Abstract
Nephrin, an important structural and signal molecule of podocyte slit-diaphragm (SD), has been suggested to contribute to the angiotensin II (Ang II)-induced podocyte injury. Caveolin-1 has been demonstrated to play a crucial role in signaling transduction. In the present study, we evaluated the role of caveolin-1 in Ang II-induced nephrin phosphorylation in podocytes. Wistar rats-receiving either Ang II (400 ng/kg/min) or normal saline (via subcutaneous osmotic mini-pumps, control) were administered either vehicle or telmisartan (3 mg/kg/min) for 14 or 28 days. Blood pressure, 24-hour urinary albumin and serum biochemical profile were measured at the end of the experimental period. Renal histomorphology was evaluated through light and electron microscopy. In vitro, cultured murine podocytes were exposed to Ang II (10−6 M) pretreated with or without losartan (10−5 M) for variable time periods. Nephrin and caveolin-1 expression and their phosphorylation were analyzed by Western-blotting and immunofluorescence. Caveolar membrane fractions were isolated by sucrose density gradient centrifugation, and then the distribution and interactions between Ang II type 1 receptor (AT1), nephrin, C-terminal Src kinase (Csk) and caveolin-1 were evaluated using Western-blotting and co-immunoprecipitation. Podocyte apoptosis was evaluated by cell nucleus staining with Hoechst-33342.
Ang II-receiving rats displayed diminished phosphorylation of nephrin but enhanced glomerular/podocyte injury and proteinuria when compared to control rats. Under control conditions, podocyte displayed expression of caveolin-1 in abundance but only a low level of phospho moiety. Nonetheless, Ang II stimulated caveolin-1 phosphorylation without any change in total protein expression. Nephrin and caveolin-1 were co-localized in caveolae fractions. AT1 receptors and Csk were moved to caveolae fractions and had an interaction with caveolin-1 after the stimulation with Ang II. Transfection of caveolin-1 plasmid (pEGFPC3-cav-1) significantly increased Ang II-induced nephrin dephosphorylation and podocyte apoptosis. Furthermore, knockdown of caveolin-1 expression (using siRNA) inhibited nephrin dephosphorylation and prevented Ang II-induced podocyte apoptosis. These findings indicate that Ang II induces nephrin dephosphorylation and podocyte injury through a caveolin-1-dependent mechanism.
Keywords: Caveolin-1, Podocyte, Angiotensin II, Nephrin
1. Introduction
Podocytes and slit diaphragm (SD) play a critical role in the maintenance of glomerular filtration barrier (GFB) [1]. Proteinuric glomerulopathies, including focal segmental glomerulosclerosis (FSGS) [2], minimal change disease (MCD) [3] and diabetic nephropathy [4], display by podocyte injury in the form of foot process effacement. In addition to leaky GFB, glomerular hypertension as a consequence of the activated renin-angiotensin-aldosterone system (RAAS) was implicated for enhanced proteinuria [5–7]. During the last decade, the direct effect of Ang II in the induction of podocyte injury is being recognized increasingly [8]. The multiple effects of Ang II are mediated predominantly through AT1 receptor, which is coupled to heterotrimeric G proteins and involved in different second-messenger signal transduction pathways [9]. Our previous studies have demonstrated that Ang II induced both podocyte injury and proteinuria [10]. However, the involved signaling mechanisms in Ang II-induced podocyte injury are not fully delineated.
Nephrin, known as the predominant molecule of slit diaphragm (SD), plays a key role in the maintenance of the GFB [11]. Nephrin belongs to the immunoglobulin superfamily of cell adhesion molecules and forms a zipper-like lattice structure in the slit diaphragm [11]. Six conservative tyrosine phosphorylation sites have been found in the intracellular domain of nephrin [12], which are phosphorylated by the Src family kinase (SFK) Fyn [12,13]. The phosphorylation events of nephrin are thought to be important for the survival status of podocytes and rearrangement of cytoskeletal proteins [14]. Some recent reports have demonstrated that Ang II can influence the nephrin expression, but the effects on phosphorylation of nephrin remain debated.
Caveolin-1 is a key component of caveolae, which are 50–100 nm membrane invaginations and thought to play important roles in cell signaling transduction. Multiple signaling molecules are located in caveolae and their functions are modified by caveolin-1 [15]. Phosphorylation of caveolin-1 on tyrosine 14 may function to facilitate caveolin-1 interaction with other proteins in a stimulus-specific fashion [16,17]. Fyn has been found residing in podocyte caveolae, in which nephrin, podocin, CD2AP, and Neph1 are also found [18,19]. Here, we study the role of caveolin-1 in podocytes and hypothesize that caveolin-1 is participating in Ang II-nephrin signaling in podocytes.
2. Materials and methods
2.1. Animals
Thirty-six male SPF Wistar rats weighing between 140 and 160 g were purchased from Hubei Research Center of Experimental Animals and were maintained at a controlled temperature (23±2 °C) and humidity (55±5%) under an artificial light cycle, with a free access to tap water and standard rat chow. Embedded with osmotic mini-pump (Alzet model 2002 or 2004, CA), rats were randomly subjected to normal saline infusion, or Ang II infusion at 400 ng/kg/min, or Ang II at 400 ng/kg/min+telmisartan at 3 mg/kg/day by means of intragastric administration for 14 or 28 days. Systolic blood pressure was measured by tail cuff plethysmography in conscious, trained, and preheated rats. 24-hour urine was collected in metabolic cages and urinary albumin excretion rate was measured at days 7, 14, 21, and 28. Animals were sacrificed at days 14 and 28, and the blood was collected for serum creatinine, Ang II concentration analysis. Kidneys were infused by vanadate (a phosphatase inhibitor) before isolated and stored at −80 °C for biochemical and renal pathological analysis.
2.2. Cell culture
A conditionally immortalized murine podocytes were kindly provided by Dr. Peter Mundel (Mount Sinai School of Medicine, New York). Podocytes were maintained in RPMI 1640 medium (HyClone, USA) containing 10% heat-inactivated fetal calf serum (Gibco, USA), 100 U/mL penicillin G, and 100 μg/mL streptomycin in an incubator with 5% CO2. During podocyte proliferation, the medium was mixed with 10 U/mL recombinant mouse interferon-γ (Sigma, USA), and the cells were maintained at 33 °C. Then podocytes were cultured at 37 °C to induce differentiation without interferon-γ for 10–14 days. 15–25 passages of podocytes were used in the following experiments. All experiments were performed in differentiated podocytes.
2.3. Isolation of caveolar membrane fractions
Cells were washed in PBS, lysed in MES-buffered saline (MBS, 25 mM Mes [pH 6.5] and 150 mM NaCl) with 1% Triton X-100 and protease/phosphatase inhibitors, then solubilized by 10 passes through a 25-G needle and sonicated for 15 s on ice. Samples were equalized for protein and mixed with equal volume of 90% sucrose in MBS, then placed above with 35, 30, 25, and 5% sucrose in MBS, and centrifuged at 100,000×g for 16 h at 4 °C. The caveolae fraction appeared at the 5 to 25% interface. From the top of each gradient, 12 equal fractions were collected and recentrifuged at 34,000×g for 45 min, and separated on a 15% gel. Fractions 2 to 5 correspond to caveolae, as confirmed by immunoblotting for caveolin-1.
2.4. Western immunoblotting
Cells were lysed in RIPA buffer (150 mM sodium chloride, 1.0% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris, pH 8.0) with protease/phosphatase inhibitors, and centrifuged at 12,000 rpm for 20 min at 4 °C. Then the protein samples were mixed with loading buffer and boiled at 95–100 °C for 5 min. The proteins were separated on 8 to 12% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) and then transferred to nitrocellulose membrane (GE Healthcare) by semidry blotting. Nephrin Guinea pig monoclonal antibody (1:1000; Progen), nephrin rabbit Phospho (pY1217) monoclonal antibody (1:1000; Epitomics), AT1 (N-10) rabbit polyclonal antibody (1:100; Santa Cruz), β-actin mouse monoclonal antibody (1:1000; Santa Cruz), caveolin-1 rabbit monoclonal antibody (1:1000; Cell Signaling Technology), phospho-caveolin-1 (Tyr14) rabbit polyclonal antibody (1:1000; Cell Signaling Technology) and Csk rabbit monoclonal antibody were used as primary antibodies. Horseradish peroxidase-conjugated secondary antibodies (1:1000; Cell Signaling Technology) were used at 1:5000. Blots were visualized by the enhanced chemiluminescence reaction (Santa Cruz) and developed on the film.
2.5. Immunofluorescence assay
The frozen kidney sections were prepared using a cryostat and covered by bovine serum for 30 min at room temperature. The cell climbing film was fixed in 4% paraformaldehyde with 0.1% Triton X-100 for 30 min at 4 °C. Nephrin/phospho-nephrin or caveolin-1/phosphor-caveolin-1 antibodies were used as primary antibodies for overnight at 4 °C. FITC/TRITC-conjugated IgG was used as secondary antibodies at 37 °C for 90 min.
2.6. Co-immunoprecipitation
Co-immunoprecipitations were carried out as per the directions of Co-immunoprecipitation kit (Pierce, USA). Briefly, caveolin-1 rabbit mAb was covalently coupled onto an amine-reactive resin. The caveolae fractions prepared before were mixed with bait and diluted with IP Lysis/Wash Buffer. Then the mixture was incubated with the appropriate resin overnight at 4 °C. The spin columns were centrifuged and the flow-through was saved. Then the samples were mixed with 5× Lane Marker Sample Buffer to make a 1× final solution and heated at 95–100 °C for 5 min. The samples were separated by SDS-PAGE and analyzed by immunoblotting of AT1, nephrin and Csk antibodies.
2.7. Transfection of pEGFP-C3-cav-1
Total RNA was isolated from mouse kidney and the first-strand complementary DNA synthesis was carried out using a reverse Transcription System kit (Fermentas, USA). The open reading frame (ORF) of caveolin-1 (NM_007616) amplification was performed using PCR with special primer pairs (sense: 5′-CCGGAATTCCGATGTCTGGGGGCAAATACG-3′, anti-sense: 5′-CGCGGATCCGCGTCATATCTCTTTCTGCGTG-3′). The fragment containing the ORF of caveolin-1 was further ligated into the pEGFP-C3 vector, which was a generous gift from Dr. Zhao Ruiming (Wuhan University, China), at the EcoR I/BamH I site and the recombinant plasmid was named as pEGFP-C3-cav-1 [20]. pEGFP-C3-cav-1 or empty vectors were infected into podocytes using Lipo2000 (Invitrogen, USA). Then the podocytes were selected using G-418 (400 mg/L) to get stably transfected cells.
2.8. Transfection of cav-1 siRNA
Cav-1 siRNA transfection was performed according to the HiPerFect transfection reagent Handbook (QIAGEN, Germany). Briefly, 2×105 cells were seeded in 6-well plates and transfected with the complexes composed of 10 nmol/L cav-1 siRNA (QIAGEN, Germany) or a negative control scrambled siRNA provided by the manufacturer. The cells were incubated with the transfection complexes under their normal growth conditions for 48 h.
2.9. Apoptosis studies
Morphologic evaluation of podocyte apoptosis was performed by staining cells with Hoechst-33342 (Sigma, USA). Cells were prepared under control and experimental conditions by staining with Hoechst-33342 (1 μg/mL) at 37 °C for 5 min, then washed by cold PBS for three times. And the cells were observed under fluorescence microscope. Percentage of apoptotic cells was recorded in ten random fields in 3 wells for each variable. Three sets of experiments were carried out, each in quadruplicate.
2.10. Statistical analyses
All the data were presented as mean±SEM. Statistically significant differences in mean values were tested by analysis of variance. The differences at p<0.05 were considered statistically significant. The data were analyzed using SPSS 13.0.
3. Results
3.1. Clinical and pathological parameters
It has been well accepted that Ang II contributes to the progression of glomerular injury through its hemodynamic and/or non-hemodynamic effects. To assess the role of Ang II in glomerular and podocyte injury, we established a rat model of Ang II infusion. As shown in Table 1, systolic blood pressure and urinary albumin in Ang II-infused rats were higher than that in control and telmisartan treated groups (at the same time points). Ang II-infused rats showed an increase of Ang II concentration both in plasma and renal tissues when compared with control and telmisartan treated rats; however, there was no difference in creatinine clearance rate (Ccr) among the three groups (Table 2). These findings were consistent with our past observations [10].
Table 1.
Systolic blood pressure and urinary albumin excretion in different groups (n=6).
| Unit | Systolic blood pressure (mm Hg)
|
Urinary albumin (mg/24 h)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 day | 7 days | 14 days | 21 days | 28 days | 0 day | 7 days | 14 days | 21 days | 28 days | |
| Control | 99.7±1.53 | 98.26±1.67 | 99.1±1.33 | 102.8±4.25 | 103.47±4.39 | 12.7±4.3 | 15.7±4.98 | 13.8±5.6 | 16.8±5.3 | 17.2±7.9 |
| Ang II | 101.3±2.9 | 128.3±3.7* | 137.4±2.05* | 147.9±2.95* | 168.11±4.97* | 13±3 | 69.1±7.33* | 115.8±12.5* | 169.7±11.7* | 200.3±12.8* |
| Telmisartan +Ang II | 99.5±1.4 | 119.3±1.1*Δ | 120.3±2.6*Δ | 127.6±1.94*Δ | 132.22±3.52*Δ | 11.5±2 | 41.9±5.42*Δ | 53.2±9.8*Δ | 88.52±8.6*Δ | 96.57±10.9*Δ |
Wistar rats received either vehicle (control), Ang II (400 ng/kg/min), or telmisartan (3 mg/kg/day)+Ang II (400 ng/kg/min) via mini-osmotic pumps for 28 days. Systolic blood pressure and urinary albumin per 24-h were measured on days 0, 7, 14, 21, 28.
p<0.05 compared with control group of respective duration;
p<0.05 compared with Ang II infused group of respective duration.
Table 2.
Endogenous creatinine clearance rate (Ccr) and concentration of Ang II in plasma or renal tissue in different groups (n=6).
| Day | Ccr (mL/min/100 g) | Concentration of Ang II
|
||
|---|---|---|---|---|
| Plasma (pg/mL) | Renal tissue (pg/mL/mg) | |||
| Control | 14 | 1.16±0.37 | 270.5±92.8 | 861.2±107.5 |
| 28 | 1.13±0.42 | 300.74±88.7 | 798.2±97.5 | |
| Ang II | 14 | 0.97±0.21 | 1026.25±148.2* | 2438.7±179.8* |
| 28 | 0.86±0.16 | 1022.3±132.7* | 2326.4±136* | |
| Telmisartan+ Ang II | 14 | 0.83±0.11 | 1381.5±151.7* | 2656.6±155.9* |
| 28 | 1.07±0.15 | 1339.3±160.3* | 2518.8±139.1* | |
Wistar rats received either vehicle (control), Ang II (400 ng/kg/min), or telmisartan (3 mg/kg/day)+Ang II (400 ng/kg/min) via mini-osmotic pumps for 14/28 days. Endogenous creatinine clearance rate (Ccr) and concentration of Ang II in plasma or renal tissue were measured on days 14 and 28.
p<0.05 compared with control group of respective duration.
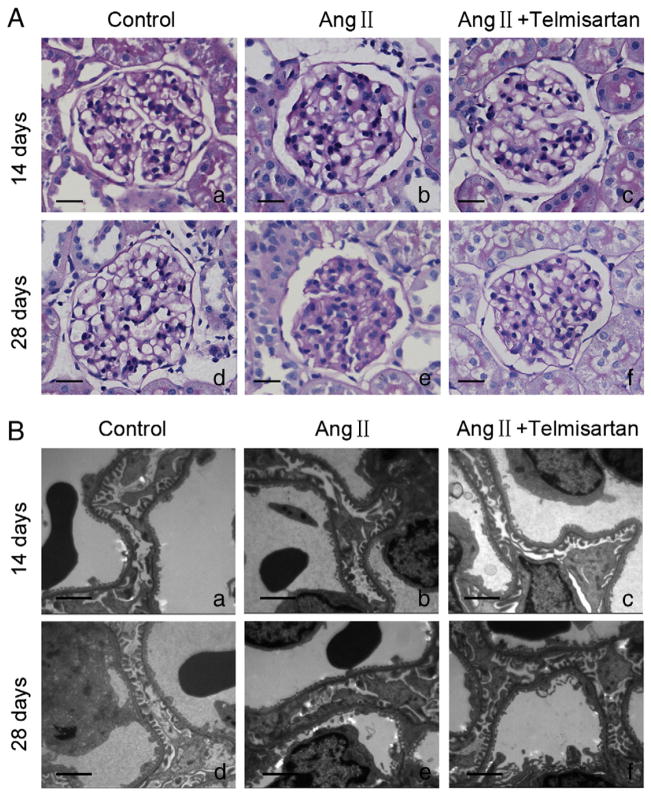
Light microscopy revealed mild mesangial and tubulointerstitial accumulation of matrix in Ang II receiving rats at 14 days (Fig. 1Ab); whereas Ang II receiving rats displayed significant mesangial and interstitial, expansion at 28 days (Fig. 1Ae). On the other hand, telmisartan provided protection against the pathological changes induced by Ang II (Fig. 1Ac and f). Electron microscopic studies displayed diffuse foot process fusion and/or effacement in Ang II-receiving rats at days 14 and 28 (Fig. 1Bb and e).
Fig. 1.
Glomerular pathological changes in different groups. (A): Light microscopy evaluation of glomerular pathological changes with PAS staining. Original magnification ×400; (B): electron microscopy evaluation of ultrastructure of capillary loops in each group. Original magnification ×10,000; (a): normal control group on day 14; (b): Ang II-infused group on day 14. (c): Telmisartan treatment group on day 14; (d): normal control group on day 28; (e): Ang II-infused group on day 28. (f): Telmisartan treatment group on day 28. PAS: Periodic Acid-Schiff.
3.2. Effect of Ang II on nephrin expression in vivo and in vitro
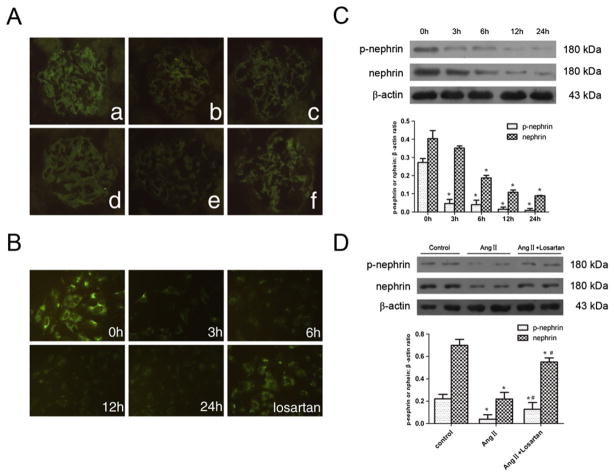
To confirm the effect of Ang II on nephrin expression, Ang II-infused rats were used. Additionally, cultured podocytes were treated with Ang II (10−6 mol/L) for several time points (0 h, 3 h, 6 h, 12 h, and 24 h). The cells were also treated with Ang II (10−6 mol/L) for 6 h in the presence of losartan (10−5 mol/L). As shown in Fig. 2, nephrin was significantly decreased after Ang II stimulation both in vivo and in vitro (Fig. 2A and B). Furthermore, phosphorylated nephrin in Ang II-treated podocytes (in the culture) was also significantly decreased (Fig. 2C). Pre-treatment of losartan reversed the expression of both nephrin and phosphorylated nephrin, which was reduced by Ang II stimulation (Fig. 2D).
Fig. 2.
Ang II stimulation reduced the expression of nephrin and phospho-nephrin in podocyte. (A): Immunofluorescence detection of glomerular nephrin expression in different groups. Original magnification ×400; (a): normal control group on day 14; (b): Ang II-infused group on day 14. (c): Telmisartan treatment group on day 14; (d): normal control group on day 28; (e): Ang II-infused group on day 28. (f): Telmisartan treatment group on day 28; (B): immunofluorescence detection of nephrin expression in cultured podocytes stimulated by Ang II (10−6 mol/L) at various time points or pretreated with losartan (10−5 mol/L). Original magnification ×400; (C): Western-blotting detection of phospho-nephrin and nephrin expression in cultured podocytes stimulated by Ang II (10−6 mol/L) at various time points. (D): Western-blotting detection of phospho-nephrin and nephrin expression in cultured podocytes pretreated with losartan (10−5 mol/L) before stimulated by Ang II (10−6 mol/L) for 6 h. In C and D, n=6. *p<0.05 compared with control group; #p<0.05 compared with Ang II treated group.
3.3. Effect of Ang II on caveolin-1 expression
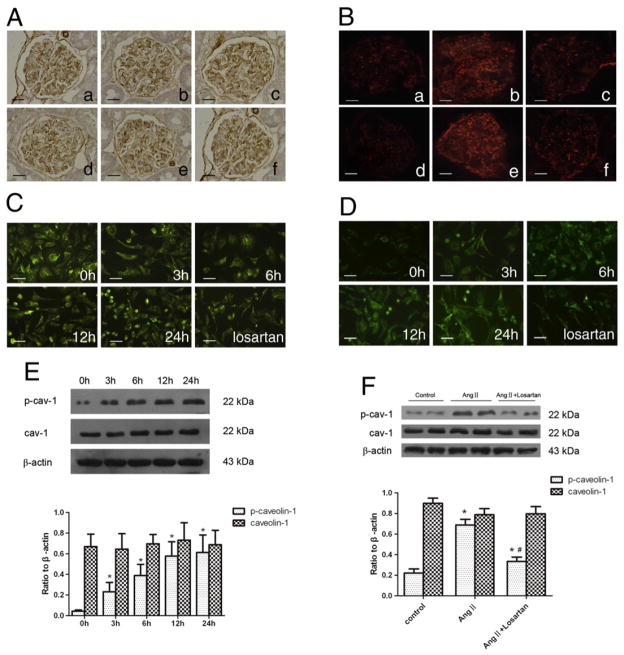
Caveolin-1 is highly expressed in podocytes and partially localized in podocyte slit-diaphragm [19]. To determine the effect of Ang II on the expression of caveolin-1 and phosphorylated caveolin-1, Ang II-infused rats and cultured podocytes were used. As shown in Fig. 3, podocytes had an abundant expression of caveolin-1 and a low level of phosphorylated caveolin-1. Ang II did not induce significant changes in total caveolin-1 expression both in vivo and in vitro. However, Ang II treatment significantly increased phosphorylated caveolin-1; whereas, both telmisartan and losartan inhibited caveolin-1 phosphorylation.
Fig. 3.
Effect of Ang II on the expression of caveolin-1 and phospho-caveolin-1. (A): Immunohistochemistry detection of glomerular caveolin-1 expression in different groups. Original magnification ×400; (B): immunofluorescence detection of glomerular caveolin-1 phosphorylation in different groups. Original magnification ×400; (a): normal control group on day 14; (b): Ang II-infused group on day 14. (c): Telmisartan treatment group on day 14; (d): normal control group on day 28; (e): Ang II-infused group on day 28. (f): Telmisartan treatment group on day 28; (C): immunofluorescence detection of caveolin-1 expression in cultured podocytes stimulated by Ang II (10−6 mol/L) at various time points or pretreated with losartan (10−5 mol/L). Original magnification ×400; (D): immunofluorescence detection of phospho-caveolin-1 expression in cultured podocytes stimulated by Ang II (10−6 mol/L) at various time points or pretreated with losartan (10−5 mol/L). Original magnification ×400; (E): Western-blotting detection of phospho-caveolin-1 and caveolin-1 expression in cultured podocytes stimulated by Ang II (10−6 mol/L) at various time points; (F): Western-blotting detection of phospho-caveolin-1 and caveolin-1 in cultured podocytes treated with losartan (10−5 mol/L) before stimulated by Ang II (10−6 mol/L) for 6 h. In E and F, n=6. *p<0.05 compared with control group; #p<0.05 compared with Ang II-treated group.
3.4. Effect of Ang II on the interaction between caveolin-1 and nephrin
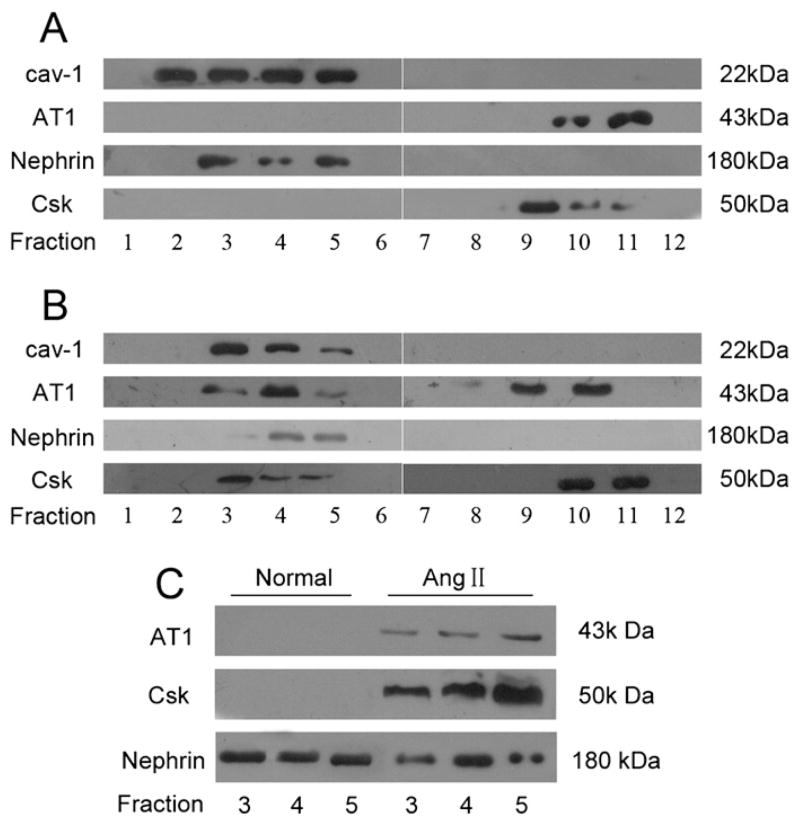
Nephrin has been shown to co-localize with caveolin-1 in caveolae in podocytes [18,19]. To test whether caveolin-1 plays a role in Ang II-induced nephrin dephosphorylation, sucrose gradient density centrifugation was performed to confirm if nephrin resides in microdomains of caveolae. It was well known that the major effects of Ang II were mediated by its type 1 receptor (AT1) [9,21] and C-terminal Src kinase (Csk), a negative regulator of Src family kinases [16]. Therefore, both AT1 and Csk were analyzed in the present study. As shown in Fig. 4A, nephrin was present in the caveolae fractions (2 to 5), which were confirmed by immunoblotting with caveolin-1. AT1 and Csk were detected in the non-caveolar cytosolic fractions (higher density fraction 9 to 11), but not in the caveolae fractions in control podocyte lysates. However, AT1 and Csk were demonstrated in both caveolae and non-caveolae fractions of Ang II-treated podocyte lysates (Fig. 4B). To further evaluate the interaction between caveolin-1 and nephrin, caveolin-1 protein was immunoprecipitated from caveolae lysates from both Ang II treated and untreated podocytes. It was noted that only nephrin was co-immunoprecipitated with caveolin-1 in the caveolae fraction of normal podocytes. When treated with Ang II for 3 h, both AT1 and Csk were co-immunoprecipitated with caveolin-1 (Fig. 4C). These findings indicated that Ang II invoked interaction among caveolin-1, AT1 and Csk.
Fig. 4.
Role of caveolin-1 in Ang II-induced nephrin dephosphorylation. Lysates from normal cultured podocytes (A) and Ang II-treated podocytes for 3 h (B) were separated by sucrose gradient density centrifugation into 12 fractions as outlined in Materials and methods. (A): Fractions 2 to 5 were identified as caveolae by the presence of caveolin-1. Nephrin was also found to be present in caveolae fractions. AT1 and Csk were present in the higher density noncaveolar cytosolic fractions but not seen in the caveolae fractions. (B): After Ang II stimulation for 3 h, AT1 and Csk were found to be present both in the caveolae fractions and noncaveolar fractions. (C): Caveolin-1 was immunoprecipitated respectively from caveolae fractions (fraction 3 to 5) of normal podocytes and Ang II-treated podocytes for 3 h. The immunoprecipitates were then analyzed by Western-blotting with antibodies of AT1, Csk and nephrin. AT1: Ang II type-1 receptor; Csk: C-terminal Src kinase.
3.5. Effect of caveolin-1 on Ang II-induced nephrin dephosphorylation
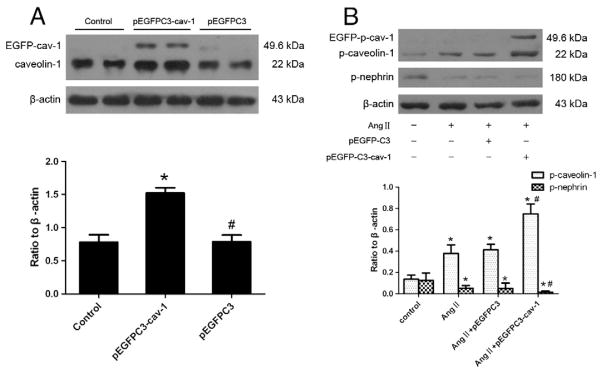
To further evaluate the effect of caveolin-1 on Ang II-induced nephrin dephosphorylation, a recombinant plasmid (pEGFPC3-cav-1) was constructed [20] and transfected into cultured podocytes to upregulate caveolin-1 expression. As shown in Fig. 5A, pEGFPC3-cav-1 transfection increased the expression of caveolin-1 approximately 2-fold more than that of control cells or cells transfected with pEGFPC3. Ang II-induced phosphorylation of caveolin-1 and dephosphorylation of nephrin were overtly increased in the caveolin-1-overexpressing podocytes when compared to podocytes untransfected or transfected with pEGFPC3 (Fig. 5B).
Fig. 5.
Caveolin-1 overexpression enhances nephrin dephosphorylation induced by Ang II. (A): Caveolin-1 protein expression in control cells, pEGFPC3-cav-1 transfected cells and pEGFPC3 transfected cells was evaluated by Western blotting, *p<0.05 compared with control cells; #p<0.05 compared with pEGFPC3-cav-1 transfected cells. (B): Podocytes were stimulated by Ang II (10−6 mol/L) for 3 h in the presence of pEGFPC3-cav-1 or pEGFPC3 transfection, or absence of both pEGFPC3-cav-1 and pEGFPC3 transfection (defined as control group). Phosphorylation of nephrin and caveolin-1 was detected using Western-blotting. *p<0.05 compared with control cells; #p<0.05 compared with cells stimulated by Ang II only or in the presence of pEGFPC3 transfection.
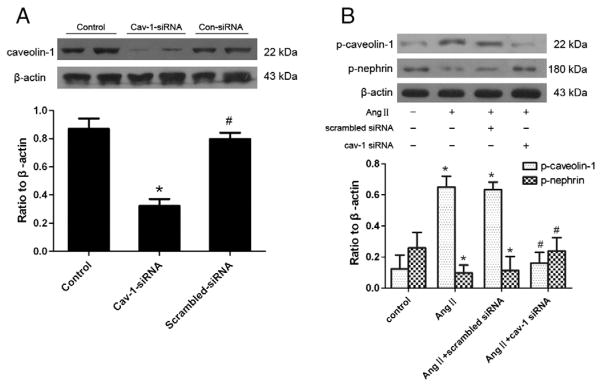
In addition, cav-1 siRNA transfection was performed to reduce caveolin-1 expression in vitro. Transfection of podocytes with cav-1 siRNA resulted in a 60% diminished expression of caveolin-1, compared with control cells or cells transfected with scrambled siRNA (Fig. 6A). Furthermore, knockdown of caveolin-1 expression using siRNA inhibited phosphorylation of caveolin-1 and dephosphorylation of nephrin induced by Ang II (Fig. 6B).
Fig. 6.
Cav-1 siRNA downregulates Ang II-induced nephrin dephosphorylation. (A): Caveolin-1 protein expressions in control cells, cav-1 siRNA transfected cells and scrambled-siRNA transfected cells were evaluated by Western blotting, respectively. *p<0.05 compared with control cells; #p<0.05 compared with cav-1 siRNA transfected cells. (B): Podocytes were stimulated by Ang II (10−6 mol/L) for 3 h with cav-1 siRNA or scrambled siRNA transfection, or without both cav-1 siRNA and scrambled siRNA transfection (defined as control group). Immunoblotting for phospho-caveolin-1 and phospho-nephrin was performed in different group cells. *p<0.05 compared with control cells; #p<0.05 compared with cells stimulated by Ang II only or with scrambled siRNA transfection.
3.6. Effect of caveolin-1 on Ang II-induced podocyte apoptosis
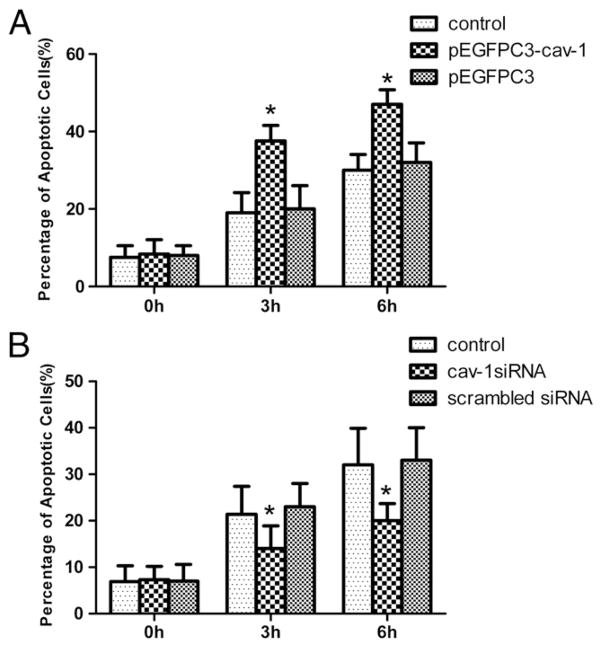
Our previous studies showed that Ang II induced podocyte apoptosis in a dose- and time-dependent manner [10,22]. To confirm the effect of caveolin-1 on Ang II-induced podocyte apoptosis, we upregulated or downregulated caveolin-1 expression using the recombinant plasmid pEGFPC3-cav-1 or siRNA of cav-1, followed by apoptosis assay. As shown in Fig. 7A, caveolin-1 overexpression significantly enhanced Ang II-induced podocyte apoptosis. In parallel experiments, we noted that knock-down of caveolin-1 using siRNA prevented podocyte apoptosis in response to Ang II (Fig. 7B).
Fig. 7.
Effect of caveolin-1 on Ang II-induced podocyte apoptosis. (A): Equal numbers of podocytes with or without pEGFPC3-cav-1 transfection (defined as control group), or with pEGFPC3 transfection were stimulated by Ang II (10−6 mol/L) for several time points (0 h, 3 h, 6 h). At the end of the stimulation period, cells were evaluated for apoptosis. *p<0.05 compared with control cells and cells transfected with pEGFPC3; (B): equal numbers of podocytes with or without cav-1 siRNA transfection (defined as control group), or with scrambled siRNA were stimulated by Ang II (10−6 mol/L) for several time points (0 h, 3 h, 6 h). At the end of the stimulation period, cells were evaluated for apoptosis. *p<0.05 compared with control cells and cells transfected with scrambled siRNA.
4. Discussion
The present study confirmed the effect of Ang II in the induction of podocyte apoptosis and albuminuria [10]. Ang II also induced nephrin dephosphorylation and caveolin-1 phosphorylation in podocytes. Interestingly, Ang II-induced podocyte caveolin-1 phosphorylation displayed a temporal relationship with the dephosphorylation of nephrin. Furthermore, transfection of recombinant plasmid pEGFP-C3-cav1 into cultured podocytes not only exhibited enhanced caveo-lin-1 expression but also diminished nephrin phosphorylation; on the other hand, knockdown of the podocyte caveolin-1 expression (using siRNA) blocked the Ang II-induced nephrin dephosphorylation. These findings indicate that Ang II-induced nephrin dephosphorylation and podocyte injury are mediated through caveolin-1 phosphorylation.
Nephrin is a structural protein in SD and is also considered to be a signaling molecule. It has been shown that phosphorylation of tyrosine residue (Y1204) of rat nephrin by Fyn kinase allows Nck adaptor protein binding to nephrin motifs, which induces actin polymerization in podocytes [23]. Several other proteins, such as CD2AP, podocin, can bind to the cytoplasmic domain of phosphonephrin through their SH2 or SH3 domains. This binding triggers downstream signaling pathways in podocyte biology [24]. However, the role of nephrin phosphorylation is still uncertain. Verma et al. [14] reported that the phosphorylation of nephrin at Y1208 was minimal in normal glomeruli, but transiently increased during foot process effacement in protamine sufate-induced podocyte injury. However, Li et al. [25] and Uchida et al. [26] demonstrated that the phosphorylation of nephrin was significantly reduced in puromycin nephropathy respectively. In the present study we used an antibody to evaluate the phosphorylation of nephrin at Y1217 and found that nephrin phosphorylation decreased rapidly after Ang II stimulation. Since podocyte nephrin dephosphorylation was associated with podocyte injury and albuminuria, it appears that nephrin phosphorylation is physiologically important for the maintenance of normal podocyte morphology and function.
Many signaling molecules, including nephrin, podocin, CD2AP, Src and RhoA have been identified as residents of caveolae in various cell types [19]. Caveolae localization of signaling molecules might provide a platform for their regulated activation [27]. Caveolin-1 is the core element of caveolae and has been shown to play a key regulatory role through its scaffolding domain (amino acids 82 to 101) [28]. Previous study has shown that caveolin-1 Y14 phosphorylation may provide a docking site for other proteins and facilitate interaction of other proteins with phosphorylated caveolin-1 in a stimulus-specific fashion [27]. Indeed, caveolin-1 phosphorylation has been demonstrated to be required for stretch-induced EGFR and Akt activation in mesangial cells [17]. Caveolin-1 Y14 phosphorylation was present in smooth muscle cells and co-immunoprecipitated with the focal adhesion protein paxillin after Ang II stimulation [29]. In the present study, Ang II-induced phosphorylation of podocyte caveolin-1 on Y14 was associated with nephrin dephosphorylation; whereas, silencing of the podocyte caveolin-1 prevented the Ang II-induced nephrin dephosphorylation. These findings indicated that Ang II-induced nephrin dephosphorylation and podocyte injury were mediated through caveolin-1 phosphorylation.
We previously reported that Ang II-induced podocyte apoptosis [22] was associated with down regulation of nephrin [10]. In the present study, the overexpression of caveolin-1 not only induced de-phosphorylation of nephrin but also promoted podocyte apoptosis; interestingly, podocytes overexpressing caveolin-1 displayed greater number of apoptotic cells when compared to Ang II-treated cells. On the contrary, knockdown of the caveolin-1 not only inhibited nephrin dephosphorylation but also prevented podocyte apoptosis in response to Ang II. These findings indicate that caveolin-1 modulates nephrin phosphorylation, and thus may be playing a critical role in the maintenance of podocyte survival.
Csk is known to be a negative regulator of Src family kinases. It specifically binds to phosphorylated caveolin-1 [16]. Caveolin-1 phosphorylation is likely to be an intermediate step in a signaling cascade occurring within caveolae. The present data indicate that Ang II-induced caveolin-1 phosphorylation not only recruit cytosolic Csk into the caveolae, but also result in an interaction with caveolin-1. The interaction between phosphorylated caveolin-1 and Csk may be an important upstream event of nephrin dephosphorylation. But how caveolin-1 phosphorylation and Csk interaction induce nephrin dephosphorylation remains to be determined.
In conclusion, our present study demonstrates an important role of caveolin-1 phosphorylation in Ang II-induced nephrin dephosphorylation and podocyte apoptosis. The effect of caveolin-1 seems to be mediated through its interaction with Csk, and thus suggests a cross-talk between nephrin and caveolin-1. Ang II-caveolin-1-nephrin signaling pathway may provide new effective therapeutic targets for the RAAS-associated podocyte injury.
Acknowledgments
These studies were supported by grants from the National Science Foundation of China (30871167 to G.D. and 30900688 to C.C.) and from the National Institutes of Health (RO1DK084910).
References
- 1.Hartleben B, Godel M, Meyer-Schwesinger C, Liu S, Ulrich T, Kobler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstadt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB. The Journal of Clinical Investigation. 2010;120(4):1084–1096. doi: 10.1172/JCI39492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook HT. Kidney International. 2011;79(6):581–583. doi: 10.1038/ki.2010.521. [DOI] [PubMed] [Google Scholar]
- 3.Wei C, Reiser J. Nephrology, Dialysis, Transplantation. 2011;26(6):1776–1777. doi: 10.1093/ndt/gfr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiggins RC. Kidney International. 2007;71(12):1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki K, Han GD, Miyauchi N, Hashimoto T, Nakatsue T, Fujioka Y, Koike H, Shimizu F, Kawachi H. American Journal of Pathology. 2007;170(6):1841–1853. doi: 10.2353/ajpath.2007.060484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HJ, Sato T, Rodriguez-Iturbe B, Vaziri ND. Journal of Pharmacology and Experimental Therapeutics. 2011;337(3):583–590. doi: 10.1124/jpet.110.175828. [DOI] [PubMed] [Google Scholar]
- 7.Yalavarthy R, Smith ML, Edelstein C. Nephrology (Carlton, Vic) 2007;12(5):529–531. doi: 10.1111/j.1440-1797.2007.00820.x. [DOI] [PubMed] [Google Scholar]
- 8.Durvasula RV, Shankland SJ. American Journal of Physiology Renal Physiology. 2008;294(4):F830–F839. doi: 10.1152/ajprenal.00266.2007. [DOI] [PubMed] [Google Scholar]
- 9.Wolf G, Butzmann U, Wenzel UO. Nephron Physiology. 2003;93(1):P3–P13. doi: 10.1159/000066656. [DOI] [PubMed] [Google Scholar]
- 10.Jia J, Ding G, Zhu J, Chen C, Liang W, Franki N, Singhal PC. American Journal of Nephrology. 2008;28(3):500–507. doi: 10.1159/000113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner MC, Rhodes G, Wang E, Pruthi V, Arif E, Saleem MA, Wean SE, Garg P, Verma R, Holzman LB, Gattone V, Molitoris BA, Nihalani D. Journal of Biological Chemistry. 2008;283(51):35579–35589. doi: 10.1074/jbc.M805507200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Lemay S, Aoudjit L, Kawachi H, Takano T. Journal of the American Society of Nephrology. 2004;15(12):3006–3015. doi: 10.1097/01.ASN.0000146689.88078.80. [DOI] [PubMed] [Google Scholar]
- 13.Lahdenpera J, Kilpelainen P, Liu XL, Pikkarainen T, Reponen P, Ruotsalainen V, Tryggvason K. Kidney International. 2003;64(2):404–413. doi: 10.1046/j.1523-1755.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- 14.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB. The Journal of Clinical Investigation. 2006;116(5):1346–1359. doi: 10.1172/JCI27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pantera B, Bini C, Cirri P, Paoli P, Camici G, Manao G, Caselli A. Journal of Neurochemistry. 2009;110(1):194–207. doi: 10.1111/j.1471-4159.2009.06123.x. [DOI] [PubMed] [Google Scholar]
- 16.Cao H, Courchesne WE, Mastick CC. Journal of Biological Chemistry. 2002;277(11):8771–8774. doi: 10.1074/jbc.C100661200. [DOI] [PubMed] [Google Scholar]
- 17.Zhang B, Peng F, Wu D, Ingram AJ, Gao B, Krepinsky JC. Cellular Signalling. 2007;19(8):1690–1700. doi: 10.1016/j.cellsig.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P. The Journal of Clinical Investigation. 2001;108(11):1621–1629. doi: 10.1172/JCI12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorensson J, Fierlbeck W, Heider T, Schwarz K, Park DS, Mundel P, Lisanti M, Ballermann BJ. Journal of the American Society of Nephrology. 2002;13(11):2639–2647. doi: 10.1097/01.asn.0000033277.32822.23. [DOI] [PubMed] [Google Scholar]
- 20.Ren Z, Liang W, Ding G. Medical Journal of Wuhan University. 2011;32(2):191–195. [Google Scholar]
- 21.Ruster C, Wolf G. Journal of the American Society of Nephrology. 2006;17(11):2985–2991. doi: 10.1681/ASN.2006040356. [DOI] [PubMed] [Google Scholar]
- 22.Ding G, Reddy K, Kapasi AA, Franki N, Gibbons N, Kasinath BS, Singhal PC. American Journal of Physiology Renal Physiology. 2002;283(1):F173–F180. doi: 10.1152/ajprenal.00240.2001. [DOI] [PubMed] [Google Scholar]
- 23.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, Huang H, Larose L, Li SS, Takano T, Quaggin SE, Pawson T. Nature. 2006;440(7085):818–823. doi: 10.1038/nature04662. [DOI] [PubMed] [Google Scholar]
- 24.Fan Q, Zhang H, Ding J, Liu S, Miao J, Xing Y, Yu Z, Guan N. Genes to Cells. 2009;14(9):1079–1090. doi: 10.1111/j.1365-2443.2009.01336.x. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Zhu J, Aoudjit L, Latreille M, Kawachi H, Larose L, Takano T. Biochemical and Biophysical Research Communications. 2006;349(1):310–316. doi: 10.1016/j.bbrc.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 26.Uchida K, Suzuki K, Iwamoto M, Kawachi H, Ohno M, Horita S, Nitta K. Kidney International. 2008;73(8):926–932. doi: 10.1038/ki.2008.19. [DOI] [PubMed] [Google Scholar]
- 27.Peng F, Wu D, Ingram AJ, Zhang B, Gao B, Krepinsky JC. Journal of the American Society of Nephrology. 2007;18(1):189–198. doi: 10.1681/ASN.2006050498. [DOI] [PubMed] [Google Scholar]
- 28.Razani B, Woodman SE, Lisanti MP. Pharmacological Reviews. 2002;54(3):431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- 29.Ushio-Fukai M, Hilenski L, Santanam N, Becker PL, Ma Y, Griendling KK, Alexander RW. Journal of Biological Chemistry. 2001;276(51):48269–48275. doi: 10.1074/jbc.M105901200. [DOI] [PubMed] [Google Scholar]