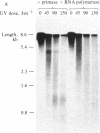
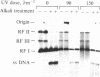
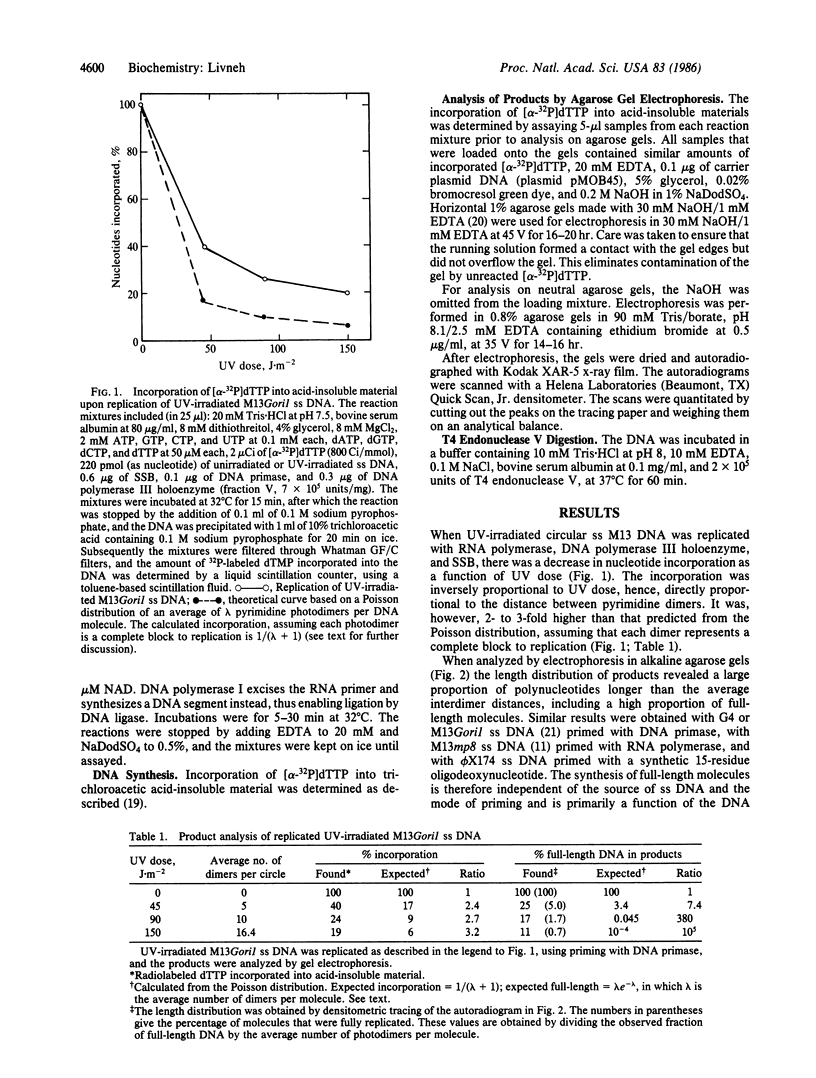
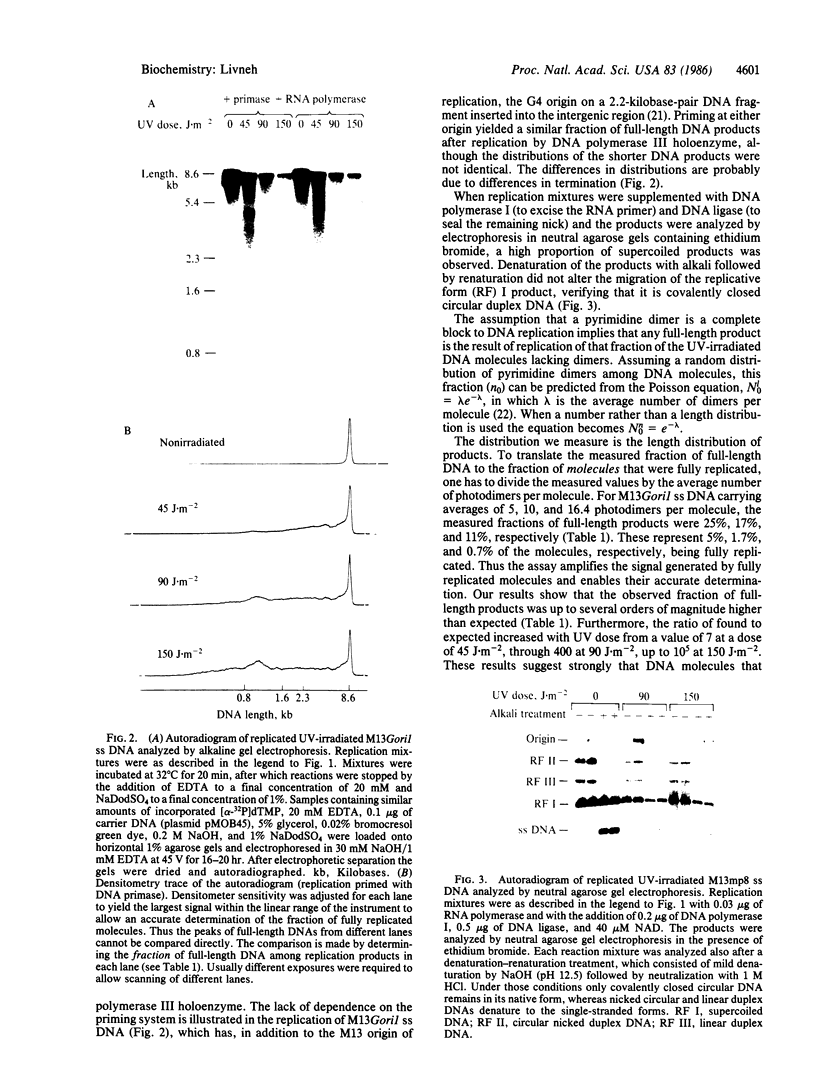
Abstract
Replication of UV-irradiated circular single-stranded phage M13 DNA by Escherichia coli RNA polymerase (EC 2.7.7.6) and DNA polymerase III holoenzyme (EC 2.7.7.7) in the presence of single-stranded DNA binding protein yielded full-length as well as partially replicated products. A similar result was obtained with phage G4 DNA primed with E. coli DNA primase, and phage phi X174 DNA primed with a synthetic oligonucleotide. The fraction of full-length DNA was several orders of magnitude higher than predicted if pyrimidine photodimers were to constitute absolute blocks to DNA replication. Recent models have suggested that pyrimidine photodimers are absolute blocks to DNA replication and that SOS-induced proteins are required to allow their bypass. Our results demonstrate that, under in vitro replication conditions, E. coli DNA polymerase III holoenzyme can insert nucleotides opposite pyrimidine dimers to a significant extent, even in the absence of SOS-induced proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brash D. E., Haseltine W. A. UV-induced mutation hotspots occur at DNA damage hotspots. Nature. 1982 Jul 8;298(5870):189–192. doi: 10.1038/298189a0. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Mottershead R. P. Mutagenic DNA repair in Escherichia coli. III. Requirement for a function of DNA polymerase III in ultraviolet-light mutagenesis. Mol Gen Genet. 1976 Feb 27;144(1):53–58. doi: 10.1007/BF00277304. [DOI] [PubMed] [Google Scholar]
- Brotcorne-Lannoye A., Maenhaut-Michel G., Radman M. Involvement of DNA polymerase III in UV-induced mutagenesis of bacteriophage lambda. Mol Gen Genet. 1985;199(1):64–69. doi: 10.1007/BF00327511. [DOI] [PubMed] [Google Scholar]
- Caillet-Fauquet P., Defais M., Radman M. Molecular mechanisms of induced mutagenesis. Replication in vivo of bacteriophage phiX174 single-stranded, ultraviolet light-irradiated DNA in intact and irradiated host cells. J Mol Biol. 1977 Nov 25;117(1):95–110. doi: 10.1016/0022-2836(77)90025-0. [DOI] [PubMed] [Google Scholar]
- Chase J. W., Whittier R. F., Auerbach J., Sancar A., Rupp W. D. Amplification of single-strand DNA binding protein in Escherichia coli. Nucleic Acids Res. 1980 Jul 25;8(14):3215–3227. doi: 10.1093/nar/8.14.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodgson J. B., Wells R. D. Action of single-strand specific nucleases on model DNA heteroduplexes of defined size and sequence. Biochemistry. 1977 May 31;16(11):2374–2379. doi: 10.1021/bi00630a010. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Walker G. C. Proteins required for ultraviolet light and chemical mutagenesis. Identification of the products of the umuC locus of Escherichia coli. J Mol Biol. 1983 Feb 25;164(2):175–192. doi: 10.1016/0022-2836(83)90074-8. [DOI] [PubMed] [Google Scholar]
- Gordon L. K., Haseltine W. A. Comparison of the cleavage of pyrimidine dimers by the bacteriophage T4 and Micrococcus luteus UV-specific endonucleases. J Biol Chem. 1980 Dec 25;255(24):12047–12050. [PubMed] [Google Scholar]
- Kaguni J., Ray D. S. Cloning of a functional replication origin of phage G4 into the genome of phage M13. J Mol Biol. 1979 Dec 25;135(4):863–878. doi: 10.1016/0022-2836(79)90516-3. [DOI] [PubMed] [Google Scholar]
- Kato T., Rothman R. H., Clark A. J. Analysis of the role of recombination and repair in mutagenesis of Escherichia coli by UV irradiation. Genetics. 1977 Sep;87(1):1–18. doi: 10.1093/genetics/87.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Shinoura Y. Isolation and characterization of mutants of Escherichia coli deficient in induction of mutations by ultraviolet light. Mol Gen Genet. 1977 Nov 14;156(2):121–131. doi: 10.1007/BF00283484. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Shearman C. W., Loeb L. A. Mutagenesis in vitro by depurination of phiX174 dna. Nature. 1981 May 28;291(5813):349–351. doi: 10.1038/291349a0. [DOI] [PubMed] [Google Scholar]
- Lackey D., Krauss S. W., Linn S. Characterization of DNA polymerase I*, a form of DNA polymerase I found in Escherichia coli expressing SOS functions. J Biol Chem. 1985 Mar 10;260(5):3178–3184. [PubMed] [Google Scholar]
- Lawrence C. W. Are pyrimidine dimers non-instructive lesions? Mol Gen Genet. 1981;182(3):511–513. doi: 10.1007/BF00293945. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Livneh Z., Lehman I. R. Recombinational bypass of pyrimidine dimers promoted by the recA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1982 May;79(10):3171–3175. doi: 10.1073/pnas.79.10.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry C., Kornberg A. DNA polymerase III holoenzyme of Escherichia coli. Purification and resolution into subunits. J Biol Chem. 1977 Sep 25;252(18):6478–6484. [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Meyer R. R., Shlomai J., Kobori J., Bates D. L., Rowen L., McMacken R., Ueda K., Kornberg A. Enzymatic conversion of single-stranded phiX174 and G4 circles to duplex forms: discontinuous replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):289–293. doi: 10.1101/sqb.1979.043.01.035. [DOI] [PubMed] [Google Scholar]
- Minton K., Durphy M., Taylor R., Friedberg E. C. The ultraviolet endonuclease of bacteriophage T4. Further characterization. J Biol Chem. 1975 Apr 25;250(8):2823–2829. [PubMed] [Google Scholar]
- Moore P. D., Bose K. K., Rabkin S. D., Strauss B. S. Sites of termination of in vitro DNA synthesis on ultraviolet- and N-acetylaminofluorene-treated phi X174 templates by prokaryotic and eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1981 Jan;78(1):110–114. doi: 10.1073/pnas.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P., Strauss B. S. Sites of inhibition of in vitro DNA synthesis in carcinogen- and UV-treated phi X174 DNA. Nature. 1979 Apr 12;278(5705):664–666. doi: 10.1038/278664a0. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y., Yamashita K., Sekiguchi M. Purification and characterization of normal and mutant forms of T4 endonuclease V. J Biol Chem. 1982 Mar 10;257(5):2556–2562. [PubMed] [Google Scholar]
- O'Connor D., Stöhrer G. Site-specifically modified oligodeoxyribonucleotides as templates for Escherichia coli DNA polymerase I. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2325–2329. doi: 10.1073/pnas.82.8.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick M. H. Studies on thymine-derived UV photoproducts in DNA--I. Formation and biological role of pyrimidine adducts in DNA. Photochem Photobiol. 1977 Apr;25(4):357–372. doi: 10.1111/j.1751-1097.1977.tb07355.x. [DOI] [PubMed] [Google Scholar]
- Piette J. G., Hearst J. E. Termination sites of the in vitro nick-translation reaction on DNA that had photoreacted with psoralen. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5540–5544. doi: 10.1073/pnas.80.18.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poddar R. K., Sinsheimer R. L. Nature of the complementary strands synthesized in vitro upon the single-stranded circular DNA of bacteriophage phiX174 after ultraviolet irradiation. Biophys J. 1971 Apr;11(4):355–369. doi: 10.1016/s0006-3495(71)86220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin S. D., Moore P. D., Strauss B. S. In vitro bypass of UV-induced lesions by Escherichia coli DNA polymerase I: specificity of nucleotide incorporation. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1541–1545. doi: 10.1073/pnas.80.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Sedat J. W., Sinsheimer R. L. Structure of the DNA of bacteriophage phiX174. VII. Methylation. J Mol Biol. 1970 Oct 28;53(2):251–259. doi: 10.1016/0022-2836(70)90298-6. [DOI] [PubMed] [Google Scholar]
- Sagher D., Strauss B. Insertion of nucleotides opposite apurinic/apyrimidinic sites in deoxyribonucleic acid during in vitro synthesis: uniqueness of adenine nucleotides. Biochemistry. 1983 Sep 13;22(19):4518–4526. doi: 10.1021/bi00288a026. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Seawell P. C., Simon T. J., Ganesan A. K. Binding of T4 endonuclease V to deoxyribonucleic acid irradiated with ultraviolet light. Biochemistry. 1980 Apr 15;19(8):1685–1691. doi: 10.1021/bi00549a026. [DOI] [PubMed] [Google Scholar]
- Sedliaková M., Brozmanová J., Masek F., Kleibl K. Evidence that dimers remaining in preinduced Escherichia coli B/r Hcr+ become insensitive after DNA replication to the extract from Micrococcus luteus. Biophys J. 1981 Nov;36(2):429–441. doi: 10.1016/S0006-3495(81)84742-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B., Carrier W. L. Pyrimidine dimers in ultraviolet-irradiated DNA's. J Mol Biol. 1966 May;17(1):237–254. doi: 10.1016/s0022-2836(66)80105-5. [DOI] [PubMed] [Google Scholar]
- Tessman I. UV-induced mutagenesis of phage S13 can occur in the absence of the RecA and UmuC proteins of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6614–6618. doi: 10.1073/pnas.82.19.6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Hondel C. A., Weijers A., Konings R. N., Schoenmakers J. G. Studies on bacteriophage M13 DNA. 2. The gene order of the M13 genome. Eur J Biochem. 1975 May 6;53(2):559–567. doi: 10.1111/j.1432-1033.1975.tb04099.x. [DOI] [PubMed] [Google Scholar]
- Villani G., Boiteux S., Radman M. Mechanism of ultraviolet-induced mutagenesis: extent and fidelity of in vitro DNA synthesis on irradiated templates. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3037–3041. doi: 10.1073/pnas.75.7.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos J. M., Rommelaere J. Are pyrimidine dimers tolerated during DNA replication of UV-irradiated parvovirus minute-virus-of-mice in mouse fibroblasts? Biochimie. 1982 Aug-Sep;64(8-9):839–844. doi: 10.1016/s0300-9084(82)80139-9. [DOI] [PubMed] [Google Scholar]
- Weiner J. H., Bertsch L. L., Kornberg A. The deoxyribonucleic acid unwinding protein of Escherichia coli. Properties and functions in replication. J Biol Chem. 1975 Mar 25;250(6):1972–1980. [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]