Abstract
Phasic changes in dopamine activity play a critical role in learning and goal-directed behavior. Unpredicted reward and reward predictive cues evoke phasic increases in the firing rate of the majority of midbrain dopamine neurons – results that predict uniformly broadcast increases in dopamine concentration throughout the striatum. However, measurement of dopamine concentration changes during reward has cast doubt on this prediction. We systematically measured phasic changes in dopamine in four striatal subregions (nucleus accumbens shell (Shell) and core (Core), dorsomedial (DMS) and dorsolateral striatum (DLS)) in response to stimuli known to activate a majority of dopamine neurons. We used fast-scan cyclic voltammetry in awake and behaving rats, which measures changes in dopamine on a similar timescale to the electrophysiological recordings that established a relationship between phasic dopamine activity and reward. Unlike the responses of midbrain dopamine neurons, unpredicted food reward and reward-predictive cues evoked a phasic increase in dopamine that was subregion specific. In rats with limited experience, unpredicted food reward evoked an increase exclusively in the Core. In rats trained on a discriminative stimulus paradigm, both unpredicted reward and reward-predictive cues evoked robust phasic dopamine in the Core and DMS. Thus, phasic dopamine release in select target structures is dynamic and dependent on context and experience. Since the four subregions assayed receive different inputs and have differential projection targets, the regional selectivity of phasic changes in dopamine has important implications for information flow through the striatum and plasticity that underlies learning and goal-directed behavior.
Keywords: nucleus accumbens, motivation, learning, rat, basal ganglia
Introduction
The striatum – dorsal and ventral – is critical for motivational processes, cognition and voluntary motor behavior. Based on cytoarchitecture and anatomical connections, the striatum is divided into subregions: the shell (Shell) and core (Core) of the nucleus accumbens (ventral division) and the dorsomedial (DMS) and dorsolateral (DLS) striatum (dorsal division) Groenewegen et al., 1999a; 1999b; Bolam et al., 2000; Voorn et al., 2004; Humphries & Prescott, 2010). Consistent with the anatomy, functional specifications have emerged in which striatal subregions differentially contribute to goal-directed behavior (Kelley, 1999; Li et al., 2010), learning (Featherstone & McDonald, 2004; Yin & Knowlton, 2004; Pennartz et al., 2009; Ragozzino et al., 2009) and motor behavior (Sabol et al., 1985; Olds et al., 2006). Although there is not universal agreement on the functional roles of striatal subregions, there is little doubt for regional specificity.
All subregions of the striatum receive input from midbrain dopamine neurons and dopamine modulates ongoing striatal activity (Nicola & Deadwyler, 2000; Bamford et al., 2004; Surmeier et al., 2009; Gerfen & Surmeier, 2010). Unpredicted primary rewards and cues that come to predict them are remarkably effective in evoking phasic increases in the firing rate of dopamine neurons (Mirenowicz & Schultz, 1996; Schultz, 1998; Matsumoto & Hikosaka, 2009). In addition, phasic changes in dopamine neuronal activity are important in the formation of cue-reward associations (Zweifel et al., 2009). Because a majority of dopamine neurons respond to reward with similar latencies, one interpretation is that there is a global increase in dopamine concentration throughout the striatum (Schultz, 1998). Even though pools of midbrain dopamine neurons project to the striatum in a topographical manner (Ikemoto, 2007), terminal fields of individual dopamine neurons extensively arborize and cover a large volume of the striatum (Matsuda et al., 2009) - lending support to the idea that reward-related phasic dopamine activity results in a widely broadcast signal throughout the striatum.
In contrast to a global striatal signal suggested by electrophysiology, dopamine measurements made in select striatal subregions suggest that changes in dopamine concentration are regionally specific (Aragona et al., 2009; Bassareo et al., 2011). Most studies examining regional specificity in dopamine concentration changes have either used techniques that lack the temporal resolution to discern phasic changes (Ito et al., 2002; Bassareo et al., 2011), focus on a subset of striatal regions (Aragona et al., 2008; 2009; Wanat et al., 2010) or both. Thus, to date, it remains unclear whether phasic dopamine, released in response to reward or during goal-directed behavior, is widely transmitted throughout the striatum or in a more regionally selective manner. To resolve this issue, we used fast-scan cyclic voltammetry to measure phasic changes in dopamine concentration in the Shell, Core, DMS or DLS in response to (i) ventral midbrain stimulation, (ii) unpredicted primary reward delivery, and (iii) reward predictive cues. We predicted that although all areas would support dopamine release in response to electrical stimulation, dopamine release evoked by reward-related stimuli would differ across striatal subregions.
Materials and Methods
Subjects
Male, Sprague Dawley rats (Charles River Laboratories) weighing 325-400 g at the time of testing were used. Animals were individually housed in plastic cages (26.5 × 50 × 20 cm) in a temperature (22°C) and humidity (30%) controlled environment on a 12/12 h light/dark cycle (lights on at 7:00 a.m.). Prior to training and during recovery from surgery rats had ad libitum access to both standard lab chow and water. During training and testing, rats were food restricted to ∼95% of their ad libitum body weight with free access to water. Animal care and use was in accordance with the National Institutes for Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of Illinois at Chicago.
Apparatus
Rats were trained and tested in a standard operant chamber (Med Associates, St. Albans, VT, USA). A houselight and two different sound generators were located on one wall of the chamber. A custom designed acrylic pellet receptacle was located in the center of the opposite wall. A retractable lever with a circular white cue light above it was positioned on either side of and equidistant to the pellet receptacle. A hole in the top of the chamber allowed for the attachment of the headstage for voltammetric measurements. The headstage, in turn, was attached to an electric swivel (Crist Instrument Company, MD, USA) mounted above which permitted free movement throughout the chamber during recording.
Surgical Procedures
Rats were anesthetized with ketamine hydrochloride (100 mg/kg, intraperitoneal) and xylazine hydrochloride (10 mg/kg, intraperitoneal). A guide cannula (Bioanalytical systems, West Lafayette, IN, USA) was implanted 2.5 mm below the skull and dorsal to one of four striatal regions according to the following coordinates relative to bregma (in mm): nucleus accumbens shell (Shell) +1.7 anterior, -0.9 lateral; nucleus accumbens core (Core) +1.3 anterior, -1.5 lateral; dorsomedial striatum (DMS) +0.6 anterior, -2.1 lateral; and dorsolateral striatum (DLS) +0.6 anterior, -4.0 lateral. A chlorinated silver wire (Ag/AgCl) reference electrode was placed contralateral to the guide cannula in the left forebrain. Stainless steel screws and dental cement secured the guide cannula and reference electrode. Next, a removable custom micromanipulator loaded with a carbon fiber electrode was attached to the guide cannula and the electrode was lowered into the selected striatal region. A bipolar stimulating electrode (Plastics One, Roanoke, VA, USA) was then implanted in the ventral tegmental area/substantia nigra pars compacta (VTA/SNpc; AP -5.2 mm, ML -1.0 mm). The stimulating electrode was lowered from -7.0 mm (relative to surface of the brain) at 0.2 mm increments. At each increment a train of current pulses was delivered (60 pulses delivered at 60 Hz, 120 μA). After stimulation evoked a phasic increase in dopamine, the position of the stimulating electrode was optimized for maximal evoked dopamine and cemented in place. The carbon fiber electrode was then removed. Rats were allowed to recover with free access to food and water until reaching pre-surgery body weight (3-5 days). After recovery, rats were food restricted as described above.
Carbon Fiber Electrodes
Electrodes were fabricated from individual carbon fibers (Goodfellow Cambridge LTD, Huntingdon, UK; 7 μm diameter). Fibers were aspirated into glass pipettes (A-M Systems, Carlsborg, WA, USA; 0.6 mm O.D., 0.4 mm I.D.) and pulled on a vertical puller (Narishege, East Meadow, NY, USA). The glass seal was evaluated under light microscopy and the carbon fiber was cut to a length of 75-100 μm using a scalpel. Electrodes were then loaded into custom-designed micromanipulators (UIC Research Resources Center), which interfaced with the guide cannula implanted in the rat. Following recording sessions, electrodes were calibrated using dopamine (1 μM) in a flow injection system to convert changes in current due to the oxidation of dopamine to concentration. However, as it was not possible to calibrate all electrodes, data are presented in nA throughout the paper. For electrodes that were calibrated, the average conversion factor was 1 nA = 66.6 nM. This conversion rate is in excellent agreement with other published reports (Park et al., 2010).
Fast Scan Cyclic Voltammetry (FSCV)
FSCV procedures used here were performed as previously described (Day et al., 2007; Roitman et al., 2008; Ebner et al., 2010). Briefly, the potential of a carbon fiber electrode, lowered into a subregion, was periodically driven from -0.4 V to +1.3 V (versus the Ag/AgCl reference electrode) and back in a triangular fashion (400 V/s; 10 Hz). Current due to oxidation and reduction of electroactive species was measured after background subtraction removed the stable contribution of current produced by oxidation and reduction of surface molecules on the carbon fiber. Prior to each experiment, the VTA/SNpc was stimulated (24 pulses, 60 Hz, 120 μA, 4 ms/pulse). Stimulation reliably evokes two responses: an increase in dopamine followed by a basic pH change (Roitman et al., 2004). Representative current by voltage plots (cyclic voltammograms) were obtained for each of these responses. Training sets were constructed from cyclic voltammograms for dopamine and pH to allow for principal component analysis (PCA) on data collected during the behavioral sessions as previously described (Heien et al., 2004; Day et al., 2007). The application of voltage changes to the electrode as well as the sampling of electrochemical data and dopamine extraction was performed using computer software written in LabVIEW (National Instruments, Austin, TX, USA; Heien et al., 2004).
Electrically evoked phasic dopamine release and reuptake
On the day of testing, rats (n=46) were placed into the operant chamber and a carbon fiber recording electrode was lowered into the selected striatal region (Shell, Core, DMS or DLS) using a custom-made micromanipulator. The Ag/AgCl reference electrode, stimulating electrode, and carbon fiber recording electrode were connected to a headstage containing a voltammetric amplifier attached via a tether to the electric swivel at the top of the operant chamber. The carbon fiber electrode was allowed to equilibrate for 40 minutes to minimize current drift. After equilibration, the VTA/SNpc was stimulated as described in the preceding section: 1) to ensure the carbon fiber electrode was well placed to measure dopamine, and 2) to obtain representative cyclic voltammograms for dopamine and pH for PCA.
Experiment 1: Phasic dopamine release to unpredicted food reward
Prior to surgery, rats (n=23) were food-restricted and trained on two separate days to retrieve 45 mg sugar pellets (Sugar Dustless Precision Pellets, #F0042; Bio-Serv, Frenchtown, NJ, USA) delivered with a variable inter-trial interval (range 30-90 s; 30 trials). Rats then underwent surgery and, after recovery, were given at least one session to retrieve pellets while connected to a headstage to acclimate for voltammetric recording. On the day of testing, a new carbon fiber electrode was lowered into a striatal subregion (Shell, n=6; Core, n=5; DMS, n=6; DLS, n=6) and voltammetric measurements were made during delivery and retrieval of sugar pellets.
Experiment 2.1: Phasic dopamine release during a discriminative stimulus paradigm
Rats (n=23) were food-restricted and trained to press a lever for a sugar pellet reward. Initially, depression of either lever resulted in both levers immediately retracting and the delivery of a sugar pellet. After 5 s, the levers were extended again into the chamber. Rats received daily 30 min sessions until 50 lever presses were made during 2 consecutive days. On the following day, the discriminative stimulus paradigm (Jones et al., 2010) began. In this task, a discrete audiovisual cue (white noise or tone plus a cue light above the lever) was presented 3 s prior to extension of one lever. A different audiovisual cue was presented 3 s prior to extension of the other lever. Presentation of one set of cues (DS+) followed by a response on the associated lever resulted in the delivery of a sugar pellet. Presentation of the other set of cues (DS-) followed by a response on the associated lever resulted only in lever retraction with no other programmed responses. Levers were retracted after 5 s if no press was made and the trial was concluded. Audiovisual stimuli and rewarded versus non-rewarded levers were counterbalanced across rats. Each training session consisted of 60 trials (30 DS+, 30 DS-) that were presented pseudorandomly such that a set of cues was never presented more than 3 consecutive trials. The inter-trial interval was randomly varied (average inter-trial interval: 15 ± 4 s). Once rats responded on >90% DS+ trials and abstained on >70% of DS- trials for 2 consecutive days they were prepared for voltammetric recordings. Following recovery from surgery, rats were again food restricted and retrained to criteria. During post-operative training, rats were connected to a headstage to acclimate for voltammetric recording procedures. Once rats reached task criteria, testing began the following day. On the test day, a carbon fiber electrode was lowered into a striatal subregion (Shell, n=5; Core, n=6; DMS, n=6; DLS, n=6) and voltammetric measurements were made during the discriminative stimulus paradigm.
Experiment 2.2: Phasic dopamine release to unpredicted food reward following the discriminative stimulus test
On the test day and immediately following the discriminative stimulus paradigm rats were presented with unpredicted sugar pellets in a manner identical to Experiment 1 while recording continued. During the session, sugar pellets were delivered with a randomly selected inter-trial interval (range 30-90 s; 30 trials).
Data Analysis
To examine regional differences in behaviorally evoked dopamine, PCA was used to extract a dopamine trace for each trial, by ascribing the amount of current attributable specifically to dopamine. For each rat, trials were then averaged across a behavioral session. Three distinct epochs within the average dopamine traces were utilized for further analysis: a Baseline epoch (average of 5 s prior to pellet delivery or cue onset), an Event epoch [average of 1 s after: Pellet delivery (Experiment 1 and 2.2), DS+, or DS- onset (Experiment 2.1)], and a Late epoch [average of 5-10 s after DS+ or DS- onset (Experiment 2.1, DLS only)]. We compared epochs within each striatal region using paired t-tests. Statistical analyses were carried out using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA) and Statistica (StatSoft, Tulsa, OK, USA) software and an alpha level of 0.05 was set for significance.
Histological Verification of Electrode Placement
After test sessions, rats were injected with a lethal dose of sodium pentobarbital (∼100 mg/kg). To determine recording location, a stainless steel electrode (A-M Systems #571500, Sequim, WA, USA) was lowered to the same depth of the carbon fiber during data collection and an electrolytic lesion was made. Rats were then transcardially perfused with 0.9% phosphate buffered saline followed by a 10% formalin solution (Sigma-Aldrich). Brains were removed and stored in 10% formalin solution before being frozen. Using a cryostat, serial coronal sections (50 μm) were made through the striatum and sections were then mounted on gelatin-coated slides. Slides were stained with cresyl violet. The location of the recording electrode was identified using a light microscope and the subregion was determined with the aid of the stereotaxic atlas by Paxinos and Watson (1998).
Results
Electrode placements resulted in selective sampling within distinct striatal subregions
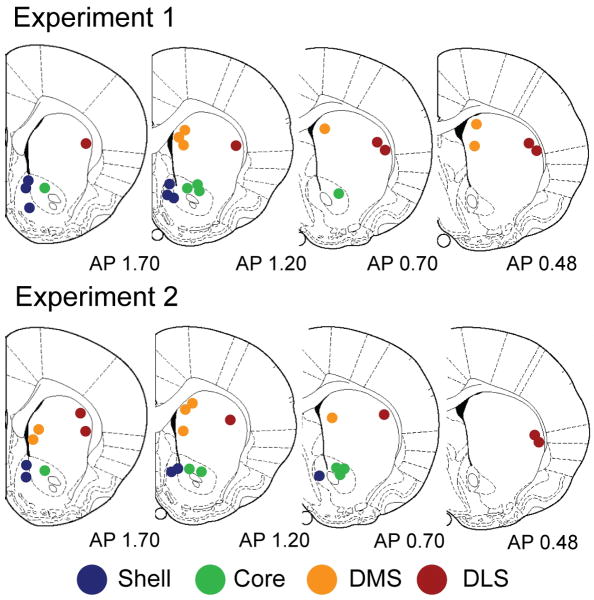
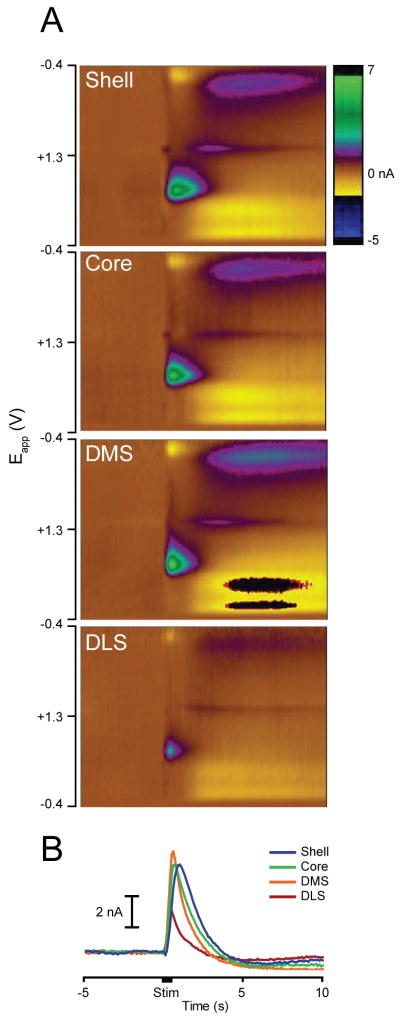
Electrode locations for all recordings are shown in Figure 1. For recordings in the nucleus accumbens Shell and Core, electrode placements were located between 0.7 and 1.7 mm anterior to bregma. Shell placements were located between 0.6 to 1.6 mm lateral to the midline and 6.5 to 8.0 mm ventral to brain surface. Core placements were located 1.0 to 2.2 mm lateral to the midline and were dorsal to the anterior commissure from 6.6 to 7.2 mm ventral to brain surface. Recordings in the DMS were located between 0.48 and 1.7 mm anterior to bregma, 1.0 to 1.8 mm lateral to the midline and from 3.8 to 5.5 mm ventral to brain surface. DLS placements were located 3.6 to 4.5 mm lateral to the midline and ventral 3.8 to 5.5 mm from the surface of the brain. Furthermore, electrical stimulation evoked a significant increase in the dopamine signal across all striatal subregions (Baseline vs. Stimulation epochs, P's < 0.01, paired t-tests; Figure 2). This result demonstrates that all recording sites were capable of supporting dopamine release and that all electrodes were capable of detecting dopamine release. Inspection of the average dopamine signals from each region suggests that the kinetics of the response varied between regions, possibly due to reuptake. However, as we were not able to calibrate all electrodes, and reuptake is concentration dependent, we were unable to formally analyze this feature.
Figure 1.
Histological verification of recording sites. Recordings were made in the Shell (blue), Core (green), DMS (orange) or DLS (red). Top: Location of recording sites for Experiment 1 examining phasic dopamine release evoked by unpredicted food reward. Bottom: Location of recording electrodes for Experiment 2 examining phasic dopamine release during the discriminative stimulus task and subsequent unpredicted food reward presentation. Numbers are distances in mm anterior to bregma. Rat atlas sections are adapted from The Rat Brain in Stereotaxic Coordinates by G. Paxinos and C. Watson, 1996, Sydney, Australia: Academic Press. Copyright 1996 by Academic Press. Adapted with permission.
Figure 2.
Electrical stimulation of VTA/SNpc (24 pulses, 60 Hz) evokes phasic dopamine release in all striatal subregions. (A) Dopamine release in each subregion is shown using color plots, which show current changes (in color) across the applied voltages (Eapp; ordinate) over time (abscissa). Dopamine is identified by its oxidation (green feature, ∼0.6 V) and reduction (dark blue/yellow feature, ∼-0.2 V) peaks that arise just after stimulation onset. (B) Average change in dopamine evoked by electrical stimulation in each subregion: Shell (blue), Core (green), DMS (orange) and DLS (red). The black bar on the x-axis denotes stimulation duration.
Unpredicted food reward selectively evokes phasic dopamine in the Core
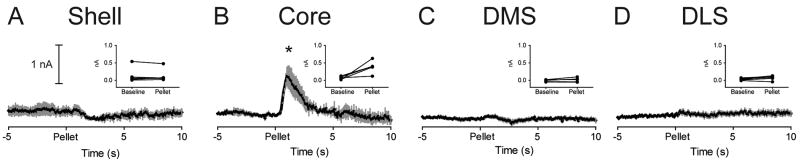
While we observed significant electrically-evoked dopamine release in all subregions of the striatum, dopamine evoked by unpredicted sugar pellet delivery (see Figure 3 for example) was not uniform (Figure 4). In the Core, dopamine (peak = 0.80 ± 0.17 nA) was significantly elevated during the Pellet epoch relative to Baseline (t4 = 3.50, P = 0.025; Figure 4B). In all other subregions, unpredicted pellet delivery failed to evoke a change in dopamine [Shell (t5 = 0.67, P = 0.667; Figure 4A); DMS (t5 = 1.61, P = 0.169 Figure 4C) and DLS (t5 = 2.41, P = 0.061; Figure 4D)]. In all panels, insets show the average baseline and pellet dopamine for individual rats.
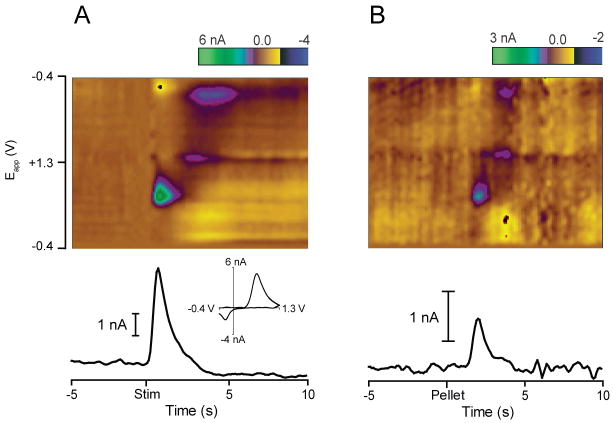
Figure 3.
Individual trial examples of phasic dopamine evoked by electrical stimulation of the VTA/SNpc and by unpredicted food reward in the Core. (A) Electrical stimulation evokes a phasic change in dopamine. Top: Color plot shows current changes (in color) as a function of applied voltage over time, as described in Fig. 2. Bottom: Change in dopamine over time extracted from color plot above using PCA. Inset: Cyclic voltammogram plotted at the time of peak dopamine release. Cyclic voltammograms for dopamine and pH obtained after stimulation are used to build a training set for PCA. (B) In the same rat, unpredicted food reward (sugar pellet) evokes a phasic increase in dopamine. Top: Color plot shows current changes as a function of applied voltage over time. Dopamine is identified by its oxidation and reduction features occurring just after pellet delivery. Bottom: Change in dopamine extracted from the color plot above using PCA.
Figure 4.
Unpredicted food reward evokes a regionally selective phasic dopamine response. Average dopamine (black line) ± SEM (gray vertical bars) in different striatal regions in response to unpredicted food reward (sugar pellet; time = 0). Insets: Average dopamine signal for each rat during both Baseline and Pellet epochs. Unpredicted food reward evokes phasic dopamine release in the Core (B) but not the Shell (A), DMS (C) or DLS (D). * P < 0.05 for Baseline versus Pellet epochs.
A reward-predictive stimulus evokes phasic dopamine release in selective striatal subregions
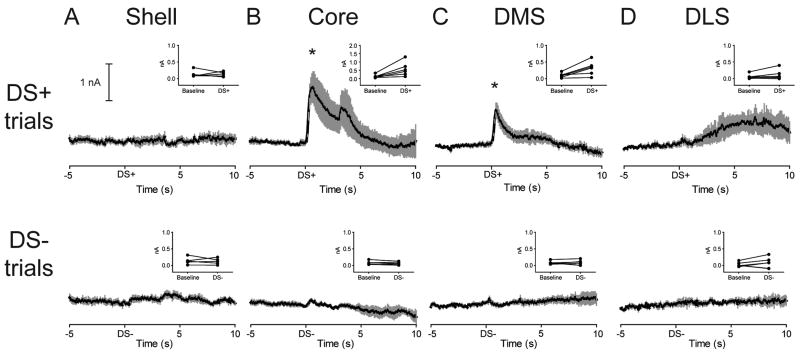
Rats trained on the discriminative stimulus paradigm, during testing, responded on 97.26 ± 0.97% of DS+ and 0.23 ± 0.00% of DS- trials. Figure 5 shows the average change in dopamine evoked by the DS+ and DS- in each subregion. Similar to unpredicted reward, the DS+ evoked an increase in dopamine in the Core relative to Baseline. The DS+ also evoked an increase in the DMS relative to Baseline. Paired t-tests (Figure 5, top row, insets) confirmed that these elevations were significant [Core (1.26 ± 0.33 nA peak dopamine for DS+, t5 = 3.19, P = 0.024; Figure 5B); DMS (0.74 ± 0.13 nA peak dopamine for DS+, t5 = 3.45, P = 0.018; Figure 5C)]. Additionally, in the Core, a second peak, corresponding in time to cue offset/lever extension, was apparent in the averaged data. Visual inspection of traces from individual rats indicated that this peak was only present in 2 out of 6 rats and was not statistically significant. The DS+ failed to evoke changes in phasic dopamine in the Shell (t4 = 0.94, P = 0.938; Figure 5A) and the DLS (t5 = 1.65, P = 0.160; Figure 5D). However, in the DLS there did appear to be a rise in dopamine occurring late in the trial. Consequently, for DLS recordings, the Baseline epoch was compared to a Late Epoch (5-10 s after DS+ onset), however, no significant difference was revealed (t5 = 1.881, P = 0.1331).
Figure 5.
Discriminative stimuli differentially evoke phasic dopamine signaling across striatal subregions. Average dopamine (black line) ± SEM (gray vertical bars) to predictive cues in striatal subregions during the discriminative stimulus test. Top: A cue predictive of reward (DS+) selectively evokes phasic dopamine release in the Core (B) and DMS (C) but not the Shell (A) or DLS (D). Insets: Average dopamine signal for each rat during both Baseline and Cue epochs. Note that in the Core (B), the scale for the ordinate is 2 nA, twice that of the other striatal regions. * P < 0.05 for Baseline versus DS+ epoch. Bottom: A cue predictive of no reward (DS-) fails to alter phasic dopamine signaling in all striatal subregions. Insets: Average dopamine concentration for each rat during both Baseline and Cue epochs.
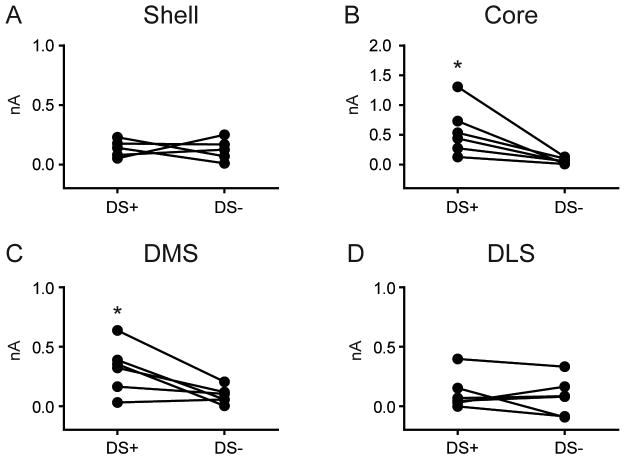
As shown in Figure 5 (bottom row), the DS- failed to evoke a change in dopamine in all striatal subregions (P's > 0.05). To determine if phasic dopamine responses were selectively evoked by a reward predictive cue, we compared, within each subregion, the difference between dopamine evoked by the DS+ versus DS- (Figure 6). Paired t-tests revealed that the DS+ evoked a greater increase in phasic dopamine than the DS- in the Core (t5 = 3.23, P = 0.023; Figure 6B), and DMS (t5 = 3.09, P = 0.027; Figure 6C). In contrast, there was no significant difference in dopamine following a DS+ cue compared to that following a DS- cue in the Shell (t4 = 0.71, P = 0.870; Figure 6A), and DLS (t5 = 0.66, P = 0.540; Figure 6D).
Figure 6.
Cue-evoked dopamine is dependent on a cue-reward association. Average dopamine signal for each rat during both Cue (DS+ versus DS-) epochs. The DS+ evoked significantly greater dopamine relative to the DS- in the Core (B) and DMS (C). Note that in the Core (B), the scale for the ordinate is 2 nA, twice that of the other striatal regions. * P < 0.05 for DS+ versus DS- epochs. No differences were observed in the Shell (A) or DLS (D).
The regional specificity of phasic dopamine evoked by unpredicted food reward is altered following discriminative stimulus training
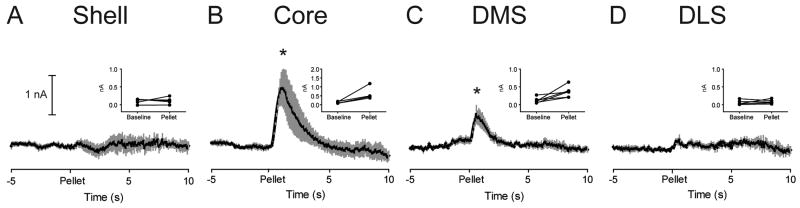
Immediately following administration of the discriminative stimulus recording session, rats were presented with unpredicted sugar pellets as described above. Average dopamine traces, aligned to pellet delivery, are shown in Figure 7. Baseline and Pellet epochs were compared using paired t-tests for each striatal subregion (Figure 7 insets). Similar to results obtained from rats without discriminative stimulus training, unpredicted food reward evoked a phasic increase in dopamine in the Core (t4 = 3.38, P = 0.028; Figure 7B) and failed to evoke a change in the Shell (t4 = 0.76, P = 0.764; Figure 7A) and DLS (t5 = 0.54, P = 0.611; Figure 7D). In contrast to results obtained in Experiment 1, after discriminative stimulus training, unpredicted food reward evoked a significant increase in phasic dopamine in the DMS (t5 = 3.29, P = 0.022; Figure 7C).
Figure 7.
In rats trained in the discriminative stimulus paradigm, unpredicted food reward evokes a different pattern of phasic dopamine release across striatal subregions. Average dopamine (black line) ± SEM (gray vertical bars) in different striatal regions in response to unpredicted food reward (sugar pellet; time = 0). Insets: Average dopamine signal for each rat during both Baseline and Pellet epochs. Unpredicted food reward evokes phasic dopamine release in the Core (B) and DMS (C) but not the Shell (A) or DLS (D). Note that in the Core (B), the scale for the ordinate is 2 nA, twice that of the other striatal regions. * P < 0.05 for Baseline versus Pellet epochs.
Discussion
Phasic changes in dopamine are critical for signaling reward presentation (Mirenowicz & Schultz, 1996; Roitman et al., 2008; Matsumoto & Hikosaka, 2009) and play a role in associating cues with reward (Waelti et al., 2001; Tsai et al., 2009; Zweifel et al., 2009) as well as approach behaviors directed at obtaining reward (Phillips et al., 2003; Roitman et al., 2004; Flagel et al., 2010). To determine if temporally comparable changes in dopamine are evoked by various reward-related conditions throughout the striatum, we measured phasic fluctuations in the Shell, Core, DMS and DLS. While robust dopamine release was evoked by electrical stimulation of the VTA/SNpc at every recording site, unpredicted food reward and a reward-predictive cue evoked phasic dopamine only in the Core and DMS – and the latter only under specific training conditions. The same stimuli failed to evoke changes in phasic dopamine in the Shell or DLS. These findings demonstrate that phasic dopamine release is not uniformly broadcast to all striatal regions in response to reward, but is selectively evoked in distinct regions. Furthermore, selectivity is dependent on the specific conditions in which a reward or associated cues are delivered.
Electrical stimulation evokes phasic dopamine release throughout the striatum and reveals regional differences in reuptake
Phasic fluctuations in dopamine in two ventral (Shell, Core) and two dorsal (DMS, DLS) striatal compartments were measured following electrical stimulation of the ventral midbrain. Electrical stimulation of the VTA/SNpc confirmed that electrodes were well positioned to measure dopamine release and histology verified electrode localization to one of the four subregions. In addition, electrically-evoked data hinted at functional differences across the four subregions. Although we were not able to formally analyze rate of reuptake as we did not have individual calibrations for all electrodes used, there did appear to be a difference in the kinetics of the dopamine response across subregions. In particular, the decay of the dopamine trace appeared longer in ventral regions than in dorsal regions, which may reflect regional differences in reuptake via the dopamine transporter. Accordingly, it has been shown that the density of the dopamine transporter varies across the striatum but comparisons have typically been made between nucleus accumbens and dorsal striatum (Richfield, 1991; Ciliax et al., 1995; Wu et al., 2001). Additionally, functional assays of dopamine reuptake rate via the dopamine transporter using FSCV have revealed differences between the nucleus accumbens and dorsal striatum in general (Jones et al., 1995; Suaud-Chagny et al., 1995). Our results, performed here in awake, behaving rats, are consistent with in vitro studies from rat (Jones et al., 1995) and non-human primate (Cragg et al., 2000), showing a ventromedial to dorsolateral gradient in the rate of dopamine reuptake where the most ventromedial placements were associated with the slowest reuptake. However, further experiments with calibrated electrodes are necessary to confirm this.
Unpredicted food reward evokes phasic dopamine release in select striatal subregions
Electrophysiological recordings have established that temporally unpredicted rewards – even during sessions in which animals receive many rewards – evoke a phasic increase in the firing rate of a majority (55-89%) of midbrain dopamine neurons (Schultz, 1998; Hyland et al., 2002; Tobler et al., 2003; Bayer & Glimcher, 2005; Matsumoto & Hikosaka, 2009). Similarly, in our study, while reward presentation may have been expected when animals were in the operant chamber, the precise timing of reward delivery remained unpredicted based on the variable inter-trial interval. As a result, we were able to systematically characterize the phasic dopamine response to unpredicted food reward across striatal subregions. Recordings made in the Core replicated previous findings and show that, in rats with limited experience receiving unpredicted food reward, delivery evokes a robust and phasic increase in dopamine (Day et al., 2007; Stuber et al., 2008; Zhang et al., 2009). Importantly, we show that phasic dopamine responses to unpredicted food reward were completely absent in all other striatal dopamine terminal regions – a finding seemingly at odds with electrophysiological recordings. Our results are consistent, however, with studies in which extracellular dopamine concentration has been assayed in the nucleus accumbens Core and Shell with in vivo microdialysis. For example, in the Shell, novel foods evoke dopamine but this response rapidly habituates upon repeated exposure (Bassareo & Di Chiara, 1999; Bassareo et al., 2011). In the Core, dopamine levels increase not in response to novel food reward but rather only after associative learning, when it can be evoked by both predictive stimuli and food itself (Bassareo et al., 2011). In our studies, rats had several sessions in which to retrieve and consume sugar pellets. Thus, the reward was not novel. It is likely, instead, that rats developed expectancies based on contextual cues and the cues associated with sugar pellet delivery.
No change in phasic dopamine release following unpredicted pellet delivery was observed in the DMS or DLS. With respect to food reward and dopamine fluctuations in the dorsal striatum, there are far fewer studies to draw on for comparison. One recent study employing FSCV examined the response to unpredicted reward and found a significantly greater increase in dopamine evoked in the NAc versus the DLS (Zhang et al., 2009). However, while there was a clear increase in the NAc, the authors combined Core and Shell placements. Moreover, the authors did not examine whether the response in the DLS itself was significant. This latter point is especially salient since the response in the DLS appeared minimal. Here, we found unpredicted reward failed to evoke a phasic dopamine response in either the DMS or DLS. Our results clearly demonstrate that with respect to phasic dopamine signaling, the quintessential stimulus that is thought to recruit the majority of dopamine neurons – unpredicted food reward – fails to evoke phasic increases in regions of the dorsal striatum.
Reward predictive cues and the evolution of phasic dopamine response in select regions
The phasic response of dopamine neurons shifts from primary food reward to the earliest reliable predictors of its delivery (Schultz, 1998; Hyland et al., 2002; Bayer & Glimcher, 2005; Matsumoto & Hikosaka, 2009). While some of these responses may reflect stimulus salience rather than reward prediction (Matsumoto & Hikosaka, 2009), once again the assumption is that a phasic rise in dopamine concentration would be observed throughout the striatum. Similar to results obtained with unpredicted food reward, predictive cues have been shown to evoke a phasic increase in dopamine in the Core (Day et al., 2007; Jones et al., 2010; Wanat et al., 2010). Again, we replicated those findings here. The DS+ (relative to baseline and the DS-) evoked a phasic increase in Core dopamine. Additionally, in the Core, we noticed a second peak that was only present in 2 of the 6 animals and so did not reach statistical significance overall. This peak was time-locked to cue offset/lever extension. Although speculative, we would suggest that this finding could reflect an inability of these two rats to accurately time the delay between initial cue presentation and lever presentation; as cue-reward delays increase (>2 s) dopamine neurons are more likely to fire to rewards, as well as reward-predictive cues (Bromberg-Martin et al., 2010). We also observed a robust increase in the DMS. The DMS has been proposed to facilitate a response selection process (Balleine et al., 2007). In the discriminative stimulus paradigm, a rat learns to select one response (“Go”) to receive a sugar pellet and selects an alternative response (“No-Go”) when no sugar pellet will be obtained. The phasic dopamine increase in the DMS to the DS+ may be critical for modulating activity in striatal output neurons to execute the optimal response choice.
Interestingly, training in the discriminative stimulus task changed rats' subsequent dopamine response to unpredicted reward. For rats not trained in the task, unpredicted reward failed to evoke phasic dopamine in the DMS. However, after discriminative stimulus training, unpredicted reward did evoke a significant phasic dopamine response. After the discriminative stimulus paradigm was concluded and unpredicted reward was administered, reward delivery contingencies changed with a concomitant change in behavioral responding from actively exploring lever locations to being more selectively positioned at the pellet receptacle. Past studies have demonstrated that neural activity in the DMS is modulated when conditions require a rapid switch or reversal of choice patterns (Ragozzino et al., 2001; Kimchi & Laubach, 2009) and thus, dopamine input to the DMS may be important for facilitating the flexible use of response patterns.
Since it is likely that phasic fluctuations in dopamine within dopamine terminal regions reflects the electrophysiological activity of midbrain dopamine neurons, to some degree (Sombers et al., 2009), then our data suggest that conditioning likely leads to the recruitment of additional dopamine neurons that respond to the DS+; in particular those that project to the DMS. Indeed, associative learning and operant responding induces long-term potentiation of glutamatergic afferents onto midbrain dopamine neurons (Stuber et al., 2008; Borgland et al., 2009). In addition, there is anatomical evidence that information may ‘spiral’ through the striatum in a ventromedial to dorsolateral manner (Haber et al., 2000). Reciprocal connections and synaptic plasticity may be a way for initial dopamine release in the Core to ultimately influence and recruit dopamine release in the DMS. Alternatively, operant responding may selectively engage VTA/SNpc afferents such that they include the population of dopamine neurons projecting to the DMS.
As with unpredicted reward, reward-predictive cues failed to evoke phasic dopamine release in the Shell. Dopamine responses to predictors of reward clearly develop in the Core (Day et al., 2007; Stuber et al., 2008; Aragona et al., 2009) and fail to develop in the Shell (Aragona et al., 2009). However, there are caveats. Recently, cues predicting the opportunity to respond for food reward were shown to evoke dopamine release in the Shell (Wanat et al., 2010), though recordings were made just ventral to the Core. Our recordings were made in the dorsomedial region of the Shell – an area thought to be critical for hedonic and affective processing of primary rewarding and aversive stimuli (Peciña & Berridge, 2005; Roitman et al., 2008; Wheeler et al., 2011). Thus, even within the Shell, regional differences between dorsomedial and ventrolateral likely exist. Finally, phasic dopamine fluctuations in the DLS were not evoked to the reward-predictive cues in this task. We did, however, note a slower onset rise in dopamine concentration occurring later in the trial (>5 s after cues), although this did not reach statistical significance. The DLS is associated with habitual behavior (Zapata et al., 2010). This burgeoning response may be reflective of the gradual engagement of the DLS as training progresses.
How to reconcile electrophysiological and electrochemical recordings?
Our results add to studies employing in vivo microdialysis (Di Chiara & Bassareo, 2007) and voltammetry (Aragona et al., 2009) or pharmacological (Besson et al., 2010; Ito & Hayen, 2011) or genetic (Palmiter, 2008) manipulations that support regional specificity for dopamine action within the striatum (Nicola, 2007). Importantly, we assayed dopamine on a timescale commensurate with electrophysiological studies that report relatively uniform responses across the medial-lateral extent of the VTA/SNpc. Our results beg the question as to why there might be dissociation between electrophysiological and electrochemical findings. There are multiple possibilities.
First, although it is well established that most dopamine neurons fire to reward, what is often unrecognized, is that a significant minority are unresponsive. The precise number varies between studies but ranges between 55 and 89% (Mirenowicz & Schultz, 1996; Waelti et al., 2001; Hyland et al., 2002; Tobler et al., 2003; Bromberg-Martin et al., 2009; Matsumoto & Hikosaka, 2009). One reason for this variance between studies, and another reason why the voltammetry and electrophysiology data may seem at odds, is sampling bias. Sampling bias can arise because one anatomical region is favored over another or because the physiological parameters used to define a neuron as dopaminergic are inaccurate. In support of these possibilities, studies that have attempted to record from the entire extent of the midbrain have identified multiple populations of neurons, which are activated by different stimuli (Brischoux et al., 2009; Matsumoto & Hikosaka, 2009; Lammel et al., 2011). Furthermore, the electrophysiological characteristics of dopamine neurons are not as strictly defined as previously thought and many studies may exclude select pools of dopamine neurons (Margolis et al., 2006; Lammel et al., 2008; 2011). Recent work has correlated a dopamine neuron's projection target with its physiological properties (Margolis et al., 2008) and with the type of stimuli it will respond to (Lammel et al., 2011). With all of this in mind, it is possible that the neurons included in classical electrophysiological studies, of which the majority exhibited a phasic increase to unpredicted reward, may have preferentially projected to the Core/DMS (Ikemoto, 2007).
Second, it is possible that dopamine release is heavily modulated by action at dopamine terminals. For example, on dopamine axons, the subunit profile of both muscarinic and nicotinic acetylcholine receptors differs between ventral and dorsal striatum (Threlfell & Cragg, 2011). Acetylcholine has been shown to exert modulatory effects on dopamine signaling (Cragg, 2006) and thus may differentially suppress release across subregions.
Third, our studies hint at increasing engagement of dorsal striatum as rats gain experience in a task. This would suggest an increasing number of dopamine neurons becoming recruited. A key feature of all of the electrophysiological studies performed in monkeys, is that the subjects were very well trained – in most experiments, thousands of trials were completed for these or other tasks. It is possible that after this much training, a larger pool of dopamine neurons is recruited by reward presentation as compared to our studies, in which rats had far fewer trials and consequently less experience.
In summary, we demonstrate that reward-related stimuli evoke dopamine in select striatal subregions. As each subregion possesses a unique complement of afferent and efferent projections, these regional differences in dopamine release permit exquisite tuning of striatal output in the service of goal-directed behaviors.
Acknowledgments
This research was supported by NIH grants HD055751 (MER) and DA025634 (MFR). We wish to thank Eric Schmidt and Matt Schuck at the UIC Research Resources Center. We also wish to thank Dr. Jamie D Roitman and Dr. Brandon J Aragona for helpful comments on earlier versions of the manuscript.
Abbreviations
- DS+
Discriminative stimulus, rewarded
- DS-
Discriminative stimulus, non-rewarded
- DMS
Dorsomedial striatum
- DLS
Dorsolateral striatum
- FSCV
Fast-scan cyclic voltammetry
- Core
Nucleus accumbens core
- Shell
Nucleus accumbens shell
- PCA
Principle component analysis
- VTA/SNpc
Ventral tegmental area/substantia nigra pars compacta
References
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28:8821–31. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Day JJ, Roitman MF, Cleaveland NA, Wightman RM, Carelli RM. Regional specificity in the real-time development of phasic dopamine transmission patterns during acquisition of a cue-cocaine association in rats. Eur J Neurosci. 2009;30:1889–99. doi: 10.1111/j.1460-9568.2009.07027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–5. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK. Dopamine modulates release from corticostriatal terminals. J Neurosci. 2004;24:9541–52. doi: 10.1523/JNEUROSCI.2891-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89:637–41. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Musio P, Di Chiara G. Reciprocal responsiveness of nucleus accumbens shell and core dopamine to food- and drug-conditioned stimuli. Psychopharmacology (Berl) 2011;214:687–97. doi: 10.1007/s00213-010-2072-8. [DOI] [PubMed] [Google Scholar]
- Bayer HM, Glimcher PW. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron. 2005;47:129–41. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M, Belin D, McNamara R, Theobald DE, Castel A, Beckett VL, Crittenden BM, Newman AH, Everitt BJ, Robbins TW, Dalley JW. Dissociable control of impulsivity in rats by dopamine D2/3 receptors in the core and shell subregions of the nucleus accumbens. Neuropsychopharmacology. 2010;35:560–9. doi: 10.1038/npp.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J Anat. 2000;196(Pt 4):527–42. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–25. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci USA. 2009;106:4894–9. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Hikosaka O. Midbrain dopamine neurons signal preference for advance information about upcoming rewards. Neuron. 2009;63:119–26. doi: 10.1016/j.neuron.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, Niznik HB, Levey AI. The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci. 1995;15:1714–23. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg SJ. Meaningful silences: how dopamine listens to the ACh pause. Trends Neurosci. 2006;29:125–31. doi: 10.1016/j.tins.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Cragg SJ, Hille CJ, Greenfield SA. Dopamine release and uptake dynamics within nonhuman primate striatum in vitro. J Neurosci. 2000;20:8209–17. doi: 10.1523/JNEUROSCI.20-21-08209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–8. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn't do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berl) 2010;210:241–52. doi: 10.1007/s00213-010-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone RE, McDonald RJ. Dorsal striatum and stimulus-response learning: lesions of the dorsolateral, but not dorsomedial, striatum impair acquisition of a stimulus-response-based instrumental discrimination task, while sparing conditioned place preference learning. Neuroscience. 2004;124:23–31. doi: 10.1016/j.neuroscience.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PEM, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Ann Rev Neurosci. 2011;34:441–66. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Galis-de Graaf Y, Smeets WJ. Integration and segregation of limbic cortico-striatal loops at the thalamic level: an experimental tracing study in rats. J Chem Neuroanat. 1999;16:167–85. doi: 10.1016/s0891-0618(99)00009-5. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–82. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien MLAV, Johnson MA, Wightman RM. Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal Chem. 2004;76:5697–704. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol. 2010;90:385–417. doi: 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JNJ, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–92. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22:6247–53. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Hayen A. Opposing roles of nucleus accumbens core and shell dopamine in the modulation of limbic information processing. J Neurosci. 2011;31:6001–7. doi: 10.1523/JNEUROSCI.6588-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Day JJ, Aragona BJ, Wheeler RA, Wightman RM, Carelli RM. Basolateral amygdala modulates terminal dopamine release in the nucleus accumbens and conditioned responding. Biol Psychiatry. 2010;67:737–44. doi: 10.1016/j.biopsych.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Garris PA, Kilts CD, Wightman RM. Comparison of dopamine uptake in the basolateral amygdaloid nucleus, caudate-putamen, and nucleus accumbens of the rat. J Neurochem. 1995;64:2581–9. doi: 10.1046/j.1471-4159.1995.64062581.x. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Functional specificity of ventral striatal compartments in appetitive behaviors. Ann N Y Acad Sci. 1999;877:71–90. doi: 10.1111/j.1749-6632.1999.tb09262.x. [DOI] [PubMed] [Google Scholar]
- Kimchi EY, Laubach M. The dorsomedial striatum reflects response bias during learning. J Neurosci. 2009;29:14891–902. doi: 10.1523/JNEUROSCI.4060-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hдckel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–62. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Cheng Y, Bian W, Liu X, Zhang C, Ye JH. Region-specific induction of FosB/ΔFosB by voluntary alcohol intake: effects of naltrexone. Alcohol Clin Exp Res. 2010;34:1742–50. doi: 10.1111/j.1530-0277.2010.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. J Neurosci. 2008;28:8908–8913. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, Kaneko T. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci. 2009;29:444–53. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–41. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–51. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berl) 2007;191:521–50. doi: 10.1007/s00213-006-0510-4. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Deadwyler SA. Firing rate of nucleus accumbens neurons is dopamine-dependent and reflects the timing of cocaine-seeking behavior in rats on a progressive ratio schedule of reinforcement. J Neurosci. 2000;20:5526–37. doi: 10.1523/JNEUROSCI.20-14-05526.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds ME, Jacques DB, Kopyov O. Relation between rotation in the 6-OHDA lesioned rat and dopamine loss in striatal and substantia nigra subregions. Synapse. 2006;59:532–44. doi: 10.1002/syn.20270. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann N Y Acad Sci. 2008;1129:35–46. doi: 10.1196/annals.1417.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Aragona BJ, Kile BM, Carelli RM, Wightman RM. In vivo voltammetric monitoring of catecholamine release in subterritories of the nucleus accumbens shell. Neuroscience. 2010;169:132–42. doi: 10.1016/j.neuroscience.2010.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Peciña S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–86. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CMA, Berke JD, Graybiel AM, Ito R, Lansink CS, van der Meer M, Redish AD, Smith KS, Voorn P. Corticostriatal interactions during learning, memory processing, and decision making. J Neurosci. 2009;29:12831–8. doi: 10.1523/JNEUROSCI.3177-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PEM, Stuber GD, Heien MLAV, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–8. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Ragozzino KE, Leutgeb S, Mizumori SJ. Dorsal striatal head direction and hippocampal place representations during spatial navigation. Exp Brain Res. 2001;139:372–6. doi: 10.1007/s002210100795. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Mohler EG, Prior M, Palencia CA, Rozman S. Acetylcholine activity in selective striatal regions supports behavioral flexibility. Neurobiol Learn Mem. 2009;91:13–22. doi: 10.1016/j.nlm.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richfield EK. Quantitative autoradiography of the dopamine uptake complex in rat brain using [3H]GBR 12935: binding characteristics. Brain Res. 1991;540:1–13. doi: 10.1016/0006-8993(91)90486-f. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PEM, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–71. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci. 2008;11:1376–7. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol KE, Neill DB, Wages SA, Church WH, Justice JB. Dopamine depletion in a striatal subregion disrupts performance of a skilled motor task in the rat. Brain Res. 1985;335:33–43. doi: 10.1016/0006-8993(85)90273-2. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Sombers LA, Beyene M, Carelli RM, Wightman RM. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci. 2009;29:1735–42. doi: 10.1523/JNEUROSCI.5562-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, Bonci A. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008;321:1690–2. doi: 10.1126/science.1160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suaud-Chagny MF, Dugast C, Chergui K, Msghina M, Gonon F. Uptake of dopamine released by impulse flow in the rat mesolimbic and striatal systems in vivo. J Neurochem. 1995;65:2603–11. doi: 10.1046/j.1471-4159.1995.65062603.x. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Plotkin J, Shen W. Dopamine and synaptic plasticity in dorsal striatal circuits controlling action selection. Curr Opin Neurobiol. 2009;19:621–8. doi: 10.1016/j.conb.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Cragg SJ. Dopamine signaling in dorsal versus ventral striatum: the dynamic role of cholinergic interneurons. Front Sys Neurosci. 2011;5:11. doi: 10.3389/fnsys.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Dickinson A, Schultz W. Coding of predicted reward omission by dopamine neurons in a conditioned inhibition paradigm. J Neurosci. 2003;23:10402–10. doi: 10.1523/JNEUROSCI.23-32-10402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–4. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJMJ, Groenewegen HJ, Robbins TW, Pennartz CMA. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–74. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412:43–8. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Kuhnen CM, Phillips PEM. Delays conferred by escalating costs modulate dopamine release to rewards but not their predictors. J Neurosci. 2010;30:12020–12027. doi: 10.1523/JNEUROSCI.2691-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Aragona BJ, Fuhrmann KA, Jones JL, Day JJ, Cacciapaglia F, Wightman RM, Carelli RM. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biol Psychiatry. 2011;69:1067–74. doi: 10.1016/j.biopsych.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J Neurosci Methods. 2001;112:119–33. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. Contributions of striatal subregions to place and response learning. Learn Mem. 2004;11:459–63. doi: 10.1101/lm.81004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A, Minney VL, Shippenberg TS. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. J Neurosci. 2010;30:15457–63. doi: 10.1523/JNEUROSCI.4072-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Doyon WM, Clark JJ, Phillips PEM, Dani JA. Controls of tonic and phasic dopamine transmission in the dorsal and ventral striatum. Mol Pharmacol. 2009;76:396–404. doi: 10.1124/mol.109.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJY, Paladini CA, Phillips PEM, Palmiter RD. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci USA. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]