Abstract
The aims of this study were: (1) to define the extent to which a high-fat (HF) diet given on a long-term basis reduces resting plasma ghrelin (total [acyl + des-acyl]) levels and the plasma ghrelin (total) response to fasting, (2) to determine whether a chronic HF diet modifies the orexigenic activity of acyl-ghrelin, (3) whether insulin pretreatment inhibits the plasma ghrelin (total) response to fasting, and (4) the extent to which pioglitazone (PIO) treatment will increase stomach and plasma ghrelin (total) levels in rats fed a HF diet. PIO is a drug given to diabetics which improves insulin resistance. Our findings show that a chronic HF diet given for either 10 or 60 weeks exerts a persistent inhibitory effect on resting plasma ghrelin (total) levels. Additionally, the plasma ghrelin (total) elevation to overnight fasting is not altered in rats fed a HF diet on a long-term basis. A HF diet does not impair the ingestive response to acyl-ghrelin. Together, these results suggest that acyl-ghrelin serves as an important orexigenic factor. Results show that insulin pretreatment does not inhibit the plasma ghrelin (total) response to fasting suggesting that meal-induced insulin secretion does not have a role in reducing ghrelin (total) secretion. In rats fed a HF diet, PIO administration increases stomach ghrelin (total) levels. Because PIO can reduce systemic glucose and lipid levels, our findings suggest that elevated glucose and lipid levels are part of the inhibitory mechanism behind reduced ghrelin (total) secretion in rats fed a HF diet.
Keywords: high fat, chronic, stomach hormone
1. INTRODUCTION
Ghrelin is an important stomach hormone that influences several metabolic activities including gastrointestinal motility, growth hormone (GH) secretion, ingestive behavior, body growth and adiposity [1, 2]. Ghrelin was identified as the endogenous ligand for the GH secretagogue receptor (GHS-R) [2, 3]. The idea of a natural GH-releasing substance in addition to hypothalamic GH-releasing hormone (GH-RH) has its bases in the synthesis of numerous artificial GH secretagogues (GHSs) and the cloning of an endogenous GHS receptor (GHS-R) [4].
Ghrelin is synthesized in stomach mucosal enteroendocrine X/A cells [5, 6]. Ghrelin is a unique gut hormone since it is modified with an n-octanoyl group at the Ser(3) residue [2, 3]. In fact, ghrelin is synthesized as either an acylated or a des-acylated form [7]. Peripheral administration of acyl-ghrelin exerts feeding activity whereas des-acyl-ghrelin is inactive [8]. In humans and rats, gastrectomy will reduce circulating ghrelin levels by approximately 85–90% indicating that hormonal ghrelin is derived predominantly from the stomach [9, 10].
Several lines of evidence show that stomach ghrelin secretion is influenced by energy levels. In humans and laboratory rodents, plasma ghrelin levels increase in response to food restriction and fasting and decline significantly upon refeeding [11, 12]. In obese humans and in rodents that consume excess calories, resting plasma ghrelin levels are reduced significantly [12–14]. Candidate inhibitory signals causing reduced ghrelin secretion following a meal or during obesity include increased plasma triglyceride, glucose and insulin levels [15]. In obese rats consuming a high-fat (HF) diet chronically, increased body fat per se has been shown not to mediate reduced ghrelin production and secretion [15].
The aims of the present study were: (1) to determine the extent to which a HF diet given on a long-term basis reduces resting ghrelin (total) levels and the plasma ghrelin (total) response to fasting, (2) to determine whether a HF diet modifies the orexigenic activity of acyl-ghrelin, (3) the extent to which insulin treatment inhibits the plasma ghrelin (total) response to fasting, and (4) whether pioglitazone (PIO) treatment will increase stomach and plasma ghrelin (total) levels in rats fed a HF diet. PIO is a drug given to diabetics to decrease insulin resistance. PIO also lowers systemic glucose and lipid levels [16–19]. The rationale behind testing the influence of PIO is that by reducing the inhibitory effects of elevated circulating glucose and lipid levels during a HF diet, PIO is expected to restore stomach ghrelin production and secretion.
2. METHODS
2.1. Animals
All animal experiments were conducted in accordance with mandated standards of humane care and were approved by the UTMB Animal Care and Use Committee. Adult male Sprague-Dawley (SD) rats were maintained in an air-conditioned and light-regulated room (lights on from 0600 to 1800 h) and given access to food and water ad libitum except in cases where rats were fasted.
2.2. Chemicals and Peptides
All chemicals were obtained from Sigma Chemical (St. Louis, MO). Synthetic peptides were purchased from either Phoenix (Belmont, CA) or Bachem (Torrance, CA). Human insulin (Novolin R, Novo Nordisk Inc., Princeton, NJ) was used. Pioglitazone was obtained from Takeda Pharmaceuticals North America (Deerfield, IL) and prepared in 0.5% methyl cellulose.
2.3. Experiments
Experiment 1
The aims of this experiment were to define the effects of long-term HF diets (10, 60 weeks) on 1) resting plasma ghrelin (total: acyl + des-acyl) levels, and 2) the plasma ghrelin (total) response to fasting. Two sets of adult male Sprague-Dawley (SD) rats (8 weeks old) were fed ad libitum either a standard AIN-76A or a HF diet (Bioserve, Frenchtown, NJ) for 10 or 60 weeks. The diet compositions (approximate percentage of calories) are as follows: 10% fat, 20% protein and 67% carbohydrate for the AIN-76A biscuit; and 48% fat (beef tallow), 16% protein and 34% carbohydrate for the HF diet. Two percent of the fat calories in the beef tallow-containing diet were derived from safflower oil to supply essential fatty acids. Plasma was harvested from the jugular vein under isoflurane or ether anesthesia from fed and overnight fasted (~18 h) rats. Plasma ghrelin (total) levels were measured by an immunoassay. During an early segment of HF feeding, body weights and daily caloric intakes were recorded. Rats fed the AIN-76 diet weighed 332-370 g and consumed an average of 69.8 ± 4.2 kcal/rat/day whereas HF fed rats weighed 371-420 g and consumed 82.1 ± 7.1 kcal/rat/day.
Experiment 2
The aim of this experiment was to define the effect of a HF diet on the orexigenic activity of acyl-ghrelin. Adult male SD rats (8 weeks old) were fed either AIN-76A or HF diets for 12 weeks as described for Experiment 1. The acute food intake response (60 min) to exogenous saline or acyl-ghrelin (3.5 nmol/kg, IP) was measured in fed rats at 1000-1100 h. Rats were acclimatized to handling and IP administration of saline before testing responses to saline and acyl-ghrelin.
Experiment 3
The aim of this experiment was to examine the influence of insulin administration on the fasting-induced elevation in plasma ghrelin (total) levels in adult male SD rats fed an AIN-76A diet for 60 weeks. Rats were given 2 injections of insulin (20U/kg, IP; 1000 and 1600 h) prior to start of fasting. Rats were then fasted overnight (~18 h, 1600-1000 h) before blood collection.
Experiment 4
A HF diet will suppress ghrelin secretion. The aim of this experiment was to examine whether PIO administration will increase stomach ghrelin synthesis and secretion in rats fed a HF diet. Adult male SD rats were fed either an AIN-76A or a HF diet for four weeks, the diets were continued and PIO (10 mg/kg) or vehicle was given by gastric lavage five days/week (Monday through Friday) for 2 weeks. Rats were sacrificed ~24 h after the last doses of PIO or vehicle. Plasma and stomach fundal mucosa were harvested. Plasma and stomach ghrelin (total) levels were measured by immunoassay. Stomach ghrelin mRNA levels were measured by Northern blotting analysis.
2.4. Ghrelin Assay
Plasma ghrelin levels were measured by a ghrelin immunoassay [20] that measures total ghrelin levels (acyl + des-acyl) [20]. Plasma ghrelin was extracted from rat plasma specimens by use of C18 Sep Pak cartridges (Waters, Milford, MA) [12]. Stomach ghrelin peptides were extracted from stomach tissue specimens as reported [12].
2.5. Ghrelin mRNA determination
Stomach ghrelin mRNA levels were measured by Northern blotting analysis as described previously [10, 12, 15].
2.6. Statistics
Values are means ± SE. Data were evaluated using an ANOVA followed by Newmann-Keuls test where appropriate. Statview software (Cary, NC) was used for data analysis.
3. RESULTS
Experiment 1
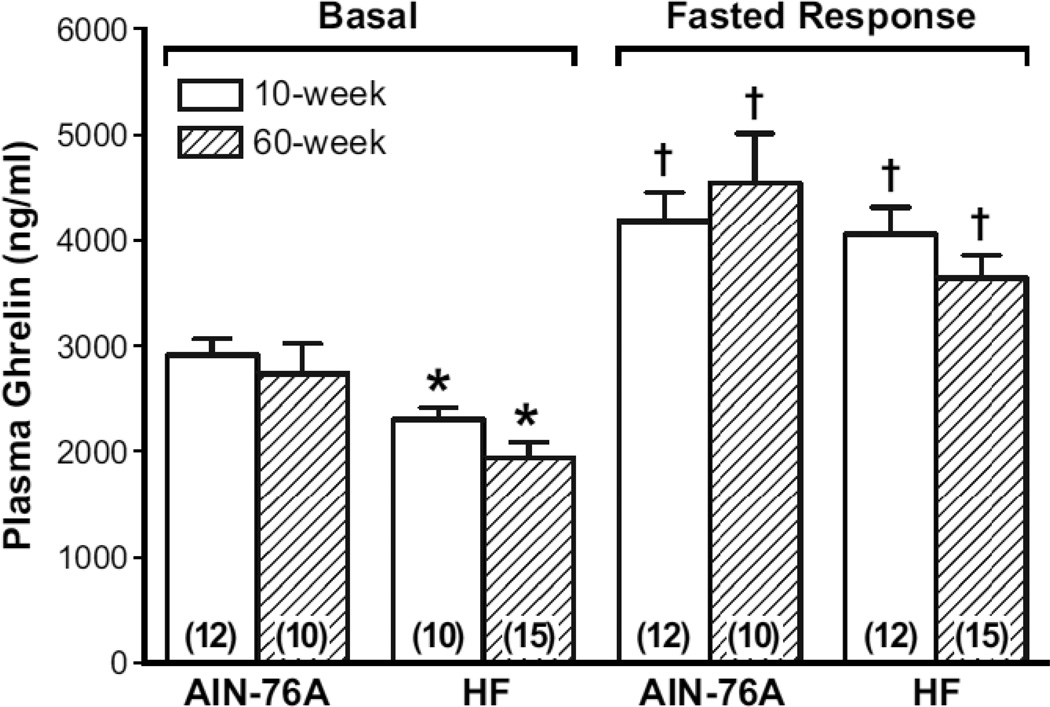
The aims of this experiment were to define the effects of a HF diet given on a long-term basis (10 or 60 weeks) on resting plasma ghrelin (total) levels and the plasma ghrelin (total) response to an eighteen hour fast (Figure 1). Previously, our laboratory reported that a HF diet given for 30 days reduced resting plasma ghrelin levels [12]. Results show that resting plasma ghrelin (total) levels were reduced significantly (p<0.05) in rats fed a HF diet either for 10 or 60 weeks when compared to rats fed a standard dietary level of fat (AIN-76A) (Figure 1). Resting plasma ghrelin (total) levels are reduced approximately 20% and 30% in rats fed a HF diet for 10 and 60 weeks, respectively.
Figure 1. Effect of chronic high-fat (HF) diet on basal plasma ghrelin levels and the plasma ghrelin response to fasting.
Male Sprague-Dawley rats were fed either a normal fat-AIN-76A (10% fat calories) or a HF diet (~45% fat calories) for either 10 or 60 weeks. Plasma was harvested either from ad lib fed or overnight fasted (~18 h) rats. Ghrelin levels were measured by immunoassay. The number inside the parenthesis is the number of rats/group. * =P <0.05 vs. respective mean in rats fed AIN-76A diet; † = P < 0.05 vs. respective basal plasma ghrelin mean.
In rats fed an AIN-76A diet, plasma ghrelin (total) levels increased significantly in response to an overnight fast. In rats fed a HF diet for either 10 or 60 weeks, plasma ghrelin (total) levels increased significantly (~1700 ng/ml) in response to an overnight fast. Plasma ghrelin (total) levels increased 1.4 to 1.9-fold in response to an overnight fast in rats fed AIN-76A and HF diets.
Experiment 2
The aim of this experiment was to determine whether a HF diet will alter the orexigenic response to exogenous acyl-ghrelin. In AIN-76A and HF-fed rats, food intake during the 1-hour period (1000-1100 h) following acyl-ghrelin administration was significantly greater when compared to saline administration (Table 1). The food intake response to acyl-ghrelin was significantly greater in rats fed HF diet when compared to rats fed the AIN-76A diet.
Table 1.
Ghrelin stimulates food intake in rats fed a high-fat diet.
| Group-diet Type | Saline or Ghrelin Challenge | Food Intake (kcal/rat) |
|---|---|---|
| AIN-76A | Saline | 2.3 ± 0.51 |
| Ghrelin | 4.6 ± 0.5* | |
| High-fat | Saline | 8.5 ± 1.2† |
| Ghrelin | 12.2 ± 1.0*† |
1 = mean food intake ± SEM; N = 8–12 rats/group,
p<0.05 vs. saline.
p<0.05 vs. respective group of AIN-76A fed rats.
Experiment 3
Systemic levels of insulin are elevated during obesity. Circulating insulin may inhibit ghrelin secretion. The aim of this experiment was to determine the extent to which insulin pretreatment will influence the fasting-induced elevation in plasma ghrelin (total) levels. Rats were given insulin or vehicle and then fasted overnight before plasma collection. Results show that insulin treatment failed to alter the fasting-induced elevation in plasma ghrelin (total) levels (Table 2).
Table 2.
Effects of insulin pretreatment on plasma ghrelin response to an overnight fast.
| Plasma Ghrelin Response (% of vehicle-treated response) |
|
|---|---|
| Group treatment | |
| Fasted + Vehicle | 103 ± 111 |
| Fasted + Insulin | 96 ± 8 |
Rats were pretreated with insulin, fasted overnight (~18h) and then plasma was collected. 1 = mean ± SEM. N =7–9 rats/group.
Experiment 4
The aim of this experiment was to determine the extent to which pioglitazone (PIO) administration will elevate stomach ghrelin (total) production and secretion in rats fed a HF diet. When compared to rats fed a normal-fat diet (AIN-76A), stomach ghrelin mRNA and peptide levels were reduced significantly in rats fed a HF diet (Table 3). Plasma ghrelin (total) levels were also reduced significantly in rats fed the HF diet. PIO administration increased plasma ghrelin (total) levels significantly when compared to plasma ghrelin (total) levels of vehicle-treated HF rats. PIO treatment also increased gastric ghrelin mRNA and ghrelin (total) peptide levels significantly in rats fed a HF diet.
Table 3.
Restoration of stomach ghrelin synthesis and secretion by pioglitazone.
| Stomach ghrelin |
|||
|---|---|---|---|
| Diet type-treatment | mRNA1 | Peptide (µg/g tissue) | Plasma ghrelin (ng/ml) |
| AIN-76A diet | 41 ± 2.52 | 67 ± 6 | 2680 ± 185 |
| HF diet + vehicle | 19 ± 2.9* | 41 ± 3* | 1596 ± 163* |
| HF + PIO | 17 ± 4.3* | 54 ± 7† | 2456 ± 260† |
N ≥ = 9/group;
ghrelin mRNA/18S ribosomal mRNA;
mean ± SEM;
p<0.05 vs. AIN-76A diet;
p<0.05 vs. HF + vehicle.
4. DISCUSSION
The aims of this present study were: (1) to determine the extent to which a HF diet given on a long-term basis will reduce resting ghrelin (total) levels and the plasma ghrelin (total) response to fasting, (2) to determine whether a HF diet modifies the orexigenic activity of acyl-ghrelin, (3) whether insulin pretreatment inhibits the plasma ghrelin (total) response to fasting, and (4) the extent to which PIO treatment will restore ghrelin synthesis and secretion in obese rats fed a HF diet. In these studies, tissue and plasma ghrelin levels were measured by an immunoassy that detects total ghrelin levels (acyl + des-acyl).
Numerous studies demonstrate that energy balance has a key role in determining fasting plasma ghrelin levels with ghrelin levels being significantly elevated by food restriction (cachexia, fasting) and reduced during obesity and refeeding in humans and laboratory animals [12–14]. In laboratory animals obesity is induced typically by feeding a HF diet chronically whereas obesity in humans occurs as a result of excessive caloric intake, physical inactivity or both. It is generally believed that changes in ghrelin secretion are adaptive responses to stimulate or suppress appetite according to the ongoing energy imbalance.
Here, we have shown that basal plasma ghrelin (total) levels remain suppressed significantly and do not escape the inhibitory effect of a HF diet administered for a prolonged period (60 weeks). Plasma ghrelin (total) levels are reduced by 20 and 29% in rats fed a HF diet for 10 and 60 weeks, respectively. We have previously reported a 40% reduction in resting plasma ghrelin (total) levels in rats fed a HF diet for 4 weeks [12]. A more significant finding is the persistent plasma ghrelin (total) elevation to overnight fasting in rats fed long-term HF diets. The magnitudes of the fasting-induced plasma ghrelin (total) elevations (~80%) are nearly identical in rats fed a HF diet for either 10 or 60 weeks. Furthermore, the fasting-induced plasma ghrelin (total) elevations in rats fed a HF and a normal fat-containing diet (AIN-76A) are of similar magnitudes. A persistent plasma ghrelin response to fasting in obese rats suggests that stomach ghrelin is an essential signal behind stimulation of food intake. The importance of ghrelin’s orexigenic activity is supported by further data showing that a HF diet does not impair the food intake response to exogenous acyl-ghrelin. In fact, the food intake response is amplified in rats fed a HF diet. We also found that the food intake response to GH-releasing hormone-6 (GHRP-6), a synthetic GHS, is enhanced in rats fed a HF diet (data not shown). Together, these findings imply that the ghrelin-GHS-R axis evolved to promote food intake during periods of food abundance in spite of excess body energy stores. Presumably, this mechanism will prevent starvation during food scarcity in animals exposed to cycles of food abundance and scarcity. A prior report showed an enhanced expression of GHS-R in vagal-nodose tissue of rats with dietinduced obesity (DIO) [21]. In the nodose ganglion, the GHS-R is thought to be part of a signaling pathway that mediates the influence of peripheral ghrelin on food intake to the brain [22]. Therefore, an increased GHS-R expression in the nodose ganglion may underlie an enhanced orexigenic response to acyl-ghrelin in obese rats. In agreement with our findings, a human study showed that obese subjects are more sensitive to the orexigenic effects of exogenous ghrelin. Ghrelin infusions increased energy intake by 70% in obese individuals compared to 20% in lean individuals [23]. An earlier rodent report also supports the idea that ghrelin signaling is a critical mechanism behind development of obesity since mice deficient in ghrelin receptors will resist development of obesity when given a HF diet [24]. In contrast to our data and these earlier human and murine findings, several studies show that a HF diet will impair ghrelin’s ingestive activity [25–27]. Because mice were used in these latter studies, the discrepant findings may simply reflect species differences. Interestingly, a rat study indicates that repetitive ghrelin treatment stimulates adiposity without a reduction in its orexigenic activity [28]. Alternatively, use of different experimental models may underlie the disparate results. For instance, one of these mouse studies showed that a HF diet reduces hyperphagia induced by chronic hyperghrelinemia [26]. In this study, a HF diet was given to mice with persistent hyperghrelinemia induced either genetically or by implantation of a ghrelin containing osmotic pump.
Another aim of the present study was to examine whether the plasma ghrelin response to fasting is inhibited by insulin treatment. Elevated systemic insulin levels may be one mechanism behind reduced ghrelin secretion during HF diets and obesity. Human studies suggest that insulin exerts an inhibitory effect on ghrelin secretion since plasma ghrelin and insulin levels show an inverse relationship at the time of food intake and during an euglycemia clamp [29]. Another human study showed a lack of significant correlation of systemic ghrelin with insulin changes in response to an OGTT in obese children with insulin resistance [30]. Additionally, in rats with streptozotocin-induced diabetes, plasma ghrelin levels decreased following a meal in spite of a blunted rise in systemic insulin levels [31]. Although our experimental model differs when compared to these earlier reports, the present results clearly show that an elevated insulin tone does not modify the fasting-induced elevation in plasma ghrelin levels. Our findings agree with a human study showing that an elevation in systemic insulin levels induced by administration of sitagliptin, an insulin enhancing agent, did not alter resting ghrelin levels [32]. Nonetheless, in view of the numerous contrasting reports, further investigation is needed to resolve the influence of insulin on ghrelin secretion.
The present study is to first to show that administration of pioglitazone (PIO), a thiazolidinedione (TZD), results in elevated stomach ghrelin peptide production and secretion in spite of an ongoing HF diet, a potent inhibitory signal on ghrelin synthesis and secretion. In terms of a mechanism, the PIO-induced elevation in stomach ghrelin homeostasis is most likely triggered by PIO-induced declines in systemic glucose and lipid levels. PIO, like other TZDs has been demonstrated to lower systemic levels of glucose and lipids [16–19].
4.1. Conclusions
Two findings support the idea that ghrelin evolved as an important orexigenic factor: an unimpaired elevation in plasma ghrelin (total) levels in response to overnight fasting and a preserved ingestive response to acyl-ghrelin challenge in rats having excess stored body energy. Our data also imply that insulin does not influence ghrelin secretion. Lastly, because PIO administration increased stomach and plasma ghrelin levels, our findings support the idea that elevated systemic glucose and lipid levels are part of the inhibitory mechanism of a HF diet and obesity on ghrelin production and secretion.
Highlights.
A long-term high-fat diet in rats has a persistent inhibitory effect on basal plasma ghrelin levels.
The plasma ghrelin elevation to an overnight fast persists in rats fed a high-fat diet for a long-term basis.
A high-fat diet does not inhibit the ingestive response to ghrelin.
Insulin does not inhibit the plasma ghrelin response to fasting.
Pioglitazone treatment increases stomach ghrelin homeostasis in rats fed a high-fat diet.
ACKNOWLEDGEMENTS
This work is supported by National Institutes of Health grant R01 DK061614.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ejskjaer N, Dimcevski G, Wo J, Hellstrom PM, Gormsen LC, Sarosiek I, et al. Safety and efficacy of ghrelin agonist TZP-101 in relieving symptoms in patients with diabetic gastroparesis: a randomized, placebo-controlled study. Neurogastroenterol Motil. 2010;22:1069-e281. doi: 10.1111/j.1365-2982.2010.01519.x. [DOI] [PubMed] [Google Scholar]
- 2.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 3.Tomasetto C, Karam SM, Ribieras S, Masson R, Lefebvre O, Staub A, et al. Identification and characterization of a novel gastric peptide hormone: the motilin-related peptide. Gastroenterology. 2000;119:395–405. doi: 10.1053/gast.2000.9371. [DOI] [PubMed] [Google Scholar]
- 4.Smith RG, Leonard R, Bailey AR, Palyha O, Feighner S, Tan C, et al. Growth hormone secretagogue receptor family members and ligands. Endocrine. 2001;14:9–14. doi: 10.1385/ENDO:14:1:009. [DOI] [PubMed] [Google Scholar]
- 5.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 6.Mizutani M, Atsuchi K, Asakawa A, Matsuda N, Fujimura M, Inui A, et al. Localization of acyl ghrelin- and des-acyl ghrelin-immunoreactive cells in the rat stomach and their responses to intragastric pH. Am J Physiol Gastrointest Liver Physiol. 2009;297:G974–G980. doi: 10.1152/ajpgi.00147.2009. [DOI] [PubMed] [Google Scholar]
- 7.Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. 2000;279:909–913. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- 8.Toshinai K, Yamaguchi H, Sun Y, Smith RG, Yamanaka A, Sakurai T, et al. Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology. 2006;147:2306–2314. doi: 10.1210/en.2005-1357. [DOI] [PubMed] [Google Scholar]
- 9.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 10.Gomez G, Englander EW, Greeley GH., Jr Nutrient inhibition of ghrelin secretion in the fasted rat. Regul Pept. 2004;117:33–36. doi: 10.1016/j.regpep.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Diz-Lois MT, Garcia-Buela J, Suarez F, Sangiao-Alvarellos S, Vidal O, Cordido F. Altered fasting and postprandial plasma ghrelin levels in patients with liver failure are normalized after liver transplantation. Eur J Endocrinol. 2010;163:609–616. doi: 10.1530/EJE-10-0508. [DOI] [PubMed] [Google Scholar]
- 12.Lee HM, Wang G, Englander EW, Kojima M, Greeley GH., Jr Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology. 2002;143:185–190. doi: 10.1210/endo.143.1.8602. [DOI] [PubMed] [Google Scholar]
- 13.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 14.Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 15.Qi X, Reed JT, Wang G, Han S, Englander EW, Greeley GH., Jr Ghrelin secretion is not reduced by increased fat mass during diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2008;295:R429–R435. doi: 10.1152/ajpregu.90329.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boden G, Homko C, Mozzoli M, Showe LC, Nichols C, Cheung P. Thiazolidinediones upregulate fatty acid uptake and oxidation in adipose tissue of diabetic patients. Diabetes. 2005;54:880–885. doi: 10.2337/diabetes.54.3.880. [DOI] [PubMed] [Google Scholar]
- 17.Kanda S, Nakashima R, Takahashi K, Tanaka J, Ogawa J, Ogata T, et al. Potent antidiabetic effects of rivoglitazone, a novel peroxisome proliferator-activated receptor-gamma agonist, in obese diabetic rodent models. J Pharmacol Sci. 2009;111:155–166. doi: 10.1254/jphs.09084fp. [DOI] [PubMed] [Google Scholar]
- 18.LeBrasseur NK, Kelly M, Tsao TS, Farmer SR, Saha AK, Ruderman NB, et al. Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues. Am J Physiol Endocrinol Metab. 2006;291:E175–E181. doi: 10.1152/ajpendo.00453.2005. [DOI] [PubMed] [Google Scholar]
- 19.Ye JM, Dzamko N, Hoy AJ, Iglesias MA, Kemp B, Kraegen E. Rosiglitazone treatment enhances acute AMP-activated protein kinase-mediated muscle and adipose tissue glucose uptake in high-fat-fed rats. Diabetes. 2006;55:2797–2804. doi: 10.2337/db05-1315. [DOI] [PubMed] [Google Scholar]
- 20.Wei W, Qi X, Reed J, Ceci J, Wang HQ, Wang G, et al. Effect of chronic hyperghrelinemia on ingestive action of ghrelin. Am J Physiol Regul Integr Comp Physiol. 2006;290:R803–R808. doi: 10.1152/ajpregu.00331.2005. [DOI] [PubMed] [Google Scholar]
- 21.Paulino G, Barbier de la Serre C, Knotts TA, Oort PJ, Newman JW, Adams SH, et al. Increased expression of receptors for orexigenic factors in nodose ganglion of diet-induced obese rats. Am J Physiol Endocrinol Metab. 2009;296:E898–E903. doi: 10.1152/ajpendo.90796.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 23.Druce MR, Wren AM, Park AJ, Milton JE, Patterson M, Frost G, et al. Ghrelin increases food intake in obese as well as lean subjects. Int J Obes (Lond) 2005;29:1130–1136. doi: 10.1038/sj.ijo.0803001. [DOI] [PubMed] [Google Scholar]
- 24.Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology. 2010;151:4745–4755. doi: 10.1210/en.2010-0556. [DOI] [PubMed] [Google Scholar]
- 26.Gardiner JV, Campbell D, Patterson M, Kent A, Ghatei MA, Bloom SR, et al. The hyperphagic effect of ghrelin is inhibited in mice by a diet high in fat. Gastroenterology. 2010;138:2468–2476. doi: 10.1053/j.gastro.2010.02.012. 76 e1. [DOI] [PubMed] [Google Scholar]
- 27.Perreault M, Istrate N, Wang L, Nichols AJ, Tozzo E, Stricker-Krongrad A. Resistance to the orexigenic effect of ghrelin in dietary-induced obesity in mice: reversal upon weight loss. Int J Obes Relat Metab Disord. 2004;28:879–885. doi: 10.1038/sj.ijo.0802640. [DOI] [PubMed] [Google Scholar]
- 28.Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- 29.Saad MF, Bernaba B, Hwu CM, Jinagouda S, Fahmi S, Kogosov E, et al. Insulin regulates plasma ghrelin concentration. J Clin Endocrinol Metab. 2002;87:3997–4000. doi: 10.1210/jcem.87.8.8879. [DOI] [PubMed] [Google Scholar]
- 30.Wang XM, Jiang YJ, Liang L, Du LZ. Changes of ghrelin following oral glucose tolerance test in obese children with insulin resistance. World J Gastroenterol. 2008;14:1919–1924. doi: 10.3748/wjg.14.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gelling RW, Overduin J, Morrison CD, Morton GJ, Frayo RS, Cummings DE, et al. Effect of uncontrolled diabetes on plasma ghrelin concentrations and ghrelin-induced feeding. Endocrinology. 2004;145:4575–4582. doi: 10.1210/en.2004-0605. [DOI] [PubMed] [Google Scholar]
- 32.Huang CL, Hsu CH, Huang KC, Su HY, Weng SF. Preprandial single oral dose of sitagliptin does not affect circulating ghrelin and gastrin levels in normal subjects. Pharmacology. 2010;85:131–135. doi: 10.1159/000280583. [DOI] [PubMed] [Google Scholar]