Abstract
Despite decades of research, the treatment and management of malignant tumors still remain a formidable challenge for public health. New strategies for cancer treatment are being developed, and one of the most promising treatment strategies involves the application of chemopreventive agents. The search for novel and effective cancer chemopreventive agents has led to the identification of various naturally occurring compounds. Xanthones, from the pericarp, whole fruit, heartwood, and leaf of mangosteen (Garcinia mangostana Linn., GML), are known to possess a wide spectrum of pharmacologic properties, including anti-oxidant, anti-tumor, anti-allergic, anti-inflammatory, anti-bacterial, anti-fungal, and anti-viral activities. The potential chemopreventive and chemotherapeutic activities of xanthones have been demonstrated in different stages of carcinogenesis (initiation, promotion, and progression) and are known to control cell division and growth, apoptosis, inflammation, and metastasis. Multiple lines of evidence from numerous in vitro and in vivo studies have confirmed that xanthones inhibit proliferation of a wide range of human tumor cell types by modulating various targets and signaling transduction pathways. Here we provide a concise and comprehensive review of preclinical data and assess the observed anticancer effects of xanthones, supporting its remarkable potential as an anticancer agent.
Keywords: Chemoprevention, xanthone, mangosteen, anticancer, natural agent, compound
INTRODUCTION
Currently, cancer remains one of the most aggressive and lethal diseases worldwide. Although surgery, chemotherapy, and radiotherapy has been practiced for many years, these anticancer therapies can only offer limited benefits to cancer patients due to metastasis, acquired chemoresistance, and toxicity issues [1–4]. Consequently, cancer prevention using non-toxic chemical entities, commonly termed ‘chemoprevention’, is a more realistic and fundamental strategy for the management of this disease. Evidence from both population-based and laboratory studies suggest that the regular consumption of fruits and vegetables reduce the incidence of degenerative diseases, including cancer, heart disease, and brain dysfunction [5–8]. Due to the limitations of current therapies, natural products may serve as chemoprevention regimens and/or novel adjunctive agents to fill a critical need in the effective, safe, and less invasive treatment of cancer.
Mangosteen (Garcinia mangostana Linn., GML), a well-known tropical fruit, is indigenous to Southeast Asia but can be found in most tropical countries. This fruit exhibits a variety of pharmacological activities. The pericarp of the fruit contains considerable amounts of biologically active compounds, such as xanthones, terpenes, anthocyanins, tannins, and phenols. For centuries, the pericarp of mangosteen was used as a medicinal agent by Southeast Asians in the treatment of skin infections and wounds, amoebic dysentery, diarrhea, and cholera [9–12]. Recently, commercial products of mangosteen added with essential minerals, containing mangosteen, green tea (Camellia sinensis), aloe vera, and multivitamins, were used by cancer patients as a dietary supplement [13]. Despite the lack of sufficient clinical evidence demonstrates that mangosteen consumption could reduce the incidence of various malignancy, these products are popular due to their perceived role in promoting health [14]. Mangosteen products are now one of the top-selling botanical dietary supplements [15]. In 2005, these products ranked sixth in single-herb dietary supplement sales, netting more than $120 million, a substantial increase compared to the previous year [16, 17].
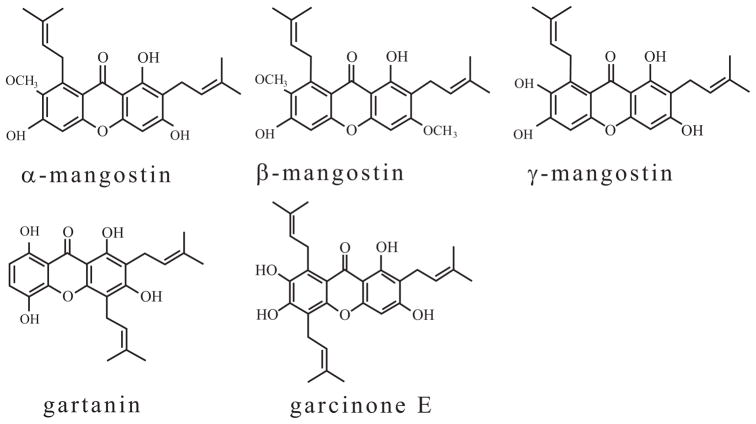
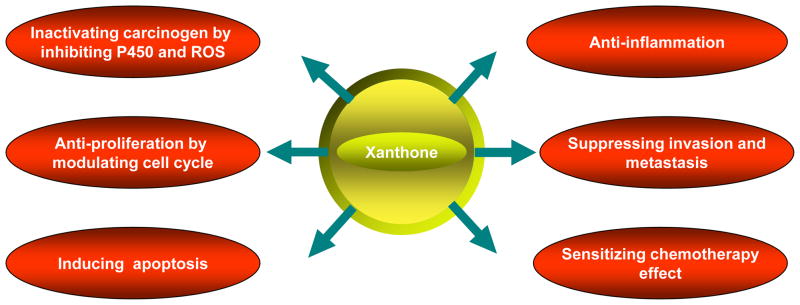
As of 2008, over 68 xanthone-type compounds had reportedly been derived from GML. Of these, α-mangostin, β-mangostin, γ-mangostin, garcinone E, and gartanin are the most abundant and most frequently studied [18] (Fig. 1). The 1H- and 13C NMR, HMBC spectrum data show that structure of xanthones compounds are comprised of a basic xanthone skeleton and several different substituents such as aromatic protons, phenolic hydroxyl groups, methoxyl prenyl group, hydroxyl proton, oxygenated methine protons, or dihydrofuran ring [19]. Several studies have shown that xanthones possess significant biological properties, including anti-oxidant, anti-tumor, anti-inflammatory, anti-allergy, anti-bacterial, anti-fungal and anti-viral activities [20, 21]. Recently, the potential chemopreventive and chemotherapeutic properties of xanthones have been extensively investigated due to their inhibitory effect on every step in the process of carcinogenesis [22]. These compounds are able to inhibit several molecular targets in the tumor cells, including kinases, cyclooxygenases, ribonucleotide reductase, and DNA polymerases [22]. The anti-cancer activities of this kind of compounds are associated with their tricyclic scaffold but vary depending on the nature and/or position of the different substituents. For example, the xanthones with high anti-cancer activity contain tetraoxygen functions with two C5 units in rings A and C described by Suksamrarn et al. [19]; the activity was generally reduced with the increase of hydroxyl groups in the C5 side chain; notably, the pyrano and furano rings bearing the hydroxyl group attached to the xanthone nucleus appear to enhance cytotoxic activity [19]. The anti-tumor activities of xanthones include cell cycle arrest, suppression of tumor cell proliferation, induction of apoptosis and differentiation, reduction of inflammation, and inhibition of adhesion, invasion, and metastasis [23–25]. Evidence for these activities is summarized in this review. The aim of this review is to elucidate the multiple molecular targets of xanthones as chemopreventive and chemotherapeutic agents and to provide rationales for testing xanthones in clinic trials (Fig. 2).
Fig. 1.
Chemical structure of major xanthones isolated from the pericarp of mangosteen (Garcinia mangostana Linn., GML).
Fig. 2.
Biochemical mechanisms responsible for chemopreventive and chemotherapeutic potential of xanthones.
CHEMOPREVENTIVE AND CHEMOTHERAPEUTIC POTENTIAL OF MANGOSTEEN
Considerable interest in xanthones has emerged due to their potent inhibition of tumor initiation, promotion, and progression. These compounds may provide an alternative approach to prevent or delay tumor onset or to alter or prevent carcinogenic progression. The antitumor activity of xanthones was first observed in Raji and P3HR-1 lymphoblastoid cells [26] and later found in HL60, K562, NB4, and U937 leukemia cells [27]. In leukemia cells, α-, β-, and γ-mangostin were effective even at a low dose (less than 10 μM) [27]. Of these compounds, α-mangostin demonstrated the strongest inhibitory activity in all cell lines tested, particularly in HL60, NB4, and U937, with complete suppression of cell growth at 72 h of treatment. As a significant cytotoxic effect was not observed in peripheral blood lymphocytes, α-mangostin appears to preferentially target leukemia cells. Subsequently, studies demonstrated that the crude methanolic extract (CME) from the pericarp of GML exhibited significant antiproliferative activity in breast cancer cells (SKBR3; ED50, 9.25±0.64 μg/ml). The observed antiproliferative activity was associated with an induction of apoptosis as assessed by detecting morphological changes and oligonucleosomal DNA fragments [28]. In addition, xanthones especially γ-mangostin could inhibit breast cancer cells growth via decreasing aromatase activity which is required for the growth of estrogen-dependent breast cancer [29]. Sato et al. examined the efficacy of eight different xanthones, including α- and γ-mangostin, on the viability of rat pheochromocytoma PC12 cells [30]. Among these compounds, α-mangostin was the most potent, exhibiting an ED50 value of 4 μM [30]. Matsumoto et al. investigated the antiproliferative effects of four structurally similar prenylated xanthones that differed in the number of hydroxyl and methoxy groups in human colon cancer DLD-1 cells [31]. With the exception of methoxy-β-mangostin, these xanthones strongly inhibited cell growth at 20 μM, and their antitumor efficacy was correlated with the number of hydroxyl groups [31]. Similar studies have demonstrated that prenylated xanthones significantly arrested tumor growth in epidermoid carcinoma of the mouth (KB), breast cancer (BC-1), small cell lung cancer (NCI-H187), CEM-SS cells, gastric adenocarcinoma cells, and B cell chronic lymphocytic leukemia (ESKOL and EHEB) [32–36]. In summary, these studies confirmed that xanthones, including α-, β-, and γ-mangostin, mangostenone C, and gartanin, inhibit cell growth at different ED50 values.
Anticarcinogenic properties of xanthones have been linked with an impressive amount of data, primarily from human cell culture systems. Although animal models may provide more convincing evidence, only a few studies with respect to cancer prevention and therapy have been conducted in vivo [37–43]. Previous work has demonstrated the chemopreventive potential of α-mangostin on putative preneoplastic lesions involved in rat colon carcinogenesis [37]. Dietary administration of crude α-mangostin significantly inhibited the induction and/or development of aberrant crypt foci (ACF), dysplastic foci (DF), β-catenin accumulated crypts (BCAC) and proliferating cell nuclear antigen (PCNA) labeling in colon epithelium [37]. These findings suggest that short-term administration of crude α-mangostin has potential chemopreventive effects on colon carcinogenesis, indicating that longer exposures to a substance may lead to the suppression of tumor development [37]. In addition to inhibiting the formation of preneoplastic lesions in a rat model of colon carcinogenesis, α- and γ-mangostin also inhibited DMBA-induced preneoplastic lesions in a mouse mammary organ culture (MMOC) assay [38]. Watanapokasin et al. characterized the antiproliferative and cytotoxic activities of xanthones both in vitro and in vivo using the human colorectal adenocarcinoma cell line, COLO 205 [39]. The results from these studies revealed that, in vitro, xanthones not only inhibit the proliferation of tumor cells but also induce cell death through activation of the caspase cascade [39]. Using a mouse subcutaneous tumor model with COLO 205 cells, researchers demonstrated that the growth of tumors was delayed following intratumoral administration of mangosteen xanthones at relatively low doses (0.024, 0.12, and 0.6 mg per tumor). When treated with a high dose of xanthones (3.0 mg per tumor), tumor size gradually decreased, and complete regressions occurred in some mice [39]. Doi et al. observed the antiproliferative activities of panaxanthone (approximately 80% α-mangostin and 20% γ-mangostin) in a mouse model using the human breast cancer cell line, BJMC3879. The results revealed that tumor volumes were significantly suppressed in mice treated with 2,500 and 5,000 ppm panaxanthone in their diet and further confirmed the effects were associated with elevation of proapoptosis and antiangiogenesis [40]. In contrast, some studies have demonstrated inactivity at the highest dose of α-mangostin (20 μg/ml) in HT-29, LNCaP, and MCF-7 cells [41–42]. Additional studies in DLD-1 cells have showed that the combination of α-mangostin and 5-FU significantly enhance growth inhibition compared to the treatment with α-mangostin or 5-FU alone in vitro experiment [43]. Taken together, these investigations suggest that mangosteen may possess marked antiproliferative activities and could act as a potential reagent for cancer chemoprevention and/or therapy. The differences in the efficacy of xanthones treatment may be due to variations in the dosage, the route of administration, the tumor origin, and/or the presence of other dietary components (Table 1).
Table 1.
Cytotoxicity of Mangosteen Xanthones In Vitro
| Cancer cells | Xanthones | ED50 (μM) | Ref |
|---|---|---|---|
| Human leukemia | |||
| HL60 | α-mangostin | 6.8 | [27] |
| HL60 | β-mangostin | 7.6 | [27] |
| HL60 | γ-mangostin | 6.1 | [27] |
| HL60 | mangostinone | 19.0 | [27] |
| HL60 | garcinone E | 15.0 | [27] |
| K562 | α-mangostin | <10.0 | [27] |
| NB4 | α-mangostin | <10.0 | [27] |
| U937 | α-mangostin | <10.0 | [27] |
| Lung cancer | |||
| NCI-H187 | mangostenone C | 8.9 | [35] |
| NCI-H187 | gartanin | 2.6 | [35] |
| A549 | α-mangostin | 12.5–15 | [80] |
| Breast cancer | |||
| SKBR3 | GML extract | 22.0 | [28] |
| BC-1 | mangostenone C | 8.5 | [35] |
| BC-1 | α-mangostin | 2.2 | [35] |
| T-lymphoblastic leukemia | |||
| CEM-SS cell | α-mangostin | 13.0 | [36] |
| CEM-SS cell | mangostanol | 23.0 | [36] |
| CEM-SS cell | γ-mangostin | 11.2 | [36] |
| CEM-SS cell | garcinone D | 7.7 | [36] |
| Mouth epidermoid carcinoma | |||
| KB cell | mangostenone C | 6.7 | [35] |
| KB cell | α-mangostin | 5.0 | [35] |
| Pheochromocytoma | |||
| PC12 | α-mangostin | 4.0 | [30] |
| Colorectal cancer | |||
| DLD-1 | α-mangostin | 7.5 | [31] |
| DLD-1 | β-mangostin | 8.1 | [31] |
| DLD-1 | γ-mangostin | 7.1 | [31] |
| HT-29 | 3-isomangostin | 4.9 | [41] |
| HT-29 | α-mangostin | 1.7 | [41] |
| HT-29 | β-mangostin | 1.7 | [41] |
| HT-29 | garcinone D | 2.3 | [41] |
| HT-29 | 9-hydroxycalabaxanthone | 9.1 | [41] |
| COLO 205 | α-mangostin | -- | [39] |
| Ovarian cancer | |||
| SK-OV3 | macluraxanthone | 4.24 | [35] |
| Glioma | |||
| C6 rat glioma cells | γ-mangostin | >30 | [37] |
Inhibition of Metabolic Activation of Carcinogens and Oxidative Damage: Implications for Suppression of Tumor Initiation
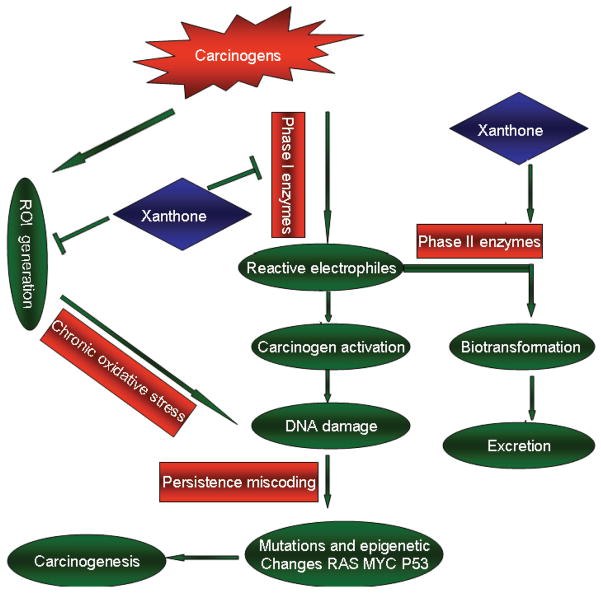
Many environmental pollutants, such as cigarette smoke, industrial emissions, and gasoline vapors can be converted to polar intermediates by undergoing biotransformation catalyzed by phase I and II enzymes such as cytochrome P450, acetyltransferases, sulphotransferases, glutathione S-transferase, glucuronyltransferase, particularly these belonging to the cytochrome P450 (CYP) superfamily. Following biotransformation, these intermediates are eliminated by undergoing conjugation reactions catalyzed by phase II enzymes that are responsible for the conjugation/detoxification of reactive metabolites. In the absence of adequate phase II enzymes, metabolically active carcinogens are more likely to target cellular DNA, causing DNA damage by forming covalent adducts with DNA nucleotides, which may ultimately initiate tumorigenesis [44, 45]. Of the various processes of carcinogenesis, blocking of tumor initiation through the induction of phase I and II enzymes such as CYP and quinone reductase (QR) is considered as an important cytoprotective mechanism. Xanthones have been reported to induce QR and inhibit P450 activity [46, 47], suggesting that these substances may have potential uses for preventing cancer initiation. Currently, however, there has been limited study regarding the inhibitive effects of xanthones on phase I and II enzymes, and warrant further investigation.
In addition to undergoing metabolic activation to electrophilic species, some carcinogens produce excessive amounts of reactive oxygen intermediates (ROI), including hydroxyl radicals (OH•), hydrogen peroxide (H2O2) and the superoxide anion (O2−•) which are involved in the development of cancer [48, 49]. These ROIs Aerobic organisms possess antioxidant systems that function to scavenge ROI. These systems include enzyme-based antioxidants, such as superoxide dismutase, glutathione peroxidase, catalase, and glutathione reductase. Tissue damage, however, can arise from an imbalance in free radicals and antioxidants, resulting in the development of a variety of degenerative disorders. The accumulation of ROI is pathogenic in that these species can react with cellular macromolecules (lipids, proteins, and nucleic acids), resulting in DNA damage, oxidative modifications, cellular disjunction, carcinogenesis, or cell death [50]. These reactions can lead to an accumulation of somatic mutations and eventually to the development of malignancies [51]. Antioxidants that inhibit or delay oxidative damage through mechanisms such as free radical scavenging are extremely important in the prevention of these malignancies [52]. As a major source of natural antioxidants, mangosteen may be an effective alternative [13].
Based on the ability to scavenge the 2, 20-azino-bis-(3- ethylbenzthiazoline-6-sulfonic acid) (ABTS) free radical and the 1, 1-diphenyl-2-picryl-hydrazyl (DPPH) radical, a recent study showed that mangosteen exhibits a high level of antioxidant activities among 27 different fruit pulps [53]. Treatment of NG108-15 neuroblastoma cells with various extracts from the fruit hull of mangosteen results in neuroprotective activity and can antagonize H2O2-induced oxidative stress [54]. In SK-N-SH neuronal cells, mangosteen successfully prevented β-amyloid (Aβ)-induced cytotoxicity, increased ROS and caspase activity [55]. In a study conducted by Chin et al., the antioxidant capacity of 16 xanthones was evaluated in a hydroxyl radical-scavenging assay, and γ-mangostin was the only active compound (IC50, 0.20 μg/mL), while other compounds were considered inactive (IC50 > 10 μg/mL) [56]. Taken together, these data suggest that xanthones may prevent the production of carcinogenic compounds and the formation of DNA adducts (Fig. 3).
Fig. 3.
Anti-carcinogenesis effects: xanthones modulate carcinogen detoxification mechanism. Many pollutants such as cigarette smoke, industrial emissions, and gasoline vapors can be converted to active carcinogens and produce excessive amounts of reactive oxygen intermediates (ROI). These mediators could cause DNA damage, genomic instability, and carcinogenesis. Xanthones can prevent the malignant conversion of precancerous cells by impacting phase I and phase II enzymes activities and inhibiting ROI generation.
Anti-Inflammatory Effects
It has been recognized that the components of the inflammatory signaling pathways are associated with carcinogenesis [44]. Mangosteen has been shown to exhibit substantial antiphlogistic activity. Shankaranarayan et al. reported that α-mangostin, 1-isomangostin and mangostin triacetate exhibited anti-inflammatory activity in a rat model [57]. Gopalakrishnan also demonstrated that α-mangostin inhibited systemic anaphylaxis and immunocyto-adherence in guinea pigs and rats [58]. Chairungsrilerd et al. showed that α- and γ-mangostins could inhibit the contractions of isolated thoracic rabbit aorta induced by histamine and serotonin, suggesting that these mangostin compounds are histaminergic and serotonergic receptor blocking agents [59]. Furthermore, α- and γ-mangostin suppressed the release of histamine from IgE-sensitized rat basophilic leukemia RBL-2H3 cells [60, 61], and the results from these studies indicate that the likely mechanism of xanthones mainly involves the suppression of the Syk/PLCγs/PKC pathway. Due to their role in carcinogenesis, apoptosis, and angiogenesis, both COX-2 and prostaglandins are excellent targets for developing new drugs to selectivity prevent and/or treat human cancers. γ-Mangostin also exhibited a potent, concentration-dependent inhibitory activity of PGE2 release induced by A23187, a Ca2+ ionophore (IC50 value of approximately 5 μM). Mechanistic studies revealed that these effects were mediated through the inhibition of both COX-1 and COX-2 activities [62]. Based on these findings, the author investigated the effect of γ-mangostin on spontaneous PGE2 release and COX-2 gene expression in C6 rat glioma cells. They found that long-term exposure to γ-mangostin significantly inhibited spontaneous PGE2 release and also inhibited lipopolysaccharide (LPS)-induced COX-2 protein expression, the role was associated with the suppression of NF-κB [63]. A similar study demonstrated that garcinone B could also inhibit both A23187-induced PGE2 release and LPS-induced stimulation of NF-κB -mediated transcription in C6 cells [64, 65]. Apart from inhibiting COX-2, pretreatment with α-mangostin significantly attenuated eNOS expression and NO levels in a rat model of isoproterenol-induced myocardial necrosis [66]. Though these findings indicate an ameliorative potential for α-mangostin in the context of isoproterenol-induced myocardial necrosis, studies should also examine tumorigenesis mediated by the NO pathway. Evaluation of methanol extracts from 32 plant parts of 19 different species of the genus Garcinia (Guttiferae) for their in vitro cytotoxic and nitric oxide inhibitory activities in three human tumor cell lines, MCF-7, NCI-H1460, and DU-145, it was found that of the 32 extracts, 27 exhibited cytotoxic activity in at least one of the three tumor cell lines. Among the tested extracts, G. bancana (stem) and G. malaccensis (stem) demonstrated the highest selectivity indices (>50) for NO inhibition [67]. The expression of pro-inflammatory cytokines appears to be differentially affected by xanthones. Although α- and γ-mangostin inhibiting PGE2 release effect is apparent (IC50 = 6.0 μg/ml), moderate effects in the release of TNF-α and IL-4 were observed with IC50 values ranging from 31.8 to 64.8 μM [68]. In primary cultures of newly differentiated human adipocytes, α- and γ-mangostin decreased LPS-induced inflammatory genes expression of tumor necrosis factor-α, IL-1b, IL-6, IL-8, monocyte chemoattractant protein-1, and Toll-like receptor-2 (TLR-2), this inhibitory effect was associated with the interference of the mitogen-activated protein kinases (MAPK) c-jun NH2-terminal kinase (JNK), extracellular signal-related kinase (ERK), p38, activator protein (AP)-1, and NF-κB [68–70]. Collectively, these data demonstrate that xanthones are effective in inhibiting the release of many inflammatory mediators releasing in non-malignant cells; however, further studies are needed to determine these xanthones’ effects in cancer cells.
Induction of Apoptosis
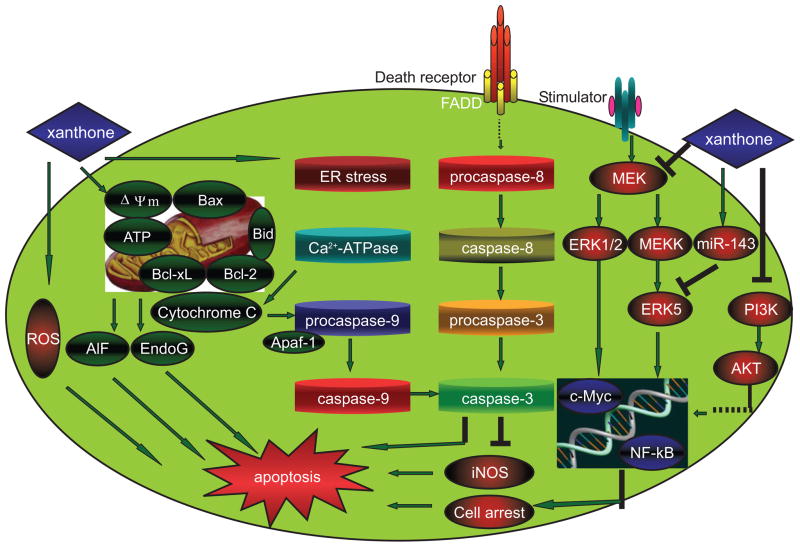
GML has shown strong anticancer properties that are mediated by several modes of actions. The most common reported anticancer mechanism of GML is its ability to induce apoptosis in cancer cells through the modulation of multiple pathways related to regulation of cell death and survival [24, 30–31].
It has been reported that GML induces apoptosis in various cancer cell types by activating pro-apoptotic signaling molecules and by inhibiting anti-apoptotic molecules of the intracellular signal transduction pathways. The induction of apoptosis in human promyelocytic leukemia (HL-60) cells by α-mangostin was mediated by the activation of caspase-9 and caspase-3, but not caspase-8, indicating that α-mangostin may be involved in the mitochondrial apoptotic pathway [31]. In this study [31], parameters of mitochondrial dysfunction, including swelling, loss of membrane potential (Δψm), decrease in intracellular ATP, ROS accumulation, and cytochrome c/AIF release, were observed within 1 or 2 h following treatment. In a similar study [30], Sato investigated the effects of eight xanthones on cell death in PC12 rat pheochromocytoma cells. PC12 cells treated with α-mangostin demonstrated typical apoptotic DNA fragmentation, caspase-3 cleavage, mitochondrial membrane depolarization, cytochrome c release, sarco-endoplasmic reticulum Ca2+-ATPase inhibition, and c-Jun NH2-terminal kinase (JNK/SAPK) activation. These results suggest that α-mangostin inhibits Ca2+-ATPase to induce apoptosis through the mitochondrial pathway in the cells [30]. GML extract-induced SKBR3 human breast cancer cell line early apoptosis was accompanied by the production of reactive oxygen species (ROS). This was not surprising because the accumulation of intracellular ROS is one of the important mechanisms leading to early apoptosis. These data suggested that ROS has effects on both intrinsic and extrinsic apoptosis pathways through modulation of expression of the major molecules in these pathways, Bcl-2 and FasL. Such conditions cause damage to various cellular components, ultimately resulting in programmed cell death or apoptosis [28]. Whether xanthones as antioxidant is contradictory to promoting apoptosis by inducing the production of ROS in cancer cell lines deserve to discuss. This interesting phenomenon is possible, when xanthones serve as antioxidant, the general choose dose is relative low (5 μg/ml) or medium (10–20 μg/ml) dose, whereas it appears that xanthones usually cause apoptosis at high (40 μg/ml) dose, indicating that the different doses of xanthones impact different roles. These probable properties of xanthones provide scope of further detail evaluation. Some constituents from xanthones may serve as a novel powerful anti-tumor agent and free radical scavenger after further detailed investigation. Moreover, the antitumor effect of γ-mangostin in high grade human malignant glioblastomas cells (U87 MG and GBM 8401) was associated with significant enhancement of intracellular peroxide production was detected by DCHDA assay [71]. The cytotoxic effect of α-mangostin on human colon cancer DLD-1 cells was found to be caused by apoptosis, but there is either activation of caspases or changes in Bax, Bcl-2 protein and apoptosis-inducing factor (AIF) following α-mangostin treatment. However, the release of endonuclease-G from the mitochondria, increased levels of microRNA-143, and reduced levels of phospho-Akt and c-Myc were detected [43]. Interestingly, the levels of phospho-extracellular signal-regulated kinase (ERK) 1/2 were increased during the early phase until 1 h after the start of treatment and thereafter decreased, and increased again in the late phase [43]. These observations suggest that the miR-143/ERK5/ c-Myc pathway and mitochondrial factors endonuclease-G may be involved in α-mangostin-induced apoptosis. These findings provide evidence for unique mechanisms of α-mangostin-induced apoptosis. Notably, Nakagawa et al. reported that xanthones not only stimulated caspase-3 activation but also simultaneously induced a marked reduction in iNOS expression and NO production, which was previously reported to play an anti-apoptotic role in B-cell chronic lymphocytic leukemia (B-CLL) cells [32]. These observations indicate that xanthones could contribute to apoptosis via the NO pathway. Recently, it was reported that combined treatment with gartanin and tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) resulted in a potential sensitization of TRAIL-resistant human gastric adenocarcinoma cells [72]. This result reveals the specificity of this molecule in its ability to exert its activity through different pathways in different types of cancer cells (Fig. 4).
Fig. 4.
Schematic diagram shows the possible effect of xanthones on the apoptosis pathways. Xanthones induce apoptosis occurrence, preferentially activate the mitochondrial pathway, support intracellular ATP decrease, cytochrome c/AIF release, caspase-9 and caspase-3 activation, endonuclease-G release. Furthermore, xanthones also influence cancer cells apoptosis via miR-143/ERK5/c-Myc pathway, NO inhibition, cell-cycle arrest, sarco-endoplasmic reticulum Ca2+-ATPase inhibition, and intracellular ROS accumulation.
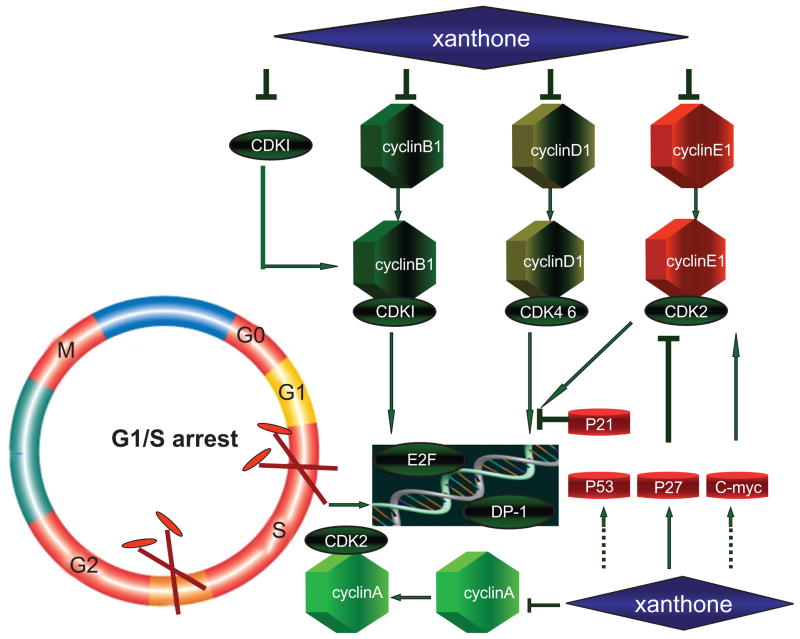
Induction of Cancer Cell Cycle Arrest
It is well known that the cell cycle is normally regulated by a number of proteins, including p53, p21waf, the cyclin-dependent kinases (cdks) and their activators, the cyclins. The dysregulation of cell cycle machinery and checkpoint signaling pathways is a hallmark of malignant cells [73, 74]. Thus, modulation of cell cycle progression is one of the major strategies for both chemoprevention and chemotherapy. Treatment of mangosteen results in a direct inhibition of the proliferation and viability of various cancer cell types in vitro, as manifested by the significant arrest of cells at various phases of the cell cycle [23]. Matsumoto et al. demonstrated that the antiproliferative effects of four structurally similar prenylated xanthones, α-mangostin, β-mangostin, γ-mangostin, and methoxy- β-mangostin, were associated with cell cycle arrest mediated by modulation of the expression of cyclin A, B1, D1, E1, cdc2 or p27 in human colon cancer DLD-1 cells. The exposure of α-mangostin and β-mangostin to DLD-1 cells resulted in G1 arrest, and treatment of γ-mangostin led to S arrest [42]. These findings provide a rational basis for the development of xanthones as agents for cancer prevention or for use in combination with anti-cancer drugs; however, further experiments are required to explore the precise molecular mechanisms underlying the observed induction of cell cycle arrest (Fig. 5).
Fig. 5.
An overview: how xanthones induce cell-cycle arrest. Xanthones block the cell cycle by activation or inhibition of cyclins, cdks, inhibitor of cdks, transcription factors or oncoproteins in cancer cells.
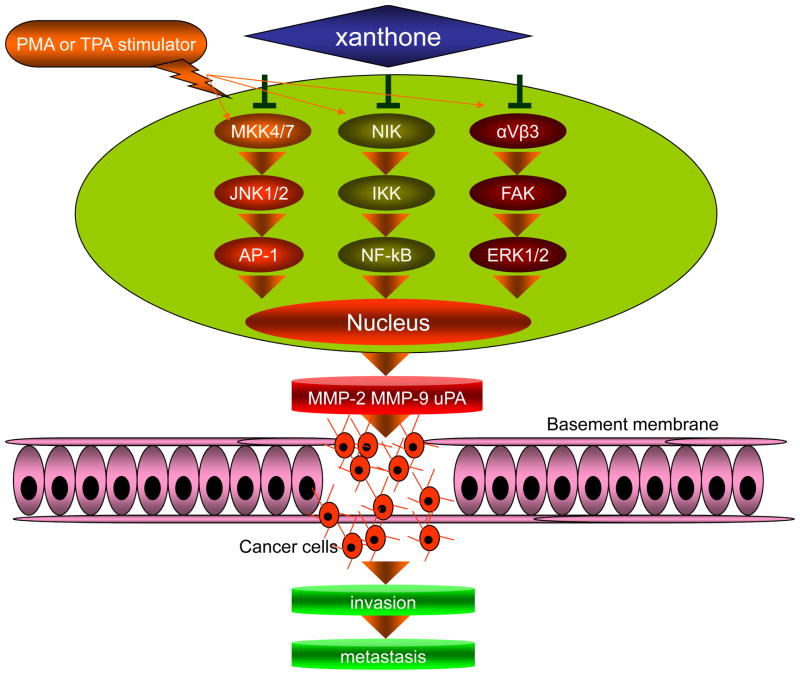
Anti-Invasive and Anti-Metastatic Effects
Metastasis of cancer cells is a complex, multistage process that involves changes in cell adhesion, migration, invasion, rearrangement of the extracellular matrix (ECM), anoikis-suppression and reorganization of cytoskeletons [75–78]. Using a cell-matrix adhesion assay, wound healing assay, and boyden chamber assay, Hung et al. were the first to report that α-mangostin exhibited an inhibitory effect on adhesion, migration, and invasion of human prostate carcinoma cells (PC-3). This effect was associated with decreased expression of matrix metalloproteinase-2 (MMP-2), matrix metalloproteinase-9 (MMP-9), and urokinase-plasminogen activator (u-PA) mediated by suppression of the JNK1/2 signaling pathway and inhibition of NF-κB and AP-1 binding activity [25]. In two similar studies using human breast adenocarcinoma cells (MCF-7) and human lung adenocarcinoma cells (A549), it has been demonstrated that α-mangostin could inhibit 12-O-tetradecanoylphorbol-13-acetate (TPA)- and phorbol 12-myristate 13-acetate (PMA)-induced cell adhesion, invasion, and migration events [80, 81]. The results indicated that α-mangostin could inhibit the activation of extracellular signal-regulated kinase 1 and 2 (ERK1/2), and downregulate the enzyme activities, protein, and messenger RNA levels of MMP-2 and MMP-9; this compound could also inhibit the degradation of inhibitor of kappaBα (IκBα) and the nuclear levels of nuclear factor kappa B (NF-κB), c-Fos, and c-Jun [79, 80]. A study in human lung adenocarcinoma A549 cells revealed that ERK1/2 inhibition occurred via blocking the activation of αvβ3 integrin and focal adhesion kinase (FAK). FAK is a non-receptor tyrosine kinase that is primarily localized to cell–matrix adhesions. It acts as a central regulator of focal adhesion, influencing cell survival, differentiation, proliferation, migration, and tissue remodeling. Briefly, α-mangostin is a novel, effective, antimetastatic agent that functions in regulating MMP-2 and MMP-9 gene expression. Promisingly, the merely in vivo study about anti-metastatic activity of xanthones had been progressed in a BALB/c mice model with breast cancer cell, BJMC3879. The results showed that lung and Lymph node metastasis tended to decrease using 5,000 ppm panaxanthone in their diet. These results suggest that the observed anti-metastatic activity of xanthone may be of clinical significance as adjuvant therapy in metastatic human breast cancer [39]. Although the anti-invasive effect of xanthones has been observed, the underlying mechanism in the invasion process remains unclear. It is known that tumor growth is dependent upon angiogenesis and that for the process of metastasis to successfully occur to different organs, tumor cells must possess sufficient blood supply. Although the effect of xanthones exposure on angiogenesis and the regulation of HIF-1a and VEGF have yet to be reported, these areas warrant investigation (Fig. 6).
Fig. 6.
Simplified model showed potential contributions of xanthones to cancer invasion and metastasis. Some external stimulator (PMA, TPA) induce the cell-matrix adhesion, invasion, and migration of cancer cells by upregulating MAPK kinases such as JNK or ERK1/2. Xanthones diminish the above induced effect, prevent NF-κB and AP-1 binding activity, and block their DNA binding site. Consequently, the expression of downstream genes (MMP-2, MMP-9, and u-PA) is downregulated.
CONCLUSION
Carcinogenesis prevention is considered to be a promising alternative strategy for the treatment of cancer. In recent years, many naturally occurring substances have demonstrated protection against experimental carcinogenesis. Based on this information, this review presents compelling evidence for the use of mangosteen not only to prevent but also to treat cancer due to the similar molecular targets that affect tumor initiation, promotion, and progression. Taken together, these results support that mangosteen can modulate various molecular pathways involved in multiple processes of carcinogenesis including the inactivation of carcinogens, the induction of apoptosis, the initiation of cell cycle arrest, and the suppression of metastasis. It may be used in combination with other chemotherapeutic agents as adjuvant therapies to achieve increased therapeutic efficacy and minimize chemotherapy-induced toxicity. Although there is compelling evidence to suggest that xanthones from mangosteen may be a remarkable candidate for chemopreventive and chemotherapeutic strategies due to its efficacy and pharmacological safety, further research must be conducted before the compounds can be employed as an agent for the chemoprevention/treatment of cancer. Extensive animal studies, long-term epidemiologic studies, and controlled clinical trials are necessary to evaluate safety and chemopreventive efficacy of mangosteen either alone or in combination with additional chemotherapeutic agents. Here, we emphasize that specific xanthone derivatives in future trials is important. α-mangostin, is the most widespread studied and exhibited the highest activity against breast cancer, human leukemia, lung cancer, pheochromocytoma, and colorectal carcinoma. Therefore, α-mangostin should be preferentially used to study for these cancers in future clinical trials. Garcinone E, the most toxic on hepatocellular carcinoma cell lines [24], was valid against gastric carcinoma cell lines [24], indicating it is good to use in alimentary system tumor trials. However, these are needed to be further explored clinically.
Acknowledgments
We wish to extend our thanks to Dr. Matthew Warner (North Dakota State University) for his thoughtful reading of the manuscript. Our research is supported by Major Scientific Grand of Shaanxi 13115 (2010ZDKG-49) (Q. Ma) and Pilot Project Grant (E. Wu) from the Centers of Biomedical Research Excellence (COBRE) grant NIH P20 RR020151 from the National Center for Research Resources (NCRR). NCRR is a component of the National Institutes of Health (NIH). The contents of this report are solely the responsibility of the authors and do not necessarily reflect the official views of the NIH or NCRR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kullmann F, Hollerbach S, Dollinger MM, et al. Cetuximab plus gemcitabine/oxaliplatin (gemoxcet) in first-line metastatic pancreatic cancer: a multicentre phase II study. Brit J Cancer. 2009;100(7):1032–6. doi: 10.1038/sj.bjc.6604983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang ZW, Li YW, Ahmad A, et al. Pancreatic cancer: understanding and overcoming chemoresistance. Nature Reviews Gastroenterology & Hepatology. 2011;8(1):27–33. doi: 10.1038/nrgastro.2010.188. [DOI] [PubMed] [Google Scholar]
- 3.Reddy LH, Marque PE, Dubernet C, et al. Preclinical toxicology (subacute and acute) and efficacy of a new squalenoyl gemcitabine anticancer nanomedicine. J Pharmacol Exp Ther. 2008;325(2):484–90. doi: 10.1124/jpet.107.133751. [DOI] [PubMed] [Google Scholar]
- 4.Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: A randomized, multicenter, phase III trial of the Swiss group for clinical cancer research and the central European cooperative oncology group. J Clin Oncol. 2007;25(16):2212–7. doi: 10.1200/JCO.2006.09.0886. [DOI] [PubMed] [Google Scholar]
- 5.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3(10):768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 6.Gordaliza M. Natural products as leads to anticancer drugs. Clin & Trans Oncol. 2007;9(12):767–76. doi: 10.1007/s12094-007-0138-9. [DOI] [PubMed] [Google Scholar]
- 7.Erbersdobler H. What role do dietary factors play in cancer prevention? Ernahrungsumschau. 2008;55(10):606–7. [Google Scholar]
- 8.Williams MT, Hord NG. The role of dietary factors in cancer prevention: beyond fruits and vegetables. Nutr Clin Pract. 2005;20(4):451–9. doi: 10.1177/0115426505020004451. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura S, Qu Y, Xu F, et al. Structures of new monoterpenes from Thai herbal medicine curcuma comosa. Chem & Pharm Bull. 2008;56(11):1604–6. doi: 10.1248/cpb.56.1604. [DOI] [PubMed] [Google Scholar]
- 10.Mahabusarakam W, Wiriyachitra P, Phongpaichit S. Antimicrobial activities of chemical -constituents from garcinia-mangostana linn. J Sci Soci Thai. 1986;12(4):239–43. [Google Scholar]
- 11.Balasubramanian K, Rajagopalan K. Novel xanthones from garcinia-mangostana, structures of xanthone-A and xanthone-B. Phytochemistry. 1988;27(5):1552–4. [Google Scholar]
- 12.Chomnawang MT, Surassmo S, Nukoolkarn VS, et al. Antimicrobial effects of Thai medicinal plants against acne-inducing bacteria. J Ethnopharmacology. 2005;101(1–3):330–3. doi: 10.1016/j.jep.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 13.Kondo M, Zhang LL, Ji HP, et al. Bioavailability and antioxidant effects of a xanthone-rich mangosteen (garcinia mangostana) product in humans. J Agr Food Chem. 2009;57(19):8788–92. doi: 10.1021/jf901012f. [DOI] [PubMed] [Google Scholar]
- 14.Yeung S. Mangosteen for the cancer patient: facts and myths. J Soc Integr Oncol. 2006;4(3):130–4. doi: 10.2310/7200.2006.022. [DOI] [PubMed] [Google Scholar]
- 15.Marcason W. What are the facts and myths about mangosteen? J Am Diet Assoc. 2006;106(6):986. doi: 10.1016/j.jada.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Foote JC. Protecting consumers in the wake of the U.S. dietary supplement health and education act. J Allied Health. 2007;36(1):57–60. [PubMed] [Google Scholar]
- 17.Garrity AR, Morton GA, Morton JC, et al. Nutraceutical mangosteen composition. 1US 6730333 Official gazette of the United States patent and trademark office patents. 2004:1282.
- 18.Ilyas M, Kamil M, Parveen M, et al. Isoflavones from garcinia-nervosa. Phytochemistry. 1994;36(3):807–9. [Google Scholar]
- 19.Suksamrarn S, Komutiban O, Ratananukul P, et al. Cytotoxic prenylated xanthones from the young fruit of Garcinia mangostana. Chem Pharm Bull. 2006;54(3):301–5. doi: 10.1248/cpb.54.301. [DOI] [PubMed] [Google Scholar]
- 20.Steinmetz KA, Potter JD. Vegetables, fruit, and cancer prevention: A review. J Am Diet Assoc. 1996;96(10):1027–39. doi: 10.1016/S0002-8223(96)00273-8. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Liu RH, Halim L. Antioxidant and antiproliferative activities of common edible nut seeds. Lwt-Food Sci Technol. 2009;42(1):1–8. [Google Scholar]
- 22.Sun J, Chu YF, Wu XZ, et al. Antioxidant and anti proliferative activities of common fruits. J Agr Food Chem. 2002;50(25):7449–54. doi: 10.1021/jf0207530. [DOI] [PubMed] [Google Scholar]
- 23.Akao Y, Nakagawa Y, Iinuma M, et al. Anti-cancer effects of xanthones from pericarps of mangosteen. Int J Mol Sci. 2008;9(3):355–70. doi: 10.3390/ijms9030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedraza-Chaverri J, Cardenas-Rodriguez N, Orozco-Ibarra M, et al. Medicinal properties of mangosteen (garcinia mangostana) Food Chem Toxicol. 2008;46(10):3227–39. doi: 10.1016/j.fct.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Hung SH, Shen KH, Wu CH, et al. Alpha-Mangostin suppresses PC-3 Human prostate carcinoma cell metastasis by inhibiting Matrix Metalloproteinase-2/9 and Urokinase-Plasminogen expression through the JNK signaling pathway. J Agr Food Chem. 2009;57(4):1291–8. doi: 10.1021/jf8032683. [DOI] [PubMed] [Google Scholar]
- 26.Sakai S, Katsura M, Takayama H, et al. The structure of garcinone-E. Chem Pharm Bull. 1993;41(5):958–60. [Google Scholar]
- 27.Matsumoto K, Akao Y, Kobayashi E, et al. Induction of apoptosis by xanthones from mangosteen in human leukemia cell lines. J Nat Prod. 2003;66(8):1124–7. doi: 10.1021/np020546u. [DOI] [PubMed] [Google Scholar]
- 28.Moongkarndi P, Kosem N, Kaslungka S, et al. Antiproliferation, antioxidation and induction of apoptosis by garcinia mangostana (mangosteen) on SKBR3 human breast cancer cell line. J Ethnopharmacology. 2004;90(1):161–6. doi: 10.1016/j.jep.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 29.Balunas MJ, Su B, Brueggemeier RW, et al. Xanthones from the botanical dietary supplement mangosteen (Garcinia mangostana) with aromatase inhibitory activity. J Nat Prod. 2008;71(7):1161–6. doi: 10.1021/np8000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato A, Fujiwara H, Oku H, et al. Alpha-Mangostin induces Ca2+-ATPase-dependent apoptosis via mitochondrial pathway in PC12 cells. J Pharmacol Sci. 2004;94:138P. doi: 10.1254/jphs.95.33. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto K, Akao Y, Yi H, et al. Preferential target is mitochondria in alpha-mangostin-induced apoptosis in human leukemia HL60 cells. Bioorg Med Chem. 2004;12(22):5799–806. doi: 10.1016/j.bmc.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 32.Menasria F, Azebaze A, Billard C, et al. Apoptotic effects on B-cell chronic lymphocytic leukemia (B-CLL) cells of heterocyclic compounds isolated from guttiferaes. Leukemia Res. 2008;32(12):1914–26. doi: 10.1016/j.leukres.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Han A, Kim J, Lantvit DD, et al. Cytotoxic xanthone constituents of the stem bark of garcinia mangostana (mangosteen) J Nat Prod. 2009;72(11):2028–31. doi: 10.1021/np900517h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kikuchi H, Ohtsuki T, Koyano T, et al. Activity of mangosteen xanthones and teleocidin A-2 in death receptor expression enhancement and tumor necrosis factor related apoptosis-inducing ligand assays. J Nat Prod. 2010;73 (3):452–5. doi: 10.1021/np900404e. [DOI] [PubMed] [Google Scholar]
- 35.Suksamrarn S, Komutiban O, Ratananukul P, et al. Cytotoxic prenylated xanthones from the young fruit of Garcinia mangostana. Chem Pharm Bull. 2006;54(3):301–5. doi: 10.1248/cpb.54.301. [DOI] [PubMed] [Google Scholar]
- 36.Ee GCL, Daud S, Izzaddin SA, et al. Garcinia mangostana: a source of potential anti-cancer lead compounds against CEM-SS cell line. J Asian Nat Prod Res. 2008;10(5–6):475–9. doi: 10.1080/10286020801948490. [DOI] [PubMed] [Google Scholar]
- 37.Nabandith V, Suzui M, Morioka T, et al. Inhibitory effects of crude alpha-mangostin, a xanthone derivative, on two different categories of colon preneoplastic lesions induced by 1, 2-dimethylhydrazine in the rat. Asian Pac J Cancer Prev. 2004;5(4):433–8. [PubMed] [Google Scholar]
- 38.Jung HA, Su BN, Keller WJ, et al. Antioxidant xanthones from the pericarp of Garcinia mangostana (mangosteen) J Agr Food Chem. 2006;54(6):2077–82. doi: 10.1021/jf052649z. [DOI] [PubMed] [Google Scholar]
- 39.Watanapokasin R, Jarinthanan F, Jerusalmi A, et al. Potential of xanthones from tropical fruit mangosteen as anti-cancer agents: caspase-dependent apoptosis induction in vitro and in mice. Appl Biochem Biotech. 2010;162(4):1080–94. doi: 10.1007/s12010-009-8903-6. [DOI] [PubMed] [Google Scholar]
- 40.Doi H, Shibata MA, Shibata E, et al. Panaxanthone isolated from pericarp of garcinia mangostana L. suppresses tumor growth and metastasis of amouse model of mammary cancer. Anticancer Res. 2009;29(7):2485–95. [PubMed] [Google Scholar]
- 41.Laphookhieo S, Syers JK, Kiattansakul R, et al. Cytotoxic and antimalarial prenylated xanthones from cratoxylum cochinchinense. Chem Pharm Bull. 2006;54(5):745–7. doi: 10.1248/cpb.54.745. [DOI] [PubMed] [Google Scholar]
- 42.Han A, Kim J, Lantvit DD, et al. Cytotoxic xanthone constituents of the stem bark of Garcinia mangostana (mangosteen) J Nat Prod. 2009;72(11):2028–31. doi: 10.1021/np900517h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakagawa Y, Iinuma M, Naoe T, et al. Characterized mechanism of alpha-mangostin-induced cell death: Caspase-independent apoptosis with release of endonuclease-G from mitochondria and increased miR-143 expression in human colorectal cancer DLD-1 cells. Bioorg Med Chem. 2007;15(16):5620–8. doi: 10.1016/j.bmc.2007.04.071. [DOI] [PubMed] [Google Scholar]
- 44.Kundu JK, Surh YJ. Cancer chemopreventive and therapeutic potential of resveratrol: Mechanistic perspectives. Cancer Lett. 2008;269(2):243–61. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 45.Delmas D, Lancon A, Colin D, et al. Resveratrol as a chemopreventive agent: A promising molecule for fighting cancer. Curr Drug Targets. 2006;7(4):423–42. doi: 10.2174/138945006776359331. [DOI] [PubMed] [Google Scholar]
- 46.Chin WY, Jung HA, Chai H, et al. Xanthones with quinone reductase-inducing activity from the fruits of Garcinia mangostana (Mangosteen) Phytochemistry. 2008;69:754–8. doi: 10.1016/j.phytochem.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 47.Foti RS, Pearson JT, Rock DA, et al. In vitro inhibition of multiple cytochrome P450 isoforms by xanthone derivatives from mangosteen extract. Drug Metab Dispos. 2009;37(9):1848–55. doi: 10.1124/dmd.109.028043. [DOI] [PubMed] [Google Scholar]
- 48.Hursting SD, Slaga TJ, Fischer SM, et al. Mechanism-based cancer prevention approaches: Targets, examples, and the use of transgenic mice. J Natl Cancer I. 1999;91(3):215–25. doi: 10.1093/jnci/91.3.215. [DOI] [PubMed] [Google Scholar]
- 49.Shukla Y, Pal SK. Dietary cancer chemoprevention: An overview. International Journal of Human Genetics. 2004;4(4):265–76. [Google Scholar]
- 50.Abdi S, Ali A. Role of ROS modified human DNA in the pathogenesis and etiology of cancer. Cancer Lett. 1999;142(1):1–9. doi: 10.1016/s0304-3835(99)00112-3. [DOI] [PubMed] [Google Scholar]
- 51.Mates JM, Sanchez-Jimenez FM. Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int J Biochem Cell B. 2000;32(2):157–70. doi: 10.1016/s1357-2725(99)00088-6. [DOI] [PubMed] [Google Scholar]
- 52.Weisburger JH. Mechanisms of action of antioxidants as exemplified in vegetables, tomatoes and tea. Food Chem Toxicol. 1999;37(9–10):943–8. doi: 10.1016/s0278-6915(99)00086-1. [DOI] [PubMed] [Google Scholar]
- 53.Leong LP, Shui G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002;76(1):69–75. [Google Scholar]
- 54.Weecharangsan W, Opanasopit P, Sukma M, et al. Antioxidative and neuroprotective activities of extracts from the fruit hull of mangosteen (Garcinia mangostana Linn. ) Med Prin Pract. 2006;15(4):281–7. doi: 10.1159/000092991. [DOI] [PubMed] [Google Scholar]
- 55.Moongkarndi P, Srisawat C, Saetun P, et al. Protective effect of mangosteen extract against beta-amyloid-induced cytotoxicity, oxidative stress and altered proteome in SK-N-SH cells. J Proteome Res. 2010;9(5):2076–86. doi: 10.1021/pr100049v. [DOI] [PubMed] [Google Scholar]
- 56.Chin YW, Kinghorn AD. Structural characterization, biological effects, and synthetic studies on xanthones from mangosteen (Garcinia mangostana), a popular botanical dietary supplement. Mini-rev Org Chem. 2008;5(4):355–64. doi: 10.2174/157019308786242223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shankaranarayan D, Gopalakrishnan C, Kameswaran L. Pharmacological profile of mangostin and its derivatives. Archives internationales de pharmacodynamie et de therapie. 1979;239(2):257–69. [PubMed] [Google Scholar]
- 58.Gopalakrishnan C, Shankaranarayanan D, Kameswaran L, et al. Effect of mangostin, a xanthone from garcinia-mangostana Linn in immunopathological and inflammatory reactions. Indian J Exp Biol. 1980;18(8):843–6. [PubMed] [Google Scholar]
- 59.Chairungsrilerd N, Furukawa K, Ohta T, et al. gamma-Mangostin, a novel type of 5-hydroxytryptamine 2A receptor antagonist. N-S Arch Pharmacol. 1998;357(1):25–31. doi: 10.1007/pl00005134. [DOI] [PubMed] [Google Scholar]
- 60.Chairungsrilerd N, Furukawa KI, Ohta T, et al. Pharmacological properties of alpha-mangostin, a novel histamine H-1 receptor antagonist. Euro J Pharmacol. 1996;314(3):351–6. doi: 10.1016/s0014-2999(96)00562-6. [DOI] [PubMed] [Google Scholar]
- 61.Itoh T, Ohguchi K, Iinuma M, et al. Inhibitory effect of xanthones isolated from the pericarp of garcinia mangostana L. on rat basophilic leukemia RBL-2H3 cell degranulation[J] Bioorgan Med Chem. 2008;16(8):4500–8. doi: 10.1016/j.bmc.2008.02.054. [DOI] [PubMed] [Google Scholar]
- 62.Nakatani K, Nakahata N, Arakawa T, et al. Inhibition of cyclooxygenase and prostaglandin E-2 synthesis by gamma-mangostin, a xanthone derivative in mangosteen, in C6 rat glioma cells. Biochem Pharmacol. 2002;63(1):73–9. doi: 10.1016/s0006-2952(01)00810-3. [DOI] [PubMed] [Google Scholar]
- 63.Nakatani K, Yamakuni T, Kondo N, et al. gamma-Mangostin inhibits inhibitor-kappa B kinase activity and decreases lipopolysaccharide-induced cyclooxygenase-2 gene expression in C6 rat glioma cells. Mol Pharmacol. 2004;66(3):667–74. doi: 10.1124/mol.104.002626. [DOI] [PubMed] [Google Scholar]
- 64.Yamakuni T, Aoki K, Nakatani K, et al. Garcinone B reduces prostaglandin E-2 release and NF-kappa B-mediated transcription in C6 rat glioma cells. Neurosci Lett. 2006;394(3):206–10. doi: 10.1016/j.neulet.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 65.Chen YC, Chen KT. Novel selective inhibitors of hydroxyxanthone derivatives for human cyclooxygenase-2. Acta Pharmacol Sini. 2007;28(12):2027–32. doi: 10.1111/j.1745-7254.2007.00663.x. [DOI] [PubMed] [Google Scholar]
- 66.Teixeira M, Cerqueira F, Barbosa CM, et al. Improvement of the inhibitory effect of xanthones on NO production by encapsulation in PLGA nanocapsules. J Drug Target. 2005;13(2):129–35. doi: 10.1080/10611860400027717. [DOI] [PubMed] [Google Scholar]
- 67.Jabit ML, Wahyuni FS, Khalid R, et al. Cytotoxic and nitric oxide inhibitory activities of methanol extracts of Garcinia species. Pharm Biol. 2009;47(11):1019–26. [Google Scholar]
- 68.Sampath PD, Vijayaragavan K. Ameliorative prospective of alpha-mangostin, a xanthone derivative from garcinia mangostana against beta-adrenergic cathecolamine-induced myocardial toxicity and anomalous cardiac TNF-alpha and COX-2 expressions in rats. Exp Toxicol Pathol. 2008;60(4–5):357–64. doi: 10.1016/j.etp.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 69.Tewtrakul S, Wattanapiromsakul C, Mahabusarakam W. Effects of compounds from Garcinia mangostana on inflammatory mediators in RAW264. 7 macrophage cells. Journal of Ethnopharmacology. 2009;121(3):379–82. doi: 10.1016/j.jep.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 70.Bumrungpert A, Kalpravidh RW, Chitchmroonchokchai C, et al. Xanthones from mangosteen prevent lipopolysaccharide-mediated inflammation and insulin resistance in primary cultures of human adipocytes. J Nutrition. 2009;139(6):1185–1191. doi: 10.3945/jn.109.106617. [DOI] [PubMed] [Google Scholar]
- 71.Chang HF, Huang WT, Chen HJ, et al. Apoptotic effects of γ-mangostin from the fruit hull of garcinia mangostana on human malignant glioma cells. Molecules. 2010;15:8953–66. doi: 10.3390/molecules15128953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kikuchi H, Ohtsuki T, Koyano T, et al. Activity of mangosteen xanthones and teleocidin A-2 in death receptor expression enhancement and tumor necrosis factor related apoptosis-inducing ligand assays. J Nat Prod. 2010;73(3):452–5. doi: 10.1021/np900404e. [DOI] [PubMed] [Google Scholar]
- 73.Collins I, Garrett MD. Targeting the cell division cycle in cancer: CDK and cell cycle checkpoint kinase inhibitors. Curr Opin Pharmacol. 2005;5(4):366–73. doi: 10.1016/j.coph.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 74.Gali-Muhtasib H, Bakkar N. Modulating cell cycle: current applications and prospects for future drug development. Curr Cancer Drug Targets. 2002;2(4):309–36. doi: 10.2174/1568009023333809. [DOI] [PubMed] [Google Scholar]
- 75.Brabek J, Mierke CT, Rosel D, et al. The role of the tissue microenvironment in the regulation of cancer cell motility and invasion. Cell Commu Sign. 2010:8. doi: 10.1186/1478-811X-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kargiotis O, Geka A, Rao JS, et al. Effects of irradiation on tumor cell survival, invasion and angiogenesis. J Neuro-Oncol. 2010;100(3):323–38. doi: 10.1007/s11060-010-0199-4. [DOI] [PubMed] [Google Scholar]
- 77.Sato H, Takino T. Coordinate action of membrane-type matrix metalloproteinase-1 (MT1-MMP) and MMP-2 enhances pericellular proteolysis and invasion. Cancer Sci. 2010;101(4):843–7. doi: 10.1111/j.1349-7006.2010.01498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mareel M, Oliveira MJ, Madani I. Cancer invasion and metastasis: interacting ecosystems. Virchows Arch. 2009;454(6):599–622. doi: 10.1007/s00428-009-0784-0. [DOI] [PubMed] [Google Scholar]
- 79.Lee YB, Ko KC, Shi MD, et al. Alpha-Mangostin, a novel dietary xanthone, suppresses TPA-mediated MMP-2 and MMP-9 expressions through the ERK signaling pathway in MCF-7 human breast adenocarcinoma Cells. J Food Sci. 2010;75(1):H13–H23. doi: 10.1111/j.1750-3841.2009.01407.x. [DOI] [PubMed] [Google Scholar]
- 80.Shih YW, Chien ST, Chen PS, et al. Alpha-Mangostin suppresses phorbol 12-myristate 13-acetate-induced MMP-2/MMP-9 expressions via alphavbeta3 integrin/FAK/ERK and NF-kappa B signaling pathway in human lung adenocarcinoma A549 cells. Cell Bio Bioph. 2010;58(1):31–44. doi: 10.1007/s12013-010-9091-2. [DOI] [PubMed] [Google Scholar]