Abstract
Internal cranial anatomy is a challenging area to study in fossilized skulls because of small sample sizes and varied post-mortem preservational alterations. This difficulty has led to the lack of correspondence between results obtained from direct osteological observation and from more indirect reconstruction methods. This paper presents corroborating evidence from direct osteological observation and from reconstruction based on computed X-ray tomography (CT) on the internal cranial anatomy of the ankylosaurid dinosaur Euoplocephalus tutus. A remarkable specimen of Euoplocephalus preserves rarely observed internal cranial structures such as vascular impressions in the nasal cavity, olfactory turbinates and possible impressions of conchae. Comparison with fossils and CT models of other taxa and other Euoplocephalus specimens adds osteological evidence for the previously reconstructed nasal cavity in this dinosaur and revises the previously described braincase morphology. A new interpretation of the ethmoidal homology identifies a mesethmoid, sphenethmoid and ectethmoid. These ethmoidal ossifications are continuous with the mineralized walls of the nasal cavity. The location of the olfactory fenestra provides further evidence that the olfactory regions of the nasal cavity are pushed to the sides of the main airway. This implies that the function of the vascular impressions in the nasal cavity and the looping of the cavity are not related to olfaction. A byproduct of the elongate, looping airway is a dramatic increase in surface area of the nasal respiratory mucosa, which in extant species has been linked to heat and water balance. A role in vocalization as a resonating chamber is another possible function of the looping and elongation of the nasal cavity. Olfaction remains as a possible function for the enlarged olfactory region, suggesting that multiple functions account for different parts of the ankylosaurid nasal cavity that underwent substantial modification. Cranial endocasts show negligible variation within Euoplocephalus, which lends some confidence to interspecific comparisons of endocranial morphology.
Keywords: Ankylosauridae, braincase, Dinosaur Park Formation, nasal cavity
Introduction
Ankylosaurs are a clade of ornithischian dinosaurs commonly called ‘armoured dinosaurs’. Due to their highly modified skulls, detailed description of the cranial anatomy is particularly important in identification of both basal and derived conditions within the clade. However, the skulls are extensively ossified and little is known about the internal cranial morphology of ankylosaurs. Cranial elements are rarely preserved individually and it is unusual to find a skull that shows the internal morphology without the aid of X-ray computed tomography (CT). Several papers describe ankylosaur braincases and cranial endocasts (Maryańska, 1977; Coombs, 1978a; Kurzanov & Tumanova, 1978; Carpenter et al. 2001; Averianov, 2002; Vickaryous & Russell, 2003; Hayakawa et al. 2005; Witmer & Ridgely, 2008; Parsons & Parsons, 2009). However, these authors provided different identifications of the foramina perforating the braincases and this makes comparison difficult. Only a handful of papers deal with other regions inside ankylosaur skulls, such as the nasal cavity. Sections of a few skulls (e.g. Euoplocephalus AMNH 5403) led to reconstruction of the ankylosaur nasal cavity as a sagittal S-shaped airway (Maryańska, 1977; Coombs, 1978b; Witmer, 1997). Results from two-dimensional CT slices supported this view (Vickaryous & Russell, 2003; Vickaryous, 2006). A three-dimensional digital reconstruction of a CT scan of the skull of the ankylosaurid Euoplocephalus recently overturned the reconstruction of a simple S-shaped airway in this genus (Witmer & Ridgely, 2008). According to the new reconstruction, the nasal cavity of Euoplocephalus follows a complex path of twists and turns that create a series of loops of the airway (Fig. 1). The nasal cavity of the nodosaurid ankylosaur Panoplosaurus also has the anterior and posterior loops, although the path of the airway is less complicated than in Euoplocephalus (Witmer & Ridgely, 2008). The complex pathways of the ankylosaur nasal cavities in Witmer & Ridgely (2008) also revealed that the internal space within the skulls previously identified as paranasal sinuses (Witmer, 1997; Vickaryous & Russell, 2003; Vickaryous, 2006) are actually part of the looping airway, although this was not readily evident in two-dimensional CT slices.
Fig. 1.
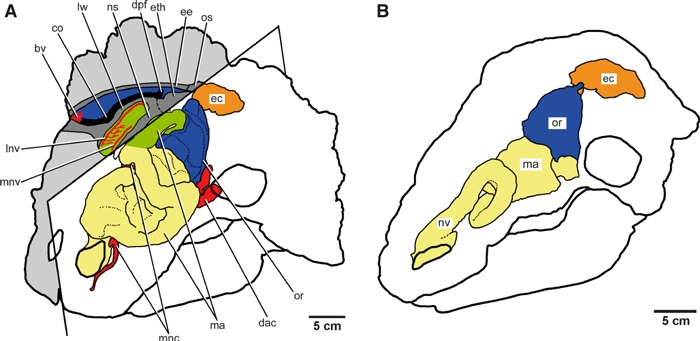
Schematic reconstructions of the nasal cavity morphology of two ankylosaur skulls. (A) The ankylosaurid Euoplocephalus tutus (AMNH 5405). The left half of the skull is derived from the reconstruction published by Witmer & Ridgely (2008), whereas the right half represents new information based on the osteological correlates of the soft tissues within and around the nasal cavity in UALVP 47977. The specimen (UALVP 47977) preserves parts of the main airway (in green), the olfactory region (in blue), and the endocranial cavity (in orange). (B) The nodosaurid Panoplosaurus mirus (ROM 1215) after Witmer & Ridgely (2008) for comparison. The nasal passage of Euoplocephalus is looped in more complex ways than that of Panoplosaurus, and the olfactory region of Euoplocephalus is pushed to the side of the main airway. bv, blood vessel trace; co, groove that possibly housed the concha; dac, dorsal alveolar canal; dpf, descending process fused to the ventral surface of the frontal; olfactory turbinate; ec, endocranial cavity; ee, ectethmoid; eth, ethmoidal complex; lnv, lateral nasal vessels; lw, lateral wall of the main airway; ma, main airway; mnv, medial nasal vessels; mnc, medial nasal canal; ns, nasal septum; nv, nasal vestibule; or, olfactory region; os, orbitosphenoid.
Witmer & Ridgely (2008) radically transformed the previous view of a straight airway in ankylosaurs because their reconstruction was possible through sophisticated CT methodology. For instance, Witmer & Ridgely (2008) note that the nodosaurid ankylosaurs Edmontonia (AMNH 3076) and Panoplosaurus (ROM 1215) are likely to differ from each other in the degree of mineralization within the nasal cavity. Whereas their reconstruction showed a looping airway for Panoplosaurus (Witmer & Ridgely, 2008), a simple, straight airway was previously reconstructed for Edmontonia due to the lack of apparent subdivision within the nasal cavity (Vickaryous, 2006). Witmer & Ridgely (2008) observed thin mineralized laminae as well as heterogeneities in the matrix within the nasal cavity of the same specimen of Edmontonia (AMNH 3076), which highlights the sensitivity of data obtained via CT scanning. For these reasons, corroborative evidence from direct observation of the skull morphology is important. In addition, the ankylosaur literature dealing with internal cranial anatomy rarely deals with comparative aspects of the braincase and nasal cavities.
Several specimens of the ankylosaurid Euoplocephalus from the Campanian (Late Cretaceous) of southern Alberta, Canada fill this gap. Two of the specimens (AMNH 5238 and UALVP 47977) reveal osteological correlates of the soft tissues within the skull, whereas the others (AMNH 5405 and UALVP 31) offer new data on braincase anatomy through three-dimensional reconstructions based on CT scanning. Cranial endocasts of these specimens establish correspondence between the cranial nerves and the foramina perforating the braincase wall in UALVP 47977.
Institutional abbreviations: AMNH (American Museum of Natural History, New York, NY, USA); MPC (Mongolian Paleontological Center, Ulanbaatar, Mongolia; followed by the collector's initials and field number); PIN (Paleontological Institute, Moscow, Russia); ROM (Royal Ontario Museum, Toronto, Canada); TMP (Royal Tyrrell Museum of Palaeontology, Drumheller, AB, Canada); UALVP (University of Alberta Laboratory for Vertebrate Paleontology, Edmonton, AB, Canada); and ZPAL (Institute of Palaeobiology of the Polish Academy of Sciences, Warsaw, Poland).
Materials and methods
A partial ankylosaurid skull roof (UALVP 47977) was collected from the Dinosaur Park Formation in Dinosaur Provincial Park in 1971. The precise location and stratigraphic level of the site was not recorded, but the site is near Happy Jack's on the north side of Red Deer River (A.L. Lindoe, personal communication, 2007). Due to erosion of the skull before field collection, the nasal cavity, the orbital region and the upper half of the braincase are exposed ventrally. Two ankylosaurid ankylosaurs are currently recognized from the Dinosaur Park Formation: Dyoplosaurus, known from a single specimen recovered from the lower part of the formation, and Euoplocephalus, known from numerous specimens throughout the formation and the overlying Horseshoe Canyon Formation (Parks, 1924; Arbour et al. 2009). The holotype specimen of Dyoplosaurus preserves the posterior part of the skull roof. The skull (along with most of the skeleton) is affixed to a panel and so the ventral side cannot be observed. At present, there are no cranial characters that separate Dyoplosaurus from Euoplocephalus; the features that distinguish Dyoplosaurus from Euoplocephalus are restricted to the pelvis and pes. As such, it is possible that isolated skulls (or skulls associated with skeletons that do not preserve the pelvis or pes) currently referred to Euoplocephalus may instead be referable to Dyoplosaurus. UALVP 47977 preserves distinct flat, polygonal osteoderms on the dorsal surface of the skull similar to those in Euoplocephalus. These osteoderms are not preserved in Dyoplosaurus, which may reflect either a taphonomic or a true diagnostic difference. As such, UALVP 47977 more closely resembles Euoplocephalus than Dyoplosaurus. For the purposes of this paper, UALVP 47977 is tentatively referred to Euoplocephalus to facilitate comparison with other skulls referred to this genus and pending a revision of the genus by V.M.A.
Computed tomography scans of two additional skulls of Euoplocephalus were available for this study. AMNH 5405 and UALVP 31 were both collected from the Steveville locality in Dinosaur Provincial Park, Alberta, Canada. CT data of AMNH 5405 used by Witmer & Ridgely (2008) was made publicly available on a website (http://www.oucom.ohiou.edu/dbms-witmer/3D-Visualization.htm) and their article may be consulted for technical details of the scanning. UALVP 31 was scanned at the University of Alberta ABACUS CT scanner, in 1-mm increments. Both skulls were digitally reconstructed using the thresholding and segmentation tools in the software program mimics Version 14 (Materialise Inc., Leuven, Belgium). Digital cranial endocasts were created for each skull and internal structures were viewed both as two-dimensional slices and as three-dimensional reconstructions. As an independent check on Witmer & Ridgely's (2008) findings on the nasal cavity, V.M.A. performed de novo segmentation of the nasal cavity of AMNH 5405. Three-dimensional models cropped to resemble the broken surfaces of UALVP 47977 were also created to compare internal structures of the different specimens. A latex cranial endocast was prepared for UALVP 47977.
Results
This description focuses primarily on UALVP 47977, as it best presents new information. This paper largely follows Witmer (1995, 1997), Evans (2006) and Witmer & Ridgely (2008) for homologies in the antorbital region (Figs 1 and 2). The nasal cavity refers to a respiratory and olfactory passage that extends between the external naris and the choana, and is equivalent to the respiratory tract, the respiratory passage and the airway in other papers.
Fig. 2.
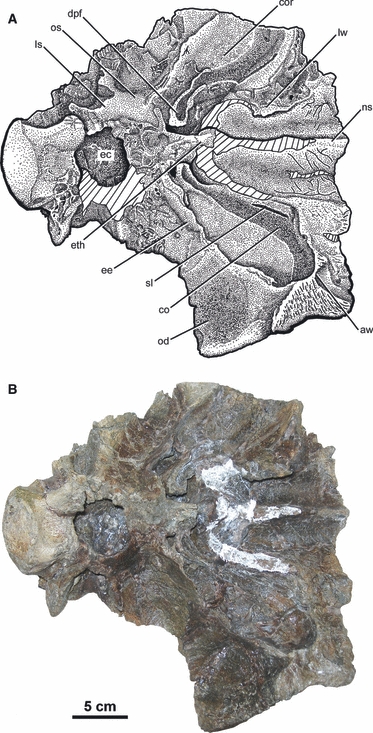
Illustration (A) and photograph (B) of the ventral view of an ankylosaurid skull roof (UALVP 47977, Euoplocephalus) from the Dinosaur Park Formation (Campanian, Upper Cretaceous), southern Alberta. Hatched lines indicate parts reconstructed with plaster. Impressions of the soft tissues, including the main airway, nasal arteries, and possible turbinates, are well defined. The ethmoidal elements are well ossified and separate regions of the nasal cavity from each other. No sutures are visible. aw, anterior wall of the cavity for the olfactory region; cor, cavity for the olfactory region; ls, laterosphenoid; od, orbital depression; sl, sulcus associated with the groove in the olfactory region. For other abbreviations, see Fig. 1.
Skull roof
In UALVP 47977, the dorsal surface of the skull is moderately weathered. The specimen preserves the top and lateral parts of the nasal cavity, which represent both the non-olfactory and olfactory regions of the nasal cavity (Fig. 2; Witmer & Ridgely, 2008). As in most ankylosaurs, but unlike Cedarpelta (Carpenter et al. 2001) and Pinacosaurus (Maryańska, 1977), no sutures can be observed. The non-olfactory dorsomedial passage of the nasal cavity under the frontals extends posteriorly behind the orbits, just anterior to the braincase. The olfactory region of the nasal cavity occupies the large cavity on both sides of the non-olfactory dorsomedial passage. A well developed bony wall separates the dorsomedial passage from the olfactory region for all its preserved length.
The dorsomedial passage of the nasal cavity (main airway) is divided into right and left passages by the nasal septum. There is no evidence for a median common chamber as reconstructed for lambeosaurine ornithopods (Evans et al. 2009). On both the lateral and medial walls of the nasal cavity proper in UALVP 47977, deep grooves extend anteroposteriorly and lead to the extensive vascular impressions in the roof of the nasal cavity (Fig. 3). The impressions branch and extend posteromedially toward the nasal septum and this pattern is bilaterally consistent. The vascular impressions are conspicuous in the anterior half of the preserved length of the main airway but are absent in the posterior half. Vascular impressions are also preserved in this region in AMNH 5238. Vascular impressions could not be reconstructed from the CT scans of AMNH 5405 and UALVP 31, but this is because of inadequate resolution of the CT scans.
Fig. 3.
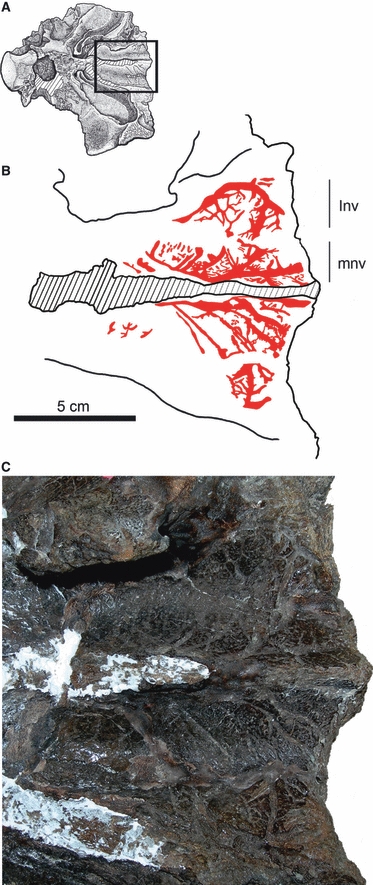
Vascular impressions in the dorsomedial part of the nasal cavity proper of UALVP 47977. (A) Drawing of skull showing region of enlargement in diagram (B) and photograph (C). These ethmoidal vessels are likely to be part of the median nasal canal system. Hatched area in B represents broken nasal septum (mesethmoid). Anterior is to the right. For abbreviations, see Fig. 1.
The cavity that housed the olfactory region of the nasal cavity occupies a large volume to the side of the dorsomedial part of the main airway. The cavity is surrounded by a thin sheet of bone (ectethmoid) laterally and by thick bony walls medially and anteriorly, and is connected posteriorly with the endocranial cavity through the olfactory fenestra. The olfactory bulb sat within this fenestra through which the olfactory nerves [cranial nerve (CN) I], ethmoidal vessels and their branches passed. This fenestra was previously identified as an olfactory tract in Talarurus (Carpenter, 2004) but the olfactory tract was located more posteriorly, well within the endocranial cavity.
In UALVP 47977, a conspicuous descending process fused to the ventral surface of the frontal develops at the front of the olfactory fenestra. The descending process accommodates a deep, spacious groove that originates from the anterior margin of the orbit. Its anterolateral site of origin is associated with vascular impressions on the medial surface of the lacrimal. The groove extends medially along the anterior wall of the olfactory region and then posteriorly along the lateral wall of the main airway, and finally ventrally along the descending process. A deep sulcus parallels the groove medially along the lateral wall of the main airway. The soft tissue that filled this groove was extensively vascularized because of the vascular impressions at the anterolateral end of the groove and because of the sulcus associated with the groove along the lateral wall of the main airway.
In another skull of Euoplocephalus (AMNH 5405), a tunnel extends anterolaterally within the roof of the olfactory region (Fig. 4) and presumably opens into the descending process. Witmer & Ridgely (2008) did not reconstruct this tunnel in AMNH 5405, but it is present on both sides in their CT data. A re-examination of the CT slices revealed that the tunnel branches laterally. Although UALVP 47977 does not have the tunnel, the vascular impression associated with the groove in the olfactory region suggests that at least the vascular component of the tissue filling the groove may correspond to the tissue filling the tunnel in AMNH 5405. The descending process and the groove could not be reconstructed from the CT scan of UALVP 31 (Fig. 5). UALVP 47977 has cracks that show the cross-sections of the frontal and the nasal. None of these cross-sections indicate pneumatization within the bones.
Fig. 4.
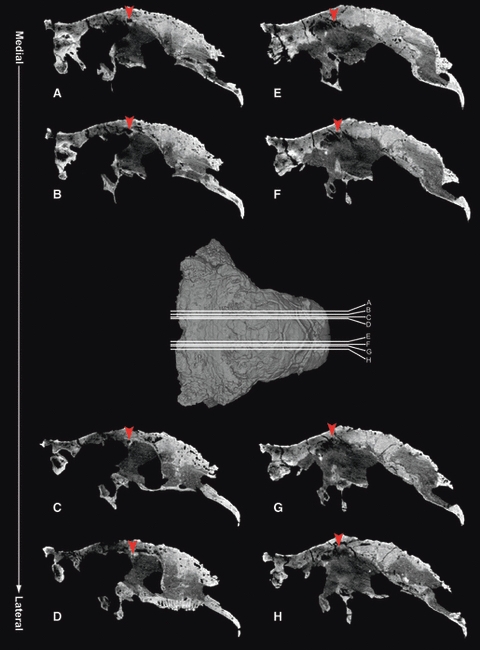
Sagittal sections of a Euoplocephalus skull (AMNH 5405) from CT data of Witmer & Ridgely (2008) show a tunnel within the frontal bone, laterally positioned and passing medially and slightly posteriorly on both sides. The most lateral sagittal section for each side is where the canal disappears into the bone. Arrowhead indicates the tunnel within the frontal, and letters A–H indicate the levels of the CT slices on the skull. Anterior is to the right in all CT slices and in the 3D model of the skull. CT data are available from the website (http://www.oucom.ohiou.edu/dbms-witmer/3D-Visualization.htm).
Fig. 5.
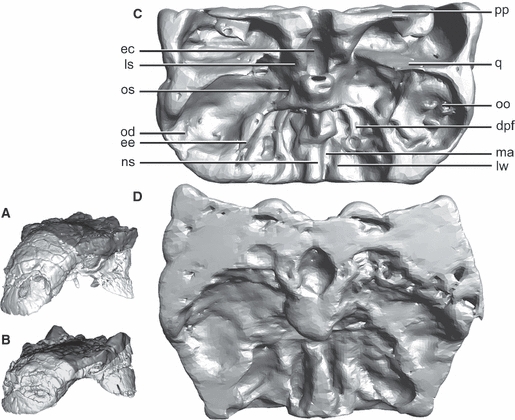
CT-based reconstruction corroborates direct osteological observation. CT renderings of the skull roofs of two Euoplocephalus specimens, AMNH 5405 (A,C) and UALVP 31 (B,D). A and B show in dark grey the portion of the skull represented in C and D in relation to the entire skull, in oblique right anterior view. C and D are sliced to mimic the areas preserved in UALVP 47977, and show internal features of the skull in ventral view that correspond to those of UALVP 47977 (Fig. 2), with anterior towards the bottom of the page. oo, ocular osteoderm; pp, paroccipital process; q, quadrate. For other abbreviations, see Figs 1 and 2.
Ethmoidal region
UALVP 47977 preserves the mesethmoid, the sphenethmoid and the ectethmoid, all of which are fully mineralized (Figs 2 and 7). The mesethmoid is a septum on the midline that separated the olfactory bulbs and is continuous anteriorly with the mineralized nasal septum. The sphenethmoid is the lateral element of the ethmoidal complex (generally referred to as a presphenoid in ornithischians: Horner, 1992; Evans, 2005, 2006) that enveloped the olfactory bulb ventrally and laterally. The sphenethmoid is continuous with the lateral wall of the main airway. The mineralized median septum of the olfactory bulbs has been described for a variety of non-avian theropods (Brochu, 2002; Coria & Currie, 2002; Sampson & Witmer, 2007; Ali et al. 2008) and is considered a homologue of the mesethmoid in birds (Ali et al. 2008). Following this position, the ossified median septum of the ethmoidal complex in UALVP 47977 is identified as the mesethmoid. It is not possible to distinguish the boundary between the mesethmoid and the sphenethmoid in UALVP 47977 and these two elements probably fused to each other early in ontogeny.
Fig. 7.
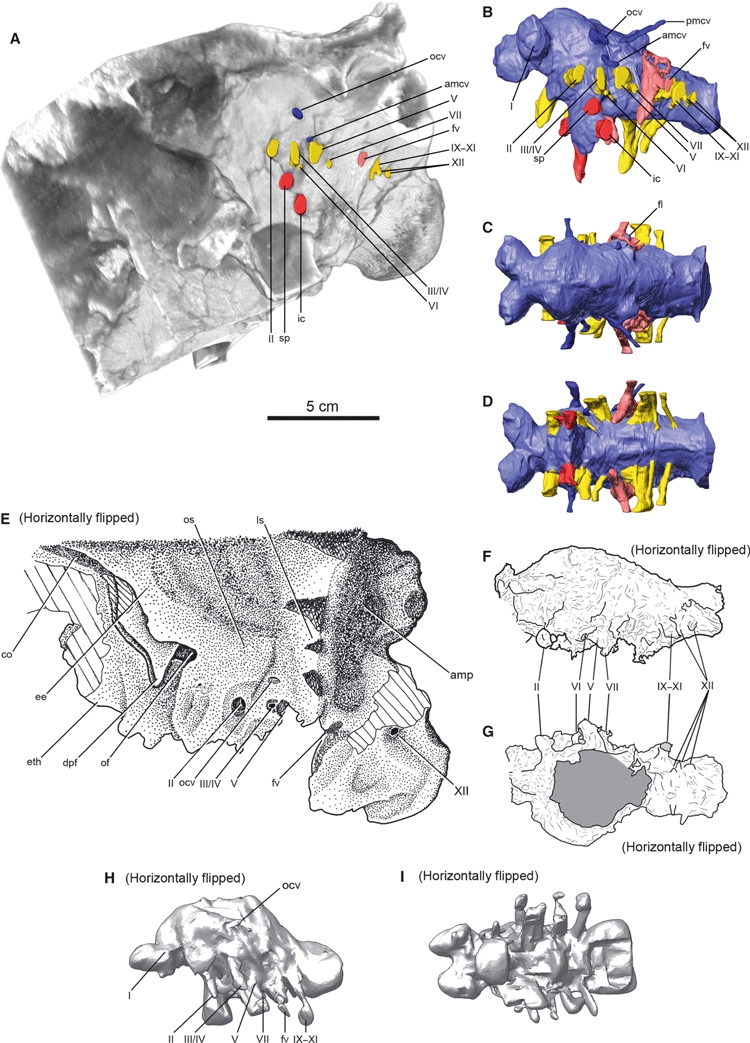
Comparison of cranial endocasts and that of braincases reveals minor variation amongst specimens referred to as Euoplocephalus. The braincase of AMNH 5405 in left lateral view (A), the cranial endocast of the same specimen in left lateral (B), dorsal (C) and ventral (D) views, the braincase of UALVP 47977 in right lateral view (E), the cranial endocast of the same specimen in right lateral (F) and ventral (G) views, and the cranial endocast of UALVP 31 in right lateral (H) and ventral (I) views. The images E–I were all inverted horizontally to show the right sides in the same orientation with the left side of AMNH 5405 for the purpose of comparison. In both UALVP 47977 and UALVP 31, the right side is better preserved. UALVP 47977 is represented by a line drawing of a latex cast, and AMNH 5405 and UALVP 31 are 3D models based on CT data. Roman numerals refer to either the foramen for, or the trunk of, the cranial nerve. amcv, anterior middle cerebral vein; amp, insertion site for M. adductor mandibulae posterior; sensuHolliday & Witmer, 2007; fl, flocculus; fv, fenestra vestibularis; ic, internal carotid artery; ocv, orbitocerebral vein; of, olfactory fenestra; pmcv, posterior middle cerebral vein; sp, sinus of pituitary. For other abbreviations, see Figs 1 and 2.
The ectethmoid forms a thin lateral wall of the olfactory region, separating it from the orbital depression laterally. It contacts the orbitosphenoid posteriorly and the lacrimal anteriorly, although the sutures are not visible at either end. Because of the skull width and the relatively more anterior placement of the orbit, the ectethmoid is elongate and oriented anterolaterally rather than transversely. The ectethmoid forms a small, medially overhanging shelf near the base of the descending process. A small foramen that pierces the ectethmoid on the left side of the skull from the orbital depression to the olfactory region may represent the orbitonasal foramen.
Anteriorly to the ethmoidal complex, the nasal septum and the lateral walls of the main airway are mineralized (Figs 2 and 3). There is no suture that distinguishes the mineralized septum and walls of the main airway from any of the cranial elements, including the ethmoidal complex, nasal, frontal and lacrimal, which they contact. The nasal septum and the lateral walls converge at the midline anterior to the ethmoidal complex. There is no opening that connects the dorsomedial passage of the main airway with the endocranial cavity. The skull roof is damaged and does not preserve the ventral part of the main airway. On the right side of the skull, however, the preserved part of the lateral wall extends ventromedially. This suggests that the mineralized wall wrapped around the dorsomedial passage of the main airway ventrally as well as laterally and medially. The anterior wall of the olfactory region extends lateromedially between the lateral wall of the main airway and the lacrimal. The anterior wall separates the olfactory region and the groove filled with the vascularized tissue from the cavity that housed the posterior loop of the main airway anteriorly (Witmer & Ridgely, 2008).
Sphenoidal region
The orbitosphenoid contacts the ectethmoid anterolaterally and the ethmoidal complex (sphenethmoid + mesethmoid) anteriorly (Figs 2 and 7). The olfactory fenestra opens between these two contacts. The olfactory nerves (CN I) would have diffused from this fenestra to both lateral and medial sides of the descending process. The orbitosphenoid contacts the laterosphenoid posteriorly and the parasphenoid ventrally. The laterosphenoid is short anteroposteriorly, but has a long, laterally oriented postorbital process that is approximately half the width of the transversely expanded ankylosaurid skull. The element is firmly fused to the skull roof. Two other sets of foramina pierce the orbitosphenoid. The foramen for the optic nerve (CN II) is larger than all other foramina for the cranial nerves except the olfactory fenestra and consists of a single exit (Fig. 7). The shared foramen for the oculomotor (CN III) and trochlear (CN IV) nerves opens posterior to the optic foramen. The foramen for the abducens nerve (CN VI) opens directly ventral to the oculomotor/trochlear foramen, which is consistent with these foramina transmitting motor nerves to the extraocular muscles.
In the laterosphenoid, the foramen for the trigeminal nerve (CN V) is posterior to the oculomotor/trochlear foramen. Just dorsal to the trigeminal foramen is an aperture for the anterior middle cerebral vein. The trigeminal foramen is anteroventral with respect to the lateral wing of the braincase (pila antotica) that contacts the postorbital laterally. In addition, the trigeminal nerve is associated with the prootic in sauropsids. Although no suture can be observed between the laterosphenoid and prootic, the topographical relationships of the trigeminal foramen with other braincase landmarks suggest that the foramen was mainly within the laterosphenoid with contribution from the prootic posteriorly. This implies that the prootic extended anteriorly below the lateral wing of the laterosphenoid. This interpretation is supported by the location of the foramen for the facial nerve (CN VII), which is located completely within the prootic in sauropsids and is just posteroventral to the trigeminal foramen.
Occipital region
Most of the elements of the occipital region are highly ossified and fused to each other. The squamosal and parietal form a roof over a chamber for M. adductor mandibulae posterior (Holliday & Witmer, 2007). The otic region is anteroposteriorly short, and the prootic and the opisthotic are indistinguishably fused together. The well developed crista interfenestralis separates the fenestra vestibularis anteriorly and the jugular foramen posteriorly (Fig. 7). The posterior foramen for the hypoglossal nerve (CN XII) opens laterally at the base of the occipital condyle, whereas the anterior foramen for the hypoglossal nerve is merged to the posteroventral corner of the jugular foramen. In Amtosaurus, there are three foramina for the hypoglossal nerve (Averianov, 2002). In occipital view, the osteoderms overhang from the skull roof elements (Fig. 6). The foramen magnum is taller than it is wide and the margin is inflated into a rim. The crescentic occipital condyle is oriented posteroventrally.
Fig. 6.

UALVP 47977 (Euoplocephalus) in occipital view. For abbreviations, see Fig. 5.
Cranial endocast
The description of the cranial endocasts focuses on AMNH 5405, which has the best preserved braincase amongst the specimens used in this study. The newly prepared cranial endocasts (AMNH 5405, UALVP 31 and UALVP 47977; Fig. 7) compare well with the published description of the cranial endocast of AMNH 5337 (Coombs, 1978a). In all specimens, the brains were anteroposteriorly short but relatively straight. The cranial endocast of UALVP 31 has a blockier, more robust appearance than that of AMNH 5405, because of the lower resolution of the CT data for UALVP 31. The endocast is also more strongly bowed dorsoventrally compared to the other specimens, but this is probably a result of taphonomic distortion of the skull. The anteroposterior shortening of the olfactory stalk partly accounts for the short anteroposterior length of the cranial endocasts of Euoplocephalus. The anteroposterior distance between the olfactory fenestra and the root of the optic nerve is less than a quarter the entire anteroposterior length of the cranial endocast in Euoplocephalus, whereas the distance is typically more than a third the length of the cranial endocast in other dinosaurs (based on figures in Hopson, 1979; Brochu, 2002; Sampson & Witmer, 2007; Witmer et al. 2008).
The olfactory bulbs diverge immediately anterior to the cerebrum at an angle of 80–100º and lead to the olfactory fenestra opening at the posteromedial end of the olfactory region. A general condition for ornithischian dinosaurs is that the olfactory tracts did not diverge as strongly anterolaterally as in Euoplocephalus (Hopson, 1979; Galton, 1983, 1988, 1989, 1997, 2001; Evans et al. 2009). The cerebral hemispheres are fairly discrete on the endocast, forming a rounded swelling immediately posterior to the olfactory tract. As is often the case in non-coelurosaurian dinosaurs (Witmer & Ridgely, 2009), however, other major neural structures such as the optics lobes and cerebellum are largely obscured by the dural envelope. An important exception is the flocculus (cerebellar auricle). The flocculus on the endocast of AMNH 5405 extends posterolaterally as a substantial, finger-like projection into the region of the inner ear and breaks the plane of the anterior semicircular canal. The presence of a flocculus in AMNH 5405 clears up the discrepancy in the reports on AMNH 5337 between Coombs (1978a: no flocculus) and Hopson (1979: large flocculus) in favor of the latter interpretation. The structure interpreted as an epiphysis cerebri (= pineal gland) projecting from the diencephalon noted in AMNH 5337 by Coombs (1978a) is also present in the endocasts of UALVP 31 and AMNH 5405, but is not visible in the endocast of UALVP 47977. Although being small, this structure is in the position to be the epiphysis. Epiphyses are present in extant birds and have been reconstructed in some dinosaurs (e.g. some theropods; Witmer & Ridgely, 2009). The lack of the epiphysis in UALVP 47977 is probably due to the presence of plaster infilling, which was used to strengthen the cracks during preparation of this specimen.
As already noted in the braincase description, the optic nerves in the endocast project almost directly laterally, such that the optic chiasm is oriented transversely rather than anterolaterally as in other archosaurs. The shared exit for the oculomotor and trochlear nerves is a large trunk directly posterior to the optic nerve. Both Coombs (1978a) and Hopson (1979) interpreted the smaller twig dorsal to the definitive oculomotor nerve canal as the trochlear nerve. This interpretation was widely accepted in the subsequent ankylosaur literature and the corresponding foramen was identified as that for the trochlear nerve in Amtosaurus (Averianov, 2002), Saichania (Maryańska, 1977), Sauropelta and Tatankacephalus (Parsons & Parsons, 2009). We instead regard their putative trochlear nerve as an orbitocerebral vein. In the endocast of AMNH 5405, the ‘trochlear nerve’ of Coombs (1978a) and Hopson (1979) is comparable to the orbitocerebral vein canals of sauropods (Sereno et al. 2007; Witmer et al. 2008), theropods (Sampson & Witmer, 2007; Witmer & Ridgely, 2009) and other dinosaurs. This feature in AMNH 5405 emerges from the lateral pole of the cerebral region and opens into the orbit well dorsal to the canals for the other nerves supplying the extraocular muscles.
The trunk of the oculomotor nerve in this study was identified by Coombs (1978a) as being associated with the pituitary vein. Indeed, this canal shared by the trochlear and oculomotor nerves seems too large to have transmitted only these two small nerves. It is likely that veins also traversed this canal. However, the term ‘pituitary vein’ is not appropriate because the venous drainage of the pituitary was almost certainly within the pituitary fossa itself and the cavernous sinus within (see Sampson & Witmer, 2007). The trunk of the abducens nerve originates from the ventral side of the brain below the trigeminal nerve and passes anterolaterally below the oculomotor and trochlear nerves.
The fossa for the pituitary gland projects more or less straight ventrally in all the specimens as a bulbous structure. In AMNH 5405, the bulbous structure is twice as wide transversely as long anteroposteriorly. Ventral to the pituitary, the endocast of the internal carotid artery is oriented ventrolaterally, whereas the artery extended anterodorsally in the cranial endocasts of other dinosaurs (Hopson, 1979; Witmer et al. 2008). The pituitary in UALVP 31 expands posteriorly, but this is most likely a result of damage to the ventral portions of the braincase. The pituitary fossa of AMNH 5405 also preserves large paired apertures dorsal to the carotid canals, which almost certainly transmitted the sphenoid branch of the carotid artery into the floor of the orbit as well as receiving ophthalmic veins.
The single trunk of the trigeminal nerve indicates that the branches diverged outside the endocranial cavity. The endocast of this nerve is dorsoventrally taller than anteroposteriorly long, suggesting that the canal housed the ganglion, as in most dinosaurs except for tyrannosaurids and birds (Witmer et al. 2008). The anterior middle cerebral vein is preserved above the trigeminal nerve in the cranial endocast of AMNH 5405 (Fig. 7C). The trunk of the facial nerve originated from the shallow recess shared with that of the vestibulocochlear nerve (CN VIII). The course of the facial nerve closely parallels that of the trigeminal nerve anteriorly and laterally, and diverges away from that of the vestibulocochlear nerve. The trunk of the vestibulocochlear nerve has two branches that separate from each other immediately outside the endocranial cavity. The dorsal branch is directed laterally toward the vestibule and the ventral one toward the cochlear ventrally. The trunks of the glossopharyngeal, vagus and accessory nerves (CNs IX–XI) exit the endocranial cavity through the jugular foramen. The jugular foramen is directly anterior to the foramen for the posterior branch of the hypoglossal nerve. AMNH 5405 has two trunks for the hypoglossal nerve, although there are possibly three trunks for this nerve in AMNH 5337 (Coombs, 1978a) and UALVP 47977 (Fig. 7). Even in the case of three trunks, the proximity and directions of the two smaller anterior trunks suggest that they likely joined to emerge from a single external foramen. In AMNH 5405, the foramen for the anterior trunk of the hypoglossal nerve is merged to the posteroventral corner of the jugular foramen. The foramen for the larger posterior trunk opens directly posterior to the jugular foramen on the lateral surface of the base of the occipital condyle.
The labyrinth of the inner ear is reasonably well preserved on the left side of AMNH 5405 and is generally similar to the one illustrated for AMNH 5337 by Hopson (1979). The lateral semicircular canal is extremely reduced, more so than in perhaps any dinosaur described to date. The anterior canal may seem somewhat elongate but this may result more from the constraint that the anterior canal must pass around the flocculus (Witmer et al. 2003). The cochlea is remarkably elongate in AMNH 5405, as illustrated also for AMNH 5337 by Hopson (1979). The elongate cochlea suggests that hearing was an important sense in Euoplocephalus.
Discussion
A combination of direct visual observation of several partial specimens (in particular AMNH 5238 and UALVP 47977) and CT-based digital reconstructions (AMNH 5405, ROM 1215, UALVP 31) makes the data gained through either technique interchangeable. The osteological data from UALVP 47977 agree with the reconstruction by Witmer & Ridgely (2008) and complement it by adding fine-scale details (such as the vascular impressions in the dorsomedial part of the nasal cavity) that cannot be imaged using most medical CT scanners. Coupled with the previous observation of large vascular canals in an ankylosaurid nasal cavity based on CT data (Witmer & Ridgely, 2008), the vascular impressions (Fig. 3) provide further evidence of extensive vascularization in the nasal cavities of ankylosaurids. The medial neurovascular canal near the nasal septum is consistent with the medial nasal vessels and nerves, which extend along the nasal septum under the skull roof in both crocodylians and birds (Sedlmayr, 2002; Witmer & Ridgely, 2008). Similarly, the lateral canal presumably represents the lateral nasal vessels and nerves. Tumanova (1987) identified a groove that extends anteroposteriorly along the dorsolateral part of the nasal septum in Talarurus as the olfactory nerve impression. The groove does not represent the olfactory nerve because the dorsomedial passage of the nasal cavity is not olfactory. The current evidence suggests that the groove in Talarurus is a channel of the medial nasal vessels and nerve. The presence of the vascular impressions on the dorsal roof of the nasal cavity proper indicates the mucosa of the cavity was appressed to the surfaces of the osseous walls. This makes it more certain that the shape and volume of the nasal cavity can be estimated from the osseous walls.
Osteological correlates in the olfactory region
Substantial evidence has now accumulated for the presence of the olfactory region in the cavity lateral to the dorsomedial passage of the main airway (Witmer & Ridgely, 2008; this paper). Based mainly on UALVP 47977, the cavity for the olfactory region can be divided into three main parts: (i) the hollow descending process; (ii) the groove along the lateral wall of the main airway associated with a sulcus along its medial margin and vascular impressions at the anterolateral end; and (iii) the space characterized by the smooth surface on the ventral side of the frontal and bound by the groove and the ectethmoid laterally. Miyashita & Arbour (2007) initially hypothesized that the descending process and the groove were occupied by the nasolacrimal canal. If the process and the groove represented an impression of the nasolacrimal canal, the canal must have extended posteriorly, which would be a novel pathway amongst vertebrates. Embryologically, the nasolacrimal canal passes anteriorly between the frontonasal process and maxillary eminence along the developing nasal cavity (Parsons, 1959; Romanoff, 1960; Witmer, 1995). This is not the case for the tissue filling the descending process and the groove within the cavity for the olfactory region. Therefore, it is unlikely that the nasolacrimal canal filled the process and the groove. Another possible explanation is that this groove housed a salt gland, such as those found anterior to the orbits in crocodilians. However, the salt gland hypothesis is also unlikely because in crocodilians this structure is typically large, teardrop-shaped and composed of many smaller lobules (Fernandez & Gasparini, 2008).
Alternatively, the descending process in UALVP 47977 may represent a mineralized posterior wall of the olfactory turbinate, and the groove associated with the process may be an impression of the mucosal concha. The position of the descending process immediately lateral to the olfactory fenestra supports this hypothesis. Witmer & Ridgely (2008) also note scroll-like olfactory turbinates in this region in both Euoplocephalus and Panoplosaurus. The olfactory nerves would have innervated the concha from both lateral and medial sides. The turbinate hypothesis is also consistent with the vascular impressions at the anterolateral end of the groove. It is uncertain whether tissues other than the concha (and its turbinate) also participated in filling in this groove. The posterior conchae of birds are likely a homologue of the conchae of crocodylians, whereas the postconchae of crocodylians are probably a neomorph (Witmer, 1995). Therefore, it is equally plausible that the descending process housed the posterior concha homologous with those of birds or the postconcha homologous with those of crocodylians.
The observed branching strongly suggests that the tunnel in AMNH 5405 (Fig. 4) was filled by blood vessels. It is likely that the same vessels were associated with the groove in the olfactory region of UALVP 47977, as the vascular impression at the anterolateral end of the groove implies. Perhaps the tunnel in AMNH 5405 is the groove in the olfactory region partly enclosed within the skull roof. If this were the case, the groove would not be an impression of the concha. Instead, the most likely candidate for filling the groove would be a venous sinus. However, the sheer size of the groove precludes the possibility that the groove was entirely an impression of the venous sinus. Furthermore, the descending process indicates that the tissue filling the groove extended ventrally, a morphology not seen in the venous system in the olfactory regions of living archosaurs. Although morphological variation in the olfactory region of Euoplocephalus and small sample size allow different interpretations, it is proposed here that the groove associated with vascular impressions in the olfactory region of UALVP 47977 represents the concha, olfactory turbinate and its associated blood vessels. There is no impression in the smooth dorsal surface along the ectethmoid that indicates tissues adjacent to the bone.
The area of the dorsal surface of the olfactory cavity in UALVP 47977 indicates that the olfactory region probably occupied a volume larger than the endocranial cavity. It is tempting to link this large volume of the cavity with increased olfactory acuity. Indeed, the olfactory bulbs of Euoplocephalus seem somewhat enlarged relative to the cerebral hemispheres but have not been subjected to the kind of quantitative analysis that has been done for theropods (Zelenitsky et al. 2009, 2011). The olfactory bulb in each of the cranial endocasts of Euoplocephalus is mediolaterally wide and dorsoventrally tall. Taken together, these findings suggest that olfaction was an important sense for Euoplocephalus, but we regard this as provisional until we can put these data in a broader comparative context.
Functional implications of the looping nasal cavity
In addition to the large cavity for the olfactory region, the looping main airways reconstructed for ankylosaurs (Witmer & Ridgely, 2008) call for functional explanations. The olfactory nerves do not exit into the dorsomedial passage of the main airway, but extend into the cavity lateral to the main airway. Therefore, the olfactory region was outside the looping pathway of the main airway (Witmer & Ridgely, 2008). This suggests that increased olfactory acuity was not the primary selective pressure for the unusual looping of the main airway of Euoplocephalus.
The looping main airway in Euoplocephalus may have evolved to increase the surface area within the nasal cavity for other functions, including thermoregulation or osmoregulation. It has previously been hypothesized that an antilopine bovid (Saiga tatarica) uses its unusually large nose as a counter-current heat exchanger (Frey & Hofmann, 1997). However, Clifford & Witmer (2004) instead supported an alternate hypothesis that the nose acts as a filter for particulate matter. This is unlikely to be the function of the looping nasal passage of ankylosaurids because the small narial opening in Saiga opens into a large chamber that slows the velocity of inhaled dust particles, a morphology not seen in Euoplocephalus. Most fundamentally, the looping nasal passage of ankylosaurs results in a dramatic increase in the surface area of the respiratory mucous membrane (Witmer & Ridgely, 2008). Extant mammals and birds expand the mucosal surface area by the development of variously branched and scrolled conchal structures, which have been shown in numerous studies to act as intermittent counter-current heat exchangers, playing a key role in heat and water balance (e.g. Schmidt-Nielsen et al. 1969, 1970; Ruben, 1996; Geist, 2000; Van Valkenburgh et al. 2011), although the situation is clearly complex (Tieleman et al. 1999; Van Valkenburgh et al. 2004; Nelson et al. 2007). Thus, it is reasonable to suggest that the increased surface area conferred by the elongate ankylosaur nasal passage may have been an alternate morphological solution with comparable physiological functions, which is consistent with the evidence for extensive nasal blood supply. UALVP 47977 shows extensive vascularization in the nasal cavity, although the vascular impressions are found in the narrow, posterior part of the airway medial to the olfactory region. The looping part of the nasal cavity was also extensively irrigated in other specimens of Euoplocephalus and in Panoplosaurus (Witmer & Ridgely, 2008). With the evidence of extensive vascularity, nasal mechanisms for regulating heat and water balance remain possible selective forces for the looping main airway of Euoplocephalus. Indeed, regardless of whether nasal elongation evolved specifically for these physiological reasons, it is hard to imagine how such an extensive, moist surface with air passing over it would not be participating in these physiological functions.
Witmer & Ridgely (2008) also suggested that the looping nasal passages may have played a role in vocal resonance. In addition to using their unusual noses for removing inhaled dust, rutting males of Saiga tense and elongate the nasal vestibulum anteriorly to lengthen the vocal tract for nasal roaring and thereby produce a lower call (Frey et al. 2007). Many birds, such as cranes and swans, have looping tracheas that achieve the same effect, which exaggerates the body size of the caller in intraspecific display during mating (Fitch, 1999). The lengthening and looping of the nasal passages while retaining relatively small olfactory areas have been used to support an acoustic function in the cranial crests of lambeosaurine hadrosaurid dinosaurs (Weishampel, 1981; Evans, 2006; Evans et al. 2009). Similarly, the complexity of the ankylosaur nasal passage may have lowered the frequency of nasal roars. Moreover, the finding here of an elongate cochlea in AMNH 5405 is consistent with this vocalization hypothesis, as argued as well for lambeosaurines (Evans et al. 2009).
In comparison with other ornithischians, the skull in ankylosaurids is shorter, but the looping of the nasal cavity more than compensated for the short skull length (Witmer & Ridgely, 2008). This strongly suggests that there is a functional advantage in maintaining or increasing high volume and surface area of the nasal cavity in ankylosaurids. This inverse correlation between skull length and nasal cavity complexity may be interpreted partly as a response to the reduction in the skull length to width ratio in ankylosaurids. The net result of the change in ratio is profound in the morphology of the ankylosaurid skull. The braincase is reduced in anteroposterior length relative to its width (Coombs, 1978a; Hopson, 1979); the trunks of the cranial nerves are oriented predominantly lateroventrally; the maxilla houses a large cavity (Coombs, 1978b; Maryańska, 1978; Witmer, 1997) for the loops of the main airway (Witmer & Ridgely, 2008); the olfactory region sits in a large cavity lateral to the dorsomedial part of the airway (Witmer & Ridgely, 2008); and the orbital depression is anteroposteriorly elongate, with the orbit in an anterior position in the skull (relative to positions in other ornithischians), whereas its medial end shifts posteriorly to align with the exit of the optic nerve from the anteroposteriorly shortened braincase. In contrast, lambeosaurine hadrosaurids achieved elongation of the main airway partly by developing a prominent crest over the skull roof (Weishampel, 1981; Evans, 2006; Evans et al. 2009). The development of different arrangements in ankylosaurids and lambeosaurines suggests widespread benefits of looping nasal passages amongst ornithischian dinosaurs.
Identification of the ethmoidal elements
The ethmoidal elements of ankylosaurids are extensively ossified. The mesethmoid and the sphenethmoid form the ethmoidal complex (Fig. 2). The ectethmoid separates the orbital depression from the olfactory region. There is no direct evidence that the ethmoidal complex consists of two mineralized elements rather than a single one. Only the sphenethmoid (generally referred to as presphenoid in ornithischians) is mineralized and forms the lateral and ventral walls of the olfactory bulbs in hadrosaurids (Evans, 2006), which lack the ossified median septum between the olfactory bulbs (= mesethmoid). A mineralized sphenethmoid and a cartilaginous median septum seem to have also been present in pachycephalosaurids (pachycephalosaurid skull caps in TMP and UALVP; e.g. TMP 84.5.1; TMP 92.88.1). Based on these observations, it is highly likely that there are two distinct centres of mineralization (a mesethmoid and a sphenethmoid) in the cartilaginous capsule enveloping the olfactory bulbs in ornithischians. Therefore, the midline ethmoidal ossification in UALVP 47977 is treated as a complex of the mesethmoid and the sphenethmoid.
The ectethmoid is part of the interorbitalis of Vickaryous & Russell (2003), the sphenethmoid of Vickaryous et al. (2004) or the anterior orbital wall of Carpenter (2004). A large part of the interorbitalis of Vickaryous & Russell (2003, Fig. 5A), however, clearly represents the orbitosphenoid, which renders the term interorbitalis redundant. In birds, an ectethmoid divides the antorbital cavity and the orbit, forming the posterior wall of the olfactory region (Witmer, 1995; Ali et al. 2008). Amongst dinosaurs, pachycephalosaurids (observed in UALVP 2, Stegoceras and UALVP casts of Prenocephale and Homalocephale holotype skulls) have ossified ectethmoids in the same position as the thin sheet of bone that forms the anteromedial wall of the orbit in UALVP 47977. The olfactory nerve passes through neither the ectethmoid nor the orbitosphenoid (both under the name ‘interorbitalis’) as Vickaryous & Russell (2003) suggested; it penetrates the sphenethmoid medial to the ectethmoid. In pachycephalosaurids (TMP 84.5.1; UALVP 2), the ectethmoid seems to contact the sphenethmoid posteriorly, but not the orbitosphenoid. Sanders & Smith (2005) used the term ectethmoid to describe an ossified element enveloping the olfactory tract in the ethmoidal region of the theropod Ceratosaurus magnicornis but this element is a sphenethmoid based on its position.
In the dorsomedial part of the anterior part of the main airway, it has been the general assumption that the ossified nasal septum in ankylosaurs is an extension of the nasal (Tumanova, 1987; Vickaryous & Russell, 2003; Vickaryous, 2006) with contributions from the premaxilla and vomer (Maryańska, 1977; Hill et al. 2003). In mammals, however, the nasal septum is largely mineralized anteriorly from the junction of the septoethmoid and septopresphenoid (Wealthall & Herring, 2006). Where mineralization occurs in tetrapods, the nasal septum is always endochondral. In birds, the nasal septum (confluent with the interorbital septum) develops from the trabecula communis (Zusi, 1993; Witmer, 1995). In crocodiles, the cartilaginous nasal septum represents a ventral part of the tectum nasi and an anterior and medial part of the planum supraseptales, within the homologue of which the mesethmoid of birds develops (Bellairs & Kamal, 1981; Klembara, 1991; Ali et al. 2008). Comparison with extant taxa suggests that the mineralized ankylosaurid nasal septum is largely the endochondral element. However, the endochondral nature of the entire nasal septum is incompatible with the observation that at least the premaxilla (a dermal bone) forms the anterior part of the nasal septum in ankylosaurids (Maryańska, 1977; Hill et al. 2003).
Comparative morphology of ankylosaurid crania
The ankylosaurids with skulls showing internal structures include: Euoplocephalus (Vickaryous & Russell, 2003; Witmer et al. 2008) from the Late Cretaceous of western North America; Pinacosaurus (Maryańska, 1971; Hill et al. 2003), Saichania (Maryańska, 1977), Talarurus (Tumanova, 1987) and an unidentified ankylosaurid (MPC PJC 2000.24), all from the Late Cretaceous of Mongolia; Gobisaurus (Vickaryous et al. 2001) from the Early Cretaceous of Asia; and Cedarpelta (Carpenter et al. 2001) and Takantacephalus (Parsons & Parsons, 2009) from the Early Cretaceous of North America. Overall, the skull is internally better ossified in UALVP 47977 (Euoplocephalus) than in the other ankylosaurid skulls. The characters discussed in this section seem to be independent of body size as some of the skulls compared here (e.g. MPC PJC 2000.14) are larger than UALVP 47977.
Talarurus has a relatively narrower skull than that of UALVP 47977. This is evident from the fact that the cavity for the olfactory region is more anterior in position than the orbital depression. In UALVP 47977, the orbital depression extends anterolaterally and separates the cavity for the olfactory region medially from the facial elements. The cavity for the main airway extends posteriorly to the orbit in UALVP 47977, whereas it is anterior to the orbit in Talarurus. The nasal septum is well developed in all the ankylosaurids that have been compared in this study. On the other hand, the lateral wall of the dorsomedial passage of the main airway is only defined by a low ridge in Saichania (Fig. 9 in Maryańska, 1977), Pinacosaurus (Pl. 27 in Maryańska, 1977) and MPC PJC 2000.14. In contrast, the thickly ossified walls separate the main airway from the olfactory region in UALVP 47977 and Talarurus. The fully mineralized lateral wall of the dorsomedial passage of the main airway is probably a universal condition in Euoplocephalus because Coombs (1978b) notes this wall and because CT images show a thick bony structure in each of the corresponding regions of TMP 1997.32.1 (Vickaryous & Russell, 2003), AMNH 5405 and UALVP 31. The variable degrees of development of the septa and walls amongst these taxa suggest that the septum mineralized independently from the lateral and posterior walls and that the mineralization of the lateral and posterior walls was regulated separately.
The descending process is less robust in an unidentified ankylosaurid from Mongolia (MPC PJC 2000.14) than in UALVP 47977. It merely amounts to a fold of a thin sheet of bone in this ankylosaurid. This is also the case for Saichania (Fig. 9 in Maryańska, 1977; labeled as ‘ethmoid’). In Talarurus (PIN 3780/1), Tumanova (1987) illustrated and described a lamina, which extends from the anterior margin of the olfactory region along the lateral wall of the dorsomedial passage of the main airway. This lamina was labeled as the anterior transverse lamina by Tumanova (1987, Fig. 5) and is also visible in a photograph of the same specimen (Carpenter, 2004; Fig. 3). The location and orientation suggests that it represents the same groove in the olfactory region as in UALVP 47977. Pinacosaurus grangeri (ZPAL MgD II/1) differs significantly from UALVP 47977 in this region. It lacks a descending process but possesses concave ridges that Maryańska (1971) interpreted as possible turbinates. The tunnel within the skull roof of Euoplocephalus (AMNH 5405) might have been present in other ankylosaurids that lack an anterolateral groove on the ventral surface of the skull roof in the olfactory region, if the groove or the tunnel is functionally associated with the descending process. However, no exits for the tunnel have been described or can be seen in illustrations of Cedarpelta, Pinacosaurus, Saichania or MPC PJC 2000.14, which suggests that the tissue filling the groove in UALVP 47977 was separate from the skull roof in each of these taxa.
Potential intraspecific variation occurs in the olfactory region of Euoplocephalus. The tunnel in AMNH 5405 (Fig. 4) cannot be identified in UALVP 47977. This suggests that a tunnel like that in AMNH 5405 may have formed as a result of partial enclosure of the groove found in the olfactory region of UALVP 47977. On the other hand, the descending process is conspicuous in UALVP 47977, whereas the process is smaller in AMNH 5405. It is uncertain if the differences were due to individual, ontogenetic, taxonomic or taphonomic variation. Although UALVP 47977 is currently best referred to Euoplocephalus, it could also be Dyoplosaurus, another ankylosaurid from the Dinosaur Park Formation (Arbour et al. 2009).
Intraspecific variation in cranial endocasts has been documented in the opossum Monodelphis domestica (Macrini et al. 2007) and oreodonts (Macrini, 2009). The proportions of cranial endocasts can vary among individuals and as a result of ontogeny (Macrini et al. 2007). Witmer et al. (2008) also showed that the morphology of the dural expansion varies in Diplodocus. The dural expansion is not conspicuous in the cranial endocasts of Euoplocephalus (Fig. 7). The variation in Euoplocephalus cranial endocasts results primarily from taphonomic distortion and limited resolution of CT scanning. None of the variations in the Euoplocephalus cranial endocasts described here are likely to be taxonomically informative. This does not entirely reject an influence of ontogeny on morphology of the endocranial cavity in Euoplocephalus, because the cranial endocasts compared here do not differ substantially in size. Nonetheless, the fact that the adult-sized cranial endocasts do not substantially vary in morphology implies that a single cranial endocast of an adult is likely sufficiently to represent a general condition for the taxon, provided that the endocranial cavity has not been taphonomically distorted.
Conclusions
A combination of direct osteological observation and CT-based reconstruction provides corroborating, complementary evidence for the nasal and endocranial soft tissues and braincase morphology of the ankylosaurid dinosaur Euoplocephalus. A partial skull roof (UALVP 47977) reveals vascular impressions in the nasal cavity, an unusual descending process (likely representing a turbinate) and deep groove possibly associated with the concha and the olfactory fenestra. The fenestra demonstrates that the cavity beside the dorsomedial passage of the main airway housed the olfactory region, which is directly anterior to the endocranial cavity in non-ankylosaur dinosaurs. The ethmoidal region preserves the ethmoidal complex (mesethmoid + sphenethmoid), the ectethmoid and mineralized walls of the nasal cavity. The neurovascular foramina of the braincase were re-interpreted. CT-based reconstructions of other specimens of Euoplocephalus show that many conspicuous osteological correlates are present in these specimens. Manually and digitally prepared cranial endocasts show minor variation within the taxon. Therefore, a single cranial endocast is likely to represent a general condition for a taxon. Two parts of the nasal cavity are unusual in ankylosaurids: the looping main airway and the large cavity for the olfactory region. The elongate, looping nasal cavity in ankylosaurid dinosaurs is not an adaptation for enhanced olfaction, but likely had thermo- and osmoregulatory benefits. An acoustic function is also possible. It is likely that the improved olfactory acuity is correlated with the increased volume of the cavity for the olfactory region in ankylosaurids, which is consistent with the size of the olfactory bulb, although the olfactory hypothesis requires corroborative evidence. These hypothesized functions suggest that multiple functional drivers may explain morphology in different parts of the ankylosaurid nasal cavity. The nasal osteological correlates are expressed or preserved differently in other ankylosaurid dinosaurs, which invites extensive interspecific comparison.
Acknowledgments
The authors thank Brandon Strilisky (TMP) for access to the specimens in his care. Allan Lindoe (University of Alberta) and David Krause (New York State University at Stony Brook) collected UALVP 47977. Michael James (University of Alberta) prepared and photographed UALVP 47977 (Figs 2 and 6). Ariana ‘Premji’ Paulina Carabajal (University of La Plata) made the latex endocast of UALVP 47977. CT scanning of UALVP 31 was conducted at the University of Alberta Hospital ABACUS Facility (G. Schaffler and R. Lambert). T.M. benefited from discussions with Ariana Paulina Carabajal, David Evans (Royal Ontario Museum), James Kirkland (Utah Geological Survey), Eric Snively (Ohio University) and François Therrien (TMP). Clint Boyd (University of Texas, Austin) carefully reviewed the earlier versions of the manuscript. Ryan Ridgely (Ohio University) worked on the 3D visualization of the endocast of AMNH 5405 and provided assistance in other ways. T.M. appreciates ongoing medical assistance from Kesia Miyashita and family. All authors acknowledge the logistical support from Eva Koppelhus (UALVP). This research was supported by NSERC, Alberta Ingenuity Fund, National Science Foundation, the Dinosaur Research Institute, and the Department of Biological Sciences at the University of Alberta.
Author contributions
T.M., V.M.A. and L.M.W. are responsible for study design, data acquisition and analysis. All authors contributed to drafting of the manuscript. T.M. drew Figs 1, 2, 4 and 7E, V.M.A prepared Figs 3, 5 and 7F–I, and L.M.W. contributed Fig. 7A–D.
References
- Ali F, Zelenitsky DK, Therrien F, et al. Homology of the ‘ethmoidal complex’ of tyrannosaurids and its implications for the reconstruction of the olfactory apparatus of non-avian theropods. J Vertbr Paleontol. 2008;28:123–133. [Google Scholar]
- Arbour VM, Burns ME, Sissons RL. A redescription of the ankylosaurid dinosaur Dyoplosaurus acutosquameus Parks, 1924 (Ornithischia: Ankylosauria) and a revision of the genus. J Vertbr Paleontol. 2009;29:1117–1135. [Google Scholar]
- Averianov AO. An ankylosaurid (Ornithischia: Ankylosauria) from the Upper Cretaceous Bissekty Formation of Uzbekistan. Bull Inst R Sci Natl Belg Sci Terre. 2002;72:97–110. [Google Scholar]
- Bellairs Ad'A, Kamal AM. The chondrocranium and the development of the skull in recent reptiles. In: Gans C, Parsons T, editors. Biology of the Reptilia, Volume 11 (Morphology F) New York: Academic Press; 1981. pp. 1–263. [Google Scholar]
- Brochu CA. Osteology of Tyrannosaurus rex: insights from a nearly complete skeleton and high-resolution computed tomographic analysis of the skull. Soc Vert Paleontol Memoir. 2002;7:1–138. [Google Scholar]
- Carpenter K. Redescription of Ankylosaurus magniventris Brown 1908 (Ankylosauridae) from the Upper Cretaceous of the Western Interior of North America. Can J Earth Sci. 2004;41:961–986. [Google Scholar]
- Carpenter K, Kirkland JI, Burge D, et al. Disarticulated skull of a new primitive ankylosaurid from the Lower Cretaceous of eastern Utah. In: Carpenter K, editor. The Armored Dinosaurs. Bloomington: Indiana University Press; 2001. pp. 211–238. [Google Scholar]
- Clifford AB, Witmer LM. Case studies in novel narial anatomy: 3. Structure and function of the nasal cavity of saiga (Artiodactyla: Bovidae: Saiga tatarica) J Zool. 2004;264:217–230. [Google Scholar]
- Coombs WP. An endocranial cast of Euoplocephalus (Reptilia, Ornithischia) Palaeontogr Abt A. 1978a;161:176–182. [Google Scholar]
- Coombs WP. The families of the ornithischian dinosaur order Ankylosauria. Palaeontology. 1978b;21:143–170. [Google Scholar]
- Coria RA, Currie PJ. The braincase of Giganotosaurus carolinii (Dinosauria: Theropod) from the Upper Cretaceous of Argentina. J Vertbr Paleontol. 2002;22:802–811. [Google Scholar]
- Evans DC. New evidence on brain-endocranial cavity relationships in ornithischian dinosaurs. Acta Palaeontol Pol. 2005;50:617–622. [Google Scholar]
- Evans DC. Nasal cavity homologies and cranial crest function in lambeosaurine dinosaurs. Paleobiology. 2006;32:109–125. [Google Scholar]
- Evans DC, Ridgely R, Witmer LM. Endocranial anatomy of lambeosaurine hadrosaurids (Dinosauria: Ornithischia): a sensorineural perspective on cranial crest function. Anat Rec. 2009;292:1315–1337. doi: 10.1002/ar.20984. [DOI] [PubMed] [Google Scholar]
- Fernandez M, Gasparini Z. Salt glands in the Jurassic metriorhynchid Geosaurus: implications for the evolution of osmoregulation in Mesozoic marine crocodyliforms. Naturwissenschaften. 2008;95:79–84. doi: 10.1007/s00114-007-0296-1. [DOI] [PubMed] [Google Scholar]
- Fitch WT. Acoustic exaggeration of size in birds via tracheal elongation: comparative and theoretical analyses. J Zool. 1999;248:31–48. [Google Scholar]
- Frey R, Hofmann RR. Skull, proboscis musculature and preorbital gland in the saiga antelope and Guenther's dikdik (Mammalia, Artiodactyla, Bovidae) Zool Anz. 1997;235:183–199. [Google Scholar]
- Frey R, Volodin I, Volodina E. A nose that roars: anatomical specializations and behavioural features of rutting male saiga. J Anat. 2007;211:717–736. doi: 10.1111/j.1469-7580.2007.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galton PM. The cranial anatomy of Dryosaurus, a hypsilophodontid dinosaur from the Upper Jurassic of North America and East Africa, with a review of the hypsilophodontids from the Upper Jurassic of North America. Geol Paleontol. 1983;17:207–243. [Google Scholar]
- Galton PM. Skull bones and endocranial casts of stegosaurian dinosaur Kentrosaurus Hennig 1915 from Upper Jurassic of Tanzania, East Africa. Geol Paleontol. 1988;22:123–143. [Google Scholar]
- Galton PM. Crania and endocranial casts from ornithopod dinosaurs of the families Dryosauridae and Hypsilophodontidae (Reptilia: Ornithischia) Geol Paleontol. 1989;23:217–239. [Google Scholar]
- Galton PM. Cranial anatomy of the basal hypsilophodontid dinosaur Thescelosaurus neglectus Gilmore (Ornithischia: Ornithopoda) from the Upper Cretaceous of North America. Rev Paléobiol. 1997;16:321–358. [Google Scholar]
- Galton PM. Endocranial casts of the plated dinosaur Stegosaurus (Upper Jurassic, western USA): a complete undistorted cast and the original specimens of Othniel Charles Marsh. In: Carpenter K, editor. The Armored Dinosaurs. Bloomington: Indiana University Press; 2001. pp. 103–129. [Google Scholar]
- Geist NR. Nasal respiratory turbinate function in birds. Physiol Biochem Zool. 2000;73:581–589. doi: 10.1086/317750. [DOI] [PubMed] [Google Scholar]
- Hayakawa H, Manabe M, Carpenter K. Nodosaurid ankylosaur from the Cenomanian of Japan. J Vertbr Paleontol. 2005;25:240–245. [Google Scholar]
- Hill RV, Witmer LW, Norell MA. A new specimen of Pinacosaurus grangeri (Dinosaur: Ornithischia) from the Late Cretaceous of Mongolia: ontogeny and phylogeny of ankylosaurs. Am Mus Novit. 2003;3395:1–29. [Google Scholar]
- Holliday CM, Witmer LM. Archosaur adductor chamber evolution: integration of musculoskeletal and topological criteria in jaw muscle homology. J Morphol. 2007;268:457–484. doi: 10.1002/jmor.10524. [DOI] [PubMed] [Google Scholar]
- Hopson JA. Paleoneurology. In: Gans C, Northcutt RG, Ulinski P, editors. Biology of the Reptilia. Vol. 9. New York: Academic Press; 1979. pp. 39–146. [Google Scholar]
- Horner JR. Cranial morphology of Prosaurolophus (Ornithischia: Hadrosauridae) with descriptions of two new hadrosaurid species and an evaluation of hadrosaurid phylogenetic relationships. Mus Rock Occas Pap. 1992;2:1–119. [Google Scholar]
- Klembara J. The cranial anatomy of early ontogenetic stages of Alligator mississippiensis (Daudin, 1802) and the significance of some of its cranial structures for the evolution of tetrapods. Palaeontogr Abt A. 1991;215:103–171. [Google Scholar]
- Kurzanov SM, Tumanova TA. On the structure of the endocranium in some ankylosaurs from Mongolia. Paleontol Zh. 1978;1978:90–96. [In Russian] [Google Scholar]
- Macrini TE. Description of a digital cranial endocast of Bathygenys reevesi (Merycoidodontidae; Oreodontoidea) and implications for apomorphy-based diagnosis of isolated, natural endocasts. J Vertbr Paleontol. 2009;29:1199–1211. [Google Scholar]
- Macrini TE, Rowe T, VandeBerg JL. Cranial endocasts from a growth series of Monodelphis domestica (Didelphidae, Marsupialia): a study of individual and ontogenetic variation. J Morphol. 2007;268:844–865. doi: 10.1002/jmor.10556. [DOI] [PubMed] [Google Scholar]
- Maryańska T. New data on the skull of Pinacosaurus grangeri (Ankylosauria) Palaeontol Pol. 1971;25:45–53. [Google Scholar]
- Maryańska T. Ankylosauridae (Dinosauria) from Mongolia. Palaeontol Pol. 1977;37:85–151. [Google Scholar]
- Miyashita T, Arbour VM. New information on the internal cranial anatomy of Euoplocephalus (Ornithischia, Ankylosauridae) J Vertbr Paleontol. 2007;27(Suppl):119A. [Google Scholar]
- Nelson JE, Christian KA, Baudinette RV. Anatomy of the nasal passages of three species of Australian bats in relation to water loss. Aust J Zool. 2007;55:57–62. [Google Scholar]
- Parks WA. Dyoplosaurus acutosquameus, a new genus and species of armored dinosaur; and notes on a skeleton of Prosaurolophus maximus. Univ Toronto Stud Geol Ser. 1924;18:1–35. [Google Scholar]
- Parsons TS. Studies on the comparative embryology of the reptilian noses. Bull Mus Comp Zool. 1959;120:101–277. [Google Scholar]
- Parsons WL, Parsons KM. A new ankylosaur (Dinosauria: Ankylosauria) from the Lower Cretaceous Cloverly Formation of central Montana. Can J Earth Sci. 2009;46:721–738. [Google Scholar]
- Romanoff AL. The Avian Embryo: Structural and Functional Development. New York: The Macmillan Company; 1960. [Google Scholar]
- Ruben JA. Evolution of endothermy in birds, mammals, and their ancestors. In: Johnston LA, Bennett AF, editors. Animals and Temperature: Phenotypic and Evolutionary Adaptation. Cambridge: Cambridge University Press; 1996. pp. 347–376. [Google Scholar]
- Sampson SD, Witmer LD. Craniofacial anatomy of Majungasaurus crenatissimus (Theropoda: Abelisauridae) from the Late Cretaceous of Madagascar. Soc Vert Paleontol Memoir. 2007;8:32–102. [Google Scholar]
- Sanders RK, Smith DK. The endocranium of the theropod dinosaur Ceratosaurus studied with computed tomography. Acta Palaeontol Pol. 2005;50:601–616. [Google Scholar]
- Schmidt-Nielsen K, Kanwisher J, Lasiewski RC, et al. Temperature regulation and respiration in the ostrich. Condor. 1969;71:341–352. [Google Scholar]
- Schmidt-Nielsen K, Hainsworth FR, Murrish D. Countercurrent heat exchange in the respiratory passages: effect on water and heat balance. Resp Physiol. 1970;9:263–276. doi: 10.1016/0034-5687(70)90075-7. [DOI] [PubMed] [Google Scholar]
- Sedlmayr JC. Unviersity of Ohio at Athens; 2002. Anatomy, evolution, and functional significance of cephalic vasculature in Archosauria. Unpublished PhD Thesis. [Google Scholar]
- Sereno PC, Wilson JA, Witmer LM, et al. Structural extremes in a Cretaceous dinosaur. PLoS ONE. 2007;2:1–9. doi: 10.1371/journal.pone.0001230. e1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieleman BI, Williams JB, Michaeli G, et al. The role of the nasal passages in the water economy of crested larks and desert larks. Physiol Biochem Zool. 1999;72:219–226. doi: 10.1086/316658. [DOI] [PubMed] [Google Scholar]
- Tumanova TA. The armoured dinosaurs of Mongolia. Sovm Sov-Mong Paleontol Eksped Trudy. 1987;32:1–80. [In Russian] [Google Scholar]
- Van Valkenburgh B, Theodor J, Friscia A, et al. Respiratory turbinates of canids and felids: a quantitative comparison. J Zool. 2004;264:281–293. [Google Scholar]
- Van Valkenburgh B, Curtis A, Samuels JX, et al. Aquatic adaptations in the nose of carnivorans: evidence from the turbinates. J Anat. 2011;218:298–310. doi: 10.1111/j.1469-7580.2010.01329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickaryous MK. New information on the cranial anatomy of Edmontonia rugosidens Gilmore, a Late Cretaceous nodosaurid dinosaur from Dinosaur Provincial Park, Alberta. J Vertbr Paleontol. 2006;26:1011–1013. [Google Scholar]
- Vickaryous MK, Russell AP. A redescription of the skull of Euoplocephalus tutus (Archosauria: Ornithischia): a foundation for comparative and systematic studies of ankylosaurian dinosaurs. Zool J Linn Soc. 2003;137:157–186. [Google Scholar]
- Vickaryous MK, Russell AP, Currie PJ, et al. A new ankylosaurid (Dinosauria: Ankylosauria) from the Lower Cretaceous of China, with comments on ankylosaurian relationships. Can J Earth Sci. 2001;38:1767–1780. [Google Scholar]
- Vickaryous MK, Maryańska T, Weishampel DB. Ankylosauria. In: Weishampel DB, Dodson P, Osmólska H, editors. The Dinosauria. 2nd edn. Berkeley: University of California Press; 2004. pp. 363–392. [Google Scholar]
- Wealthall RJ, Herring SW. Endochondral ossification of the mouse nasal septum. Anat Rec. 2006;288:1163–1172. doi: 10.1002/ar.a.20385. [DOI] [PubMed] [Google Scholar]
- Weishampel DB. The nasal cavity of lambeosaurine hadrosaurids (Reptilia, Ornithischia): comparative anatomy and homologies. J Paleontol. 1981;55:1046–1057. [Google Scholar]
- Witmer LM. Homology of facial structures in extant archosaurs (birds and crocodilians), with special reference to paranasal pneumaticity and nasal conchae. J Morphol. 1995;225:269–327. doi: 10.1002/jmor.1052250304. [DOI] [PubMed] [Google Scholar]
- Witmer LM. The evolution of the antorbital cavity of archosaurs: a study in soft-tissue reconstruction in the fossil record with an analysis of the function of pneumaticity. Soc Vert Paleontol Memoir. 1997;3:1–73. [Google Scholar]
- Witmer LM, Ridgely RC. The paranasal air sinuses of predatory and armored dinosaurs (Archosauria: Theropoda and Ankylosauria) and their contribution to cephalic structure. Anat Rec. 2008;291:1362–1388. doi: 10.1002/ar.20794. [DOI] [PubMed] [Google Scholar]
- Witmer LM, Ridgely RC. New insights into the brain, braincase, and ear region of tyrannosaurs (Dinosauria, Theropoda), with implications for sensory organization and behavior. Anat Rec. 2009;292:1266–1296. doi: 10.1002/ar.20983. [DOI] [PubMed] [Google Scholar]
- Witmer LM, Chatterjee S, Franzosa J, et al. Neuroanatomy of flying reptiles and implications for flight, posture and behavior. Nature. 2003;425:950–953. doi: 10.1038/nature02048. [DOI] [PubMed] [Google Scholar]
- Witmer LM, Ridgely RC, Dufeau DL, et al. Using CT to peer into the past: 3D visualization of the brain and ear regions of birds, crocodiles, and nonavian dinosaurs. In: Endo H, Frey R, editors. Anatomical Imaging: Towards a New Morphology. Tokyo: Springer; 2008. pp. 67–87. [Google Scholar]
- Zelenitsky DK, Therrien F, Kobayashi Y. Olfactory acuity in theropods: palaeobiological and evolutionary implications. Proc Biol Sci. 2009;276:667–673. doi: 10.1098/rspb.2008.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenitsky DK, Therrien F, Ridgely RC, et al. Evolution of olfaction in non-avian theropod dinosaurs and birds. Proc Biol Sci. 2011 doi: 10.1098/rspb.2011.0238. doi: 10.1098/rspb.2011.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusi RL. Patterns of diversity in the avian skull. In: Hanken J, Hall BK, editors. The Skull: Patterns of Structural and Systematic Diversity. Vol. 2. Chicago: University of Chicago Press; 1993. pp. 391–437. [Google Scholar]


