Abstract
The neutralization of alpha 4 integrin is currently used as treatment in several autoimmune diseases and is thought to prevent the entry of most immune cells in target tissues. Here, we showed that selective deletion of alpha4 integrin in T cells did not prevent but delayed the development of experimental autoimmune encephalomyelitis (EAE). Whereas both Th1 and Th17 cells infiltrate the central nervous system (CNS) of wild type mice, T cells present in the CNS of mice lacking alpha4 integrin were mainly enriched in Th17 cells suggesting that this T cell subset uses other integrins to access the CNS. In contrary, alpha4 integrin expression is important for Th1 cells to enter the CNS and for the stability of their Th1 associated genetic program. Therefore, our data suggest that anti-alpha4 integrin antibody treatment may be more efficient in the treatment of Th1 rather than Th17 mediated disease.
Introduction
Experimental Autoimmune Encephalomyelitis (EAE), is an animal model of multiple sclerosis (MS), characterized by multifocal areas of leukocyte infiltration, demyelination, and axonal damage (1). Two subsets of myelin specific CD4+ T cells have been implicated in the pathogenicity of MS and EAE: Th1 cells Th17 cells (2–5).
Trafficking of autoreactive CD4+ T cells from the systemic compartment into the central nervous system (CNS) are crucial early events in the development of MS and EAE lesions, and involve specific adhesion molecules (1). Alpha4 (Itga4) beta1 (Itgb1) integrin or very late activation antigen 4 (VLA4) has been proposed as the major adhesion molecule allowing the entry of T cells in the CNS. Treatment of mice with an anti-alpha 4 integrin (Itga4) antibody has been shown to dramatically reduced leukocyte adhesion and to prevent the development of EAE in most animal models (6, 7) except in C57BL/6 mice MOG-induced EAE where it has limited effect on clinical disease (8, 9). Based on these observations, a humanized anti-Itga4 monoclonal antibody was generated to treat patients with MS. While clinical trials showed a drastic reduction in the relapse rate in MS, a significant number of patients developed a life threatening condition called lethal progressive multifocal leukoencephalopathy (PML) (10). Therefore, it is important to determine the effect of Itga4 blockade on the migration of different subsets of immune cells in the CNS.
Here, we show that mice with selective deletion of Itga4 on CD4+ T cells are still susceptible to EAE development. While the number of CNS-infiltrating Th1 cells was significantly decreased, the number of CNS-infiltrating Th17 cells was not impaired in these mice. Using adoptive transfer experiments, we further show that the lack of Itga4 expression on Th1 cells impaired their phenotypic stability and their capacity to infiltrate the CNS. Together, our results suggest the efficacy of Itga4 neutralization for Th1-mediated EAE but distinct and new molecular requirements for Th17 cells entry into the CNS during EAE.
Materials and methods
Mice
All mice are on the C57BL/6 background and used at 8–12 weeks. WT, CD4Cre, TCRβδ deficient and 2D2 Tg mice were purchased from Jackson laboratories. Itga4fl/fl mice were provided by Dr. Papayannopoulou (11). All animals were bred and maintained under specific pathogen-free conditions at the Benaroya Research Institute (Seattle, WA) and all experiments were performed in accordance with the guidelines of the Benaroya Research Institute Animal Care and Use Committee.
EAE induction
Active EAE was induced as previously described (12). For passive transfer of EAE, 2D2 naïve CD62L+ CD4+ T cells were cultured with irradiated splenocytes, anti-CD3 antibody (2.5μg/ml, clone 145-2C11) and either IL-12 (10ng/ml) for Th1 cells or IL-6 (30ng/ml), TGFβ (5ng/ml) and anti-IFNγ (10μg/ml) for Th7 cells. After 3 days, 50×106 cells were injected i.v. into TCRβδ deficient recipients with pertussis toxin at day 0 and 2. EAE was scored according to the following criteria: 0, no signs of disease; 1, loss of tail tone; 2, hind limb paresis; 3, hind limb paralysis; 4, front and hind limb paralysis.
Flow cytometry
Cell suspensions from brain and spinal cord were prepared as previously described (12). Antibodies for CD4 (GK1.5), IFNγ (XMG1.2), IL17A (TC11-18H10.1), Itga4 (R1-2) were purchased from eBioscience and Biolegend. For surface cytokine staining, CD4+ T cells were stained with Miltenyi secretion assay following the manufacturer’s instructions. Cells were acquired on LSRII (BD Biosciences), and data were analyzed with FlowJo software.
T-cell proliferation
dLN were collected 8 days after immunization. Cells were cultured at 5×106 cells/ml in the presence of different concentrations of MOG for 72h. During the last 16h, cells were pulsed with 1μCi of [3H] thymidine. [3H] thymidine incorporation was measured using a β-counter.
Results and Discussion
Itga4 is expressed at higher levels in Th1 cells compared to Th17 cells
T helper cell subsets express distinct chemokine receptors and adhesion molecules conferring specific homing properties. Analysis of Itga4 expression has been previously performed on T cell clones or in vitro polarized cells, but culture conditions could modulate Itga4 expression. Here, we analyzed Itga4 expression on ex vivo effector Th1 and Th17 cell subsets isolated from draining LN after MOG35–55 immunization using a secretion assay for IL-17A and IFNγ (Fig. 1A, 1B). Itga4 was expressed at higher levels on Th1 than Th17 cells (Fig. 1A, 1B). In contrast, there was no difference in the expression of Itgb1 and Itga7 between Th1 and Th17 cells (Supplemental Fig. 1A, 1B). Therefore, the selective higher expression of Itga4 on Th1 cells raises the possibility that these cells might be more dependent on Itga4 for their entry in the CNS than Th17 cells.
FIGURE 1. Itga4 expression by Th cell subsets.

WT mice were immunized for EAE development. LNs were collected at day 8. (A) Cells were stained by intracellular (left) or surface (right) cytokine staining. (B, left) Itga4 expression on Th1 (black) and Th17 (grey) cells identified by cytokine surface staining (as shown in A) and compared to isotype control staining (Filled histograms). Data are representative of three independent experiments with 5 mice per group. (B, Right) Mean expression of the Itga4 (MFI) by Th1 (black) and Th17 (grey) populations (p<0.01).
Itga4 deletion on CD4+ T cells does not prevent the development of EAE
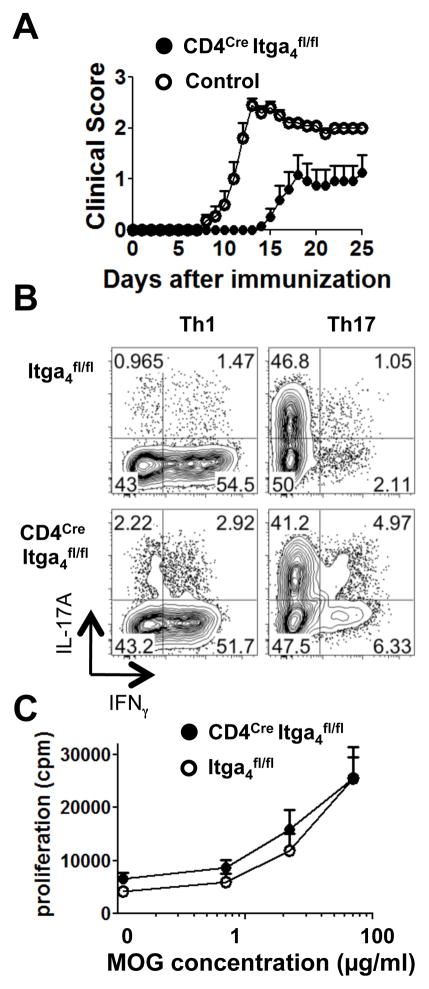
To determine whether the differential expression of Itga4 on helper T cell subsets could affect the development of EAE we used mice with specific deletion of Itga4 in T cells (CD4Cre Itga4fl/fl). Cre-mediated deletion of Itga4 in CD4+ T cells was efficient at the genomic level and resulted in more than 95% of CD4+ T cells lacking Itga4 in these animals (Supplemental Fig. 1C, 1D). The majority of WT control mice (95%) developed a monophasic disease with ascending paralysis between 10 and 14 days after immunization (Fig. 2A and Supplemental Table I). Although the mean clinical EAE score in CD4Cre Itga4fl/fl mice (2.5 ± 0.7) was similar to the one observed in WT control mice (2.7 ± 0.7); fewer CD4Cre Itga4fl/fl mice developed disease (incidence: 50% compared to 95% in WT mice, Fig. 2A, Supplemental Table I) and had a delay in disease onset (19.3 ± 4.3 days in CD4Cre Itga4fl/fl mice vs. 12.3 ± 2.3 days in WT mice, Fig. 2A, Supplemental Table I). Thus, CD4Cre Itga4fl/fl mice developed EAE with delayed onset and with less incidence that WT control mice. However, in agreement with previous report showing blockade of leukocyte adhesion but limited effect of anti-Itga4 treatment in C57BL/6 mice MOG-induced EAE (8, 9), specific deletion of Itga4 on T cells did not abolish EAE susceptibility in our model. This suggest that pathological mechanisms including but not limited to the dominant type of CNS-infiltrating effector T cells, may differ between mice strains.
FIGURE 2. Deletion of Itga4 in T cells does not prevent the development of EAE.
(A) EAE was induced in CD4Cre Itga4fl/fl mice and control mice. Shown is the mean clinical score for each group over time (± SEM). One representative experiment out of three independent experiments is shown. (B) CD4+ T cells from Itga4fl/fl mice and CD4Cre Itga4fl/fl mice were differentiated in Th1 and Th17 polarizing conditions. Plots are gated on CD4+ T cells and are representative of two independent experiments. (C) Proliferative response of dLN cells isolated from MOG-immunized Itga4fl/fl (open circles) and CD4Cre Itga4fl/fl (filled circles) mice was measured by [3H] thymidine incorporation. Results are representative of 4 independent experiments.
Itga4 deletion does not affect T cell priming and differentiation
VLA4 has been proposed to play an important role in several immune functions such as lymphopoesis, costimulation and T cell migration (11, 13–17). Therefore, we compared these parameters between CD4Cre Itga4fl/fl and Itga4fl/fl mice. We did not detect any difference in the number, percentage and phenotype of CD4+ and CD8+ T cells present in the lymphoid organs of CD4Cre Itga4fl/fl mice compared to controls (Data not shown). In addition, levels of Itgb1 which pairs with Itga4 to form VLA4 were not affected in CD4Cre Itga4fl/fl mice (Supplemental Fig. 1E). Next, we investigated whether the lack of Itga4 expression on T cells could interfere with the differentiation of naïve T cells into pathogenic Th1 and Th17 cells. We determined that CD4+ T cells from CD4Cre Itga4fl/fl and Itga4fl/fl mice could differentiate equally well in Th1 and Th17 subsets (Fig. 2B) and that Itga4 deletion was equivalent in Th1 and Th17 cells (Supplemental Fig. 1F). MOG specific T cell proliferation was also similar between CD4Cre Itga4fl/fl mice and Itga4fl/fl mice (Fig. 2C). Collectively, these results indicate that specific deletion of Itga4 on T cells does not interfere with T cell priming; but instead may affect the homing of pathogenic T cells in the CNS.
Itga4 deletion decreases Th1 cells but not Th17 homing into the CNS
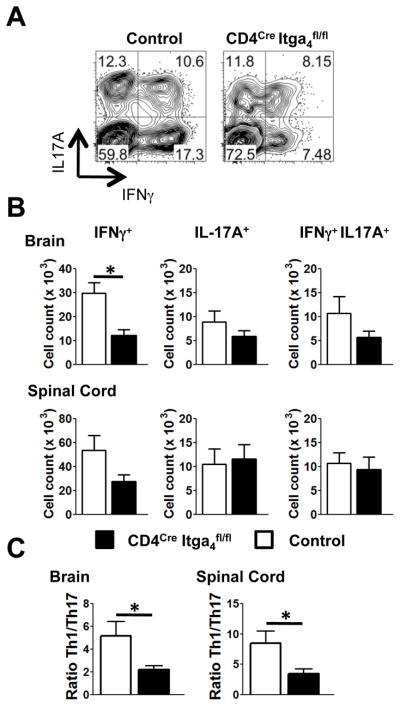
To evaluate this hypothesis, we determined the numbers of CD4+ T cells infiltrating the CNS of CD4Cre Itga4fl/fl mice during EAE. At the peak of the disease, there was a decrease in the number of CNS-infiltrating CD4+ T cells in CD4Cre Itga4fl/fl (52.9 ×103 ± 8.4 ×103) compared to control mice (126.5×103 ± 17.2 ×103). Before EAE onset and at the peak of the disease, there was a similar proportion of IL-17A+ IFNγ+ and IL-17A+ CD4+ T cells in the brain of CD4Cre Itga4fl/fl and WT control mice (Fig. 3A–C, Supplemental Fig. 1G). However, we detected a significant decrease in the percentage and absolute number of CD4+ T cells that produced only IFNγ in the CNS of CD4Cre Itga4fl/fl mice compared to control mice (Fig. 3A–C, Supplemental Fig. 1G). Importantly, deletion of Itga4 was equivalent in Th1 and Th17 cells (Supplemental Fig. 1F) and the residual proportion of CD4+ T cells which remained Itga4+ (4%) did not increase in CNS-infiltrating CD4+ T cells (Supplemental Fig. 1C). Of note, because a recent report suggests that IFN-γ+ T cells present in the CNS during EAE represent ex-Th17 cells (18), the tracking of T cell subsets by cytokine secretion might underestimate the number of CNS infiltrating Th17 cells in our model. Despite this possibility, there was still a drastic decreased Th1/Th17 ratio in the CNS of CD4Cre Itga4fl/fl mice compared to WT mice (Fig. 3C). This suggests two important mechanisms of Itga4 action: 1) Itga4 costimulation of T cells could promote Th1 cells switching to a Th17-phenotype in the CNS or 2) fewer Th1 cells entered the CNS because their trafficking there is dependent on Itga4.
FIGURE 3. CNS infiltrating T cell subsets in CD4Cre Itga4fl/fl and control mice with EAE.
EAE was induced in control and CD4Cre Itga4fl/fl mice. At the peak of the disease for each group (and at clinical score 2 or 3), brain and spinal cords were removed and intracellular cytokine staining was performed. (A) Plots show IL-17A and IFNγ expression in CD4+ T cells (A, B, C). (B) Mean numbers of T cell subsets infiltrating the brain and spinal cords of control (white) and CD4Cre Itga4fl/fl mice (black) with EAE were calculated from three independent experiments with 11 mice per group (p< 0.005). The total number of cells recovered from the CNS was 0.67 ×106 ± 0.10 ×106 for CD4Cre Itga4fl/fl mice and 1.01 ×106 ± 0.12 ×106 for WT mice. (C) Ratio of Th1/Th17 cells infiltrating the brain and spinal cord in each group of mice (p< 0.01). Data are the mean of three independent experiments with 11 mice per group and were analyzed using Student’s t test.
Loss of Itga4 delays Th1-induced EAE
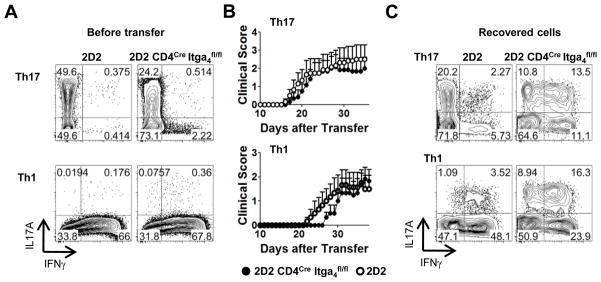
To address some of these possibilities, we differentiated CD4+ T cells from 2D2 CD4Cre Itga4fl/fl and 2D2 mice into either Th17 or Th1 cells and transferred them into T cell deficient recipient mice (Fig. 4A, 4B). Th17 cells derived from either 2D2 or 2D2 CD4Cre Itga4fl/fl mice induced a severe disease (Fig. 4B) and a significant percentage of Th17 transgenic cells recovered from the CNS had maintained IL-17A expression (Fig. 4C, upper panel). The transfer of Th1 cells lacking Itga4, in contrast, resulted in delayed disease onset compared to the disease induced by Itga4 sufficient Th1 cells (Fig. 4B). Surprisingly, cells recovered from the CNS of animals transferred with Itga4 deficient Th1 cells were mainly IL-17A producing cells (Fig. 4C, lower panel) indicating that in the absence of Itga4 on CD4+ T cells, the disease was mainly Th17, but not Th1 mediated. These results suggest the intriguing possibility that in the absence of Itga4, Th1 cells become unstable and revert to a Th17-like phenotype. The other possibility is that uncommitted cells from the pool of naïve donor cells may have differentiated into Th17 cells, and have overgrown Th1 cells in vivo causing EAE independently of Itga4 and in the absence of Th1 cell entry in the CNS (Fig. 4B, 4C, lower panel).
FIGURE 4. Itga4 deficient Th1 cells do not induce EAE.
CD4+ T cells from CD4Cre Itga4fl/fl 2D2 (right) and 2D2 (left) mice were polarized in vitro into Th17 (top) and Th1 cells (bottom). (A) IL-17 and IFNγ expression in Th1 and Th17 cells prior adoptive transfer. (B) EAE was induced by transfer of CD4Cre Itga4fl/fl 2D2 and 2D2 Th17 cells (top) or Th1 cells (bottom). Shown is the mean clinical score for each group over time (n=6 mice per group ± SEM). In each group, disease incidence was equivalent (68%). (C) CNS from transferred mice were removed and intracellular cytokine staining was performed. The plots show the percentage of CNS-infiltrating Th1 and Th17 and are representative of 4 mice per group.
Conclusions
In this study, we demonstrate that elimination of Itga4 on CD4+ T cells limited the access of Th1 cells in the CNS and affected their phenotypic characteristics, but had lesser effect on the trafficking of Th17 cells. In accordance with these observations, the selective deletion of Itga4 in CD4+ T cells did not prevent the development of Th17-induced EAE but delayed Th1-induced EAE. These findings suggest that anti-Itga4 might be more efficient at treating Th1 rather than Th17-mediated MS. A differential effect of immunomodulatory drugs on effector T cell subsets and disease development has been reported recently in another study (19). These observations together with ours suggest that the Th1 cells-induced CNS autoimmunity might be fairly well controlled by current available drugs but that more specific drugs are required for Th17-induced and dominated CNS pathologies.
Supplementary Material
Acknowledgments
This work was funded by US National Institutes of Health grant R01 NS 059996 to E.B.
We would like to thank Dr. Papayannopoulou for providing the Itga4fl/fl mice and Alice Yuan for technical assistance.
Footnotes
Competing financial interests
The authors declare no competing financial interest.
References
- 1.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 2.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162:1–11. doi: 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovett-Racke AE, Yang Y, Racke MK. Th1 versus Th17: are T cell cytokines relevant in multiple sclerosis? Biochim Biophys Acta. 2011;1812:246–251. doi: 10.1016/j.bbadis.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brucklacher-Waldert V, Stuerner K, Kolster M, Wolthausen J, Tolosa E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain. 2009;132:3329–3341. doi: 10.1093/brain/awp289. [DOI] [PubMed] [Google Scholar]
- 6.Theien BE, Vanderlugt CL, Eagar TN, Nickerson-Nutter C, Nazareno R, Kuchroo VK, Miller SD. Discordant effects of anti-VLA-4 treatment before and after onset of relapsing experimental autoimmune encephalomyelitis. J Clin Invest. 2001;107:995–1006. doi: 10.1172/JCI11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 8.Kerfoot SM, Norman MU, Lapointe BM, Bonder CS, Zbytnuik L, Kubes P. Reevaluation of P-selectin and alpha 4 integrin as targets for the treatment of experimental autoimmune encephalomyelitis. J Immunol. 2006;176:6225–6234. doi: 10.4049/jimmunol.176.10.6225. [DOI] [PubMed] [Google Scholar]
- 9.Doring A, Pfeiffer F, Meier M, Dehouck B, Tauber S, Deutsch U, Engelhardt B. TET inducible expression of the alpha4beta7-integrin ligand MAdCAM-1 on the blood-brain barrier does not influence the immunopathogenesis of experimental autoimmune encephalomyelitis. Eur J Immunol. 2011;41:813–821. doi: 10.1002/eji.201040912. [DOI] [PubMed] [Google Scholar]
- 10.Ransohoff RM. Natalizumab for multiple sclerosis. N Engl J Med. 2007;356:2622–2629. doi: 10.1056/NEJMct071462. [DOI] [PubMed] [Google Scholar]
- 11.Scott LM, Priestley GV, Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol. 2003;23:9349–9360. doi: 10.1128/MCB.23.24.9349-9360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 13.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 14.Mittelbrunn M, Molina A, Escribese MM, Yanez-Mo M, Escudero E, Ursa A, Tejedor R, Mampaso F, Sanchez-Madrid F. VLA-4 integrin concentrates at the peripheral supramolecular activation complex of the immune synapse and drives T helper 1 responses. Proc Natl Acad Sci U S A. 2004;101:11058–11063. doi: 10.1073/pnas.0307927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niino M, Bodner C, Simard ML, Alatab S, Gano D, Kim HJ, Trigueiro M, Racicot D, Guerette C, Antel JP, Fournier A, Grand’Maison F, Bar-Or A. Natalizumab effects on immune cell responses in multiple sclerosis. Ann Neurol. 2006;59:748–754. doi: 10.1002/ana.20859. [DOI] [PubMed] [Google Scholar]
- 16.Sato T, Tachibana K, Nojima Y, D’Avirro N, Morimoto C. Role of the VLA-4 molecule in T cell costimulation. Identification of the tyrosine phosphorylation pattern induced by the ligation of VLA-4. J Immunol. 1995;155:2938–2947. [PubMed] [Google Scholar]
- 17.Engelhardt B, Laschinger M, Schulz M, Samulowitz U, Vestweber D, Hoch G. The development of experimental autoimmune encephalomyelitis in the mouse requires alpha4-integrin but not alpha4beta7-integrin. J Clin Invest. 1998;102:2096–2105. doi: 10.1172/JCI4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P, Naves R, Han M, Zhong F, Castellanos JG, Mair R, Christakos A, Kolkowitz I, Katz L, Killestein J, Polman CH, de Waal Malefyt R, Steinman L, Raman C. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.