injury site to serve as bioreactors for protein production to locally provide these therapeutic factors. Nevertheless, a low survival rate for transplanted cells can complicate this strategy [19]. Gene delivery targeting host cells can induce the expression of neurotrophic factors from within the injured microenvironment [23]. The direct injection of vectors has been employed with some benefit [23, 34]; however, expression may not be localized to the injury. In this report, we have attempted to immobilize lentivirus in a multiple channel bridge to capture the synergizing effects of both delivering neurotrophic factors and physical guidance of axonal growth via a bioengineered scaffold.
We have previously reported axonal growth after SCI through a multichannel bridge, which provides a porous structure that facilitates cellular infiltration to mechanically stabilize the injury. Multiple channels within the bridge orient infiltrating cells and direct axonal elongation [4, 5, 35]. The delivery of non-viral vectors from the bridge resulted in localized transfection [4, 35]. However, the expression level obtained via non-viral vectors may have been insufficient for functional effects. Herein, we demonstrate that lentivirus delivery resulted in localized protein expression within the injury site, with lower expression persisting throughout the adjacent tissue. The expression level with lentivirus delivery was approximately 1000 fold greater in the bridge than for non-viral vector delivery. The decreased expression in the adjacent tissue relative to the bridge would be expected to create a concentration gradient in a secreted factor such as neurotrophins, with the greatest concentration at the injury and lower concentrations in the adjacent tissue. Axons are known to respond to changes in the concentration of growth factors, therefore, the gradient produced by lentivirus delivery may facilitate directed axonal growth toward the injury and into the bridge.
The cells transduced by lentivirus delivery include macrophages, Schwann cells, and fibroblasts that had infiltrated the pores of the bridge, consistent with our previous report using plasmid and lipoplex-loaded bridges [4, 35]. However, lentivirus delivery also resulted in transduction of astrocytes in the tissue adjacent to the bridge. All of these cells are commonly present after injury, and can serve diverse functions to enhance or inhibit regeneration. Macrophages can secrete soluble factors that can alter the regenerative microenvironment of the bridge, both aiding and inhibiting regeneration [36, 37]. Schwann cells, which are not endogenous to the spinal cord yet enter through the damaged root entry zones after injury, may function to support and promote regeneration by releasing growth-promoting factors and provide remyelination of the axons [38, 39]. Astrocytes, which are responsible for the formation of the glial scar and are recognized as a barrier to regeneration by inhibiting the crossing of axons though the tissue, were localized within the adjacent tissue and the tissue/bridge boundary.
Lentivirus encoding for NT-3 and BDNF were delivered, which target descending tracts responsible for recovery of motor function. NT3 has been associated with a robust and consistent outgrowth of corticospinal axons [6, 40]. Administration of exogenous BDNF can improve survival of axotomized motoneurons in neonatal rats [41]. Lentivirus induced expression of NT3 and BDNF enhanced axonal growth into the channels of the bridge relative to empty bridges. This increase observed at the rostral end of the bridge is consistent with the action of NT3 and BDNF on descending CNS tracts [6, 8, 12]. Future studies will combine these neurotrophin encoding lentivirus loaded bridges with strategies to degrade the glial scar that will enhance re-entry into the host tissue, which is necessary to promote functional recovery from the regenerated axons.
Myelinated axons were observed within the channels of the bridge, and delivery and induced expression of NT-3 and BDNF significantly increased this number of myelinated axons. Demyelination of spared axons and lack of myelination of regenerating axons are considered limiting factors in the recovery of neural function after injury. In chemically induced demyelination, endogenous oligodendrocyte precursor cells have been shown to migrate and remyelinate spared axons [42], though these studies suggest that these cells are able to migrate a fixed distance. Cell transplantation has been employed as a means to promote remyelination with some reports indicating that progenitor cells can develop into oligodendrocytes and remyelinate axons in vivo [43]. Transplantation of PN grafts and Schwann cells has resulted in myelination of spared and regenerating axons by the Schwann cells that are delivered alone or as a part of the graft [44–46]. Alternatively, cells have been transplanted that express NT3 and BDNF, which have enhanced oligodendrocyte survival and axon myelination [20, 28, 47]. NT3 and BDNF may also enhance a number of other processes. For example, BDNF has been associated with a reduction in the inflammatory response, including a reduction in astrocyte numbers [48], which further aids axon regeneration. Herein, we report an increase in the number of myelinated axons without cell transplantation. Interestingly, the percentage of myelinated axons was similar between empty and lentivirus loaded bridges (~13%). This manuscript is the first report of the novel finding that endogenous cells migrate into the channels to myelinate regenerating axons. Ongoing studies will further characterize the source and extent of myelination and investigate strategies to further enhance the process.
CONCLUSIONS
This study investigated lentivirus delivery from multiple channel PLG bridges in a rat spinal cord hemisection injury model. Transgene expression was greatest at the injury site, with transduced cells inside the bridge and in the adjacent tissue. Astrocytes, macrophages, fibroblasts, and Schwann cells were identified as some of the cell types transduced. The bridge induced axonal growth down the channels. Delivery of lentivirus vectors encoding for the neurotrophic factors NT3 and BDNF enhanced the number of regenerating axons, and also the extent of myelination. This study demonstrates that lentivirus delivery of neurotrophic factors, combined with the permissive environment of the bridge, enhances nerve regeneration after injury. We report a significant increase in the number of axons and myelinated axons after SCI. Gene delivery is a versatile strategy in which multiple vectors can be delivered from one vehicle to promote growth or to block inhibition. Delivering multiple vectors encoding for distinct proteins is straightforward as each vector will have similar physical properties, and may provide a mechanism to address the complexity microenvironment that does not support regeneration.
Supplementary Material
(a) Hematoxylin and eosin stain of a lateral section of a bridge in the spinal cord, 1 week post implantation. The image shows good tissue apposition with cells infiltration into the pores and channels. (c,e) ED-1 stain (macrophages) of bridges retrieved 1 week post implantation (b,d) counterstained with Hoechst for cell nuclei. The bridges were either (b,c) empty or loaded (d,e) with a lentivirus. No considerable differences in inflammation are observed by adding lentivirus to the bridge. Scale bar is 400 µm.
Neurofilament (NF200) staining of one channel at 4 weeks for empty, NT3, and BDNF in the rostral (300 µm), middle (2000 µm), and caudal (3500µm) locations. Scale bar is 100 µm.
ACKNOWLEDGMENTS
Financial support for this research was provided by the NIH (RO1 EB005678, R21 EB006520, RO1 EB 003806) and the Christopher and Dana Reeve Foundation spinal cord injury core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.David S, Aguayo AJ. Axonal elongation into peripheral nervous system "bridges" after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- 2.Xu XM, Guenard V, Kleitman N, Bunge MB. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol. 1995;351:145–160. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- 3.Geller HM, Fawcett JW. Building a bridge: engineering spinal cord repair. Exp Neurol. 2002;174:125–136. doi: 10.1006/exnr.2002.7865. [DOI] [PubMed] [Google Scholar]
- 4.De Laporte L, Yang Y, Zelivyanskaya ML, Cummings BJ, Anderson AJ, Shea LD. Plasmid releasing multiple channel bridges for transgene expression after spinal cord injury. Mol Ther. 2009;17:318–326. doi: 10.1038/mt.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, De Laporte L, Zelivyanskaya ML, Whittlesey KJ, Anderson AJ, Cummings BJ, et al. Multiple channel bridges for spinal cord injury: cellular characterization of host response. Tissue Eng Part A. 2009;15:3283–3295. doi: 10.1089/ten.tea.2009.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnell L, Schneider R, Kolbeck R, Barde YA, Schwab ME. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994;367:170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- 7.Malcangio M, Ramer MS, Boucher TJ, McMahon SB. Intrathecally injected neurotrophins and the release of substance P from the rat isolated spinal cord. Eur J Neurosci. 2000;12:139–144. doi: 10.1046/j.1460-9568.2000.00890.x. [DOI] [PubMed] [Google Scholar]
- 8.Namiki J, Kojima A, Tator CH. Effect of brain-derived neurotrophic factor, nerve growth factor, and neurotrophin-3 on functional recovery and regeneration after spinal cord injury in adult rats. J Neurotrauma. 2000;17:1219–1231. doi: 10.1089/neu.2000.17.1219. [DOI] [PubMed] [Google Scholar]
- 9.Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, et al. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- 10.Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinh P, Bhatia N, Rasouli A, Suryadevara S, Cahill K, Gupta R. Transplantation of preconditioned Schwann cells following hemisection spinal cord injury. Spine. 2007;32:943–949. doi: 10.1097/01.brs.0000261408.61303.77. [DOI] [PubMed] [Google Scholar]
- 12.Olson HE, Rooney GE, Gross L, Nesbitt JJ, Galvin KE, Knight A, et al. Neural stem cell- and Schwann cell-loaded biodegradable polymer scaffolds support axonal regeneration in the transected spinal cord. Tissue Eng Part A. 2009;15:1797–1805. doi: 10.1089/ten.tea.2008.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5:1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- 14.Chopp M, Zhang XH, Li Y, Wang L, Chen J, Lu D, et al. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport. 2000;11:3001–3005. doi: 10.1097/00001756-200009110-00035. [DOI] [PubMed] [Google Scholar]
- 15.Dasari VR, Spomar DG, Gondi CS, Sloffer CA, Saving KL, Gujrati M, et al. Axonal remyelination by cord blood stem cells after spinal cord injury. J Neurotrauma. 2007;24:391–410. doi: 10.1089/neu.2006.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Kim D, Himes BT, Chow SY, Schallert T, Murray M, et al. Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function. J Neurosci. 1999;19:4370–4387. doi: 10.1523/JNEUROSCI.19-11-04370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin Y, Fischer I, Tessler A, Houle JD. Transplants of fibroblasts genetically modified to express BDNF promote axonal regeneration from supraspinal neurons following chronic spinal cord injury. Exp Neurol. 2002;177:265–275. doi: 10.1006/exnr.2002.7980. [DOI] [PubMed] [Google Scholar]
- 18.Blesch A, Tuszynski MH. Transient growth factor delivery sustains regenerated axons after spinal cord injury. J Neurosci. 2007;27:10535–10545. doi: 10.1523/JNEUROSCI.1903-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill CE, Moon LD, Wood PM, Bunge MB. Labeled Schwann cell transplantation: cell loss, host Schwann cell replacement, and strategies to enhance survival. Glia. 2006;53:338–343. doi: 10.1002/glia.20287. [DOI] [PubMed] [Google Scholar]
- 20.McTigue DM, Horner PJ, Stokes BT, Gage FH. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci. 1998;18:5354–5365. doi: 10.1523/JNEUROSCI.18-14-05354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young C, Miller E, Nicklous DM, Hoffman JR. Nerve growth factor and neurotrophin-3 affect functional recovery following peripheral nerve injury differently. Restor Neurol Neurosci. 2001;18:167–175. [PubMed] [Google Scholar]
- 22.Zhou L, Baumgartner BJ, Hill-Felberg SJ, McGowen LR, Shine HD. Neurotrophin-3 expressed in situ induces axonal plasticity in the adult injured spinal cord. J Neurosci. 2003;23:1424–1431. doi: 10.1523/JNEUROSCI.23-04-01424.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor L, Jones L, Tuszynski MH, Blesch A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci. 2006;26:9713–9721. doi: 10.1523/JNEUROSCI.0734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor SJ, Rosenzweig ES, McDonald JW, 3rd, Sakiyama-Elbert SE. Delivery of neurotrophin-3 from fibrin enhances neuronal fiber sprouting after spinal cord injury. J Control Release. 2006;113:226–235. doi: 10.1016/j.jconrel.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koda M, Hashimoto M, Murakami M, Yoshinaga K, Ikeda O, Yamazaki M, et al. Adenovirus vector-mediated in vivo gene transfer of brain-derived neurotrophic factor (BDNF) promotes rubrospinal axonal regeneration and functional recovery after complete transection of the adult rat spinal cord. J Neurotrauma. 2004;21:329–337. doi: 10.1089/089771504322972112. [DOI] [PubMed] [Google Scholar]
- 26.Koda M, Murakami M, Ino H, Yoshinaga K, Ikeda O, Hashimoto M, et al. Brain-derived neurotrophic factor suppresses delayed apoptosis of oligodendrocytes after spinal cord injury in rats. J Neurotrauma. 2002;19:777–785. doi: 10.1089/08977150260139147. [DOI] [PubMed] [Google Scholar]
- 27.Kwon BK, Liu J, Lam C, Plunet W, Oschipok LW, Hauswirth W, et al. Brain-derived neurotrophic factor gene transfer with adeno-associated viral and lentiviral vectors prevents rubrospinal neuronal atrophy and stimulates regeneration-associated gene expression after acute cervical spinal cord injury. Spine. 2007;32:1164–1173. doi: 10.1097/BRS.0b013e318053ec35. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki M, Radtke C, Tan AM, Zhao P, Hamada H, Houkin K, et al. BDNF-hypersecreting human mesenchymal stem cells promote functional recovery, axonal sprouting, and protection of corticospinal neurons after spinal cord injury. J Neurosci. 2009;29:14932–14941. doi: 10.1523/JNEUROSCI.2769-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blesch A, Tuszynski MH. Cellular GDNF delivery promotes growth of motor and dorsal column sensory axons after partial and complete spinal cord transections and induces remyelination. J Comp Neurol. 2003;467:403–417. doi: 10.1002/cne.10934. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Ma Z, Smith GM, Wen X, Pressman Y, Wood PM, et al. GDNF-enhanced axonal regeneration and myelination following spinal cord injury is mediated by primary effects on neurons. Glia. 2009;57:1178–1191. doi: 10.1002/glia.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi NR, Fan DP, Giehl KM, Bedard AM, Wiegand SJ, Tetzlaff W. BDNF and NT-4/5 prevent atrophy of rat rubrospinal neurons after cervical axotomy, stimulate GAP-43 and Talpha1-tubulin mRNA expression, and promote axonal regeneration. J Neurosci. 1997;17:9583–9595. doi: 10.1523/JNEUROSCI.17-24-09583.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scarisbrick IA, Isackson PJ, Windebank AJ. Differential expression of brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4/5 in the adult rat spinal cord: regulation by the glutamate receptor agonist kainic acid. J Neurosci. 1999;19:7757–7769. doi: 10.1523/JNEUROSCI.19-18-07757.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blesch A, Yang H, Weidner N, Hoang A, Otero D. Axonal responses to cellularly delivered NT-4/5 after spinal cord injury. Mol Cell Neurosci. 2004;27:190–201. doi: 10.1016/j.mcn.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Abdellatif AA, Pelt JL, Benton RL, Howard RM, Tsoulfas P, Ping P, et al. Gene delivery to the spinal cord: comparison between lentiviral, adenoviral, and retroviral vector delivery systems. J Neurosci Res. 2006;84:553–567. doi: 10.1002/jnr.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Laporte L, Yan AL, Shea LD. Local gene delivery from ECM-coated poly(lactide-co-glycolide) multiple channel bridges after spinal cord injury. Biomaterials. 2009;30:2361–2368. doi: 10.1016/j.biomaterials.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz M. Immunological approaches to the treatment of spinal cord injury. BioDrugs. 2001;15:585–593. doi: 10.2165/00063030-200115090-00003. [DOI] [PubMed] [Google Scholar]
- 37.Anderson AJ. Mechanisms and pathways of inflammatory responses in CNS trauma: spinal cord injury. J Spinal Cord Med. 2002;25:70–79. doi: 10.1080/10790268.2002.11753604. discussion 80. [DOI] [PubMed] [Google Scholar]
- 38.King VR, Phillips JB, Hunt-Grubbe H, Brown R, Priestley JV. Characterization of non-neuronal elements within fibronectin mats implanted into the damaged adult rat spinal cord. Biomaterials. 2006;27:485–496. doi: 10.1016/j.biomaterials.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 39.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 40.Grill R, Murai K, Blesch A, Gage FH, Tuszynski MH. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J Neurosci. 1997;17:5560–5572. doi: 10.1523/JNEUROSCI.17-14-05560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vejsada R, Sagot Y, Kato AC. Quantitative comparison of the transient rescue effects of neurotrophic factors on axotomized motoneurons in vivo. Eur J Neurosci. 1995;7:108–115. doi: 10.1111/j.1460-9568.1995.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 42.Mi S, Miller RH, Tang W, Lee X, Hu B, Wu W, et al. Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells. Ann Neurol. 2009;65:304–315. doi: 10.1002/ana.21581. [DOI] [PubMed] [Google Scholar]
- 43.Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nomura H, Baladie B, Katayama Y, Morshead CM, Shoichet MS, Tator CH. Delayed implantation of intramedullary chitosan channels containing nerve grafts promotes extensive axonal regeneration after spinal cord injury. Neurosurgery. 2008;63:127–141. doi: 10.1227/01.NEU.0000335080.47352.31. discussion 41–3. [DOI] [PubMed] [Google Scholar]
- 45.Weidner N, Blesch A, Grill RJ, Tuszynski MH. Nerve growth factor-hypersecreting Schwann cell grafts augment and guide spinal cord axonal growth and remyelinate central nervous system axons in a phenotypically appropriate manner that correlates with expression of L1. J Comp Neurol. 1999;413:495–506. doi: 10.1002/(sici)1096-9861(19991101)413:4<495::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 46.Tuszynski MH, Weidner N, McCormack M, Miller I, Powell H, Conner J. Grafts of genetically modified Schwann cells to the spinal cord: survival, axon growth, and myelination. Cell Transplant. 1998;7:187–196. doi: 10.1177/096368979800700213. [DOI] [PubMed] [Google Scholar]
- 47.Girard C, Bemelmans AP, Dufour N, Mallet J, Bachelin C, Nait-Oumesmar B, et al. Grafts of brain-derived neurotrophic factor and neurotrophin 3-transduced primate Schwann cells lead to functional recovery of the demyelinated mouse spinal cord. J Neurosci. 2005;25:7924–7933. doi: 10.1523/JNEUROSCI.4890-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jain A, Kim YT, McKeon RJ, Bellamkonda RV. In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials. 2006;27:497–504. doi: 10.1016/j.biomaterials.2005.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Hematoxylin and eosin stain of a lateral section of a bridge in the spinal cord, 1 week post implantation. The image shows good tissue apposition with cells infiltration into the pores and channels. (c,e) ED-1 stain (macrophages) of bridges retrieved 1 week post implantation (b,d) counterstained with Hoechst for cell nuclei. The bridges were either (b,c) empty or loaded (d,e) with a lentivirus. No considerable differences in inflammation are observed by adding lentivirus to the bridge. Scale bar is 400 µm.
Neurofilament (NF200) staining of one channel at 4 weeks for empty, NT3, and BDNF in the rostral (300 µm), middle (2000 µm), and caudal (3500µm) locations. Scale bar is 100 µm.