Abstract
CARMA1 is a lymphocyte-specific scaffold protein necessary for T cell activation. Deletion of CARMA1 prevents the development of allergic airway inflammation in a mouse model of asthma due to a defect in naïve T cell activation. However, it is unknown if CARMA1 is important for effector and memory T cell responses after the initial establishment of inflammation, findings which would be more relevant to asthma therapies targeted to CARMA1. In the current study, we sought to elucidate the role of CARMA1 in T cells that have been previously activated. Using mice in which floxed CARMA1 exons can be selectively deleted in T cells by OX40 driven Cre recombinase (OX40+/CreCARMA1F/F), we report that CD4+ T cells from these mice have impaired T cell reactivation responses and NF-κB signaling in vitro. Furthermore, in an in vivo recall model of allergic airway inflammation that is dependent on memory T cell function, OX40+/CreCARMA1F/F mice have attenuated eosinophilic airway inflammation, T cell activation and Th2 cytokine production. Using MHC class II tetramers, we demonstrate that the development and maintenance of antigen-specific memory T cells is not affected in OX40+/CreCARMA1F/F mice. In addition, adoptive transfer of Th2-polarized OX40+/CreCARMA1F/F antigen-specific CD4+ T cells into wild-type mice induces markedly less airway inflammation in response to antigen challenge than transfer of wild-type Th2 cells. These data demonstrate a novel role for CARMA1 in effector and memory T cell responses and suggests that therapeutic strategies targeting CARMA1 could help treat chronic inflammatory disorders such as asthma.
INTRODUCTION
Caspase recruitment domain-containing membrane-associated guanylate kinase protein-1 (CARMA1), a member of the MAGUK family of kinases, is specifically expressed in lymphocytes, and is essential for lymphocyte activation via T cell receptor (TCR) or B cell receptor signaling (1–6). Upon TCR engagement, CARMA1 is phosphorylated by protein kinase C θ and recruited to the immune synapse where it forms a complex with Bcl10 and MALT1 (7–11). This complex then initiates a signaling cascade that ultimately leads to T cell activation, in part via NF-κB activation and through other signaling pathways such as c-jun N terminal kinase 2 (JNK2) (1, 12–14). Previous studies have demonstrated that deletion of CARMA1 in T cells markedly attenuates TCR induced T cell activation, proliferation and survival (5, 6), and it is thought that CARMA1 likely works to amplify the strength of TCR signaling following stimulation (15, 16). Consistent with this, we have demonstrated that CARMA1-deficient mice have impaired immune responses in the lung due to a defect in T cell activation (17). Although CARMA1 has been extensively investigated in the context of naïve T cell activation, there are no data describing its role in effector or memory T cell responses. TCR signaling mechanisms may be different for memory and effector T cell activation compared to naïve T cells, and indeed studies have shown that the activation of effector/memory T cells requires less stimulation than naïve cells (18, 19). Furthermore, a prior study of Bcl10, a binding partner of CARMA1 and another essential component of the TCR signaling cascade, demonstrated that it was not necessary for activation of memory-phenotype cells (20). Thus, it is unclear if inhibition of CARMA1 in these T cell subsets will necessarily affect their activity.
Although T lymphocytes are critical for the integrity of the host defense against lung infections, inappropriate activation of T cells in the lung against inhaled environmental antigens can lead to non-infectious chronic inflammatory lung diseases such as allergic asthma. Allergic asthma is characterized by eosinophilic airway inflammation and airway hyper-responsiveness (AHR) in response to inhaled allergens (21). The disease is initiated when allergens are captured by airway dendritic cells (DC) and presented to naïve T cells in the regional lymph nodes. When the DCs are properly matured the T cells are primed and can develop into effector Th2 cells. These cells then migrate to the lung where they produce pro-inflammatory cytokines such as IL-4, IL-5, and IL-13 that drive allergic airway inflammation (22). As part of this process, long-lived memory T cells are also generated. These cells occupy the lung and draining lymph nodes where they continue to survey for their cognate antigens. Upon re-exposure to allergen these memory T cells rapidly respond with cytokine production and proliferation leading to a re-exacerbation of allergic airway inflammation (23, 24). Previous data has demonstrated that mice lacking components of the NF-κB pathway including CARMA1 are protected from developing experimental allergic asthma (25, 26). However, there have been no studies addressing the role of CARMA1 in recall responses to allergens in experimental models of asthma. These data would be relevant to therapeutic approaches in asthma that target CARMA1 and NF-κB, since allergen-specific T cells have already been established in asthmatics and exacerbations of inflammation will largely depend on effector and memory T cell responses.
We hypothesized that deletion of CARMA1 after T cell activation would impair effector and memory T cell responses and attenuate allergic inflammation following re-exposure to allergen. CARMA1-deficient mice do not form effector or memory T cells due to a global defect in T cell activation. Thus, to address our hypothesis and circumvent this problem, we investigated the development of allergic airway inflammation using conditional CARMA1 mutant mice (OX40+/CreCARMA1F/F) in which CARMA1 expression is disrupted after T-cell activation by an OX40 promoter driven Cre recombinase that mediates deletion of exons 3 and 4 of CARMA1 (4, 27). Using a combination of in vitro and in vivo studies, we demonstrate that CARMA1 is necessary for optimal T cell responses to TCR engagement and the development of allergic airway inflammation after the initial activation of T cells. These data suggest that decreasing the levels or activity of CARMA1 even after the establishment of chronic asthma may have therapeutic potential.
METHODS
Mice
Mice with floxed CARMA1 (CARMA1F/F) were provided by Dr. Dan Littman (New York University) (4). Mice that express Cre recombinase under control of the OX40 promoter (OX40+/Cre) were provided by Dr. Nigel Killeen (University of California, San Francisco) (27). Mice with a constitutively expressed modified form of Cre recombinase that translocates to the nucleus in the presence of tamoxifen (Rosa26CreERT2/+) were provided by Dr. Thomas Ludwig (Columbia University) (28–30). Mice with a transgenic TCR specific for a peptide of chicken ovalbumin (OVA323–339) bound to H-2b (OT-II) in the C57BL/6-Thy1.2 background, C57BL/6-Thy1.1 mice, and double fluorescent-reporter mice (mT/mG) that express GFP in Cre recombinase expressing tissues (31) were all purchased from Jackson Laboratory (Bar Harbor, ME). All mice were housed and bred in a specific pathogen free animal facility at Massachusetts General Hospital. In house breeding was performed to generate OX40+/CreCARMA1F/F,X40+/+CARMA1F/Fand OX40+/CreCARMA1+/+ mice on a C57BL/6 background (at least 7 generations) or C57BL/6 OT-II background (at least 6 generations). In house breeding was also performed to generate OX40+/CremT/mG mice and CreERT2/CARMA1F/F OT-II mice. Six to 8 week old mice were used in all experiments. All protocols were approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital.
CD4+ cell activation and reactivation
CD4+ T cells were isolated from single cells suspensions of pooled lymph nodes (inguinal, brachial, axillary and cervical) and spleen from mice using negative magnetic selection (Easysep, Stemcell Technologies, Vancouver, Canada). Purity of the CD4+ cell preparations were routinely evaluated and were >90–95% pure. For some experiments, naïve CD4+ cells were isolated using a mouse CD4+ CD62L+ isolation kit (Miltenyi Biotec, Auburn, CA). On day 0, isolated CD4+ T cells were activated with anti-CD3 and anti-CD28 antibody coated beads (Dynabeads, Invitrogen, Carlsbad, CA) in a ratio of 1:1 in media supplemented with IL-2. On day 3, beads were removed from the cells and total RNA was isolated from an aliquot of cells. The remaining cells were allowed to rest at a concentration of 1×106 cells/ml in fresh media supplemented with IL-2. Cells were periodically fed fresh media supplemented with IL-2. On day 8, cells were left untreated or were reactivated with anti-CD3 and anti-CD28 antibody coated beads in the presence of IL-2 and analyzed 24h later using flow cytometry. In a separate set of experiments, cytoplasmic and nuclear protein was isolated from cells (NE-PER Cytoplasmic and Nuclear protein extraction kit, Pierce, Rockford, IL) left untreated or reactivated for 2 hours with anti-CD3 and anti-CD28 coated beads. For experiments with CreERT2/CARMA1F/F OT-II mice, isolated CD4+ cells were cultured with irradiated splenic antigen presenting cells (APCs) pulsed with 100ng/mL OVA323–339 and 1µg/mL anti-CD28 on day 0. On day 3, live CD4+ T cells were separated using Lympholyte (Cedarlane Laboratories, Burlington, NC) and treated with 1µM 4-hydroxytamoxifen (Sigma, St. Louis, MO) or an equal volume of vehicle (ethanol) for 5 days. On day 8, RNA was collected from an aliquot of cells, and the remaining cells were left unstimulated or restimulated with anti-CD3 and anti-CD28 coated beads. On day 9, activation of CD4+ T cells was evaluated by flow cytometry.
Asthma Models
Acute allergic airway inflammation was induced in mice as previously described (17). Briefly, male mice from OX40+/+CARMA1F/F, OX40+/CreCARMA1F/F and OX40+/CreCARMA1+/+ strains were sensitized with an intraperitoneal injection of 10µg alum-conjugated ovalbumin (OVA, Sigma-Aldrich) on days 0 and 7. Mice were then challenged with 1% OVA aerosol for 20 minutes on days 14, 15 and 16. For asthma recall experiments, mice were sensitized with an intraperitoneal injection of 10µg alum-conjugated OVA on days 0 and 7, challenged with 1% OVA aerosol for 20 minutes on days 14, 15, 16 and rechallenged again on days 58, 59 and 60. In acute and recall asthma experiments, data was collected 24 hours following the last OVA challenge. In some recall experiments, lungs and thoracic lymph nodes (TLN) were collected on day 58 prior to OVA rechallenge. Tissues collected from euthanized mice include bronchoalveolar lavage (BAL) fluid, right upper lobe of the lungs for RNA isolation and formalin-fixed left lung for histological evaluation. Tissues were analyzed as previously described (17).
The adoptive transfer model of allergic airway inflammation was performed as previously described (17). Briefly, CD4+ T cells were isolated from pooled lymph nodes and spleens of TCR transgenic (OT-II) OX40+/CreCARMA1F/F, OX40+/CreCARMA1+/+, and OX40+/+CARMA1F/F mice. Isolated CD4+ T cells were incubated with irradiated splenic APCs (1:4 CD4+:APC ratio) pulsed with 100ng/mL OVA323–339, 1µg/mL anti-CD28 antibody, 1µg/mL anti-IFNγ antibody and 100ng/mL recombinant mouse IL-4. From day 2, cells were maintained in media supplemented with IL-2. On day 6, an aliquot of cells were separated and stimulated with 50ng/mL PMA and 500ng/mL Ionomycin for 6 hours. Golgi stop (eBioscience, San Diego, CA) was added during the last 4 hours of incubation, and the stimulated cells were analyzed for production of intracellular cytokine IL-4. Five x 106 Th2 polarized cells from OX40+/CreCARMA1F/F, OX40+/CreCARMA1+/+, or OX40+/+CARMA1F/F mice were intraperitoneally injected into naïve C57BL/6-Thy1.1 mice on day 0 and recipient mice were rested on days 1 and 2. Mice were then nebulized with an aerosol of 5% OVA on days 3, 4 and 5. Tissues were collected on day 6 to assess BAL cellularity, cytokines, RNA expression and cell proliferation.
Flow cytometry
CD4+ T cells from in vitro experiments were stained with fluorochrome conjugated antibodies against CD4, CD69 and CD44 to evaluate activation and memory responses. Activation and memory responses were analyzed in cells by gating on live cells based on forward and side scatter properties. BAL cells from in vivo experiments were stained with antibodies against CD4, CD69, CD62L and CD44. BAL CD4+ T cells were assessed by gating on lymphocyte population based on forward and side scatter properties. All antibodies were purchased from BD Biosciences (San Diego, CA). Flow cytometry data was acquired by an Accuri C6 flow cytometer (Accuri, Ann Arbor, MI) and analyzed using CFlow software (Accuri, Ann Arbor, MI) and FlowJo (TreeStar, Ashland, OR).
Western immunoblot
Five µg nuclear protein from isolated and treated cells was separated on a SDS-PAGE gel, transferred to PVDF membranes and probed with primary antibodies specific against the p65 subunit of NF-κB (Cell Signaling, Danvers, MA). Immunoblotted membranes were stripped and reprobed with a primary antibody against Lamin-B (Santa Cruz Biotechnologies, Santa Cruz, CA) to ensure equal nuclear protein loading. For CARMA1 blots, cells were resuspended in ice-cold RIPA lysis buffer (Thermo Scientific, Rockford, IL) containing protease inhibitors, sonicated for 30 seconds, incubated on ice for 15 minutes and centrifuged at 14,000 × g for 15 minutes. The supernatants were then used for protein analysis. Equal amounts of total protein were loaded for each blot. A rabbit polyclonal antibody against CARMA1 (Abcam, Cambridge, MA) was used to detect CARMA1. Immunoblotted membranes were stripped and reprobed with a primary antibody against β-actin to ensure equal protein loading.
Quantitative real-time PCR
RNA was purified from cells or lung and analyzed by quantitative RT-PCR (QPCR) as previously described (17). In brief, samples were analyzed using SYBR Green (Applied Biosystems, Carlsbad, CA) based realtime quantitative PCR in a Mastercycler EP Realplex thermal cycler (Eppendorf, Hamburg, Germany). Transcript copy numbers of each gene deduced from the cycle threshold (CT) values were normalized against copy numbers of an endogenous control gene (GAPDH and/or β-actin) in the corresponding samples. Primer sequences used were obtained from the Massachusetts General Hospital (MGH) Primerbank (pga.mgh.harvard.edu/primerbank/) or from National Cancer Institute (NCI) Primerdepot (http://mouseprimerdepot.nci.nih.gov/).
Detection OVA-specific CD4+ T cells by class II tetramers
Lungs and TLNs were collected from mice. Single cell suspensions were prepared from pooled TLNs within each group in each experiment and individual lung samples in each experiment. OVA-specific T cells were enriched with a previously described tetramer-based enrichment procedure (32, 33) involving the combined use of two tetramers, OVA-2C and OVA-3C, specific for two immunodominant sub-epitopes of OVA, QAVHAAHAEIN and VHAAHAEINEA, respectively (34, 35). Following enrichment, tetramer bound and unbound cells were analyzed by flow cytometry. Cells were gated for lymphocytes, and OVA-specific T cells were identified and enumerated by a Tetramer+ CD4+ CD8− CD11c− CD19− phenotype.
Statistical Analysis
Results are shown as mean ± SEM values. Graphpad Prism software was used to analyze the results. Two groups were compared using a Student’s t-test. Multiple between-group comparisons of means were performed by one-way ANOVA with Neuman-Keuls post-hoc method. A value of p<0.05 was regarded as a significant difference.
RESULTS
Deletion of CARMA1 from OX40 expressing activated T cells
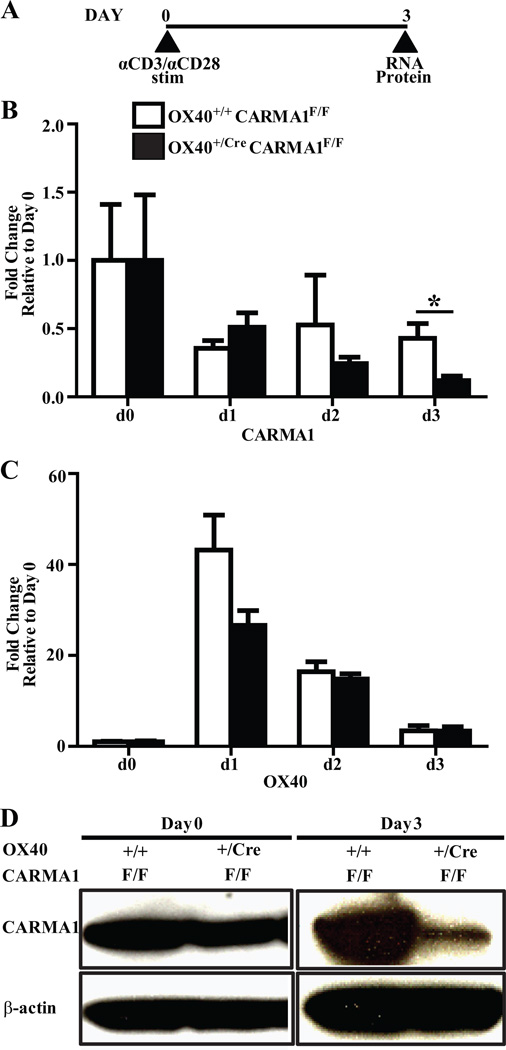
Deficiency of CARMA1, Bcl10 or MALT1 reduces NF-κB activation following TCR engagement and thus inhibits the activation of naïve T lymphocytes. This makes it difficult to study the role of CARMA1 in T cell functions following activation in CARMA1-deficient cells. To address this issue, we utilized genetically modified mice with loxP sites inserted flanking exons 3 and 4 of CARMA1 (4). In the original description of this mouse an intact Neomycin expression cassette was inserted into intron 4 of the CARMA1 gene leading to disruption in the expression of CARMA1 (CARMA1FN/FN mice). We used a modified strain derived from these mice in which the Neomycin cassette was removed, leaving behind a loxP site in intron 4 along with a predesigned, preexisting loxP site in intron 2 (CARMA1F/F mice). CARMA1F/F mice are fully competent in producing full-length CARMA1 protein, and Cre recombinase expression in these mice leads to the deletion of exons 3 and 4 of CARMA1. Exons 3 and 4 encode the C-terminal portion of the CARD and the N terminus of the coiled-coil domain and their deletion leads to disruption in functional CARMA1 expression. These mice were bred to mice that express a Cre allele under control of the OX40 promoter (27) to generate Ox40+/CreCARMA1F/F mice. Previous work has established that replacement of one of the OX40 alleles with Cre does not affect the functional responses of T cells (27, 36). OX40 is not expressed in naïve CD4+ or CD8+ cells but is upregulated within 24 hours in the majority of T cells following activation via TCR engagement (37). Thus, these mice will delete CARMA1 in a large portion of T cells only following activation. To ensure that CARMA1 is efficiently deleted in the OX40+/CreCARMA1F/F mice after T cell activation, naïve CD4+ T cells were isolated from OX40+/+CARMA1F/F and OX40+/CreCARMA1F/F mice and stimulated with beads coated with anti-CD3 and anti-CD28 antibodies (Fig 1A). The T cells from both mouse strains activated normally (data not shown). Analysis of RNA expression by QPCR demonstrated that the expression levels of CARMA1 were decreased 1 day after activation in CD4+ T cells from both OX40+/CreCARMA1F/F mice and OX40+/+CARMA1F/F control mice, however 3 days after activation the CARMA1 RNA levels in T cells from OX40+/CreCARMA1F/F mice decreased more than in T cells from Ox40+/+CARMA1F/F mice (Fig 1B). This was consistent with the OX40 pattern of expression which demonstrated a similar peak of RNA expression in T cells from both strains of mice 1 day after activation with a subsequent decline in levels over the next 2 days (Fig 1C), suggesting that OX40+/CreCARMA1F/F mice, which carry one functional OX40 allele, expresses OX40 RNA in levels comparable to OX40+/+CARMA1F/F mice which carry two functional OX40 alleles. Expression levels of Bcl10 and MALT1 also did not differ in the T cells from both strains of mice (data not shown). Western blot analyses confirmed that in unstimulated CD4+ cells, CARMA1 protein levels were comparable between OX40+/CreCARMA1F/F and OX40+/+CARMA1F/F mice (Fig 1D). However, 3 days following activation, CARMA1 protein was markedly reduced in CD4+ T cells from OX40+/CreCARMA1F/F mice compared to CD4+ T cells from OX40+/+CARMA1F/F mice (Fig 1D). Residual CARMA1 RNA and protein expression in these cells is likely because OX40 may not be upregulated in all T cells following activation. These data demonstrate that CARMA1 can be selectively and efficiently deleted from OX40 expressing activated CD4+ T cells following activation.
Figure 1. Deletion of CARMA1 from OX40 expressing activated CD4+ T cells.
A) CD4+ T cells isolated from mice were stimulated with anti-CD3 and anti-CD28 antibody coated beads for three days. B and C) Transcript expression of CARMA1 (B) and OX40 (C) in CD4+ T cells 0, 1, 2, and 3 days following activation, as analyzed by real-time quantitative PCR. Pooled data from 3–9 mice from 3 independent experiments are shown as mean ± SEM. *p<0.05 by t-test. D) Protein levels of CARMA1 in naïve CD4+ T cells and CD4+ T cells 3 days following activation, as analyzed by Western blot. CD4+ T cells, isolated from mouse splenocytes were activated for 3 days with anti-CD3 and anti-CD28 coated antibodies. Thirty µg total cell lysate protein was loaded for the day 0 blot and 50 µg total cell lysate protein for the day 3 blot. Following immunoblotting for CARMA1, membranes were stripped and reprobed for β-actin to ensure equal loading.
Deletion of CARMA1 after T cell activation reduces T cell reactivation in vitro
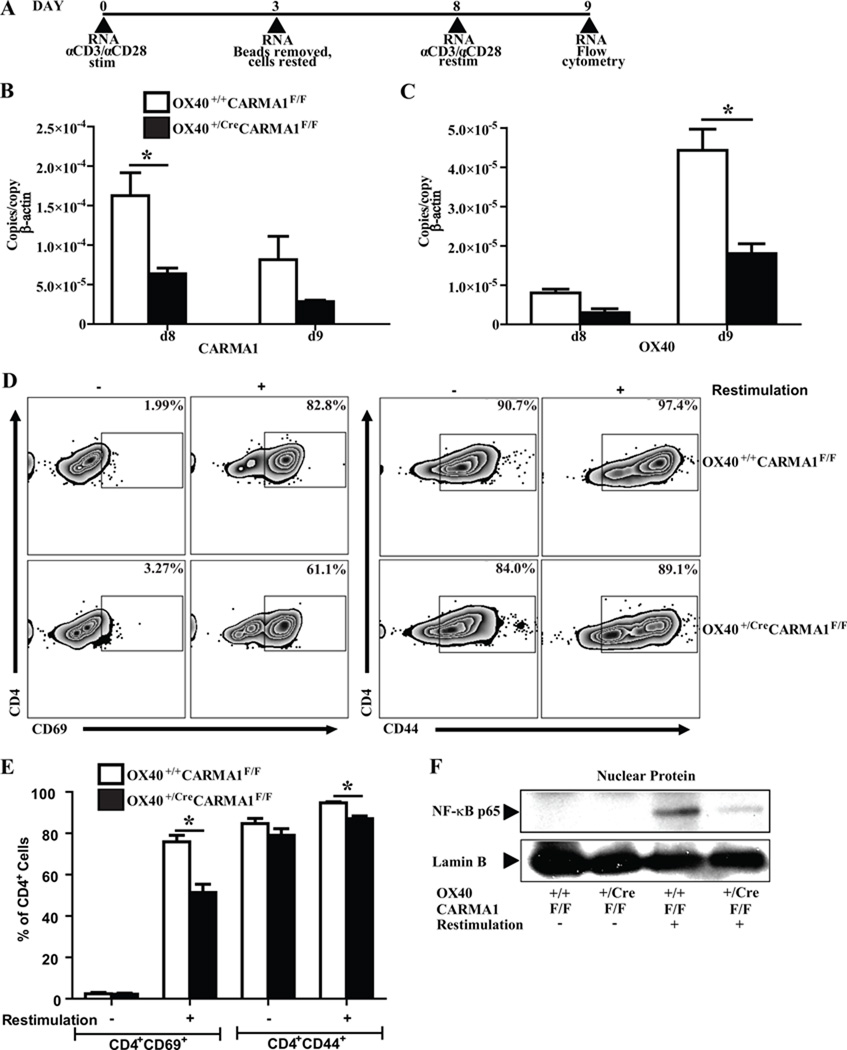
The effects of CARMA1 deletion after T cell activation remains unknown. We hypothesized that deletion of CARMA1 following T cell activation would impair the response of these cells to re-stimulation via the TCR by inhibiting NF-κB signaling. To address this question, CD4+ T cells were activated with anti-CD3 and anti-CD28 coated beads for 3 days, rested for 5 days in media, and then restimulated with anti-CD3 and anti-CD28 (Fig 2A). Eight days after activation, CARMA1 RNA levels remained reduced in the CD4+ T cells from OX40+/CreCARMA1F/F mice compared to OX40+/CreCARMA1F/F mice following stimulation (Fig 2B). The expression of OX40 RNA was similar in T cells from both strains of mice 8 days after activation but did not increase as much in CD4+ T cells from OX40+/CreCARMA1F/F mice following reactivation compared to T cells from OX40+/+CARMA1F/F mice (Fig 2C). Following the resting period, very few cells expressed CD69, while a majority of T cells from both strains of mice expressed CD44, indicative of prior activation (Fig 2D and 2E). However, following restimulation, there was a lower percentage of CD69+ and CD44+ CD4+ T cells isolated from OX40+/CreCARMA1F/F mice compared to CD4+ T cells from OX40+/+CARMA1F/F mice (Fig 2D and 2E). These data suggest that deletion of CARMA1 after activation impairs subsequent responses of these cells to restimulation via the TCR. To address if the decrease in reactivation is associated with a concomitant decrease in NF-κB activity, we measured the levels of NF-κB p65 in the nuclear protein extracts isolated from CD4+ T cells 2h following reactivation with anti-CD3 and anti-CD28 antibodies. Decreased nuclear translocation of p65 subunit of NF-κB was observed in the CD4+ T cells from OX40+/CreCARMA1F/F mice compared to CD4+ T cells from OX40+/+CARMA1F/F mice (Fig 2F). As expected from our findings in Fig 1, nuclear translocation of NF-κB was only partially abrogated in activated CD4+ T cells from OX40+/CreCARMA1F/F mice.
Figure 2. Deletion of CARMA1 after T cell activation reduces T cell reactivation in vitro.
A) CD4+ T cells isolated from mice were stimulated with anti-CD3 and anti-CD28 coated antibodies, rested and reactivated. B and C) Transcript expression levels of CARMA1 (B) and OX40 (C) on days 8 and 9 of the experimental protocol, as analyzed by real-time quantitative PCR. Data from 3 mice per genotype are shown as mean ± SEM. *p<0.05 by t-test. D) Representative flow cytometry plots demonstrating CD69 and CD44 expressing CD4+ T cells without (−) or with (+) anti-CD3 and anti-CD28 restimulation, assessed on day 9. All plots depict events in live lymphocyte gate. E) Percentage of activated (CD4+CD69+) and memory-phenotype (CD4+CD44+) T cells without (−) or with (+) anti-CD3 and anti-CD28 restimulation, assessed on day 9. Data reported as mean ± SEM from 9–10 mice in 4 separate experiments. *p<0.05 by t-test. F) Nuclear translocation of NF-κB p65 subunit in CD4+ T cells without (−) or with (+) anti-CD3 and anti-CD28 restimulation. Each lane was loaded with 5 µg of nuclear protein extracted from cells 2 hours following restimulation. Following immunoblotting for CARMA1, membranes were stripped and reprobed for Lamin-B to ensure equal nuclear protein loading. Blot representative of 2 independent experiments with 2 different samples per genotype.
Given the incomplete deletion mediated by the OX40 controlled Cre, we also tested the effects of CARMA1 deletion post-activation using an inducible Cre system. Mice that constitutively express (via the ROSA26 promoter) a tamoxifen-inducible Cre-ERT2 fusion protein (29, 30) were crossed to the CARMA1F/F line and then to the OT-II line to generate Rosa26CreERT2/+/CARMA1F/FOT-II mice. Use of tamoxifen in vivo with ROSA26CreERT2/+ mice can lead to bone marrow toxicity (38) so we chose to isolate T cells from naïve Rosa26CreERT2/+/CARMA1F/F OT-II and wild-type OT-II mice and then study them in vitro. Isolated CD4+ T cells were activated with OVA in the presence of irradiated splenocytes for 3 days as previously described (17). Naïve T cells from both strains of mice activated and proliferated normally (data not shown). Live cells were then isolated and then rested for 5 days in media with IL-2 and 1µM 4-hydroxytamoxifen or vehicle (Suppl. Fig 1A). QPCR analysis of the cells following tamoxifen treatment demonstrated effective deletion of CARMA1 in the tamoxifen treated Rosa26CreERT2/+/CARMA1F/F OT-II cells (Suppl. Fig 1B). Cells were then split and half were restimulated with anti-CD3/CD28 beads for 24 h and then analyzed for activation. As expected both vehicle and tamoxifen treated wild-type OT-II cells upregulated CD69 upon restimulation. Vehicle treated Rosa26CreERT2/+/CARMA1F/F OT-II CD4+ T cells upregulated CD69 in a similar manner to wild-type OT-II cells, however after exposure to tamoxifen there was almost no upregulation of CD69 consistent with a defect in TCR mediated reactivation (Suppl. Fig 1C). Taken together, these data suggest that deletion of CARMA1 in effector T cells impairs TCR mediated NF-κB signaling and T cell reactivation.
Deletion of CARMA1 by Ox40-driven Cre does not reduce acute allergic airway inflammation
Previous studies from our laboratory have demonstrated that deletion of CARMA1 from naive T cells abrogates allergic airway inflammation by preventing T cell activation (17). We first wanted to determine when OX40-Cre activity would be induced in an OVA-induced model of acute allergic airway inflammation. For these experiments, we used a fluorescent-reporter (mT/mG) mouse that expresses an enhanced GFP in tissues following Cre-mediated excision of the mT cassette (31). OX40+/CremT/mG mice were immunized with i.p injections of alum-conjugated OVA and challenged with OVA (Suppl. Fig 2A). OX40 driven Cre-recombinase activity, indicated by the expression of GFP, was evaluated in CD4+ T cells from the lungs, spleen and BAL from these mice following OVA immunization or following OVA immunization and challenge. Naïve mice had very few GFP+ CD4+ T cells in the lung and spleen (data not shown). Immunization alone resulted in generation of small numbers of GFP+ CD4+ T cells in the lungs and spleens of mice and almost no GFP+ cells in the BAL (Suppl. Fig 2B and data not shown). However, OVA immunization and challenge resulted in substantial generation of GFP+ CD4+ T cells in the lungs and BAL, but not the spleen (Suppl. Fig 2D and data not shown). These studies demonstrated that immunization and challenge with OVA led to Cre activity in activated CD4+ T cells from OX40+/Cre mice. Hence, we would expect OX40+/CreCARMA1F/F mice would effectively delete CARMA1 from activated T cells in an OVA induced model of allergic airway inflammation.
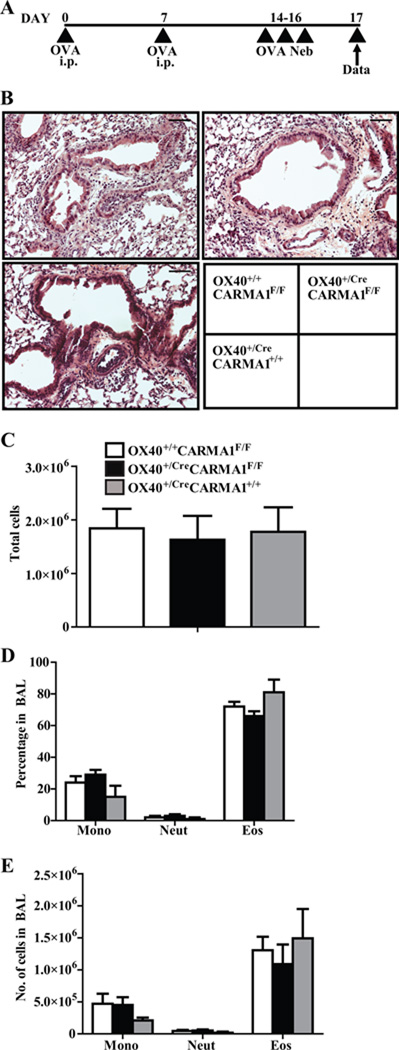
To determine if deletion of CARMA1 after T cell activation reduces allergic airway responses, OX40+/CreCARMA1F/F, OX40+/+ CARMA1F/F and OX40+/CreCARMA1+/+ mice were sensitized and challenged with OVA in an acute model of allergic asthma (Fig 3A). We utilized OX40+/CreCARMA1+/+ mice to control for any effects of the deletion of one OX40 allele and for the expression of Cre recombinase in activated T cells. Development of allergic airway inflammation was comparable across all three strains of mice tested. Histology of lung sections did not demonstrate a marked difference in cellular infiltration between OX40+/CreCARMA1F/F, OX40+/+CARMA1F/F and OX40+/CreCARMA1+/+ mice (Fig 3B) Furthermore, all three strains demonstrated similar cell counts and cell differential in the bronchoalveolar lavage (BAL) (Fig 3C, 3D, and 3E). In addition, similar numbers of CD4+CD69+ T cells were observed in the BAL recovered from OX40+/CreCARMA1F/F and OX40+/+CARMA1F/F mice (data not shown). These data suggest that OX40-Cre driven deletion of CARMA1 does not impair acute allergic airway responses.
Figure 3. Deletion of CARMA1 by OX40-Cre does not reduce acute allergic airway inflammation.
A) Protocol used to induce acute allergic airway inflammation. B) Representative Hematoxylin & Eosin stained lung sections demonstrating cellular infiltration. Black bars are 50 µM C) Total cell number in BAL fluid. D, E) Percentages and numbers of individual cell types in BAL fluid. Data reported as mean ± SEM from 3–6 mice in 2 separate experiments.
Deletion of CARMA1 by Ox40-driven Cre attenuates allergic airway inflammation following antigen rechallenge
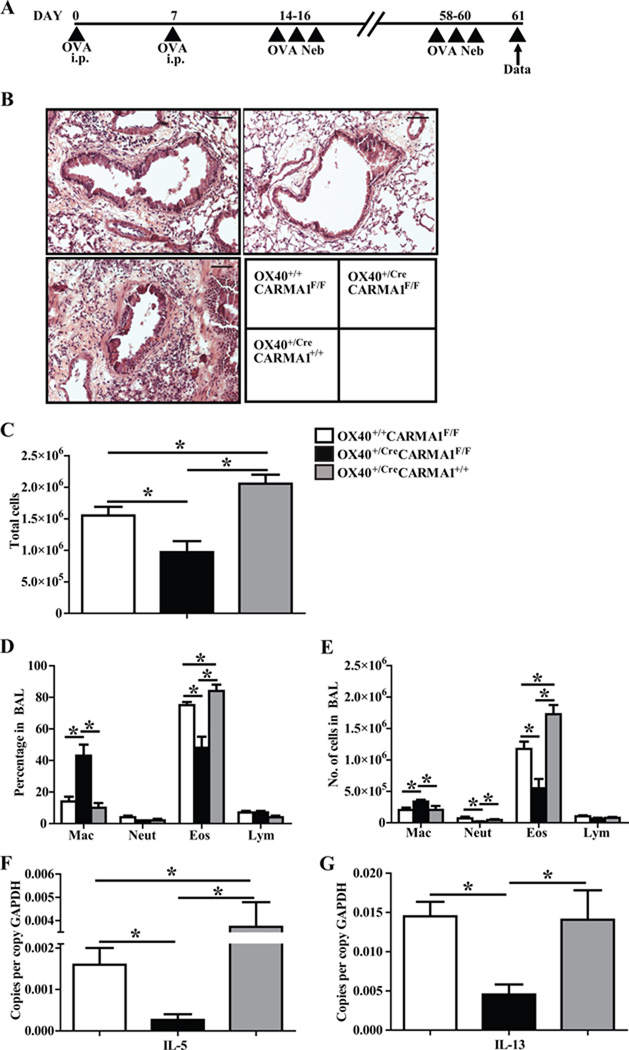
To determine if deletion of CARMA1 following T cell activation reduced T cell memory responses in allergic asthma, we used OX40+/CreCARMA1F/F, OX40+/+CARMA1F/F and OX40+/CreCARMA1+/+ mice in a modified protocol where mice were sensitized and challenged with OVA as in the acute model of allergic asthma, rested for six weeks, and then rechallenged with OVA (Fig 4A). Histology of lung sections demonstrated a marked decrease in cellular infiltration in OX40+/CreCARMA1F/F mice compared to OX40+/+CARMA1F/F and OX40+/CreCARMA1+/+ mice (Fig 4B). Evaluation of BAL revealed decreased number of total cells in OX40+/CreCARMA1F/F mice compared to controls (Fig 4C). Differential cell counts of BAL fluid also demonstrated a decrease in the percentage and total number of eosinophils in OX40+/CreCARMA1F/F mice compared to controls (Fig 4D, and 4E). In addition, the percentage and total number of macrophages were increased in OX40+/CreCARMA1F/F mice (Fig 4D, and 4E). Finally, QPCR performed on lung RNA revealed that transcript expression of the pro-allergic cytokines IL-5 and IL-13 were reduced in the lungs of OX40+/CreCARMA1F/F mice compared to OX40+/+CARMA1F/F mice and OX40+/CreCARMA1+/+ mice (Fig 4F and 4G). We also assessed the levels of IL-4 but the expression was extremely low in all strains of mice (data not shown).
Figure 4. Deletion of CARMA1 by OX40-Cre attenuates allergic airway inflammation following OVA rechallenge.
A) Protocol used to induce allergic airway inflammation. B) Representative Hematoxylin & Eosin stained lung sections demonstrating cellular infiltration. Black bars are 50 µM C) Total cell number in BAL fluid. D and E) Percentages and numbers of individual cell types in BAL fluid. F and G) Real-time quantitative PCR analysis of IL-5 and IL-13 mRNA expression in mouse lungs. Data for C to G reported as mean ± SEM from 5–10 mice in three separate experiments. *p<0.05 by one-way ANOVA.
Deletion of CARMA1 by Ox40-driven Cre attenuates memory T cell reactivation in allergic airway inflammation following allergen rechallenge
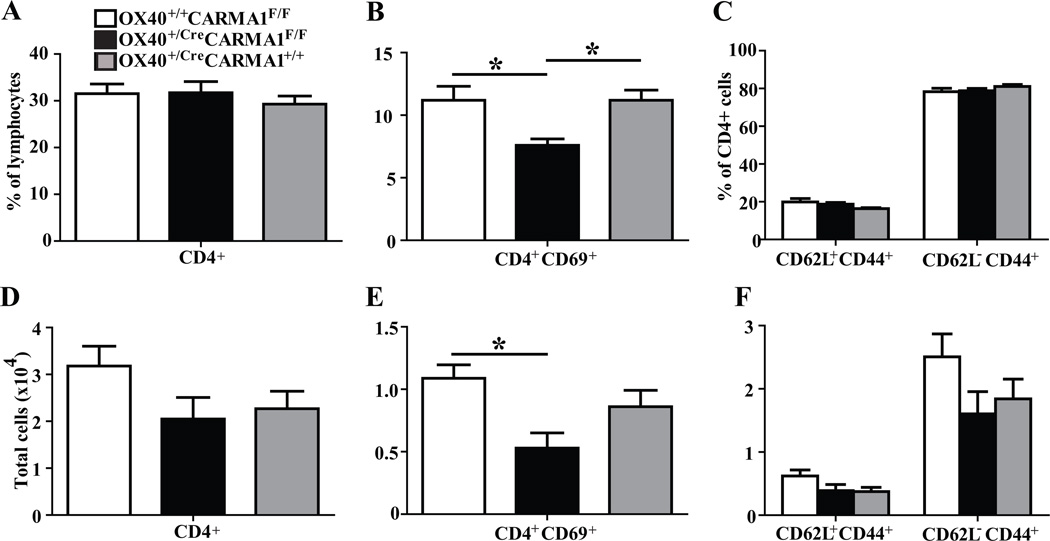
To determine if the reduced eosinophil influx and cytokine production in the lungs of OX40+/CreCARMA1F/F mice is due to reduced memory T cell reactivation, BAL cells from mice subjected to OVA rechallenge were analyzed by flow cytometry for T cell activation and memory markers. The percentage (Fig 5A) and total number (Fig 5D) of CD4+ T cells were comparable across the three strains tested. There was a decreased percentage (Fig 5B) of CD4+CD69+ cells in the BAL from OX40+/CreCARMA1F/F mice compared to both OX40+/+CARMA1F/F and OX40+/CreCARMA1+/+ mice. There was also a decreased total number (Fig 5E) of CD4+CD69+ cells in the BAL from OX40+/CreCARMA1F/F mice compared to the OX40+/+CARMA1F/F mice. However, there was no difference in the percentage (Fig 5C) or number (Fig 5F) of central memory (CD4+CD62L+CD44+) or effector memory (CD4+CD62L−CD44+) T cells in the BAL fluid among the three strains. Reduced number of activated (CD4+CD69+) T cells following allergen rechallenge with preserved number of memory-phenotype T cells suggests a functional reactivation defect in the memory T cell pool.
Figure 5. Deletion of CARMA1 by OX40-Cre attenuates T cell activation in vivo.
A and B) Percentage and number of total (A, D), activated (B, E), and memory (C, F) CD4+ T cell subsets recovered in BAL fluid from mice subjected to the OVA rechallenge protocol depicted in Fig 4A. Data for A to F reported as mean ± SEM from 5–10 mice in three separate experiments. *p<0.05 by one-way ANOVA.
Deletion of CARMA1 by OX40-driven Cre does not impair the development of memory T cells in the lungs and lymph node
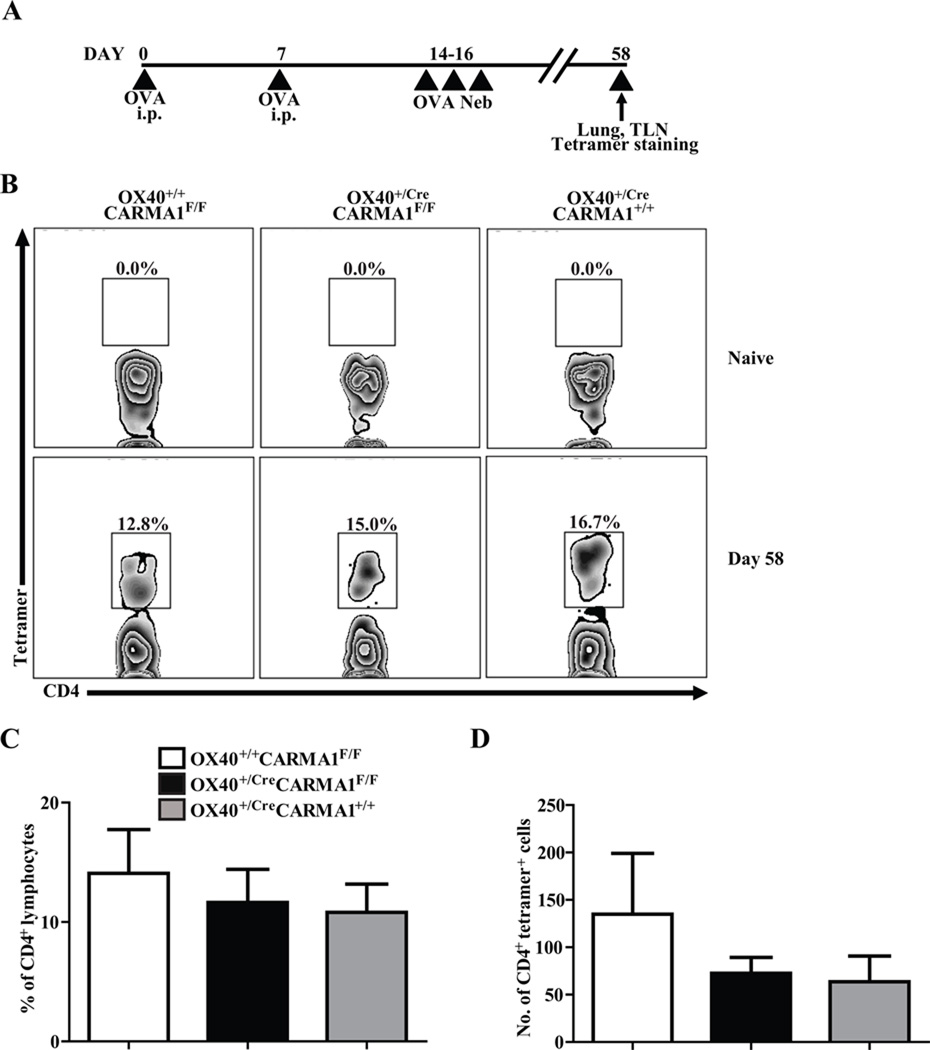
It has been previously demonstrated that activated T cells from mice lacking the CARD domain of CARMA1 undergo increased levels of apoptosis compared to T cells from wild-type mice, a defect rescued by the addition of IL-2 in cell cultures (6, 39, 40). These data suggest that deletion of CARMA1 could lead to enhanced apoptosis of activated T cells and impair the formation of memory CD4+ T cells. In order to test if there was impaired development of memory T cells in the OX40+/CreCARMA1F/F mice in vivo, we used MHC class II tetramers to measure the levels of OVA-specific CD4+ T cells in the lungs and draining lymph nodes of OX40+/CreCARMA1F/F, OX40+/+CARMA1F/F, and OX40+/CreCARMA1+/+ mice in the naïve state and following OVA sensitization and challenge (Fig 6A). Consistent with data from others (32), naïve mice had nearly undetectable numbers of OVA-specific T cells in the lungs (Fig 6B). Six weeks following OVA sensitization and challenge, there was a marked increase in the percentage and number of OVA-specific CD4+ T cells in the lungs (Fig 6B) and lymph nodes (Suppl. Fig 3) of all three strains of mice without differences between the genotypes (Fig 6C and 6D). Comparable levels of OVA-specific CD4+ T cells in the three strains of mice suggest that the reduced inflammation observed in OX40+/CreCARMA1F/F mice is not due to a reduced size of antigen-specific memory T cell pool.
Figure 6. Deletion of CARMA1 by Ox40-Cre does not impair the development of memory T cells in the lungs.
A) Protocol used to evaluate the number of OVA-specific T cells in the lungs of mice prior to allergen rechallenge. B) Detection of OVA-specific CD4+ T cells in naïve and OVA-exposed lungs by MHC Class II tetramers. Presented data are representative flow cytometry plots that indicate the percentage of CD4+/tetramer+/CD8/CD11c/CD19 cells following bead selection for tetramer-bound lymphocytes from the lungs of mice. C and D) Percentage and number of CD4+/tetramer+/CD8/CD11c/CD19 cells following bead selection for tetramer-bound lymphocytes from the lungs of mice on day 58. Data reported as mean ± SEM from 6–10 mice in 3 separate experiments.
Deletion of CARMA1 by OX40-driven Cre attenuates allergic airway inflammation following adoptive transfer of in vitro Th2-polarized CD4+ T cells from OX40+/CreCARMA1F/F mice
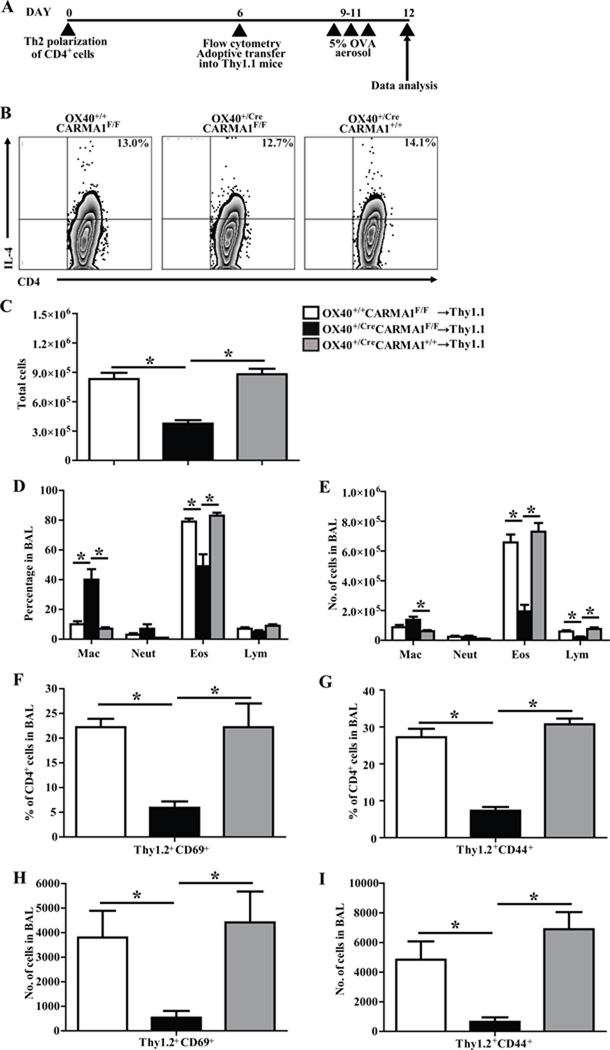
In order to isolate the defect to CD4+ effector T cells we utilized an adoptive transfer model of allergic airway inflammation that is dependent on transferred OVA-specific Th2 cells to generate inflammation. For these experiments, Thy1.2+ CD4+ T cells were isolated from OX40+/CreCARMA1F/F, OX40+/+CARMA1F/F and OX40+/CreCARMA1+/+ OT-II mice and polarized into Th2 cells in vitro according to a standard 6 day protocol (Fig 7A) as previously described (17). As expected, Western blot analysis revealed reduced CARMA1 protein in Th2 polarized CD4+ T cells from OX40+/CreCARMA1F/F OT-II mice compared to cells from OX40+/+CARMA1F/F OT-II mice (data not shown). The viability and number of cells obtained after Th2 polarization was comparable between the three strains of mice and analysis of intracellular cytokine production revealed comparable IL-4 production in Th2 polarized CD4+ T cells from all three strains of mice(Fig 7B). + T cells from OX40+/CreCARMA1F/F, OX40+/+CARMA1F/F and OX40+/CreCARMA1+/+ mice were then adoptively transferred into Thy1.1+ wild-type mice which were subsequently challenged with OVA (Fig 7A). A reduction in the total cell numbers (Fig 7C), percentage of eosinophils (Fig 7D), and total eosinophil and lymphocyte cell numbers (Fig 7E) was observed in BAL fluid recovered from recipient mice that received OX40+/CreCARMA1F/F OT-II cells compared to recipient mice that received OX40+/+CARMA1F/F OT-II cells or OX40+/CreCARMA1+/+ OT-II cells. Furthermore, there was a reduced percentage and number of Thy1.2+CD69+ (Fig 7F and 7H) and Thy1.2+CD44+ (Fig 7G and 7I) in the BAL fluid recovered from recipient mice that received OX40+/CreCARMA1F/F OT-II cells compared to recipient mice that received OX40+/+CARMA1F/F OT-II or OX40+/CreCARMA1+/+ OT-II cells.
Figure 7. Deletion of CARMA1 by OX40-Cre impairs the function of memory T cells.
A) Experimental protocol used for in vitro Th2 polarization and adoptive transfer of CD4+ T cells from OX40+/CreCARMA1F/F, OX40+/+CARMA1F/F, and OX40+/CreCARMA1+/+ mice. B) Intracellular IL-4 production following PMA and Ionomycin restimulation of Th2 polarized CD4+ T cells on day 6. C, D and E) Total cell number, percentage and number of individual cell types in the BAL fluid recovered from Thy1.1+ recipient mice. F and G) Percentage and (H and I) number of Thy1.2+ (donor) activated, effector and memory CD4+ T cell subsets recovered in the BAL fluid from recipient Thy1.1+ mice. Data for C to I reported as mean ± SEM from 5–10 recipient mice per donor genotype from 2 separate experiments. *p<0.05 by one-way ANOVA.
DISCUSSION
CARMA1 is an important component of the TCR-mediated signaling cascade that leads to NF-κB activation (1, 11–14) and naïve T cell activation (2, 4, 5). In the study presented here, we demonstrate that deletion of CARMA1 from T cells after activation reduces their responses to TCR restimulation, including NF-κB activation and expression of activation markers. We then demonstrate that deletion of CARMA1 after T cell activation in vivo leads to attenuated recall responses in a model of allergic airway inflammation due to a failure to reactivate memory T cells. These data characterize a novel role for CARMA1 in mediating CD4+ effector/memory T cell reactivation and functions in response to TCR stimulation.
Memory CD4+ T cells that develop with initial exposure to pathogens and reside in the lung are the first responders to a secondary infection with the same or similar pathogen (41). Although these memory CD4+ T cells serve to counter the detrimental effects of secondary infections, aberrant activation of T cells specific for non-pathogenic antigens can lead to inflammatory diseases such as allergic asthma (42, 43). Allergen-specific MHC class II restricted CD4+ T cells are thought to be the primary mediators of allergic airway inflammation (23, 24, 44–46). Specifically, CD4+ Th2-type lymphocytes seems to be central to the pathogenesis of allergic asthma, as the levels of these cells and the cytokines they secrete (IL-4, IL-5 and IL-13) are elevated in the airways of human allergic asthma patients (45, 47–50). Thus, understanding the mechanisms that control TCR signaling in effector and memory T cells may reveal potential therapeutic targets that can inhibit T cell driven chronic inflammatory disorders such as asthma.
In our prior work, we demonstrated that CARMA1-deficient mice do not develop allergic airway inflammation in response to OVA sensitization and challenge due to a defect in naïve T cell activation (17), suggesting that CARMA1 could be an effective therapeutic target in asthma. However, this does not fully mimic established allergic asthma in humans where the allergen-specific T cells that drive allergic inflammation have already been activated and reside in the lung as memory or effector T cells. Reactivation of effector and memory T cells requires engagement of the TCR by MHC–peptide-antigen complexes, but the activation of these cells requires less stimuli and secondary signals than naïve T cells (18, 19). Since CARMA1 likely acts as an amplifier of TCR stimulation (15, 16), it may not be necessary for reactivation of effector and memory T cell subsets. Consistent with this, a prior study of Bcl10, a binding partner of CARMA1, demonstrated that it was not necessary for activation of memory-phenotype cells (20). These data suggest that TCR signaling in memory and effector T cells may not require components of the pathway that are necessary for activation in naïve T cells. Thus, prior to the study presented here it was unclear if inhibition of CARMA1 in these T cell subsets would affect their activity.
In the murine model used in our experiments, immunization with OVA leads to the priming and expansion of OVA-specific T cells (51). These cells are then poised to react if re-exposed to OVA in organs and lymphoid tissue (52). When mice are challenged with OVA in the airways, the protein is taken up by antigen presenting cells and presented to these OVA-specific T cells in the lung and draining lymph nodes. The T cells are activated, migrate to the airways, and then help initiate and orchestrate the allergic inflammatory response (53). Following the initial acute response, memory T cells are formed and remain in the lung and draining lymph nodes to provide surveillance for further re-exposure to antigen. This mimics the situation in humans with established allergic asthma where re-exposure in the lung to allergens rapidly leads to an exacerbation of allergic airway inflammation. Central to the model is the activation and reactivation of T cells via TCR stimulation.
In order to study the role of CARMA1 in effector and memory T cells, we used Cre recombinase based systems to delete CARMA1 in T cells following the initial activation of these cells. For these experiments, we primarily used mice that express Cre recombinase driven by the OX40 promoter. OX40 (CD134, TNFRSF4) is a member of the TNF receptor-superfamily and functions as a secondary co-stimulatory molecule on T cells. It is expressed 24 to 72 hours following T cell activation and is not expressed on naïve T cells or most resting memory T cells (37). It is also expressed on a majority of thymic derived regulatory T cells and a small proportion of memory-phenotype cells in the periphery of naïve mice (27, 54). In our study, anti-CD3 and anti-CD28 driven activation of isolated CD4+ T cells from these mice over a 3-day period was effective at decreasing CARMA1 RNA and protein expression, although the deletion was not complete. Upon restimulation, the OX40+/CreCARMA1F/F T cells had reduced but not absent expression of activation and memory markers along with defective NF-κB signaling compared to control cells. Since OX40 was only upregulated on a proportion of the cells, we suspect that the residual CARMA1 was from cells that did not express OX40 and thus did not induce Cre recombination of CARMA1. This may also explain the attenuated but not complete inhibition of TCR signaling in these cells. Given the heterogeneity of OX40 expression, we confirmed our findings using Rosa26CreERT2/+/CARMA1F/F OT-II cells that contain a Cre recombinase that is active only in the presence of tamoxifen. When these cells were activated with OVA and then restimulated after tamoxifen exposure there was more complete deletion of CARMA1 and nearly no upregulation of CD69. These results suggest that the partial, but incomplete reactivation responses observed in vitro are likely caused by the incomplete deletion of CARMA1 in the OX40-Cre based system.
We next sought to corroborate our findings in vivo. Using a mouse model of allergic airway inflammation, we found that acute allergic airway responses were not attenuated in OX40+/CreCARMA1F/F mice that were immunized and challenged over 3 days with OVA. Given that OX40 is upregulated over 24 to 72 hours, it is likely that CARMA1 expression was not significantly decreased in the majority of effector T cells until the end of the challenge phase and thus it is not surprising that we did not see differences in acute response to OVA challenge. In contrast to the effects seen in the acute model, when OX40+/CreCARMA1F/F mice were immunized, challenged, rested to allow memory T cell formation, and then rechallenged with OVA there was marked attenuation of allergic airway responses compared to the responses in OX40+/+CARMA1F/F mice and OX40+/CreCARMA1+/+ mice. The attenuated response was manifested by reduced numbers of airway eosinophils and activated CD4+ T cells as well as decreased RNA expression of the Th2 cytokines IL-5 and IL-13. There are two potential mechanisms that explain our findings: 1) the CARMA1-deficient memory cells have impaired reactivation in response to OVA and thus do not induce as intense inflammation as wild-type T cells; or 2) since CARMA1 may influence T cell survival (39, 40), CARMA1-deficient T cells may undergo apoptosis and thus are not available to react to the recall challenge. Using MHC class II tetramers specific for TCRs that recognize OVA peptides, we were able to demonstrate similar numbers of OVA specific T cells just prior to OVA rechallenge in the lung and draining lymph nodes of OX40+/CreCARMA1F/F mice compared to control mice, thus suggesting that the impaired inflammatory response is due to impaired memory T cell function rather than apoptosis. It is interesting that the deletion of CARMA1 after T cell activation did not affect the size of the memory T cell population, suggesting that optimal TCR signaling (via CARMA1 amplification) is not necessary for memory T cell formation and maintenance. Despite the attenuated response with recall challenge, the allergic inflammation was not completely eliminated. This may be due to the existence of residual memory T cells with intact CARMA1 expression in the OX40-Cre driven system as seen in vitro.
The recall experiments also did not give us specific information about the effects of CARMA1-deletion on effector T cell functions. The experiment utilizing adoptive transfer of Th2-polarized OX40+/CreCARMA1F/F OT-II cells limited the CARMA1-deletion to effector T cells and clearly demonstrated that these cells were less effective than control cells in inducing allergic inflammation. In addition, the reduction in inflammation was more dramatic than seen in the recall model suggesting that either CARMA1 is more important for effector T cell function than for memory T cell function or that the OX40 driven Cre-mediated deletion of CARMA1 was more efficient in vitro than in vivo.
An important consideration is that in the OX40+/CreCARMA1F/F mice CARMA1 is likely deleted from a majority of regulatory T cells given the expression of OX40 on most thymic derived regulatory T cells (27, 55). This does not result in a decrease in the number of regulatory T cells in these mice at baseline (data not shown). It is unclear whether this will affect regulatory T cell function, however in vitro experiments with T cells from a mouse line with a mutant form of CARMA1 that results in very low level expression suggested that CARMA1 was not necessary for suppression by regulatory T cells (16). Furthermore, a regulatory T cell functional defect would not explain our findings as this would be expected to lead to more inflammation rather than less. However, it is possible that a defect in regulatory T cell function will partially counter the effects of CARMA1-deficiency in memory and effector T cells by enhancing inflammation. The result from the adoptive transfer of Th2-polarized CARMA1-deficient OT-II cells into naïve mice addresses this issue by limiting CARMA1-deficiency to effector T cells.
Finally, it is important to note that CARMA1 likely participates in non-TCR signaling pathways. For example, a recent report demonstrated that the CARMA1/Bcl10/MALT1 complex also binds to the OX40 signalosome and drives TCR-independent NF-κB activation in T cells after antigen clearance (33). It remains to be determined if the participation of CARMA1 in other T cell signaling pathways contributes to effector and memory T cell function.
In conclusion, our results demonstrate that CARMA1 is necessary for optimal effector and memory T cell function in response to antigen challenge. The effect seems to be mediated in part by impaired TCR signaling in these cells. These data have implications for the treatment of chronic T cell-mediated disorders such as allergic asthma where T cells have already been sensitized and are poised to react to antigen reexposure. Inhibition of CARMA1 or other components of the TCR signaling pathway could limit the intensity of the recall response initiated by memory T cells. In addition, the inhibition of CARMA1 in effector T cells could limit the amount of inflammation generated by these cells in response to antigen.
Supplementary Material
Acknowledgements
The authors would like to thank Barry Sandall for technical support with this research, Dr. Nigel Killeen for providing the OX40+/Cre mice, and Dr. Dan Littman for providing the CARMA1F/F mice.
Footnotes
Grant Support: This work was supported by National Institutes of Health grants R01HL088297-04 (B.D.M and R.J.X) and T32HL007874 (B.D.M).
Nonstandard abbreviations used: AHR, airway hyperresponsiveness; BAL, bronchoalveolar lavage; TCR, T cell receptor.
Conflict of interest: The authors have declared that no conflict of interest exists.
REFERENCES
- 1.Blonska M, Lin X. CARMA1-mediated NF-kappaB and JNK activation in lymphocytes. Immunol Rev. 2009;228:199–211. doi: 10.1111/j.1600-065X.2008.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thome M. CARMA1, BCL-10 and MALT1 in lymphocyte development and activation. Nat Rev Immunol. 2004;4:348–359. doi: 10.1038/nri1352. [DOI] [PubMed] [Google Scholar]
- 3.Pomerantz JL, Denny EM, Baltimore D. CARD11 mediates factor-specific activation of NF-kappaB by the T cell receptor complex. Embo J. 2002;21:5184–5194. doi: 10.1093/emboj/cdf505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egawa T, Albrecht B, Favier B, Sunshine MJ, Mirchandani K, O’Brien W, Thome M, Littman DR. Requirement for CARMA1 in antigen receptor-induced NF-kappa B activation and lymphocyte proliferation. Curr Biol. 2003;13:1252–1258. doi: 10.1016/s0960-9822(03)00491-3. [DOI] [PubMed] [Google Scholar]
- 5.Hara H, Wada T, Bakal C, Kozieradzki I, Suzuki S, Suzuki N, Nghiem M, Griffiths EK, Krawczyk C, Bauer B, D’Acquisto F, Ghosh S, Yeh WC, Baier G, Rottapel R, Penninger JM. The MAGUK family protein CARD11 is essential for lymphocyte activation. Immunity. 2003;18:763–775. doi: 10.1016/s1074-7613(03)00148-1. [DOI] [PubMed] [Google Scholar]
- 6.Newton K, Dixit VM. Mice lacking the CARD of CARMA1 exhibit defective B lymphocyte development and impaired proliferation of their B and T lymphocytes. Curr Biol. 2003;13:1247–1251. doi: 10.1016/s0960-9822(03)00458-5. [DOI] [PubMed] [Google Scholar]
- 7.Bertin J, Wang L, Guo Y, Jacobson MD, Poyet JL, Srinivasula SM, Merriam S, DiStefano PS, Alnemri ES. CARD11 and CARD14 are novel caspase recruitment domain (CARD)/membrane-associated guanylate kinase (MAGUK) family members that interact with BCL10 and activate NF-kappa B. J Biol Chem. 2001;276:11877–11882. doi: 10.1074/jbc.M010512200. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto R, Wang D, Blonska M, Li H, Kobayashi M, Pappu B, Chen Y, Lin X. Phosphorylation of CARMA1 Plays a Critical Role in T Cell Receptor-Mediated NF-kappaB Activation. Immunity. 2005;23:575–585. doi: 10.1016/j.immuni.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Thome M, Charton JE, Pelzer C, Hailfinger S. Antigen receptor signaling to NF-kappaB via CARMA1, BCL10, and MALT1. Cold Spring Harb Perspect Biol. 2010;2 doi: 10.1101/cshperspect.a003004. a003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sommer K, Guo B, Pomerantz JL, Bandaranayake AD, Moreno-Garcia ME, Ovechkina YL, Rawlings DJ. Phosphorylation of the CARMA1 Linker Controls NF-kappaB Activation. Immunity. 2005;23:561–574. doi: 10.1016/j.immuni.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Rueda D, Thome M. Phosphorylation of CARMA1: The link(er) to NF-kB activation. Immunity. 2005;23:551–555. doi: 10.1016/j.immuni.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Gaide O, Martinon F, Micheau O, Bonnet D, Thome M, Tschopp J. Carma1, a CARD-containing binding partner of Bcl10, induces Bcl10 phosphorylation and NF-kappaB activation. FEBS Lett. 2001;496:121–127. doi: 10.1016/s0014-5793(01)02414-0. [DOI] [PubMed] [Google Scholar]
- 13.Gaide O, Favier B, Legler DF, Bonnet D, Brissoni B, Valitutti S, Bron C, Tschopp J, Thome M. CARMA1 is a critical lipid raft-associated regulator of TCR-induced NF-kappa B activation. Nat Immunol. 2002;3:836–843. doi: 10.1038/ni830. [DOI] [PubMed] [Google Scholar]
- 14.Wang D, You Y, Case SM, McAllister-Lucas LM, Wang L, DiStefano PS, Nunez G, Bertin J, Lin X. A requirement for CARMA1 in TCR-induced NF-kappa B activation. Nat Immunol. 2002;3:830–835. doi: 10.1038/ni824. [DOI] [PubMed] [Google Scholar]
- 15.Altin JA, Tian L, Liston A, Bertram EM, Goodnow CC, Cook MC. Decreased T-cell receptor signaling through CARD11 differentially compromises forkhead box protein 3-positive regulatory versus T(H)2 effector cells to cause allergy. J Allergy Clin Immunol. 2011;127:1277–1285. doi: 10.1016/j.jaci.2010.12.1081. e1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes MJ, Krebs P, Harris N, Eidenschenk C, Gonzalez-Quintial R, Arnold CN, Crozat K, Sovath S, Moresco EM, Theofilopoulos AN, Beutler B, Hoebe K. Commitment to the regulatory T cell lineage requires CARMA1 in the thymus but not in the periphery. PLoS Biol. 2009;7:e51. doi: 10.1371/journal.pbio.1000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medoff BD, Seed B, Jackobek R, Zora J, Yang Y, Luster AD, Xavier R. CARMA1 is critical for the development of allergic airway inflammation in a murine model of asthma. J Immunol. 2006;176:7272–7277. doi: 10.4049/jimmunol.176.12.7272. [DOI] [PubMed] [Google Scholar]
- 18.Floyd TL, Koehn BH, Kitchens WH, Robertson JM, Cheeseman JA, Stempora L, Larsen CP, Ford ML. Limiting the amount and duration of antigen exposure during priming increases memory T cell requirement for costimulation during recall. Journal of Immunology. 2011;186:2033–2041. doi: 10.4049/jimmunol.1003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalia V, Sarkar S, Ahmed R. Fine-tuning CD4+ central memory T cell heterogeneity by strength of stimulation. European Journal of Immunology. 2008;38:15–19. doi: 10.1002/eji.200738044. [DOI] [PubMed] [Google Scholar]
- 20.Zeng H, Chen Y, Yu M, Xue L, Gao X, Morris SW, Wang D, Wen R. T cell receptor-mediated activation of CD4+CD44hi T cells bypasses Bcl10: an implication of differential NF-kappaB dependence of naive and memory T cells during T cell receptor-mediated responses. The Journal of biological chemistry. 2008;283:24392–24399. doi: 10.1074/jbc.M802344200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemanske RF, Jr, Busse WW. Asthma: clinical expression and molecular mechanisms. J Allergy Clin Immunol. 2010;125:S95–102. doi: 10.1016/j.jaci.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finkelman FD, Hogan SP, Hershey GK, Rothenberg ME, Wills-Karp M. Importance of cytokines in murine allergic airway disease and human asthma. J Immunol. 2010;184:1663–1674. doi: 10.4049/jimmunol.0902185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Afshar R, Medoff BD, Luster AD. Allergic asthma: a tale of many T cells. Clin Exp Allergy. 2008;38:1847–1857. doi: 10.1111/j.1365-2222.2008.03119.x. [DOI] [PubMed] [Google Scholar]
- 24.Medoff BD, Thomas SY, Luster AD. T Cell Trafficking in Allergic Asthma: The Ins and Outs. Annu Rev Immunol. 2008;26:205–232. doi: 10.1146/annurev.immunol.26.021607.090312. [DOI] [PubMed] [Google Scholar]
- 25.Yang L, Cohn L, Zhang DH, Homer R, Ray A, Ray P. Essential role of nuclear factor kappaB in the induction of eosinophilia in allergic airway inflammation. J Exp Med. 1998;188:1739–1750. doi: 10.1084/jem.188.9.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das J, Chen CH, Yang L, Cohn L, Ray P, Ray A. A critical role for NF-kappa B in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nat Immunol. 2001;2:45–50. doi: 10.1038/83158. [DOI] [PubMed] [Google Scholar]
- 27.Klinger M, Kim JK, Chmura SA, Barczak A, Erle DJ, Killeen N. Thymic OX40 expression discriminates cells undergoing strong responses to selection ligands. J Immunol. 2009;182:4581–4589. doi: 10.4049/jimmunol.0900010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shapiro-Shelef M, Lin KI, Savitsky D, Liao J, Calame K. Blimp-1 is required for maintenance of long-lived plasma cells in the bone marrow. J Exp Med. 2005;202:1471–1476. doi: 10.1084/jem.20051611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC., Jr Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest. 2005;115:3484–3493. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo K, McMinn JE, Ludwig T, Yu YH, Yang G, Chen L, Loh D, Li C, Chua S, Jr, Zhang Y. Disruption of peripheral leptin signaling in mice results in hyperleptinemia without associated metabolic abnormalities. Endocrinology. 2007;148:3987–3997. doi: 10.1210/en.2007-0261. [DOI] [PubMed] [Google Scholar]
- 31.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 32.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moon JJ, Chu HH, Hataye J, Pagan AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nat Protoc. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robertson JM, Jensen PE, Evavold BD. DO11.10 and OT-II T cells recognize a C-terminal ovalbumin 323–339 epitope. J Immunol. 2000;164:4706–4712. doi: 10.4049/jimmunol.164.9.4706. [DOI] [PubMed] [Google Scholar]
- 35.Chu HH, Moon JJ, Kruse AC, Pepper M, Jenkins MK. Negative selection and peptide chemistry determine the size of naive foreign peptide-MHC class II-specific CD4+ T cell populations. J Immunol. 2010;185:4705–4713. doi: 10.4049/jimmunol.1002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165:3043–3050. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 37.Croft M, So T, Duan W, Soroosh P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev. 2009;229:173–191. doi: 10.1111/j.1600-065X.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higashi AY, Ikawa T, Muramatsu M, Economides AN, Niwa A, Okuda T, Murphy AJ, Rojas J, Heike T, Nakahata T, Kawamoto H, Kita T, Yanagita M. Direct hematological toxicity and illegitimate chromosomal recombination caused by the systemic activation of CreERT2. Journal of immunology. 2009;182:5633–5640. doi: 10.4049/jimmunol.0802413. [DOI] [PubMed] [Google Scholar]
- 39.Schulze-Luehrmann J, Ghosh S. Antigen-receptor signaling to nuclear factor kappa B. Immunity. 2006;25:701–715. doi: 10.1016/j.immuni.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Moreno-Garcia ME, Sommer K, Haftmann C, Sontheimer C, Andrews SF, Rawlings DJ. Serine 649 phosphorylation within the protein kinase C-regulated domain down-regulates CARMA1 activity in lymphocytes. Journal of Immunology. 2009;183:7362–7370. doi: 10.4049/jimmunol.0902438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nature reviews.Immunology. 2009;9:153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 42.D’Ambrosio D, Mariani M, Panina-Bordignon P, Sinigaglia F. Chemokines and their receptors guiding T lymphocyte recruitment in lung inflammation. Am J Respir Crit Care Med. 2001;164:1266–1275. doi: 10.1164/ajrccm.164.7.2103011. [DOI] [PubMed] [Google Scholar]
- 43.Herrick CA, Bottomly K. To respond or not to respond: T cells in allergic asthma. Nat Rev Immunol. 2003;3:405–412. doi: 10.1038/nri1084. [DOI] [PubMed] [Google Scholar]
- 44.Ray A, Khare A, Krishnamoorthy N, Qi Z, Ray P. Regulatory T cells in many flavors control asthma. Mucosal Immunol. 2010;3:216–229. doi: 10.1038/mi.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meiler F, Zimmermann M, Blaser K, Akdis CA, Akdis M. T-cell subsets in the pathogenesis of human asthma. Curr Allergy Asthma Rep. 2006;6:91–96. doi: 10.1007/s11882-006-0045-0. [DOI] [PubMed] [Google Scholar]
- 46.Robinson DS. The role of the T cell in asthma. J Allergy Clin Immunol. 2010;126:1081–1091. doi: 10.1016/j.jaci.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 47.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 48.Del Prete GF, De Carli M, D’Elios MM, Maestrelli P, Ricci M, Fabbri L, Romagnani S. Allergen exposure induces the activation of allergen-specific Th2 cells in the airway mucosa of patients with allergic respiratory disorders. Eur J Immunol. 1993;23:1445–1449. doi: 10.1002/eji.1830230707. [DOI] [PubMed] [Google Scholar]
- 49.Walker C, Bode E, Boer L, Hansel TT, Blaser K, Virchow JC., Jr Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis. 1992;146:109–115. doi: 10.1164/ajrccm/146.1.109. [DOI] [PubMed] [Google Scholar]
- 50.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kool M, Soullie T, van Nimwegen M, Willart MA, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 53.Eisenbarth SC, Cassel S, Bottomly K. Understanding asthma pathogenesis: linking innate and adaptive immunity. Curr Opin Pediatr. 2004;16:659–666. doi: 10.1097/01.mop.0000145920.00101.e4. [DOI] [PubMed] [Google Scholar]
- 54.Kim JK, Klinger M, Benjamin J, Xiao Y, Erle DJ, Littman DR, Killeen N. Impact of the TCR signal on regulatory T cell homeostasis, function, and trafficking. PLoS ONE. 2009;4:e6580. doi: 10.1371/journal.pone.0006580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takeda I, Ine S, Killeen N, Ndhlovu LC, Murata K, Satomi S, Sugamura K, Ishii N. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172:3580–3589. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.