Abstract
Measuring multiple hormones simultaneously in a single assay saves sample volume, labor, time, reagents, money and consumables. Thus, multiplex arrays represent a faster, more economically and ecologically sound alternative to singleton assays.
Objectives
To validate a new, commercially-available multiplex female array produced by Quansys Biosciences against individual immunoassays for the quantification of six hormones in urine samples from women in different reproductive stages.
Methods
Urine samples were analyzed using the new Quansys multiplex female hormone array and compared with well-established individual immunoassays for adiponectin, free cortisol, c-peptide, estrone-3-glucuronide (E1G), follicle stimulating hormone beta-subunit (FSH-beta), and human chorionic gonadotropin beta-subunit (hCG-beta). Correlations between assays were assessed using Pearson correlation, linear regression and Bland-Altman analysis. The temporal profiles of free cortisol, E1G, FSH-beta and hCG-beta were also compared.
Results
The multiplex array was highly correlated with the individual immunoassays for five of the tested hormones (Pearson’s correlation coefficient ≥ 0.75), and yielded temporal patterns of hormone profiles consistent with the individual immunoassays for free cortisol, E1G, FSH-beta and hCG-beta.
Conclusions
The Quansys multiplex female hormone array is a valid alternative method to individual immunoassays for the quantification of stress, reproductive and energetic hormones and metabolites in human urine samples and can be used to examine the dynamic interactions between these hormones.
Keywords: multiplex assay validation, cortisol, reproductive hormones, urine, repetitive measurements
INTRODUCTION
Evaluating how everyday psychosocial, energetic and environmental challenges affect growth, development, reproduction and health can be best achieved by using longitudinal naturalistic designs. These designs often involve the collection of a large number of specimens in difficult field conditions. Those specimens need to be properly stored and later transported to facilities where they are analyzed. In non-urban field sites appropriate storage space is frequently scarce and transport is logistically difficult and economically costly. Thus, specimens collected in those types of settings are both expensive and very valuable commodities. Methods that help reduce the expenses related to their storage and transport are therefore important to fields such as human biology, ecology and epidemiology.
Another major expense associated with longitudinal naturalistic studies is that they usually involve the measurement of multiple biomarkers in each of the numerous bio-specimens collected. Traditionally, quantification of multiple biomarkers has been performed using individual immunoassays for each biomarker. This process requires the collection, storage and preservation of enough sample volume to assess all the metabolites of interest in separate assays. Individual analyses are also costly in terms of personnel time, amount of reagents and consumables.
To reduce these costs we commissioned Quansys Biosciences (Logan, UT, USA) to develop a custom-designed multiplex array for the concurrent quantification of physiological biomarkers of stress (free cortisol), energy balance (adiponectin and c-peptide), and reproductive function [estrone-3-glucuronide (E1G), follicle stimulating hormone beta-subunit (FSH-beta), and human chorionic gonadotropin beta-subunit (hCG-beta)] in first morning urine samples. To that aim Quansys produced a 96-well array based on sandwich and competitive immunoassays which uses 6 antibodies bound in 3 × 2 spot array format to the bottom of each well of a microtiter plate. Here we present our validation of the Quansys multiplex immunoassay array based on a comparison with previously established individual immunoassays.
The simultaneous quantification of multiple biomarkers allows researchers to store and transport smaller sample volumes and saves time, reagents and consumables, reducing the amount of waste produced, providing a faster, greener and more economic alternative to individual assays.
METHODS
Two sets of urine samples were used in this validation study: 1. archived samples collected in the context of the Society, Environment and Reproduction (SER) study (Nepomnaschy et al., 2004; 2006) and 2. samples collected for the calibration of laboratory equipment. This research was approved by the Research Ethics Board of Simon Fraser University.
1. Archived Samples
Background on the SER study’s location, participants, specimen collection and sample preparation has been previously published (Nepomnaschy et al., 2004; 2006). Briefly, urine specimens were collected thrice weekly from women in a rural Kaqchikel Mayan community located in the southwest highlands of Guatemala who were parous, not pregnant, not using any form of chemical contraceptive method, and at least 6 months from the birth of their last child. We restricted our current analysis to a subset of samples collected from 15 participants in the SER study who had resumed ovarian cyclicity post-partum and who had provided at least 8 morning urine specimens (range: 8 to 39 specimens). A minimum of 8 samples allowed us to generate individual hormone/metabolite profiles for each participant. Samples were preserved at −10°C for up to 6 months in the field and then shipped on dry ice from Guatemala to the CLASS laboratory at the University of Michigan where they were archived at −80°C. The specimens were originally assayed between 2002 and 2004 in the CLASS laboratory for urinary free cortisol, estrogen conjugates (E1C), FSH-beta and hCG-beta (details below). In 2010 samples were shipped on dry ice to the Maternal and Child Health laboratory at Simon Fraser University, where they were stored again at −80°C until analysis using the multiplex assay.
2. Calibration Samples
First morning urine specimens were collected from volunteers (3 males ages 35–40; 13 females ages 20–40 in the follicular phase of the ovarian cycle; 15 females ages 20–40 in the luteal phase; 4 menopausal females ages 55–75) for the purpose of calibrating laboratory equipment. All female volunteers were not using any form of chemical contraceptive method. Aliquots from the original specimens were stored at −80°C until analysis using individual and multiplex immunoassays. Two-ml aliquots of each sample were transported on dry ice to the Center for Studies in Demography and Ecology (CSDE) at the University of Washington and stored at −20°C until analysis using individual immunoassays.
Immunoassays
Concentrations of urinary free cortisol and reproductive hormones (E1C, FSH-beta, and hCG-beta) were measured in the archived samples using immunoassays developed for use with urine samples and the Bayer Automated Chemiluminescence System (ACS-180) immunoassay analyzer in the CLASS laboratory (Nepomnaschy et al., 2004; Santoro et al., 2003).
Commercial immunoassay kits were used to determine urinary concentrations of adiponectin (Cat # 40-055-200002; GenWay Biotech, San Diego, CA, USA), free cortisol (Cat # ADI-901-071; ENZO Life Sciences, Plymouth Meeting, PA) and c-peptide (Cat # CP179S; Calbiotech, Spring Valley, CA, USA) in the calibration samples. All three commercial immunoassay kits were designed for use with human serum, plasma and urine samples. Concentrations of E1G and FSH-beta in the calibration samples were measured using in-house immunoassays developed for use with urine samples by the CSDE laboratory (Brindle et al., 2006; O’Connor et al., 2004).
A variety of different multiplex technologies exist for the simultaneous identification and quantification of multiple biomarkers in the same sample. ELISA/EIA microarrays, including the Q-plex technology used by Quansys, involve the spotting of antibodies created against different biomarkers onto glass, nitrocellulose or 96-well plates. Flow cytometry-based assays involve conjugating these antibodies to microsphere beads and mixing different combinations of antibody-bound beads together to create a multiplex assay. Both technologies are essentially micro-ELISA/EIAs within a well or on a bead. Many different companies sell multiplex arrays for a variety to analytes (e.g., cytokines, reproductive hormones, angiogenesis biomarkers). Thus, the technology for creating ELISA/EIA microarrays is widely available. The specific array we have validated is commercially available from the aforementioned company.
Each archived and calibration urine sample was analysed using the Quansys multiplex female hormone array at the Maternal and Child Health Laboratory at SFU. Each of the assays within the multiplex array works either as a sandwich or competitive immunoassay. Each of the six spots within each well contains a distinct capture antibody population. For the sandwich immunoassays (adiponectin, c-peptide, FSH-beta, hCG-beta) the biomarkers in each sample bind to their distinct capture antibody spots and are subsequently bound by biomarker-specific, horseradish peroxidise (HRP)-bound secondary antibodies. For the competitive assays (free cortisol, E1G), the biomarkers in each sample compete with HRP-conjugated forms of the biomarkers to bind to their distinct capture antibody spots. Following the addition of substrate to each well, the HRP activity and resulting chemiluminescence of each spot is directly (sandwich assay) or indirectly (competitive assay) proportional to the concentration of biomarker in the sample. To determine the biomarker concentration in each sample, an image of the plate is taken by the Quansys Q-view imager system, which includes a high-resolution Canon digital camera, and the intensity of the chemiluminescence of each spot is compared to that of a 7-point standard curve generated using 5-parameter logistic regression for that particular biomarker using Quansys Q-view software.
For all multiplex and individual assays, samples were run in duplicate wells. Outliers were identified as data points for which the coefficient of variation was > 12% between duplicates, and the samples were rerun. The standard ranges, sensitivities and precision of the Quansys multiplex array and the individual immunoassays are presented in Table 1.
Table 1.
Comparison of the Quansys multiplex immunoassay array and individual immunoassays.
| Quansys multiplex array range sensitivity and precision |
Individual immunoassay range, sensitivity and precision |
Comparison of the Quansys multiplex array with individual immunoassays |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hormone | Standard range | Sensitivity | Intra- assay CV |
Inter- assay CV |
Company | Standard range | Sensitivity | Intra- assay CV |
Inter- assay CV |
n (urine set) |
Trendlines | Pearson correlation (r) |
| adiponectin | 0.039–28.300 ng/mL |
0.023 ng/mL |
L8.7% M 11.0% H 10.4% |
L4.7% M7.1% H 8.9% |
Genway | 1.00–50.0 ng/mL |
0.156 ng/mL |
4.4% | 6.2% | 10 (calib) |
log y = 1.469(log x) + 0.315 |
0.935* |
| free cortisol |
1.018–742.000 ng/mL |
0.343 ng/mL |
L 7.4% M 6.7% H 7.3% |
L6.7% M 6.7% H 12.1% |
ENZO | 0.156–10.0 ng/mL |
0.057 ng/mL |
10.5% | 13.4% | 35 (calib) |
log y = 0.802(log x) + 1.759 |
0.780* |
| Bayer ACS: 180 |
2.0–750.0 ng/mL |
2.0 ng/mL |
2.0% | 6.5% | 320 (archiv) |
log y = 1.247(log x) + 0.312 |
0.922* | |||||
| c-peptide | 0.054–39.700 ng/mL |
0.090 ng/mL |
L 9.0% M 8.7% H 10.2% |
L 2.8% 5.7% H 14.7% |
Cal- Biotech |
0.20–9.80 ng/mL |
0.045 ng/mL |
3.9% | 10.8% | 27 (calib) |
log y = 0.722(log x) + 0.706 |
0.934* |
| E1G | 0.716–522.000 ng/mL |
0.252 ng/mL |
L 11.3% M 9.5% H 8.2% |
L 8.4% M 6.5% H.9.7% |
In-house EIA |
0.3125–40.0 ng/mL |
1.45 ng/mL |
L 3.6% H 12.2% |
L 3.2% H 13.7% |
33 (calib) |
log y = 1.403(log x) + 0.418 |
0.940* |
| Bayer ACS:180 (E1C) |
5.1–408.0 ng/mL |
0.1 ng/mL |
3.8% | 6.5% | 335 (archiv) |
log y = 1.745(log x) −0.319 |
0.914* | |||||
| FSH-beta | 0.023–16.720 ng/mL |
0.017 ng/mL |
L 4.4% M 5.5% H 11.7% |
L 8.3% M 4.6% H 9.0% |
In-house IEMA |
0.125–8.0 ng/mL |
0.143 ng/mL |
L 3.9% M 4.6% H 5.6% |
L 14.5% M 14.5% H 17.0% |
31 (calib) |
log y = 0.601(log x) + 0.212 |
0.694* |
| 0.229–167.200 mIU/mL |
0.171 mIU/mL |
Bayer ACS: 180 |
0.3–144.0 mIU/mL |
0.3 mIU/mL |
2.3% | 5.8% | 371 (archiv) |
log y = 0.836(log x) + 0.345 |
0.890* | |||
| hCG-beta | 0.058–42.500 ng/mL |
0.035 ng/mL |
L 4.7% M 5.6% H 11.1% |
L 4.1% M 5.4% H 13.4% |
Bayer ACS: 180 |
0.003–1.0 ng/mL |
0.003 ng/mL |
3.5% | 5.8% | 342 (archiv) |
log y= 0.322(log x)−0.235 |
0.427* |
Assays are considered similar if the regression analysis is significant (p < 0.05) and the Pearson correlation coefficient (r) is ≥ 0.75.
Quansys assay sensitivities are reported as the lower limit of detection (LDD) which is based on the mean plus two-times the standard deviation of the non-specific binding sample tested 20 times.
Quansys assay precision is reported as intra-assay (20 replicates of a low- (L), middle- (M) and high-concentration (H) sample) and inter-assay (low-, middle- and high-concentration samples run in duplicate across 20 plates) coefficient of variation.
Sensitivities of the in-house assays (E1G, FSH-beta) are reported as the lowest dose giving results that are significantly different from zero (p < 0.05) based on duplicate wells in 20 plates. Precision values of the in-house assays precision were calculated across 10 (FSH-beta) or 20 (E1G) plates.
Sensitivity and precision values for the individual, commercially-available immunoassays are provided by the manufacturer.
n = sample size. Urine set indicates whether the calibration (calib) or archived (archiv) urine samples were used for analysis. For all trendlines, y = Quansys values and x = individual immunoassay values.
p < 0.0001.
Assay Comparison and Statistical Analysis
The biomarker results from the multiplex assay and the individual immunoassays were not normally distributed. Therefore, they were log-transformed prior to comparison using linear regression (JMP, version 8, SAS Corporation, Cary, NC) and Bland-Altman analysis (Bland and Altman, 1999). Samples whose concentrations fell below the sensitivity of the Quansys or individual assay were not included in the analysis, resulting in different sample sizes across analytes. The assays were considered similar if the correlation between the assays was significant (two-tailed p-value <0.05) and the Pearson correlation coefficient (r) was ≥0.75.
We also determined whether the multiplex array returned hormone profile patterns consistent with the individual immunoassays by plotting and comparing log-transformed cortisol, E1G, FSH-beta and hCG-beta profiles measured using both the Quansys multiplex and the Bayer ACS:180 assays.
RESULTS AND DISCUSSION
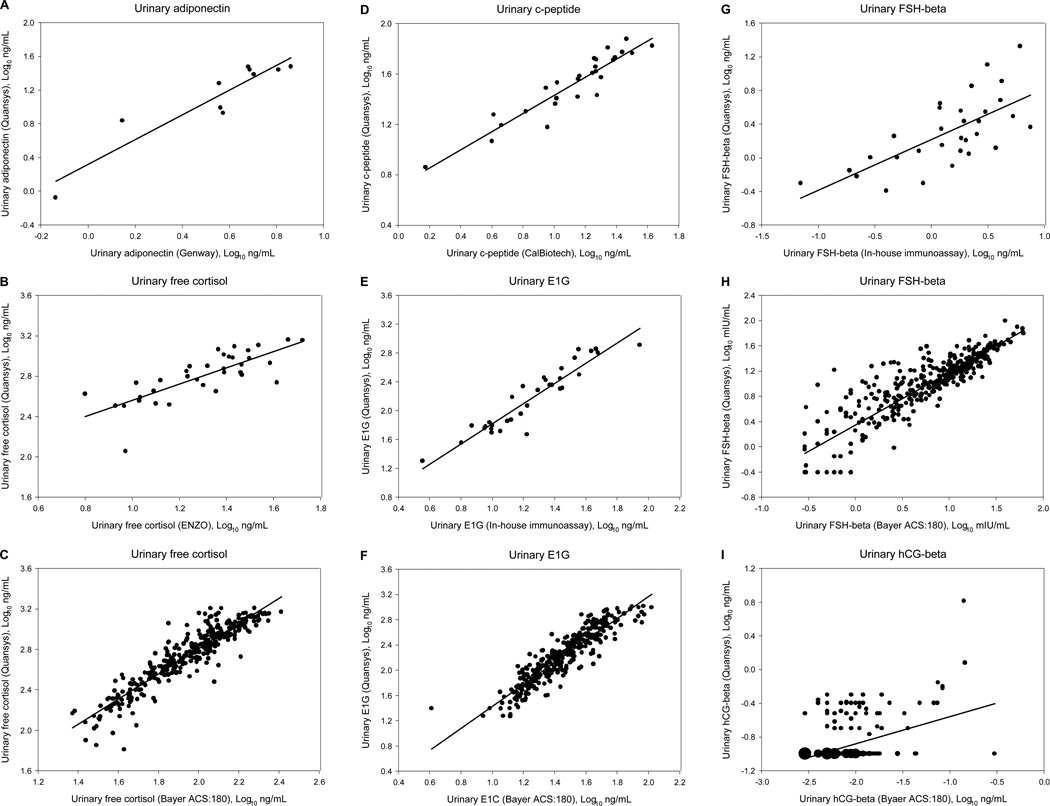
Pearson correlation coefficients and the equations for the best-fit lines are presented for all of the hormones in Table 1. Linear regression analysis indicated that five of the multiplexed assays were not statistically different from the established individual immunoassays (Table 1; Figure 1).
Figure 1.
Linear regressions of the log-transformed results from each of the six hormone measures using the multiplex assay versus the corresponding results obtained from the individual immunoassays. Each individual immunoassay comparison is shown on a separate graph (A–I). The size of each point is proportional to the number of urine samples it represents.
One comparison, between the multiplex assay and the in-house immunoassay for FSH-beta, fell below our pre-determined correlation coefficient threshold (r = 0.694 < 0.75; Table 1; Figure 1G). This relatively weaker correlation could be the result of using different antibody pairs that recognize different epitopes on the relatively large FSH-beta molecule. Furthermore, the use of different antigen standards in the multiplex and individual FSH-beta immunoassays may have also influenced the absolute values of the results. The multiplex uses intact FSH as the standard for the FSH-beta assay (with antibodies directed against FSH-beta), while the in-house IEMA uses FSH-beta as the standard. In contrast, the multiplex FSH-beta assay was highly correlated with the Bayer ACS:180 FSH-beta assay (Table 1; Figure 1H).
When all of the data for hCG-beta were included (n = 362), the Quansys multiplex and Bayer hCG-beta assays were highly correlated (r = 0.878; p < 0.001). However, the Bayer assay had a more limited range than the multiplex assay (Table 1). Subsequently, while the levels of hCG-beta associated with pregnancy maintenance were measureable by the multiplex assay, they maxed out the Bayer assay and are reported as the maximum value of the assay’s range (1.0 ng/mL hCG-beta). This resulted in two distinct groups of Bayer hCG-beta data: low values representing samples from non- or newly-pregnant women and high values representing samples from pregnant women. When only the data from non- or newly-pregnant women were included in the analysis (i.e., data within the measurable range of both assays; n = 342) the correlation between the two assays was significant, but fell below our pre-determined correlation coefficient threshold (r = 0.427 < 0.75; Table 1; Figure 1I). Thus, the hCG-beta assay was the only multiplexed assay that differed from the corresponding individual immunoassay. The use of different antibody pairs that recognize different epitopes on the hCG-beta molecule may explain the relatively weaker correlation we observed between the hCG-beta immunoassays. Therefore, for hCG-beta, hormone profile assessment (below) may be a more appropriate comparison between the two assays.
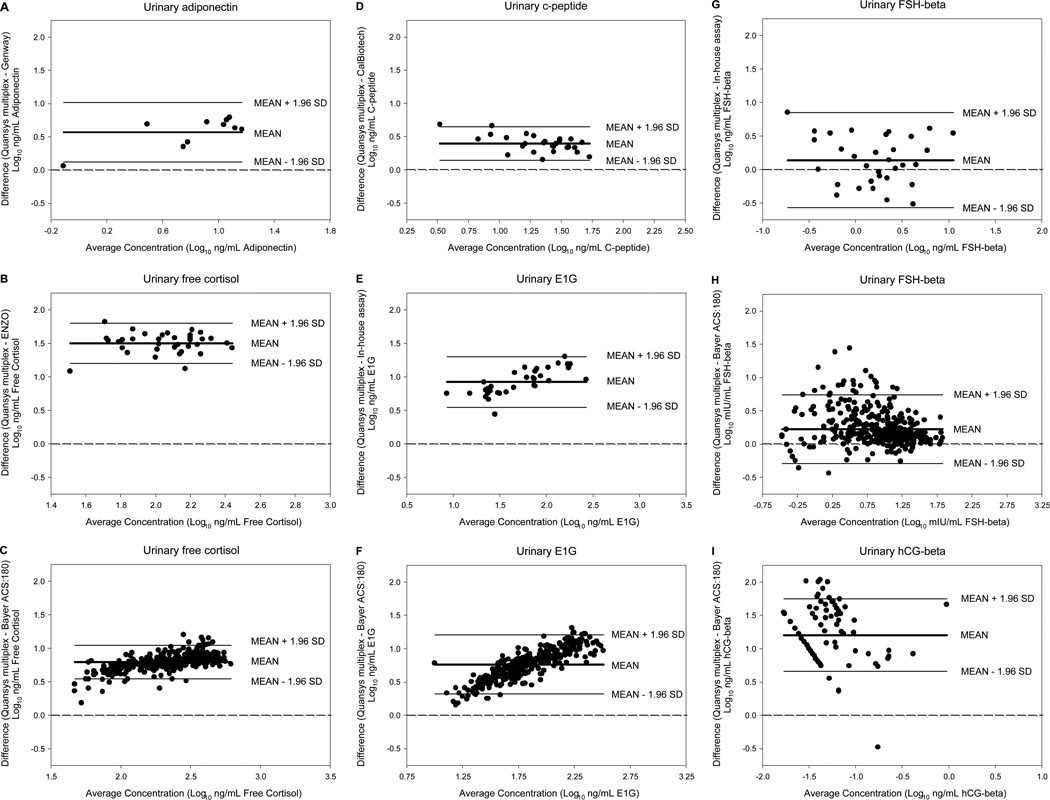
Bland-Altman analysis shows the magnitude of the differences between the multiplex and individual immunoassays for each biomarker (Figure 2). The urinary biomarker results from the individual immunoassays were consistently lower than the corresponding concentrations measured by the multiplex assay, but the differences were generally consistent across the hormone concentration ranges, with a few exceptions. When comparing the multiplexed E1G assay with both the in-house E1G EIA and the Bayer ACS:180 E1C immunoassay (Figure 2E and F, respectively), there was an increase in the difference between the assays as the magnitude of E1G concentration increased, but no corresponding change in the variability of the differences. In contrast, there was an increase in the variability of the differences as the magnitude of FSH-beta concentration decreased in the comparison between urinary FSH-beta as measured by multiplex and the Bayer ACS:180 immunoassay (Figure 2H).
Figure 2.
Bland-Altman plots of the differences between log-transformed urinary biomarker concentration measured by the multiplex and individual immunoassays plotted against the average log-transformed urinary biomarker concentration. Each individual immunoassay comparison is shown on a separate graph (A–I). Each point represents a urine sample. The center solid line indicates the mean difference. The upper and lower solid lines indicate the 95% confidence intervals, or limits of agreement. The dashed line indicates the line of equality.
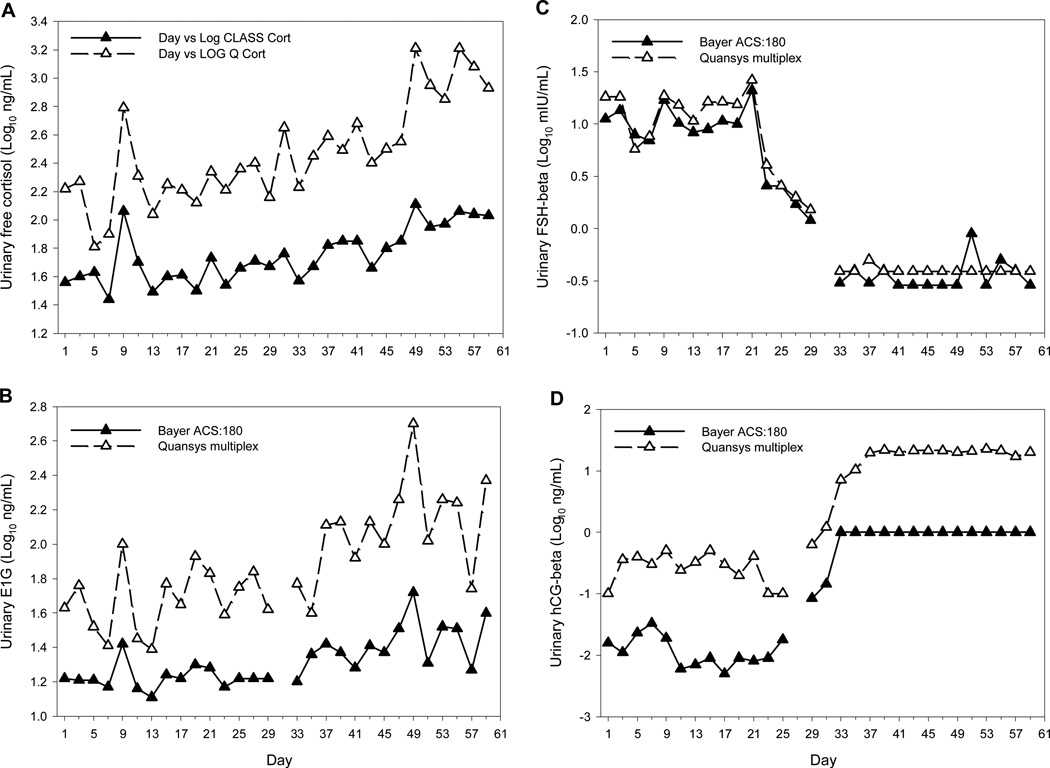
Free cortisol, E1G, FSH-beta and hCG-beta profiles obtained for a representative participant measured using the multiplex and Bayer assays are shown in Figure 3. The multiplex and Bayer ACS:180 immunoassays resulted in similar temporal patterns for urinary free cortisol (Figure 3A), E1G (Figure 3B), FSH-beta (Figure 3C) and hCG-beta (Figure 3D), with similar peaks and troughs throughout the sampling period. These results were similar for the other 14 participants. As both methods consistently detected the same patterns in the excretion rates of all four hormones, both assays are valid when assessing temporal changes in these hormones.
Figure 3.
Free cortisol (A), E1G (B), FSH-beta (C) and hCG-beta (D) profiles for subject 3 as determined by Bayer ACS:180 (▲) and Quansys multiplex (∆) immunoassays.
Our validation results confirm that the Quansys multiplex female array is a valid alternative to individual immunoassays for the quantification of adiponectin, free cortisol, c-peptide, E1G, FSH-beta and hCG-beta in human urine samples and can be used to assess daily changes in the urinary excretion of these hormones and their dynamic interactions. This multiplex technology provides a more economic, rapid and ecologically sound alternative to individual assays for studies requiring the measurement of multiple biomarkers per bio-specimen.
ACKNOWLEDGEMENTS
We thank the personnel of the CLASS Laboratory for assistance with the Bayer ACS-180 hormone analyses and our Guatemalan assistants for their dedicated help during fieldwork.
Grant information: This research was supported by a Canadian Institutes for Health Research Operating Grant (CIHR 106705) and a Simon Fraser University Start-up Grant to P.A.N. and a National Institutes of Health, Eunice Kennedy Shriver Nation Institute of Child Health & Human Development Center Grant (NICHD 5R24HD042828-09) to the Center for Studies in Demography and Ecology of the University of Washington.
ABBREVIATIONS
- E1G
estrone-3-glucuronide
- E1C
estrogen conjugates
- FSH-beta
follicle stimulating hormone beta-subunit
- hCG-beta
human chorionic gonadotropin beta-subunit
LITERATURE CITED
- 1.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Meth Med Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 2.Brindle E, Miller RC, Shofer JB, Klein NA, Soules MR, O’Connor KA. Urinary beta-luteinizing hormones and beta-follicle stimulating hormone immunoenzymometric assays for population research. Clin Biochem. 2006;39:1071–1079. doi: 10.1016/j.clinbiochem.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nepomnaschy PA, Lee TCK, Zeng L, Dean CB. (under review) Using first morning cortisol levels to compare physiologic stress levels between individuals: Ranking methods and sample sizes. Submitted to Proc Roy Soc B. 2011 August [Google Scholar]
- 4.Nepomnaschy PA, Welch KB, McConnell DS, Low BS, Strassmann BI, England BG. Cortisol levels and very early pregnancy loss in humans. Proc Natl Acad Sci USA. 2006;103:3938–3942. doi: 10.1073/pnas.0511183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nepomnaschy PA, Welch K, McConnell D, Strassmann BI, England BG. Stress and female reproductive function: A study of daily variations in cortisol, gonadotrophins, and gonadal steroids in a rural Mayan population. Am J Hum Biol. 2004;16:523–532. doi: 10.1002/ajhb.20057. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor KA, Brindle E, Shofer JB, Miller RC, Klein NA, Soules MR, Campbell KL, Mar C, Handcock MS. Statistical correction for non-parallelism in a urinary enzyme immunoassay. J Immunoassay Immunochem. 2004;25:259–278. doi: 10.1081/ias-200028078. [DOI] [PubMed] [Google Scholar]
- 7.Santoro N, Crawford SL, Allsworth JE, Gold EB, Greendale GA, Korenman S, Lasley BL, McConnell D, McGaffigan P, Midgely R, Schocken M, Sowers M, Weiss G. Assessing menstrual cycles with urinary hormone assays. Am J Phys Endocrinol Metab. 2003;284:E521–E530. doi: 10.1152/ajpendo.00381.2002. [DOI] [PubMed] [Google Scholar]