Abstract
Rationale
Mitochondria constitute 30% of myocardial mass. Mitochondrial fusion and fission appear essential for health of most tissues. Mitochondrial fission occurs in neonatal cardiomycyte and is implicated in cardiomyocyte death. Mitochondrial fusion has not been observed in post-mitotic myocytes of adult hearts, and its occurrence and function in this context are controversial.
Objective
Determine the consequences on organelle and organ function of disrupting cardiomyocyte mitochondrial fusion in vivo.
Methods and Results
The murine mfn1 and mfn2 genes, encoding mitofusins (Mfn) 1 and 2 that mediate mitochondrial tethering and outer mitochondrial membrane fusion, were interrupted by Cre-mediated excision of essential exons in neonatal (Nkx2.5-Cre) and adult (MYH6 MER-Cre-MER plus tamoxifen or Raloxifene) hearts. Embryonic combined Mfn1/Mfn2 ablation was lethal after e9.5. Conditional combined Mfn1/Mfn2 ablation in adult hearts induced mitochondrial fragmentation, cardiomyocyte and mitochondrial respiratory dysfunction, and rapidly progressive and lethal dilated cardiomyopathy. Before heart failure developed, cardiomyocyte shortening and calcium cycling were unaffected by absence of Mfn1 and Mfn2. Based on the time course over which fusion-defective mitochondrial size decreases, a mitochondrial fusion/fission cycle in adult mouse hearts occurs approximately every 16 days.
Conclusions
Mitochondrial fusion in adult cardiac myocytes is necessary to maintain normal mitochondrial morphology and is essential for normal cardiac respiratory and contractile function. Interruption of mitochondrial fusion causes lethal cardiac failure at a time corresponding to 3 or 4 cycles of unopposed mitochondrial fission.
Keywords: Mitochondrial fusion, mitochondrial fragmentation, dilated cardiomyopathy
Introduction
Mitochondrial fusion and fission are evolutionarily conserved mechanisms that promote mitochondrial health via exchange of mitochondrial proteins, lipids, and genomes. Dilution of senescent enzymes and mutated mitochondrial DNA is a reparative process for dysfunctional organelles. Subsequent mitochondrial fission restores normal mitochondrial morphology and contributes to mitochondrial health by packaging damaged mitochondrial components into a daughter organelle that is eliminated 1. Mitochondrial fusion is mediated by several GTPases: mitofusins (Mfn) 1 and 2 on the outer mitochondrial membrane, and optic atrophy 1 (Opa1) on the inner mitochondrial membrane. Loss of function mutations of these mitochondrial fusion proteins produces the degenerative neurological disease Charcot Marie Tooth Syndrome Type 2A (Mfn2 mutations) and autosomal dominant optic atrophy (Opa1 mutations) 2. Heart disease is not a factor of these conditions 3, suggesting that mitochondrial fusion may be dispensable in the heart. Indeed, whereas mitochondrial fission has been observed and implicated in programmed death or differentiation of cardiac myocytes 4, 5, mitochondrial fusion has not been described in adult cardiac myocytes, and there is controversy over whether it occurs 6. Cardiac deletion of Mfn2 had little effect on normal hearts, and appeared protective after ischemic damage 7, but Mfn1 can induce mitochondrial fusion in the absence of Mfn2 8; without both mitofusins organelle fusion does not occur. Thus, we engineered genetic mouse models in which both mitofusins are ablated in cardiac myocytes of either mouse early embryos or adults.
Methods
Detailed methods are in the Online Supplement.
Results
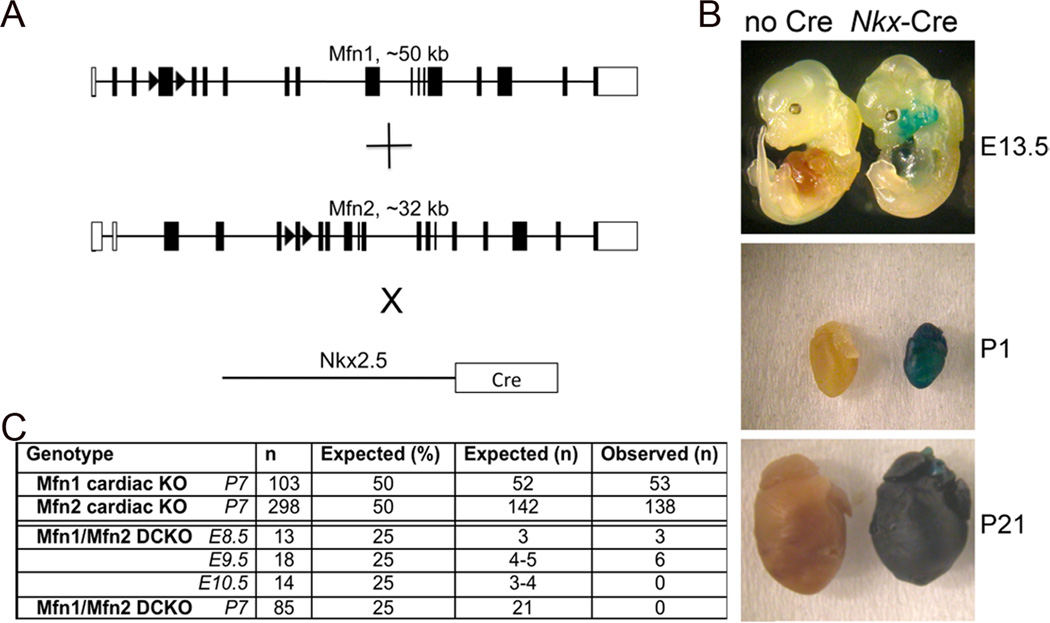
We ablated murine cardiomyocyte mfn1 and mfn2 using floxed allele mice 9 (Figure 1a) crossed onto Nkx2.5 Cre 10 that promotes gene recombination in the embryonic mouse heart (Figure 1b). Mfn1 and Mfn2 single cardiac null mice were born at expected Mendelian ratios (Figure 1c). In contrast, Mfn1/Mfn2 double cardiac knockout mice (DKO) were not observed at birth, with lethality between e9.5 and e10.5 (Figure 1c). Thus, expression of either Mfn1 or Mfn2 in embryonic hearts is sufficient for viability, but absence of both is incompatible with life.
Figure 1. Combined ablation of Mfn1 and Mfn2 in the early embryonic heart is lethal.
(A) Schematic representation of Cre-Lox strategy for combined cardiomyocyte-specific deletion of mfn1 exon 4 and mfn2 exon 6 using Nkx2.5-Cre knockin. (B) Cardiomyocyte gene recombination by Nkx-2.5 Cre knock-in crossed to ROSA-26 LacZ reporter line and assayed for recombination (blue staining) at 13.5 days p.c. (E13.5), the second day post birth (P2) and three weeks of age (P21). (C) Yield of Nkx2.5-Cre driven cardiac Mfn1, Mfn2, and Mfn1/Mfn2 double cardiac knockout (DCKO) mice.
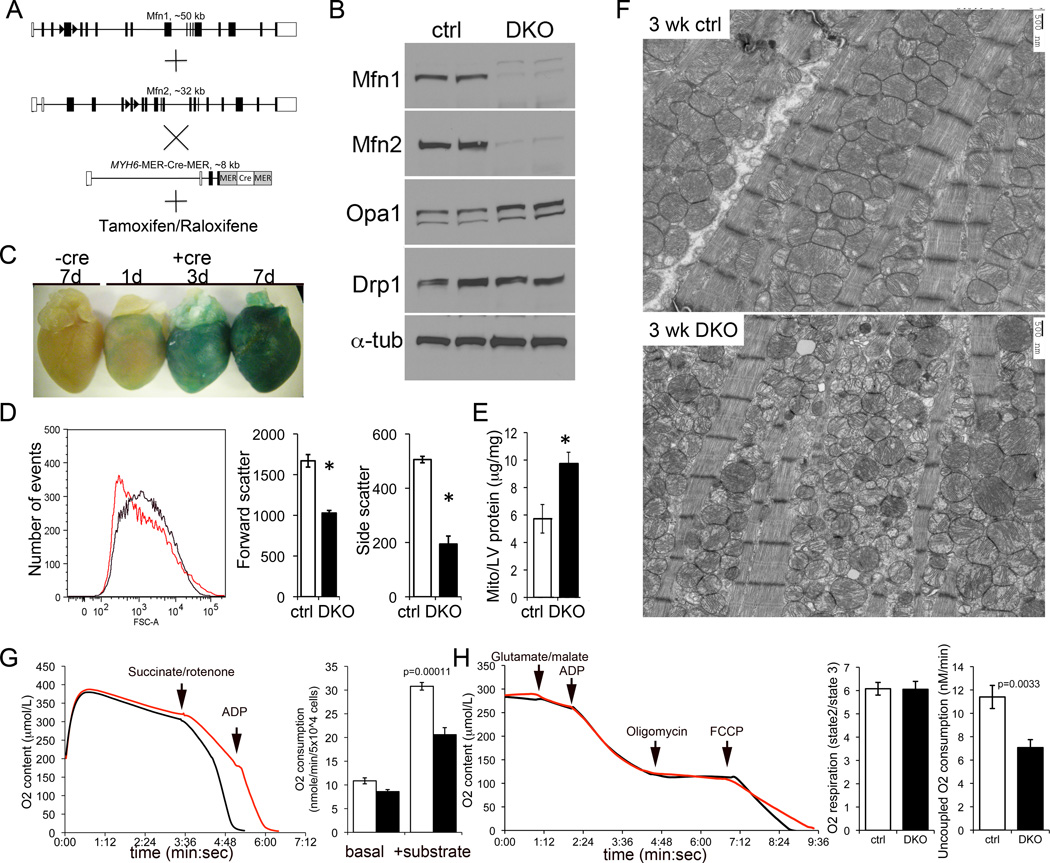
To avoid embryonic lethality, cardiac Mfn1/Mfn2 DKO mice were generated using a tamoxifen-inducible modified estrogen receptor (MER) cardiac-specific MYH6-Cre transgene 11 (Figure 2a). Mfn cardiac DKO mice were born at expected Mendelian ratios and survived normally. Three weeks after tamoxifen induction (8 week old mice), cardiac Mfn1 and Mfn2 immunoreactivities were decreased by >80% (Figure 2b). The inner mitochondrial fusion protein Opa1 was modestly upregulated in Mfn1/Mfn2 DKO hearts, whereas the mitochondrial fission protein DRP1 was unaffected (Figure 2b).
Figure 2. Conditonal combined ablation of Mfn1 and Mfn2 in adult hearts induces mitochondrial fragmentation and cardiomyocyte respiratory dysfunction.
(A) Schematic representations of conditional Cre-Lox strategy for combined cardiomyocyte-specific deletion of mfn1 and mfn2 using MYH6-driven modified estrogen receptor-Cre and tamoxifen or Raloxifene. (B) Immunoblot analysis of mitochondrial fusion and fission proteins 3 weeks after tamoxifen administration. Control (Ctrl) is mfn1fl/fl + mfn2fl/fl without Cre treated with tamoxifen. Each column is a separate mouse heart. (C) Time course of gene recombination induced by tamoxifen, assayed by LacZ staining of ROSA-26 crosses as in Figure 1b. (D) Flow cytometric analysis of isolated cardiac mitochondria size (forward scatter; FSC) and sphericity (side scatter) in Mfn1/Mfn2 double cardiac KO mice 3 weeks after tamoxifen. Left graph shows representative mitochondrial size (Forward scatter) distribution from identically treated ctrl (black) and Mfn1/Mfn2-DKO (red) hearts. Group mean data for forward and side scatter are to the right (n=3; *=P<0.05 vs ctrl). (E) Mitochondrial mass measured as protein content indexed to heart weight (n=4 ctrl and 3 DKO; * is P=0.0026 vs ctrl). (F) Transmission electron microscopic examination of cardiomyocyte mitochondria (5,000x). (G) Time-dependent oxygen consumption of digitonin-permeabilized cardiomyocytes from ctrl (black) and Mfn1/Mfn2 DKO (red) mice. Right panel shows group data; white is ctrl, black is DKO. n=3. (H) Isolated cardiac mitochondrial respiration studies. Data are plotted as in (G). The respiratory control index (state 3 ADP-stimulated/state 2 ADP-limited) is shown immediately to the right, and maximal uncoupled respiration in response to FCCP at the extreme right (n=3 each).
The time course of tamoxifen-mediated cardiac gene ablation was assessed using the ROSA26 lacZ marker gene (Figure 2c). Gene recombination (blue-stained myocardium) was evident 1 day after the first tamoxifen dose, was observed throughout the heart on day 3, and was uniform and intense on day 7.
We determined the consequence of mitofusin deficiency on mitochondrial morphology using flow cytometry of cardiac mitochondria three weeks after tamoxifen-induced mfn1/mfn2 gene deletion. Forward scattered light measures mitochondrial size and side-scattered light measures complexity of shape. Histograms of forward scatter showed the typical normal distribution of mitochondrial size in control hearts (Figure 2d, left; black line), but a leftward shift and loss of normal size distribution in Mfn cardiac DKO hearts (Figure 2d, left; red line). Mitochondrial size was decreased ~40% (Forward scatter; P=0.002) (Figure 2d, middle) and shape complexity was decreased ~60% (Side scatter; P=0.001) (Figure 2d, right). Mitochondrial protein content of Mfn cardiac DKO hearts was approximately twice that of controls (Figure 2e, P=0.0026), similar to findings in skeletal muscle Mfn DKO mice16; the mechanism for “proliferation” of fusion-defective mitochondria is currently unknown.
These results suggest that loss of adult cardiomyocyte Mfn proteins results in a plethora of small, round mitochondria. Ultrastructural studies confirmed this interpretation, and further revealed abnormal or degenerated mitochondrial cristae in Mfn cardiac DKO cardiomyocytes (Figure 2f). This form of mitochondrial dysmorphology is referred to as mitochondrial “fragmentation”, although it is actually the result of mitochondrial fission unopposed by normal fusion 9. Mfn1/Mfn2-deficient cardiomyocytes exhibited diminished ADP-stimulated O2 consumption and decreased maximal O2 consumption of uncoupled isolated DKO mitochondria (Figure 2h), revealing impaired respiration (Figure 2g). Thus, mitochondrial fusion mediated by Mfn1 or Mfn2 is essential for normal cardiac mitochondrial morphology and respiratory function.
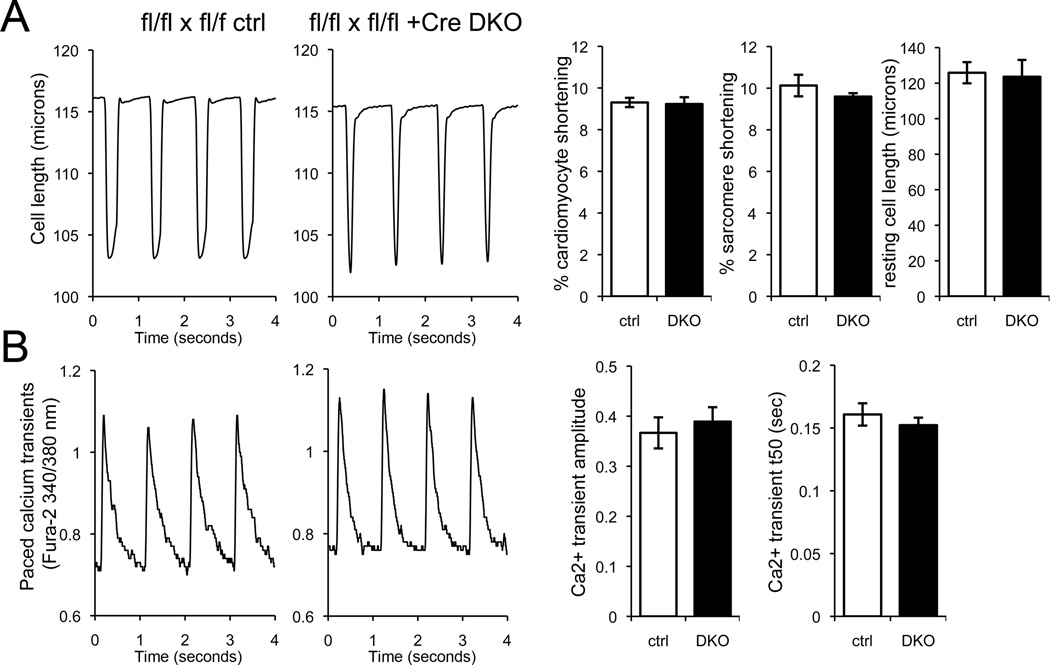
Our studies were designed to interrupt mitochondrial fusion in adult mouse hearts. Because Mfn2 is implicated in tethering mitochondria to endoplasmic reticulum and inter-organelle calcium cross-talk 8, 12, we investigated the possibility that altered calcium caused the Mfn cardiac DKO cardiomyopathy. Both contractility (Figure 3a) and cytosolic calcium transients (Figure 3b) were normal in Mfn cardiac DKO cardiomyocytes 1 week after tamoxifen treatment. Thus, combined Mfn1 and Mfn2 ablation does not primarily affect cardiomyocyte excitation-contraction coupling.
Figure 3. Cardiomyocyte contraction and calcium cycling are normal 1 week after combined cardiac Mfn1 and Mfn2 ablation.
(A) Unloaded cardiomyocyte shortening with field stimulation at 1Hz. Representative tracings show time-dependent change in cell length. Left tracing is tamoxifen-treated mfn1fl/fl, mfn2fl/fl without Cre; right tracing is identically treated mfn1fl/fl, mfn2fl/fl Cre+. Bar graphs show group quantitative data (ctrl is white, DKO is black). (B) Phasic calcium transients in Fura-2 loaded cardiomyocytes as above. Group quantitative data for transient amplitude and time for 50% normalization of the transient (t50) are shown to the right. Data are mean ± SEM of n= 3 paired hearts, with each experimenting averaging 8–12 cardiomyocytes myocytes/heart. There were no differences between DKO and ctrl.
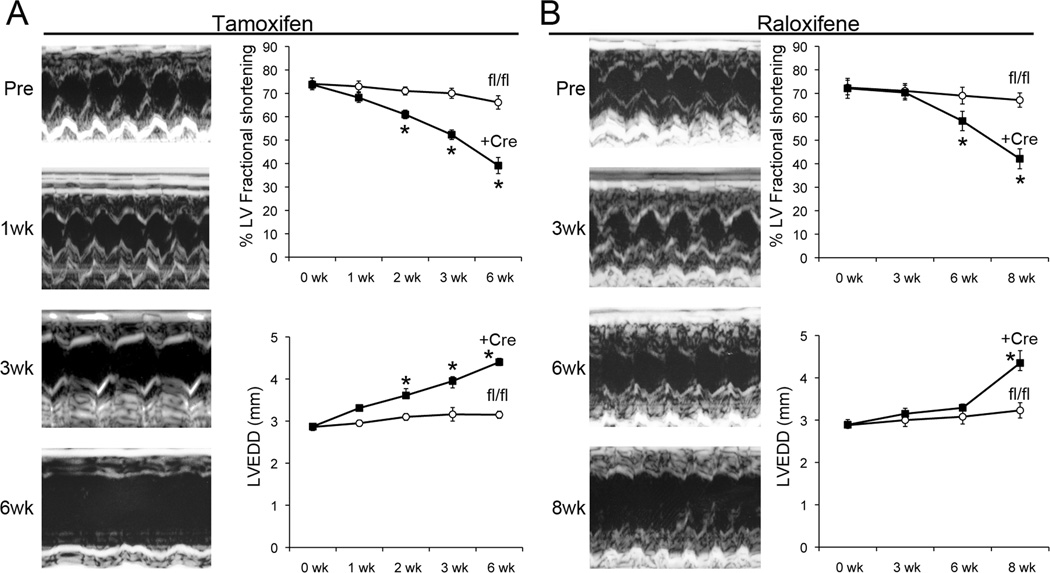
Cardiomyocyte mitochondrial dysmorphology, impaired cellular respiration, and the early lethal phenotype of embryonic cardiac Mfn1/Mfn2 ablation suggested that disrupting cardiomyocyte mitochondrial fusion might have pathological consequences, given sufficient time. Therefore, we serially interrogated cardiac structure and function after combined mfn1/mfn2 gene ablation induced by tamoxifen. Compared to littermate controls (tamoxifen-treated floxed allele mice without Cre), cardiac Mfn DKO hearts were normal 1 week after tamoxifen, but progressively dilated during the subsequent five weeks (Figure 4a). Conditional combined mfn1 and mfn2 ablation with Raloxifene 13 resulted in a similar progressive dilated cardiomyopathy, although the time course was slightly delayed (Figure 4b). Signs of overt heart failure (rapid respirations, decreased movement) were observed after seven to eight weeks, and the mice succumbed shortly thereafter.
Figure 4. Combined Mfn1 and Mfn2 ablation in adult hearts induces rapidly progressive dilated cardiomyopathy.
(A) Representative M-mode echocardiograms of un-anesthetized mouse left ventricles before (Pre) and at intervals after conditional ablation of Mfn1 and Mfn2 with tamoxifen. Group data for fractional shortening (top) and LV end diastolic dimension (LVEDD, bottom) are to the right (n = 4–5 per group). (B) Same as in (A), but using Raloxifene to activate MER-Cre-MER (n=4 per group).
Discussion
We used conditional cardiac-specific combined ablation of Mfn1 and Mfn2 to demonstrate that mammalian hearts have the same requirement for mitochondrial fusion as Drosophila heart tubes and murine brain and skeletal muscle 9, 14–16. When the mitochondrial fusion apparatus was disrupted in adult hearts, cardiomyocyte mitochondria “fragmented”, whole cardiomyocyte and isolated mitochondrial respiration were compromised, and the hearts dilated and failed over a period of weeks. These results prove that mitochondrial morphological and functional integrity requires an intact fusion/fission apparatus. Although mitochondrial fission can be increased in programmed cardiomyocyte death 17, unopposed mitochondrial fission was not associated with increased cardiomyocyte TUNEL positivity or sensitivity of the permeability transition pore to calcium (Online Figure I). Our findings are similar to a recent report of combined Mfn1/Mfn2 deletion in murine skeletal muscle 16. Indeed, mitochondrial fragmentation, cellular respiratory dysfunction, and myopathy are features of both the cardiac and skeletal muscle-specific Mfn1/Mfn2 double knockout mice. Thus, although adult cardiac myocytes are post-mitotic and their mitochondria are limited in motility and physically constrained between myofilaments, the heart is not an exception to the general rule that mitochondrial fusion is important to cellular and tissue health.
Because we used inducible double gene ablation, our data permit us to estimate the rate of in vivo mitochondrial fusion in adult cardiac myocytes. This calculation assumes that steady-state mitochondrial fusion and fission are balanced in normal hearts and that the rate of mitochondrial fission does not change after Mfn1/Mfn2 ablation. Unopposed fission will decrease mitochondrial population size over time in a geometric relationship. Cre-mediated gene excision was complete 1 week after tamoxifen, and mitochondrial size decreased by ~40% two weeks thereafter. Thus, a cardiomyocyte mitochondrial fusion/fission cycle takes approximately 16 days. Lethal heart failure observed 7–8 weeks after Mfn1/Mfn2 ablation therefore corresponds to 3–4 cycles of unopposed mitochondrial fission.
Mitofusins are regulated in cardiac disease or cardiomyocyte injury 18–20. Our results suggest that decreased mitofusin expression or function has the potential to contribute to cardiac pathology by interfering with essential mechanics of mitochondrial fission/fusion.
Novelty and Significance.
What is known?
Myocardium is the most mitochondria-rich tissue.
In most tissues mitochondria periodically tether, fuse, exchange organelle contents, and divide.
Mitochondrial fusion has not been observed in vertebrate hearts; its existence in this context is disputed and its role (if any) is unknown.
What new information does this article contribute?
By combinatorially ablating the genes encoding outer mitochondrial membrane fusion proteins, Mfn1 and Mfn2, in embryonic or adult mouse hearts, we interrupted mitochondrial fusion in cardiomyocytes.
Mitofuscin-deficient adult heart cells contained unusually small mitochondria with structurally abnormal cristae and exhibited respiratory compromise.
Interrupting mitochondria fusion in either embryonic or adult hearts proved lethal, in the latter case inducing rapidly progressive cardiac remodeling.
Mitochondria are engines that fuel cardiac contraction and mediators of programmed cardiomyocyte death. In many tissues, mitochondria cyclically tether to each other, fuse, and divide, thereby exchanging constituent proteins and nucleic acids. The highly organized intracellular architecture of cardiomyocytes enforces close mitochondrial approximation, suggesting that tethering may be irrelevant and fusion functionally unimportant. Compound genetic deletion of mitofusins that mediate mitochondrial tethering and fusion in embryonic and adult hearts proved lethal, inducing organelle fragmentation from un-opposed fission, respiratory compromise, and rapidly progressive dilated cardiomyopathy without programmed cardiomyocyte death. Thus, mitochondrial fusion is essential to vertebrate cardiac health.
Supplementary Material
Acknowledgments
Sources of Funding
National Institutes of Health R01 HL59888.
Non-standard abbreviations and acronyms
- Mfn
mitofusin
- MER
modified estrogen receptor
- DKO
double (cardiac) knockout
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Westermann B. Mitochondrial fusion and fission in cell life and death. Nature reviews Molecular cell biology. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 2.Liesa M, Palacin M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 3.Cartoni R, Martinou JC. Role of mitofusin 2 mutations in the physiopathology of Charcot-Marie-Tooth disease type 2A. Exp Neurol. 2009;218:268–273. doi: 10.1016/j.expneurol.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Chung S, Dzeja PP, Faustino RS, Perez-Terzic C, Behfar A, Terzic A. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat Clin Pract Cardiovasc Med. 2007;4 Suppl 1:S60–S67. doi: 10.1038/ncpcardio0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 6.Hom J, Sheu SS. Morphological dynamics of mitochondria--a special emphasis on cardiac muscle cells. J Mol Cell Cardiol. 2009;46:811–820. doi: 10.1016/j.yjmcc.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papanicolaou KN, Khairallah RJ, Ngoh GA, Chikando A, Luptak I, O'Shea KM, Riley DD, Lugus JJ, Colucci WS, Lederer WJ, Stanley WC, Walsh K. Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol. 2011;31:1309–1328. doi: 10.1128/MCB.00911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorn GW, 2nd, Scorrano L. Two close, too close: sarcoplasmic reticulum-mitochondrial crosstalk and cardiomyocyte fate. Circ Res. 2010;107:689–699. doi: 10.1161/CIRCRESAHA.110.225714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 10.Moses KA, DeMayo F, Braun RM, Reecy JL, Schwartz RJ. Embryonic expression of an Nkx2-5/Cre gene using ROSA26 reporter mice. Genesis. 2001;31:176–180. doi: 10.1002/gene.10022. [DOI] [PubMed] [Google Scholar]
- 11.Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89:20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 12.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 13.Koitabashi N, Bedja D, Zaiman AL, Pinto YM, Zhang M, Gabrielson KL, Takimoto E, Kass DA. Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced MerCreMer gene deletion models. Circ Res. 2009;105:12–15. doi: 10.1161/CIRCRESAHA.109.198416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorn GW, 2nd, Clark CF, Eschenbacher WH, Kang MY, Engelhard JT, Warner SJ, Matkovich SJ, Jowdy CC. MARF and Opa1 control mitochondrial and cardiac function in Drosophila. Circ Res. 2011;108:12–17. doi: 10.1161/CIRCRESAHA.110.236745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141:280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, Gong Q, Stice JP, Knowlton AA. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res. 2009;84:91–99. doi: 10.1093/cvr/cvp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang L, Moore XL, Gao XM, Dart AM, Lim YL, Du XJ. Down-regulation of mitofusin-2 expression in cardiac hypertrophy in vitro and in vivo. Life Sci. 2007;80:2154–2160. doi: 10.1016/j.lfs.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Shen T, Zheng M, Cao C, Chen C, Tang J, Zhang W, Cheng H, Chen KH, Xiao RP. Mitofusin-2 is a major determinant of oxidative stress-mediated heart muscle cell apoptosis. J Biol Chem. 2007;282:23354–23361. doi: 10.1074/jbc.M702657200. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Yin R, Liu J, Wang P, Wu S, Luo J, Zhelyabovska O, Yang Q. Peroxisome proliferator-activated receptor delta regulates mitofusin 2 expression in the heart. J Mol Cell Cardiol. 2009;46:876–882. doi: 10.1016/j.yjmcc.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.