Abstract
MRL/MpJ (MRL) mice, commonly used as a model for autoimmune disease, have a high frequency of ovarian cysts originating from the rete ovarii. In the present study, to clarify how the rete ovarii, which are remnants of mesonephric tubules during embryogenesis, progress to cystic formation with aging, the morphology of MRL rete ovarii was analyzed and compared with that of normal C57BL/6N (B6) mice. In B6 mice, the rete ovarii consisted of a series of tubules, including the extraovarian rete (ER), the connecting rete (CR), and the intraovarian rete (IR), based on their location. Whereas the ER of B6 mice was composed of highly convoluted tubules lined by both ciliated and non-ciliated epithelia, the tubules in the CR and IR had only non-ciliated cells. In MRL mice, dilations of the rete ovarii initiated from the IR rather than the ER or CR. Although the histological types of cells lining the lumen of the rete ovarii were the same as those in B6 mice, the ER in MRL mice showed a variety in morphology. In particular, the connections between the ER and ovary tended to disappear with increasing age and the development of ovarian cysts. Furthermore, the epithelium lining the large ovarian cysts in MRL mice had ciliated cells forming the cluster. On the basis of these findings, it is suggested that cystic changes of the rete ovarii in MRL mice are caused by the dilations of the IR with invasion of the ER and CR into the ovarian medulla. These data provide new pathological mechanisms for ovarian cyst formation.
Keywords: mesonephric tubules, MRL/MpJ mice, ovarian cysts, rete ovarii
Introduction
The rete ovarii is homologous with the male rete testis and is found in normal adult ovaries of various species, including humans. The rete ovarii is formed by mesodermal cells participating in differentiation of the mesonephros that migrate to the developing gonad during embryogenesis (Byskov & Lintern-Moore, 1973). In the adult female, the rete ovarii is described as a tubular structure that connects with the ovary and is present throughout life (Wenzel & Odend'hal, 1985). In adult mice, tubules of the rete ovarii are usually localized in the hilus of the ovary, but there have been some cases where they extend through the medulla or are isolated in the parovarian tissues (adipose tissue and connective tissue around the ovary) adjacent to the hilus (Byskov & Lintern-Moore, 1973). In detail, the rete ovarii is divided into three parts in adult mice based on location and morphology: (i) the extraovarian rete (ER), surrounded by the parovarian tissues, is composed of convoluted tubules, (ii) the connecting rete (CR) is associated with the smooth muscles of the ovarian ligament, forming a network of convoluted tubules that connects the ER and ovary, and (iii) the intraovarian rete (IR) within the ovary (Long, 2002).
In mice, although the presumed function of the rete ovarii is to control the meiosis of germ cells and differentiation of granulosa cells in both the fetus and post-natal female, their function in adults is still unknown (Byskov et al. 1977; Maitland & Ullmann, 1993). Some authors have suggested a secretory function of the rete ovarii in adults because secretory materials were observed in the lumen of the rete ovarii, which showed cystic changes in various species such as the cow, camel, cat, dog, and guinea pig (Byskov, 1975; Jiang et al. 2004). Furthermore, some researchers have hypothesized that the rete ovarii is a common source of ovarian cysts and epithelial tumors in aged mice (Tan & Fleming, 2004; Tan et al. 2005; Burdette et al. 2007; Fleming et al. 2007).
Cysts originating from the rete ovarii in humans are relatively rare compared with follicular cysts; however, ovarian cysts from the rete ovarii could be the source of gynecological problems and result in a medical emergency. In fact, the physical pressure from large cysts on ovarian tissue could induce the interruption of female reproductive functions and hemorrhaging of ruptured cysts (Jain, 2002).
In a previous study, we reported a high incidence of ovarian cysts derived from the rete ovarii in the MRL/MpJ (MRL) mouse strain, a representative model for autoimmune diseases, including dermatitis, vasculitis, arthritis, and glomerulonephritis (Ichii et al. 2008; Kon et al. 2008). Ovarian cysts in CD-1 mice, another model for rete-derived ovarian cysts, showed epithelial malformations of cysts such as hyperplasia, metaplasia, stratification, and the appearance of lengthened columnar cells with compact cilia or vacuolated cytoplasm (Long, 2002; Fleming et al. 2007). In contrast, the rete ovarian cysts of MRL mice showed dilation of the IR, which is mainly lined with a single-layered ciliated or non-ciliated squamous or cuboidal epithelium (Kon et al. 2008), with considerably fewer pathological changes than the IR epithelium of CD-1 mice described by Long (2002). Additionally, a significant genetic linkage was obtained from the analysis of quantitative trait loci (QTL) between specific loci on chromosomes 4 or 14 and the development of ovarian cysts in MRL mice (Lee et al. 2010). However, there is no evidence as to how the rete ovarii changes sequentially into an ovarian cyst in this mouse model (Kon et al. 2008; Lee et al. 2010).
In the present study, to clarify the morphological dynamics during ovarian cyst formation in the MRL strain, the three-dimensional features and ultrastructure of the rete ovarii were compared between MRL and normal C57BL/6N (B6) mice at various ages. Our findings indicate a new pathological mechanism: the CR and ER as well as the IR could be a source of ovarian cysts in MRL mice, and results from our study might contribute to the elucidation of the pathology of this significant female genital problem.
Materials and methods
Animals
Specific pathogen-free mouse strains of MRL and B6 mice were purchased from Japan SLC (Shizuoka, Hamamatsu, Japan). These animals were maintained in a controlled environment with a room temperature of 22 ± 4 °C, a relative humidity of 55 ± 20%, and a 12-h light and dark cycle in the animal facility of the Graduate School of Veterinary Medicine, Hokkaido University. The mice were allowed free access to tap water and an adequate diet. All mice were treated according to the Guidelines for the Care and Use of Laboratory Animals, Hokkaido University, Graduate School of Veterinary Medicine accredited facility by the Association for Assessment and Accreditation of Laboratory Animal Care.
Histology
After inhalation anesthesia of the mice, ovaries with the parovarian tissues in each animal were removed and fixed by a mixture of formalin and acetic acid (70% ethanol : formalin : acetic acid, 15 : 5 : 1). After overnight fixation, specimens were stained with hematoxylin, processed through graded alcohol, and replaced with xylene. The rete ovarii or ovarian cysts were observed and photographed using a stereoscopic microscope, and then embedded in paraffin using a routine procedure. Serial sections (4-μm thick) were cut at 30-μm intervals and stained with periodic acid-Schiff (PAS) and observed under a light microscope. On observing the serial sections, the CR was identified by its continuity with the ER or IR and its epithelial morphology, which was different from that of adjacent lymphatic ducts, ovarian surface epithelium, and uterine tubes.
Histoplanimetry
To examine the morphological changes of the rete ovarii with age, 35 ovaries from B6 mice and 61 ovaries from 2-week-old to 12-month-old MRL mice were analyzed. For histoplanimetry, these mice were divided into three groups according to their age: 2 weeks to 3 months (before sexual maturation), 4–8 months (after sexual maturation), and 9–12 months (after the critical age when MRL mice develop ovarian cysts; Kon et al. 2008). The ER phenotype was categorized into three types according to the connection with the ovary and complexity of tubular structures. In detail, absence of tubular structures or undeveloped tubules isolated in the paraovarian tissue was categorized as the regressive type (Fig. 5A). The ER with a single tubule that preserves the connection with the ovary was defined as the simple-tubule type (Fig. 5B). Finally, the ER with well developed convoluted tubules divided by the lobule was defined as the developed type (Fig. 5C). Using histological sections of the rete ovarii, the ratio of each type of the ER in the total ovary number was calculated in the B6 and MRL mice.
Fig. 5.
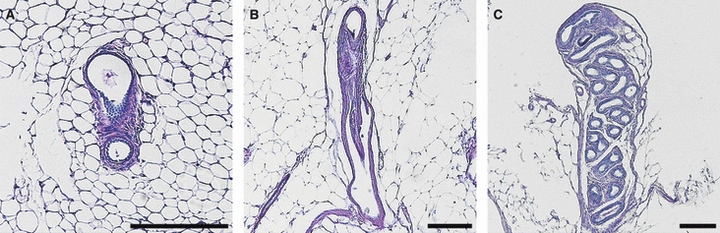
Histological typing of the extraovarian rete (ER) with PAS-hematoxylin staining. (A) Discontinuous ER was categorized as ‘regressive type’. (B) A single convoluted ER was categorized as ‘simple type’. (C) Complicated ER was categorized as ‘developed type’. Bars: 200 μm.
To assess the age-related changes of the IR or intraovarian cysts, the maximum sizes of circumference in the serial sections were measured using imagej software (http://rsb.info.nih.gov/ij; National Institute of Health, Bethesda, MD, USA), and the average of each group was calculated. We have previously confirmed by QTL analysis that the maximum circumference of cysts in the serial sections is the most appropriate histoplanimetric index, compared with other indices such as area or diameter (Lee et al. 2010).
Each nucleus : cytoplasm area ratio in ER, CR, and IR epithelia was measured from the histological sections using imagej software.
Scanning electron microscopy
Mice were euthanized by cutting the vena cava under deep anesthesia and perfused thoroughly by heart perfusion with 2.5% glutaraldehyde in 0.1 m phosphate buffer (PB; pH 7.2). The removed ovaries were then fixed with 2.5% glutaraldehyde in 0.1 m PB for 4 h. Fixed ovaries were cut in half, rinsed three times in 0.1 m PB, and kept in 2% tannic acid for 4 h at 4 °C. After washing with 0.1 m PB, these tissues were post-fixed with 1% osmium tetroxide in 0.1 m PB for 4 h. After rinsing with 0.1 m PB, specimens were dehydrated through graded alcohol, transferred into 2-methyl-2-propanol, and freeze-dried. The dried specimens were sputter-coated with a Hitachi E-1030 ion sputter coater (Hitachi Co., Ltd., Tokyo, Japan), and then examined on a JEOL S-4100 SEM (Hitachi High-Tech Fielding Corporation, Tokyo, Japan) with an accelerating voltage of 20 kV.
To classify the cell types of the cysts originating from the rete ovarii in MRL mice, the lengths of the primary cilia and the cilia on the cell surface were measured using imagej software; the mean value of each group was calculated (n > 20 cells in each group).
Immunohistochemistry
To examine the proliferation of the rete ovarii epithelium, ovaries with their parovarian tissues were fixed with 4% paraformaldehyde and cut into 2-μm paraffinized sections. In brief, the endogenous peroxidase of deparaffinized sections was eliminated by incubating the sections in absolute methanol containing 0.3% H2O2 for 30 min at room temperature. After blocking solution treatment (Mouse stain kit, Histofine, Nichirei, Tokyo, Japan), the sections were incubated overnight at 4 °C with the primary antibody for the proliferating cell nuclear antigen (PCNA; Calbiochem, San Diego, CA, USA) diluted in 0.01 m phosphate-buffered saline (PBS). The sections for negative controls were incubated in 0.01 m PBS without primary antibody. Finally, the rete ovarii was visualized with 3, 3′-diaminobenzidine tetrahydrochloride-H2O2 solution for 4 min and counterstained with hematoxylin.
Statistics
The non-parametric Kruskal–Wallis test (Scheffé's method) was applied to examine the significance of values among each group.
Results
Structure of the ER in B6 mice
To clarify the normal structure and localization of the rete ovarii in the parovarian tissues, the ovaries with adipose tissue in B6 mice were observed using hematoxylin-stained whole-mount specimens and PAS-stained semi-serial sections. As shown in Fig. 1A, most of the rete ovarii was observed outside the ovary. This unique structure was the ER part of the rete ovarii and prominent from the adipose tissue into the peritoneal cavity. In the distal part of the ER, remarkably convoluted tubules were observed (Fig. C,D). These tubular structures of the ER showed a clear lumen in whole-mount specimens (Fig. 1D,E). By changing the depth of focus in the stereomicroscope, these tubular structures were determined as closed and cecum (Fig. 1E). The branches of the ovarian vein ran toward the middle of the ER and adipose tissue of the ovary (Fig. 1A). However, arteries could not be observed due to a lack of blood in the lumen. These morphological findings of the rete ovarii with the parovarian tissues in B6 mice are summarized in Fig. 1B.
Fig. 1.
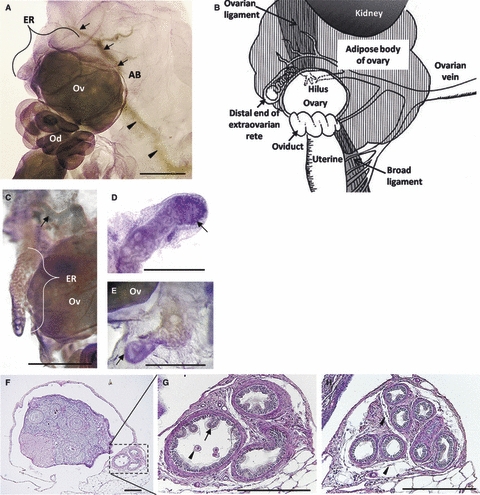
Structures of the extraovarian part of the rete ovarii in B6 mice. (A) In the whole-mount preparation at 2 months, the extraovarian rete (ER) structure was observed beside the ovary (Ov). Ovarian vein (arrowheads) and its branches (arrows) were observed in the adipose body (AB) running toward the ER. The hilus where the rete system starts is located on the opposite side from the front. Bar: 1 mm. Od, oviduct. (B) Schematic drawing of comparative localization of the rete ovarii tubules in B6 mice. From the ventral to the dorsal aspect, the ER turns out of the ovary forming an arch; almost all parts are buried deep in the adipose body, except for the limited area of the distal end. (C) Adipose tissues were removed from the ER in a 2-month-old mouse. Highly convoluted tubules were contained within the ER structure. The rete ovarii branch of the ovarian vein (arrow) was closely associated with the ER. Hematoxylin stain. Bar: 1 mm. (D,E) In the whole-mount preparation of adipose tissue removed at 12 months of age, the distal ends of the ER (arrows) were ramified into several convoluted tubules and surrounded by thick connective tissues densely stained with hematoxylin. Bars:1 mm. (F–H) Microscopic features of the ER in the same 12-month-old mouse. PAS-hematoxylin stain. Bars: 200 μm. (F) The ER (dotted box) was localized outside of the ovarian bursa. (G) Magnified, ciliated pseudostratified, or stratified columnar epithelia formed mucosal folds toward the lumen (arrow), in which cell debris (arrowhead) was observed. (H) Proximal parts of the rete ovarii were more convoluted and lobular in appearance and were surrounded by loose connective tissue (arrowheads).
In the paraffinized sections, ciliated and non-ciliated epithelial cells (from cuboidal to columnar) lined the convoluted tubules of the ER, and each tubule was separated by a septum of loose connective tissue (Fig. 1G,H). Cellular debris with vacuolated cytoplasm was observed in the lumen, likely from the epithelium (Fig. 1G). Most notably, the mucosal fold, composed of aggregations of stratified or pseudostratified columnar epithelium with cilia, was prominent into the lumen. These tubules of the ER were surrounded by independent serosa that continued to the serosa of the ovary (Fig. 1F–H).
Structure of the CR and IR in B6 mice
As described by Long (2002), the ER in the parovarian tissues continued to the CR and IR. Since the CR, without independent serosa, was embedded within the smooth muscle layers of the ovarian ligament, they could only be observed by paraffin sections (Fig. 2A). The epithelium of the CR was composed of stratified or pseudostratified cuboidal epithelium without cilia and a high nucleus to cytoplasm area ratio in B6 mice (see Fig. 2A, inset).
Fig. 2.
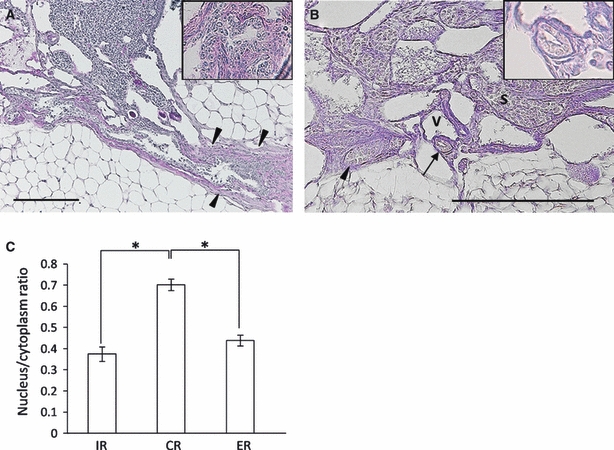
Microscopic features of the rete ovarii in B6 mice. (A) Longitudinal section of the connecting rete (CR) in a 12-month-old mouse. The CR was closely associated with the ovarian ligament (arrowheads), and its epithelial border showed an irregular line with a discontinuous basement membrane. The inset shows high magnification of the epithelium. PAS-hematoxylin stain. Bar: 200 μm. (B) Cross- (arrow) and longitudinal sections (arrowhead) of the intraovarian rete (IR) in a 5-month-old mouse. Lumen of the IR was narrow and the basement membrane was clear. The inset shows high magnification of the epithelium. PAS-hematoxylin stain. S, stroma cells; V, small vein. Bar: 200 μm. (C) Nucleus to cytoplasm area ratio in each part of the IR, CR, and ER. *P<0.0001 by the Kruskal–Wallis test, Scheffé's method, n > 30 cells in each group; value = mean ± SE.
The IR was the shortest part of the rete ovarii. The IR localized in the medullary interstitium, hilus or serosa of the ovary, and the vessels of medulla were occasionally observed (Fig. 2B). Single-layered non-ciliated cuboidal epithelia of the IR were encircled by the basement membrane, distinguishing them from stroma cells (see Fig. 2B, inset). Small tubules of the IR showed a clear cytoplasm similar to that of the ER and a nucleus to cytoplasm area ratio lower than that of the CR; these findings were confirmed by histoplanimetry experiments (Fig. 2C).
Structure of the ER in MRL mice
Whereas the ER in B6 mice was composed of several convoluted tubules, the ER of MRL mice showed variations in the number of tubules as well as histological features. Furthermore, the connection between the ER and ovary tended to be lost with aging (Fig. 3A–D). In 2-month-old MRL mice, most of the ER was composed of convoluted tubules continuing to the ovary, and their convolution was simpler than those in B6 mice (Fig. 3A). After sexual maturation, the rete ovarii tended to be dilated (Fig. 3B,C). Slightly dilated ER was connected to the ovarian hilus (Fig. 3B); the connection between the ER and cyst was missing in some mice (Fig. 3C). In older mice that developed large cysts, the parovarian tissues containing vessels were severely pressed by the ovarian cysts, and the ER structures were unclear (Fig. 3D).
Fig. 3.
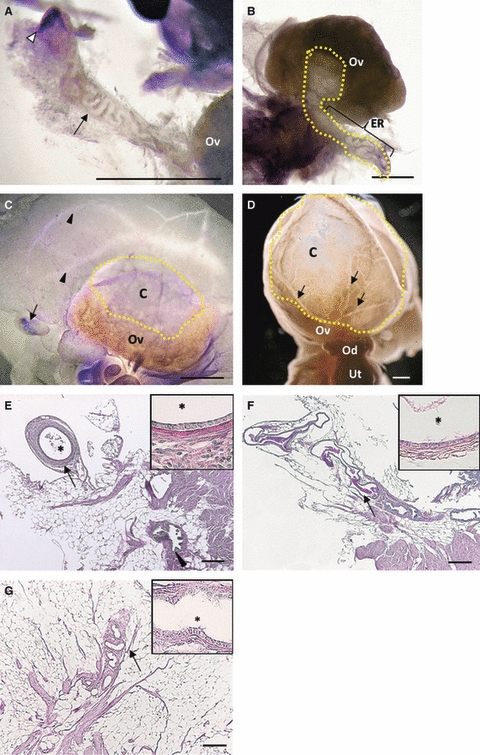
Structures of the extraovarian rete (ER) and ovarian cysts in MRL mice. (A–D) Whole-mount preparations stained by hematoxylin. Bars: 1 mm. (A) At 2 months, a singular tubule of the ER (black arrow) connected the distal end of the ER (white arrowhead), surrounded by thick connective tissue. (B) At 4 months, the ER was dilated and continued to the small cyst near the hilus. The yellow dotted line shows the ovarian rete system. (C) At 3 months, the rete ovarii branch of the ovarian vein (arrowheads) and the disconnected distal end of the ER (arrow) were observed in the parovarian tissues. (D) At 10 months, the parovarian tissues and ovarian venous branches (arrows) seemed to be attached to the cyst wall due to significant cyst development. C, cysts; Od, oviduct; Ov, ovary; Ut, uterine. (E–G) Histological sections stained by PAS-hematoxylin. Bars: 200 μm; asterisks: the lumen of rete tubule. (E) At 3 months, the distal end of the ER was surrounded by thick connective tissue and independent serosa and was composed of non-ciliated cuboidal epithelium (arrow and inset). Arrowhead indicates the CR associated with the ovarian ligament. Some parts of the adipose tissue were removed. (F) At 5 months, PAS-positive material (arrows) is shown in the ER lumen. (G) The epithelia were highly ciliated (inset). The serosa and adipose tissues were removed. At 7 months, cuboidal to low columnar epithelia with or without cilia were observed at a single tubular ER (arrow and inset).
Histologically, the ER tubules in MRL mice were lined by flattened or low columnar epithelium with or without cilia. These features differed among individuals and with age; however, younger mice tended to have the simple-tubule type that was connected to the ovary, whereas older mice tended to have the cecal type that was separated from the ovary. The ER, having a comparatively wide lumen, was occasionally separated from the ovary and lined by cuboidal epithelium with a lot of cell debris or PAS-positive materials in the lumen (Fig. 3E–G). The ER with tubules was composed of cuboidal or low columnar epithelium (Fig. 3G). On the other hand, the ER in MRL mice tended to have an undeveloped mucosal fold structure, compared to that in B6 mice (Fig. 3E–G).
Structures of the CR and IR in MRL mice
The CR in MRL mice was associated with the irregular smooth muscles of ovarian ligaments beside the ovarian hilus and it also dilated with aging (Fig. 4A). The epithelium of the CR was composed of non-ciliated cells, and the expanded lumen of the CR contained cell debris (Fig. 4A).
Fig. 4.
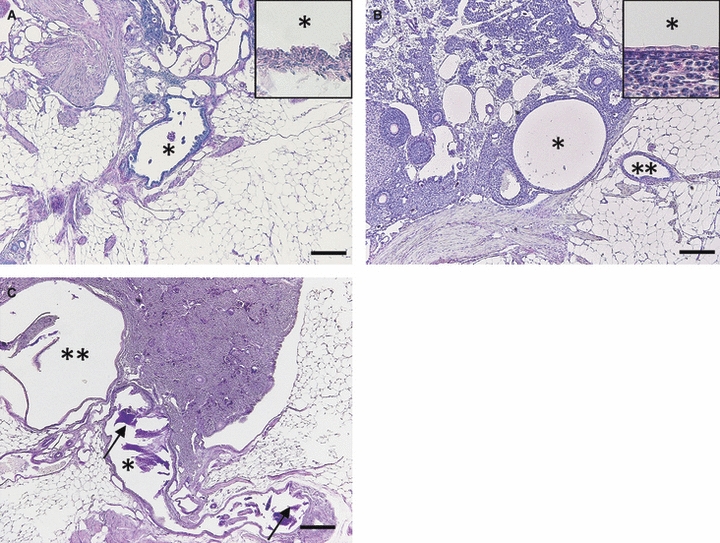
Microscopic features of the connecting rete (CR) and IR in MRL mice. (A–C) Histological sections stained by PAS-hematoxylin. Bars: 200 μm. (A) At 6 months, cell debris in the lumen of the dilated CR (asterisk) was detected. The inset shows high magnification of the epithelium. (B) At 2 months, expansion of the IR (single asterisk) and slightly dilated extraovarian rete (ER; double asterisks) were observed. The inset shows high magnification of flattened epithelium of the IR. (C) At 4 months, amorphous cyst (single asterisk) with dense PAS-positive materials (arrows) expanded toward the ovarian ligament and adipose tissues, and invaded into an intraovarian cyst (double asterisks).
In young mice (< 2 months old), the IR started to dilate near the hilus, and its cystic change could not be detected by gross observations (Fig. 4B). Similar to those of B6 mice, the dilated IR was composed of single-layered flattened epithelium without cilia. In mice with large IR cysts, the connection from the IR to CR and ER could be observed, which showed slight dilations (Fig. 4C).
Although young MRL mice without ovarian cysts tended to show a specific nuclear/cytoplasm ratio among the ER, CR, and IR similar to those in B6 mice, clear identification of the nuclear/cytoplasm ratio in each part of the rete ovarii was impossible due to cell stretching and deformation of the border between the hilus and parovarian tissues in the adult MRL mice.
Correlations between the appearance of the ER and cyst formation of the IR
Based on the histological observations of MRL rete ovarii, we hypothesized that there is a correlation between the morphological varieties of ER and cyst formation of the IR in MRL mice. For histoplanimetric analysis, the ER was divided into three types by its morphology: (i) the regressive type, where the ER was isolated in parovarian tissues and was composed of undeveloped tubules (Fig. 5A); (ii) the simple-tubule type, where the ER was composed of single tubules connecting to the ovary (Fig. 5B); and (iii) the developed type, where the ER was composed of multiple convoluted tubules (Fig. 5C).
As shown in Table 1, B6 mice usually had the developed ER type that was composed of complicated convoluted tubules at all ages examined. In B6 mice, no relationship between luminal size of the IR and ER types was found, and the IR did not dilate with age (Table 1). On the other hand, in MRL mice, there was an increase in the percentage of the regressive ER type with age, indicating that in animals with large cysts the tubular structures tended to be absent or separated from the ovary. MRL mice showed significant dilation of the IR with age. In addition, mice with the regressive ER type had a greater IR circumference (Table 1).
Table 1.
The relationship between ER morphology and circumference of the IR in B6 and MRL mice at various ages
| Ages | |||||
|---|---|---|---|---|---|
| Strain | Rete ovarii | 2 weeks–3 months | 4–8 months | 9–12 months | |
| B6 | ER morphology | Regressive (%) | ND | 9 | ND |
| Simple (%) | 15 | ND | 30 | ||
| Developed (%) | 85 | 91 | 70 | ||
| IR or intraovarian cysts | Circumference (mm) | 1 ± 0.2 | 0.9 ± 0.1 | 0.78 ± 0.1 | |
| MRL | ER morphology | Regressive (%) | 6 | 18 | 66 |
| Simple (%) | 79 | 77 | 17 | ||
| Developed (%) | 15 | 5 | 17 | ||
| IR or intraovarian cysts | Circumference (mm) | 1.3 ± 0.1 | 4.3 ± 0.6 | 22.2 ± 5.3*†‡ | |
Total of 35 ovaries in B6 and 61 ovaries in MRL mice were used.
ND, not detected.
Significantly different with ‘2 weeks–3 months’.
Significantly different with ‘4–8 months’.
P<0.0001 by the Kruskal–Wallis test, Scheffé's method, values = means ± SE.
Ultrastructural characteristics of the rete ovarii epithelium and cysts
Under SEM observation, the epithelia of the extremely expanded ovarian cysts contained non-ciliated cells and numerous clusters of ciliated cells, which were characteristic of the ER (Fig. 6A,B). The surface of the cyst in aged MRL mice was composed of three cell types: Type I cells (non-ciliated), Type II cells (ciliated), and Type III cells. Non-ciliated (Type I) and ciliated cells (Type II) were the same as the cell types observed under a light microscope. Type I cells, which usually possessed primary cilia and sparse microvilli, were divided into dome-like cells and flat-surfaced cells (Fig. 6C,D). Type II cells, with numerous cilia, were usually clustered in a particular space (Fig. 6E), lining the inside of cysts or forming the papillary projections (Fig. 6b). Type III cells, with one or two markedly long curled cilia and relatively longer microvilli, were observed with SEM (Fig. 6F). The morphology of the cilia in type III cells was different from that in type II cells, with spiral winding (Fig. 6F). A comparison of cilia length among the three cell types indicated that the cilia of type III cells were markedly the longest (Fig. 6G).
Fig. 6.
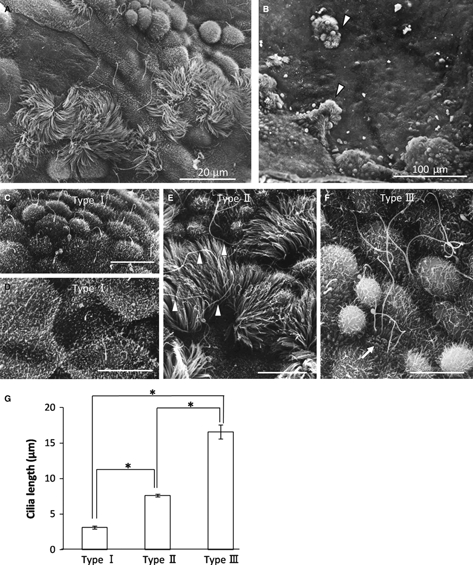
Scanning electron microscopic observation of ovarian cysts in 12-month-old MRL mice. (A) Cyst epithelium was composed of ciliated and non-ciliated cells. (B) Papillary structures (white arrowheads) were observed in the cyst wall, in which ciliated and non-ciliated cells were co-localized. (C–F) Epithelial cells making up the ovarian cyst wall were classified into three groups. Bars: 10 μm. (C,D) Type I (non-ciliated) was composed of dome-like cells (C) and flattened cells (D) with primary cilia and low microvilli. (E) Typical ciliated cells were categorized as type II cells. Long cilia indicated by the white arrowheads were different from type II cells. (F) One or two curly cilia longer than normal cilia of ciliated cells were discriminated as type III cells, in which dense and tall microvilli were observed in the cell surface (white arrow). (G) Comparison of cilia length of type I cells, type II cells, and type III cells. *P<0.0001 by the Kruskal–Wallis test, Scheffé's method, n > 20 cells in each group; value = mean ± SE.
Detection of cell proliferation in the rete ovarii epithelium of dilated cysts
To examine whether cyst formation was due to the in situ proliferation of the epithelium, immunohistochemistry for PCNA was performed in 5-month-old and 12-month-old MRL mice (Fig. 7). Results from these experiments indicated that no proliferating cells were observed in the single-layered cyst epithelium from the rete ovarii of MRL mice < 12 months of age (Fig. 7A–E).
Fig. 7.
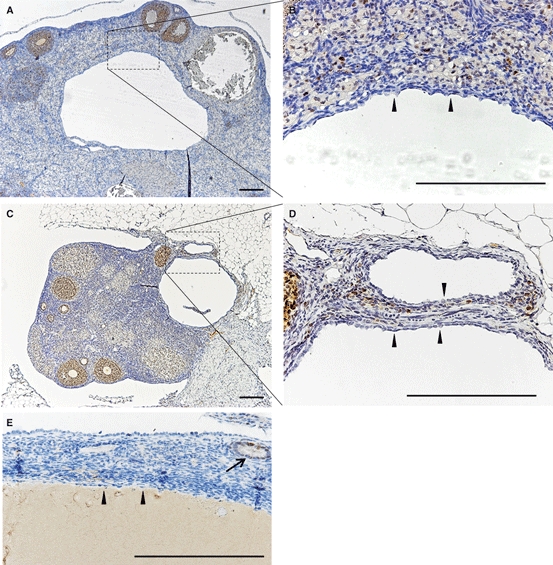
Detection of PCNA-positive cells in the MRL ovarian cysts. (A,B) Five months old. (C–E) Twelve months old. PCNA-positive epithelial cells were not detected at 5 months (A,B) or at 12 months (C–E). Arrowheads indicate the epithelium lining the ovarian cysts derived from rete ovarii (A–E). Bars: 200 μm.
Discussion
Normal mice preserve the structures of the rete ovarii throughout their life
In embryogenesis, some part of mesonephrogenic cells forms the mesonephric tubules at the cranial pole of the developing ovary, which remains as the rete ovarii in the adult female (Waterberg, 1982). Similar to previous reports (Long, 2002), by observation of the three-dimensional morphology of the rete ovarii in the B6 strain, we confirmed that the rete ovarii structure in normal adult mice is divided into the ER, CR, and IR by their localization in the ovary and parovarian tissues. Whereas ruminants have connections between the rete ovarii and infundibulum of the uterine tubule (Odend'hal et al. 1986), we did not find these connections between the rete ovarii and uterine tube in B6 or MRL mice in this study. The epithelium lining the rete ovarii is composed of non-ciliated cuboidal to columnar cells, non-ciliated pseudostratified or multilayered cuboidal cells, and ciliated and non-ciliated columnar cells in the IR, CR, and ER, respectively. Furthermore, the ER had highly convoluted tubules and prominent mucosal folds, and these structures were not dramatically changed throughout the examined ages of B6 mice. Our findings indicate that normal B6 mice, resistant to cystic rete ovarii, tend to preserve the structures of the rete ovarii, rather than have it regressed. Although the fetal rete ovarii cells influence development of the ovary through interaction with other cellular constituents and differentiate to form follicular cells (Upadhyay et al. 1979; Wenzel & Odend'hal, 1985), the function of the remaining rete ovarii in adult females is unclear. In the present study, we clarified the PAS-positive materials, papillary mucosal fold, and prominent ciliated columnar cells in the ER lumen of normal B6 mice, similar to previous reports in various species (Archbald et al. 1971; Byskov, 1975, 1978; Gelberg et al. 1984). These findings suggest a secretory function of the rete ovarii; however, further studies using real-time observation, such as video recording of cilia and fluid movement in the organ culture medium, would be needed to investigate this hypothesis further.
The CR and ER structures participate in intraovarian cyst formation in MRL mice
The majority of rete ovarii cysts in MRL mice are located within or adjacent to the hilus of the ovary (Kon et al. 2008; Lee et al. 2010). In addition to these findings, we clarified that the initial cyst formation in MRL mice was identified as IR expansion. Usually, the dilations of the IR in MRL mice begin after sexual maturation (Table 1). Furthermore, MRL mice showed polymorphism in the ER, which could be divided into the regressive, simple, and developed types. In MRL mice, the structure of ER tubules regressed with age, in contrast to those in B6 mice. From these findings, we hypothesized that there was a correlation between ER phenotype and IR dilation. Indeed, data from our correlation analysis between ER phenotype and IR size demonstrated that the ovaries with a less-developed ER structure tended to have larger cysts in MRL mice.
Furthermore, although the IR in MRL mice was lined by non-ciliated cells similar to those observed in B6 mice, large ovarian cysts occupying over half of the ovarian stroma had patchy clusters of ciliated columnar cells, which were a typical feature of the ER. Similar to these findings, Kon et al. (2008) clarified the ciliated cells in the ovarian cyst epithelium by both light microscopy and transmission electron microscopy. Based on the localization of ciliated cells in ovarian cysts, we propose possible pathological changes of ovarian cyst formation in MRL mice (Fig. 8). In the early phase, the IR epithelium stretches and forms a small cyst because of an increase in internal pressure by unknown causes such as excessive secretion or defective re-absorption and abnormal fluid movement in the rete ovarii tubules (Fig. 8B). Then, the CR epithelium continuing from the IR becomes part of the intraovarian cyst (Fig. 8B). With involution of the CR, the convoluted tubular structure of the ER inevitably becomes less convoluted and contributes to the cyst formation (Fig. 8B,C). Furthermore, cyst epithelium in ovarian stroma within 1-year-old MRL mice was not proliferative, based on PCNA immunohistochemistry data. These data indicate that the formation of cysts was not caused by the proliferation of epithelium in the rete ovarii. However, a minority of MRL mice with multiple rete tubules seemed to form ovarian cysts yielding to internal pressure at the critical point, because there were some cases of multiple cysts with apparent septum structures (Fig. 8D,E).
Fig. 8.
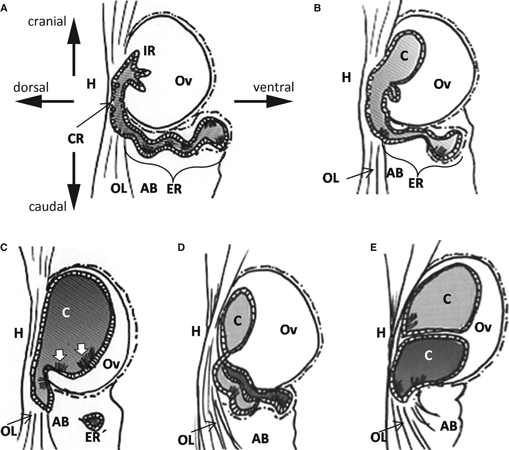
Schematic drawings of the time-interval change of the rete ovarii in MRL mice, from the lateral to the medial aspect. The direction of the arrows indicates the relative ovary position. AB, adipose body of the ovary; C, cyst; CR, the connecting rete; ER, the extraovarian rete; ER′, separated ER; H, hilus; IR, the intraovarian rete; OL, ovarian ligament. (A) Mice < 2 months old typically did not have a dilated IR. (B,C) MRL mice from 3 to 12 months of age. (B) Distal part of the ER becomes tracked towards the ovarian ligament and was disconnected in some mice. (C) In large cysts, ciliated cells (white arrows) appeared in the rete epithelium around the hilus. (D–E) Multiple cyst formation in a few MRL mice. (D) In the early phase, MRL mice with several ER tubules, as in the B6 mice, would be more resistant to ER inclusion than MRL mice with a singular and disconnected ER. (E) Eventually, several tubules of the ER form multiple ovarian cysts in the ovary and ovarian ligament.
Possible primary causes of ovarian cysts in MRL mice
Although we have proposed a mechanism for ovarian cyst formation, the primary causes remain unclear. Recently, it was noted that embryonic metabolism was retained in adult MRL mice, and this unique phenotype is associated with tissue remodeling (Naviaux et al. 2009). By 10.5 embryonic days in mice, tubules emerge from the mesonephric duct; then between 11.5 and 13.5 embryonic days, convoluted mesonephric tubules are bound to the mesonephric duct (Vuzquez et al. 1998). It has been reported that slight misalignment in embryogenesis might be involved in late mesonephric duct tissue remodeling, dilated epididymal ducts, rete testis dilation, and multicystic kidneys (Smith et al. 2008; Nistal et al. 2010). From these findings, abnormal cellular metabolism in the MRL strain might affect tissue organization from mesenchymal origin and the differentiation of the mesonephric duct in embryogenesis. Therefore, polymorphism of the ER associated with the formation of cysts in MRL mice might be a result of abnormal events in embryogenesis.
In addition to ciliated and non-ciliated cell types lining the cyst lumen in histological sections, we found a third cell type that had one or two curly cilia in cells and a dense and long microvilli population. Although the significant functions and origins were not clear in this study, the developmental role of cilia has been emphasized in the cystic diseases of various organs. Hydrocephalus and polycystic kidney diseases are interpreted as cilia-associated diseases such as primary ciliary dyskinesia or immotile cilia syndrome, which involve a malfunction of the primary cilia or the cilium of the epithelia enclosing the ventricles or urinary tubules (Lechtreck et al. 2008; Boehlke et al. 2010; Gallagher et al. 2010). From these findings, we hypothesize that the primary cilium or cilia of the rete ovarii epithelium have a role in the homeostasis of the rete ovarii in adults, and dysfunctions of these cilia might cause abnormal fluid mechanics. In addition to the functions of the epithelium associated with the regulation of fluid mechanics, chronic inflammation associated with autoimmune traits of adult MRL mice might result in abnormal fluid excretion of the rete ovarii epithelium. In mucosal epithelium such as the gastrointestinal tract, inflammatory cytokines stimulated the secretion of mucus and fluid (Rege, 1999).
In summary, we clarified that MRL ovarian cysts initiated from dilation of the IR, and these pathological events involved the CR and ER as a part of the cysts. This result might provide fundamental information about the pathogenesis of ovarian cysts, which is a major problem for the female reproductive system.
References
- Archbald LF, Schultz RH, Fahning ML, et al. Rete ovarii in heifers: a preliminary study. J Reprod Fertil. 1971;26:413–414. doi: 10.1530/jrf.0.0260413. [DOI] [PubMed] [Google Scholar]
- Boehlke C, Kotsis F, Patel V, et al. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat Cell Biol. 2010;12:1115–1122. doi: 10.1038/ncb2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette JE, Oliver RM, Ulyanov V, et al. Ovarian epithelial inclusion cysts in chronically superovulated CD1 and Smad2 dominant-negative mice. Endocrinology. 2007;148:2598–2604. doi: 10.1210/en.2007-0030. [DOI] [PubMed] [Google Scholar]
- Byskov AG. The role of the rete ovarii in meiosis and follicle formation in the cat, mink, and ferret. J Reprod Fertil. 1975;45:201–209. doi: 10.1530/jrf.0.0450201. [DOI] [PubMed] [Google Scholar]
- Byskov AG. The anatomy and ultrastructure of the rete system in the fetal mouse ovary. Biol Reprod. 1978;19:720–735. doi: 10.1095/biolreprod19.4.720. [DOI] [PubMed] [Google Scholar]
- Byskov AG, Lintern-Moore S. Follicle formation in the immature mouse ovary: the role of the rete ovarii. J Anat. 1973;116:207–217. [PMC free article] [PubMed] [Google Scholar]
- Byskov AG, Skakkebaek NE, Stafanger G, et al. Influence of ovarian surface epithelium and rete ovarii on follicle formation. J Anat. 1977;123:77–86. [PMC free article] [PubMed] [Google Scholar]
- Fleming JS, McQuillan HJ, Millier MJ, et al. E-cadherin expression and bromodeoxyuridine incorporation during development of ovarian inclusion cysts in age-matched breeder and incessantly ovulated CD-1 mice. Reprod Biol Endocrinol. 2007;5:1–14. doi: 10.1186/1477-7827-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher AR, Germino GG, Somlo S. Molecular advances in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17:118–130. doi: 10.1053/j.ackd.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelberg HB, McEntee K, Heath EH. Feline cystic rete ovarii. Vet Pathol. 1984;21:304–307. doi: 10.1177/030098588402100307. [DOI] [PubMed] [Google Scholar]
- Ichii O, Konno A, Sasaki N, et al. Autoimmune glomerulonephritis induced in congenic mouse strain carrying telomeric region of chromosome 1 derived from MRL/MpJ. Histol Histopathol. 2008;23:411–422. doi: 10.14670/HH-23.411. [DOI] [PubMed] [Google Scholar]
- Jain KA. Sonographic spectrum of hemorrhagic ovarian cysts. J Ultrasound Med. 2002;21:879–886. doi: 10.7863/jum.2002.21.8.879. [DOI] [PubMed] [Google Scholar]
- Jiang J, Take Y, Kobayashi Y, et al. Adenomatous hyperplasia of the rete ovarii in beagle. J Toxicol Pathol. 2004;17:127–128. [Google Scholar]
- Kon Y, Konno A, Hashimoto Y, et al. Ovarian cysts in MRL/MpJ mice originate from rete ovarii. Anat Histol Embryol. 2008;36:172–178. doi: 10.1111/j.1439-0264.2006.00728.x. [DOI] [PubMed] [Google Scholar]
- Lechtreck KF, Delmotte P, Robinson ML, et al. Mutations in Hydin impair ciliary motility in mice. J Cell Biol. 2008;11:633–643. doi: 10.1083/jcb.200710162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Ichii O, Otsuka S, et al. Quantitative trait locus analysis of ovarian cysts derived from rete ovarii in MRL/MpJ mice. Mamm Genome. 2010;21:162–171. doi: 10.1007/s00335-010-9254-x. [DOI] [PubMed] [Google Scholar]
- Long GG. Apparent mesonephric duct (rete anlage) origin for cysts and proliferative epithelial lesions in the mouse. Toxicol Pathol. 2002;30:592–598. doi: 10.1080/01926230290105785. [DOI] [PubMed] [Google Scholar]
- Maitland P, Ullmann SL. Gonadal development in the opossum, Monodelphis domestica: the rete ovarii does not contribute to the steroidogenic tissues. J Anat. 1993;183:43–56. [PMC free article] [PubMed] [Google Scholar]
- Naviaux RK, Le TP, Bedelbaeva K, et al. Retained features of embryonic metabolism in the adult MRL mouse. Mol Genet Metab. 2009;96:133–144. doi: 10.1016/j.ymgme.2008.11.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistal M, González-Peramato P, Sousa G, et al. Cystic dysplasia of the epididymis: a disorder of mesonephric differentiation associated with renal maldevelopment. Virchows Arch. 2010;456:695–702. doi: 10.1007/s00428-010-0906-8. [DOI] [PubMed] [Google Scholar]
- Odend'hal S, Wenzel JS, Player EC. The rete ovarii of cattle and deer communicates with the uterine tube. Anat Rec. 1986;216:40–43. doi: 10.1002/ar.1092160107. [DOI] [PubMed] [Google Scholar]
- Rege RK. Inflammatory cytokines alter human gallbladder epithelial cell absorption/secretion. J Gastrointest Surg. 1999;4:185–192. doi: 10.1016/s1091-255x(00)80055-4. [DOI] [PubMed] [Google Scholar]
- Smith PJ, DeSouza R, Roth DR. Cystic dysplasia of the rete testis. Urology. 2008;72:237–240. doi: 10.1016/j.urology.2007.11.132. [DOI] [PubMed] [Google Scholar]
- Tan OL, Fleming JS. Proliferating cell nuclear antigen immuno-reactivity in the ovarian surface epithelium of mice of varying ages and total lifetime ovulation number following ovulation. Biol Reprod. 2004;71:1501–1507. doi: 10.1095/biolreprod.104.030460. [DOI] [PubMed] [Google Scholar]
- Tan OL, Hurst PR, Fleming JS. Location of inclusion cysts in mouse ovaries in relation to age, pregnancy, and total ovulation number: implications for ovarian cancer? J Pathol. 2005;205:483–490. doi: 10.1002/path.1719. [DOI] [PubMed] [Google Scholar]
- Upadhyay S, Luciani JM, Zamboni L. The role of the mesonephros in the development of indifferent gonads and ovaries of the mouse. Ann Biol Anim Biochim Biophys. 1979;19:1179–1196. [Google Scholar]
- Vuzquez MD, Bouchet P, Mallet JL, et al. 3D reconstruction of the mouse's mesonephros. Anat Histol Embryol. 1998;27:283–287. doi: 10.1111/j.1439-0264.1998.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Waterberg H. Development of the early human ovary and role of the mesonephros in the differentiation of the cortex. Anat Embryol. 1982;165:253–280. doi: 10.1007/BF00305481. [DOI] [PubMed] [Google Scholar]
- Wenzel JG, Odend'hal S. The mammalian rete ovarii: a literature review. Cornell Vet. 1985;75:411–425. [PubMed] [Google Scholar]


