Abstract
Background
Chronic inflammation caused by hepatitis B virus infection, hepatitis C virus infection, and/or heavy alcohol use can lead to fibrosis, cirrhosis, and eventually hepatocellular carcinoma (HCC). FIB-4 is an index score calculated from platelet count, alanine transaminase, aspartate transaminase, and age that predicts fibrosis and cirrhosis. We hypothesized that high FIB-4 would be associated with development of HCC in HIV-infected persons, who are at high risk due to high prevalence of viral hepatitis and alcohol consumption, and possibly due to HIV infection itself.
Methods
Using proportional hazards models, we tested this hypothesis among 22,980 HIV-infected men from the Veterans Aging Cohort Study. We identified incident HCC cases from the VA Central Cancer Registry.
Results
During follow-up, there were 112 incident HCC diagnoses. The age-and race/ethnic group-adjusted HR was 4.2 (95% CI: 2.4, 7.4)for intermediate FIB-4 and 13.0 (95% CI: 7.2, 23.4) for high FIB-4, compared to low FIB-4. After further adjustment for enrollment year, CD4 count, HIV-1 RNA level, antiretroviral therapy use, hepatitis B and C virus infection, alcohol abuse/dependency, and diabetes, FIB-4 remained a strong, significant, independent risk factor for HCC. The multivariate-adjusted HR was 3.6 (95% CI: 2.1, 6.4) for intermediate FIB-4 and 9.6 (95% CI: 5.2, 17.4) for high FIB-4.
Conclusions
Calculated from routine, non-invasive laboratory tests, FIB-4 is a strong, independent HCC risk factor in HIV-infected patients.
Impact
FIB-4 might prove valuable as an easily measured index to identify those at highest risk for HCC, even prior to development of clinical cirrhosis.
Keywords: hepatocellular carcinoma, FIB-4, HIV, liver neoplasms, hepatic fibrosis
Introduction
Primary liver cancer, mainly hepatocellular carcinoma (HCC), is the third leading cancer cause of death worldwide(1)and the fifth among men in the United States(2), where HCC incidence and mortality rates continue to increase(3). Chronic inflammation associated with hepatitis B virus (HBV) infection, hepatitis C virus (HCV)infection, and/or heavy alcohol use can lead to fibrosis, cirrhosis and eventually to HCC (4, 5). FIB-4 is an index score calculated from platelet count, alanine transaminase (ALT), aspartate transaminase (AST), and age that is predictive of advanced hepatic fibrosis and cirrhosis (6). We hypothesized that high FIB-4 would be associated with subsequent development of HCC among HIV-infected persons, who are at high risk due to high prevalence of viral hepatitis (7)and alcohol consumption (8), and possibly due to HIV infection itself (9).
Materials and Methods
The study sample included all HIV-infected men who enrolled in the Veterans Aging Cohort Study (VACS) Virtual Cohort (VC) between January 1, 1996 and December 31, 2007 with available FIB-4 index score within the year prior to or one month after the cohort enrollment date and with greater than one year of follow-up (N=22,980). The number of otherwise eligible VACS VC subjects who did not have an available FIB-4 at baseline was 11,499. The cohort, which was 98% male, included no female subjects diagnosed with HCC; therefore females were excluded from the analysis. The VACS VC is based on national Veterans Affairs (VA) medical databases, with no contact with patients, and is described in detail elsewhere (10). It is an open cohort that enrolls veterans when they begin HIV care in the VA medical system. The cohort was approved by the Institutional Review Boards of the VA Connecticut Healthcare System and Yale University and has been granted a waiver of informed consent.
We defined baseline as date of enrollment into the cohort. Follow-up time extended from one year post-baseline to the earliest of date of HCC diagnosis, death, loss to follow-up, or December 31, 2008. Incident HCC cases were identified by linkage with the VA Central Cancer Registry (VACCR), which aggregates data provided by local cancer registries at VA medical centers nationwide. We defined HCC as International Classification of Diseases for Oncology, third edition [ICD-O-3] topography code C22.0 (liver)in combination with behavior code 3 (malignant) and ICD-O-3 morphology codes 8170–8180 (hepatocellular carcinoma) (11). To reduce the possibility of reverse causality, we excluded cases and person-years at risk in the first year of follow-up.
Baseline FIB-4 ( ) was calculated from laboratory results closest to baseline within the prior year, or during the month after baseline for persons with no prior year results. We defined low (<1.45), intermediate (1.45–3.25), and high FIB-4 (>3.25) as previously established (6). To rule out the possibility that age, a component of FIB-4, was the main driver of our findings, we removed age to create a “modified FIB-4” ( ) and classified subjects into quartiles of modified FIB-4. We also examined the relationship between HCC risk and the other two components of FIB-4, platelet count (<100,000/μL versus ≥100,000/μL) and AST/ALT ratio (≥1 versus <1), which are known in themselves to be associated with cirrhosis (12, 13).
We included as covariates the following demographic variables, HIV-related variables, and known and suspected risk factors for HCC: age, race/ethnic group, year of enrollment, baseline CD4 count, baseline HIV-1 RNA level, baseline antiretroviral therapy (ART)use, history of active HBV infection, history of HCV infection(not necessarily chronic), alcohol abuse and dependency, and diabetes status, which was included because it is an emerging suspected HCC risk factor (5). History of active HBV infection was defined by positive HBV surface antigen, e antigen, or DNA test result up to one year post-baseline. Since >90% of anti-HCV-positive HIV-infected persons have HCV viremia(14), history of HCV infection (not necessarily chronic) was defined by positive anti-HCV antibody test, recombinant immunoblot assay, and/or HCV RNA up to one year post-baseline. Subjects with two outpatient or one inpatient alcohol abuse/dependence-related ICD-9 diagnostic code (ICD-9 code 291: alcohol-induced mental disorders, 303: alcohol dependence syndrome, 305.0: nondependent alcohol 4 abuse) between five years before and one year post-baseline were classified as positive for alcohol abuse/dependence. Baseline diabetes, diagnosed at any time before baseline, was defined as described previously (15).
Statistical analyses were performed using SAS 9.2. We created Kaplan-Meier cumulative incidence plots (PROC LIFE TEST)and used the log-rank test to compare plots. Cox proportional hazards regression models (PROC PHREG) estimated hazard ratios (HR) and two-sided 95% confidence intervals (CI). We tested the proportional hazards assumption using martingale residuals (ASSESS statement in PROC PHREG) and found no meaningful violations. To calculate a p-value for trend, FIB-4 was included as a continuous variable, with an upper limit to address outliers, in a Cox model. Missing values were classified as “unknown” in primary analyses. Results were similar when we used multiple imputation (PROC MI)for missing values; we therefore only report the primary results.
Results
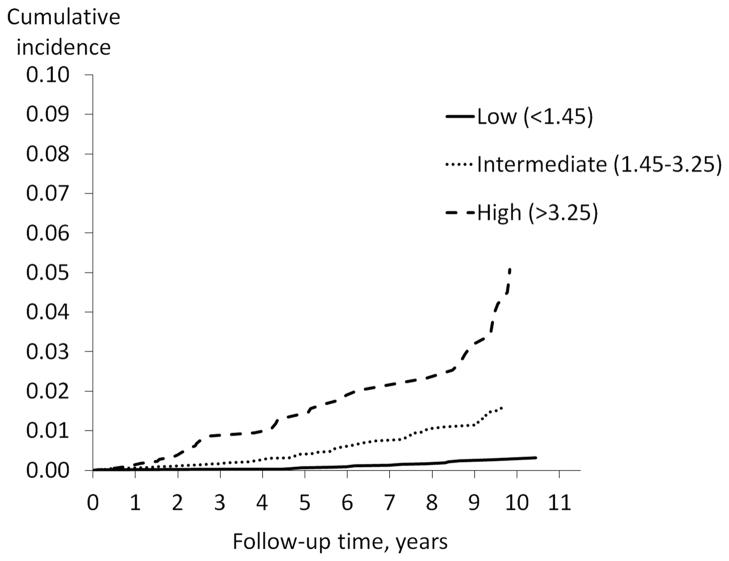
After excluding diagnoses prior to enrollment into the cohort and diagnoses during the first year of follow-up, there were 112 incident HCC cases(66% microscopically confirmed)with an average of 6.7 years (range 1.1–12.4)from baseline to HCC diagnosis. All of the HCC diagnoses had the ICD-O-3 histology code 8170. Of the 112 incident HCC cases, 17 had low FIB-4, 54 had intermediate FIB-4, and 41 had high FIB-4 at baseline (Table 1). The Kaplan-Meier plots (Figure 1) showed cumulative incidence of HCC to be lowest among those with low baseline FIB-4 and highest among those with high FIB-4. The 10-year cumulative incidence was 0.27%, 1.6%, and 5.1% for low, intermediate, and high FIB-4, respectively (p < 0.001).
Table 1.
Distribution of cohort baseline characteristics and Cox proportional hazard regression models for FIB-4 in relation to hepatocellular carcinoma, among 22,980 HIV-infected veterans from the Veterans Aging Cohort Study Virtual Cohort
| Hepatocellular Carcinoma | Cox Regression Models | ||||
|---|---|---|---|---|---|
| Yes (N=112) N (%) |
No (N=22868) N (%) |
Age-and race-adjusted* HR(95% CI)† |
Multivariate-adjusted‡ HR(95% CI) |
||
| FIB-4 | Low (<1.45) | 17 (15%) | 12802 (56%) | 1.0 (ref) | 1.0 (ref) |
| Intermediate(1.45–3.25) | 54 (48%) | 7543 (33%) | 4.2 (2.4,7.4) | 3.6 (2.1,6.4) | |
| High (>3.25) | 41 (37%) | 2523 (11%) | 13.0 (7.2,23.4) | 9.6 (5.2,17.4) | |
| Age | <35 | 3 (3%) | 2545 (11%) | 1.0 (ref) | 1.0 (ref) |
| 35–39 | 8 (7%) | 3104 (14%) | 2.2 (0.6,8.5) | 1.5 (0.4,5.9) | |
| 40–44 | 14 (13%) | 4801 (21%) | 2.7 (0.8,9.3) | 1.2 (0.3,4.5) | |
| 45–49 | 37 (33%) | 5261 (23%) | 7.2 (2.2,23.5) | 2.9 (0.9,10.1) | |
| 50–54 | 21 (19%) | 3373 (15%) | 6.7 (2.0,22.7) | 2.6 (0.7,9.3) | |
| 55–59 | 16 (14%) | 1906 (8%) | 13.5 (3.9,46.5) | 5.2 (1.4,18.8) | |
| ≥60 | 13 (12%) | 1878 (8%) | 10.1 (2.9,35.7) | 4.0 (1.1,14.6) | |
| Race/ethnic group | Non-Hispanic white | 47 (42%) | 8253 (36%) | 1.0 (ref) | 1.0 (ref) |
| Non-Hispanic black | 51 (46%) | 11722 (51%) | 0.9 (0.6,1.3) | 0.7 (0.4,1.0) | |
| Hispanic | 13 (12%) | 1748 (8%) | 1.1 (0.6,2.1) | 0.9 (0.5,1.8) | |
| Other/unknown | 1 (1%) | 1145 (5%) | 0.3 (0,2.4) | 0.3 (0,2.2) | |
| Enrollment year | 1996 | 49 (44%) | 6663 (29%) | 1.0 (ref) | 1.0 (ref) |
| 1997–1999 | 32 (29%) | 6378 (28%) | 0.9 (0.6,1.4) | 0.9 (0.6,1.4) | |
| 2000–2002 | 22 (20%) | 4073 (18%) | 1.8 (1.0,3.1) | 2.0 (1.0,3.6) | |
| 2003–2005 | 7 (6%) | 4011 (18%) | 1.5 (0.6,3.6) | 1.4 (0.4,4.2) | |
| 2006–2007 | 2 (2%) | 1743 (8%) | 5.1 (1.0,25.5) | 5.0 (0.9,27.9) | |
| CD4 count (cells/μl) | ≥500 | 20 (18%) | 4667 (20%) | 1.0 (ref) | 1.0 (ref) |
| 200–499 | 54 (48%) | 8348 (37%) | 1.3 (0.8,2.2) | 1.1 (0.7,1.9) | |
| <200 | 29 (26%) | 7730 (34%) | 1.0 (0.5,1.7) | 0.7 (0.4,1.3) | |
| Unknown | 9 (8%) | 2123 (9%) | 1.0 (0.5,2.3) | 1.1 (0.5,2.5) | |
| HIV-1 RNA copies/mL | <500 | 32 (29%) | 5265 (23%) | 1.0 (ref) | 1.0 (ref) |
| ≥500 | 69 (62%) | 15485 (68%) | 0.8 (0.5,1.3) | 0.8 (0.5,1.2) | |
| Unknown | 11 (10%) | 2118 (9%) | 0.8 (0.4,1.6) | 0.7 (0.3,1.5) | |
| Antiretroviral therapy use | No | 99 (88%) | 17774 (78%) | 1.0 (ref) | 1.0 (ref) |
| Yes | 13 (12%) | 5094 (22%) | 1.7 (0.9,3.3) | 1.3 (0.6,3.1) | |
| Hepatitis B virus infection | Negative | 74 (66%) | 18226 (80%) | 1.0 (ref) | 1.0 (ref) |
| Positive | 30 (27%) | 1412 (6%) | 5.9 (3.9,9.1) | 5.8 (3.7,9) | |
| Unknown | 8 (7%) | 3230 (14%) | 0.6 (0.3,1.2) | 0.9 (0.4,2.0) | |
| Hepatitis C virus infection | Negative | 35 (31%) | 12133 (53%) | 1.0 (ref) | 1.0 (ref) |
| Positive | 65 (58%) | 6847 (30%) | 3.4 (2.2,5.2) | 2.9 (1.8,4.6) | |
| Unknown | 12 (11%) | 3888 (17%) | 0.7 (0.3,1.4) | 0.9 (0.4,2.0) | |
| Alcohol abuse/dependence | No | 89 (79%) | 18190 (80%) | 1.0 (ref) | 1.0 (ref) |
| Yes | 23 (21%) | 4678 (20%) | 1.2 (0.8,2.0) | 0.9 (0.6,1.5) | |
| Diabetes | No | 105 (94%) | 21759 (95%) | 1.0 (ref) | 1.0 (ref) |
| Yes | 7 (6%) | 1109 (5%) | 2.1 (1.0,4.6) | 1.6 (0.7,3.5) | |
Race is age-adjusted and age is race-adjusted.
HR, hazard ratio;
CI, confidence interval.
Adjusted for age, race/ethnic group, enrollment year, CD4 count, viral load, ART use, HBV and HCV infection, alcohol abuse/dependency, and diabetes
Figure 1.
Kaplan-Meier cumulative incidence function: time to incident hepatocellular carcinoma (HCC) by FIB-4 category. Kaplan-Meier cumulative incidence curves display time to incident HCC diagnoses, stratified by FIB-4 category (low, intermediate, and high). To reduce the possibility of reverse causality, follow-up time began one year after baseline. Therefore, time 0 on the x-axis is equivalent to one year post-baseline.
About half of the subjects were non-Hispanic black and more than one-third were non-Hispanic white (Table 1). Among the subjects who developed HCC, 65(58%) had a history of HCV infection, and 30 (27%) had a history of HBV infection. Of subjects who did not develop HCC, only 30% and 6% had a history of HCV and HBV infection, respectively.
After adjustment for age and race/ethnic group(Table 1), the HR was 4.2 (95% CI: 2.4, 7.4)for intermediate FIB-4 and 13.0 (95% CI: 7.2, 23.4) for high FIB-4, compared to low FIB-4(p-value for trend < 0.0001). After further adjustment for enrollment year, CD4 count, HIV-1 RNA level, ART use, HBV and HCV infection, alcohol abuse/dependency, and diabetes, FIB-4 remained a strong, significant independent HCC risk factor (Table 1). The multivariate-adjusted HR was 3.6(95% CI: 2.1, 6.4)for intermediate FIB-4 and 9.6(95% CI: 5.2, 17.4)for high FIB-4 (p-value for trend < 0.0001). When we restricted the analysis to the 74 microscopically-confirmed cases, the multivariate-adjusted HR was 2.9 (95% CI: 1.5, 5.5) for intermediate FIB-4 and 7.0 (95% CI: 3.5, 14.2) for high FIB-4 (p-value for trend < 0.0001).
To determine if high FIB-4 was associated with long-term risk, we calculated HRs excluding the first five years of follow-up. With 78 incident HCC cases, the multivariate-adjusted HR was 3.1 for intermediate FIB-4 (95% CI: 1.7, 5.8) and 7.4 for high FIB-4 (95% CI: 3.7, 14.8) (p-value for trend <0.0001). When we evaluated the association between the “modified FIB-4” score and HCC, we found a significantly elevated risk of HCC in the third and fourth “modified FIB-4” quartiles compared to the first quartile (p-value for trend <0.0001). The multivariate-adjusted HR was 3.1 (95% CI: 1.3, 7.7) for the third quartile and 8.6 (95% CI: 3.7, 20.3)for the fourth quartile, similar to HRs for FIB-4 itself, even after removing age from the index. With respect to the other two individual components of FIB-4, in a multivariate model that included all covariates and platelet count, the multivariate-adjusted HR for low platelet count (<100,000/μL) was 2.4 (95% CI: 1.3, 4.3). In a separate multivariate model that included all covariates and the AST/ALT ratio, the multivariate-adjusted HR for AST/ALT ratio ≥1 was 1.2 (95% CI: 0.8, 1.8).
We performed multivariate analyses stratified by HCV infection status at baseline (negative, positive, or unknown). Among HCV-negative subjects, the multivariate-adjusted HR was 5.7 (95% CI: 2.0, 15.9) for intermediate FIB-4 and 9.8 (95% CI: 3.0, 31.9) for high FIB-4 (p-value for trend= 0.0020). Among HCV-positive subjects, the multivariate-adjusted HR was 2.5 (95% CI: 1.2, 5.2) for intermediate FIB-4 and 7.0 (95% CI: 3.3, 15.1) for high FIB-4 (p-value for trend <0.0001). Finally, among subjects with unknown HCV status, the multivariate-adjusted HR was 8.6 (95% CI: 1.0, 76.9) for intermediate FIB-4 and 27.9 (95% CI: 2.7, 290.4) for high FIB-4 (p-value for trend= 0.045).
Interestingly, high FIB-4 was associated with subsequent development of HCC among the subjects who were HBV-negative, HCV-negative, and with no diagnosis of alcohol abuse/dependence at baseline. The multivariate-adjusted HR was 7.5 (95% CI: 1.5, 38.8) for intermediate FIB-4 and 9.6 (95% CI: 1.2, 77.1) for high FIB-4 (p-value for trend =0.065).
Finally, to assess the possibility that our results were influenced by selection bias stemming from differences between the VACS-VC cohort members with baseline FIB-4 values (N=22,980), who were included in our analyses, and those without baseline FIB-4 values (N=11,499), who were excluded from our analyses, we included all 34,479 subjects in a multivariate model to calculate a multivariate-adjusted HR for those with a FIB-4 value at baseline compared with those without a FIB-4 value at baseline. The multivariate-adjusted HR was 1.0 (95% CI: 0.7, 1.4).
Discussion
FIB-4, a marker of advanced fibrosis and cirrhosis, was a strong risk factor for HCC in our HIV-infected cohort. After adjustment for demographic variables, HIV-related variables, and known and suspected HCC risk factors, persons with high FIB-4 had a 10-fold greater risk of developing HCC than did persons with low FIB-4. Even FIB-4 scores in the intermediate range were associated with almost fourfold risk. FIB-4 was a strong risk factor for HCC in all subgroups examined, including HCV-infected and HCV uninfected subjects and subjects with none of the three main known HCC risk factors (HCV infection, HBV infection, and alcohol abuse/dependence, although the latter was not a significant risk factor in our data). Furthermore, high FIB-4 was associated with both short and long-term HCC risk, and the risk persisted when age was removed from the FIB-4 index.
Although the efficacy of HCC screening is still being explored (16), there is much interest in developing algorithms that identify high-risk populations for screening. For example, investigators in Taiwan recently validated a risk score including sex, age, ALT, hepatitis B e antigen status, and serum HBV DNA level for predicting risk of HCC for Asian patients with chronic HBV infection (17). Although platelet count and the AST/ALT ratio, two components of the FIB-4 index, have been shown to be associated with cirrhosis in HCV infected patients (12, 13), we found FIB-4, which combines these two markers, to be better at identifying persons at high risk for HCC than were either of these two markers alone. The ultimate cost-effectiveness of screening for HCC will depend upon several factors, including the ability to identify a high-risk population and the ability to accurately diagnose patients at a point where early intervention can be beneficial. We are proposing that FIB-4, or further refinements of FIB-4, may help to identify a high-risk population. This is evinced by the 19-fold higher 10-year cumulative incidence for subjects with high FIB-4 (5.1%) compared to subjects with low FIB-4, who were very unlikely to develop HCC (0.27%).
This study had the strengths of a large sample size, laboratory test results to determine hepatitis co-infections, and cancer registry-confirmed cancer diagnoses. Although only two-thirds of the otherwise eligible cohort members had an available FIB-4 value at baseline, selection bias would distort the results only if the relationship between FIB-4 and HCC was different between those with and without a FIB-4 value, which is unlikely. Furthermore, the similar HCC risk among subjects with and without FIB-4 values at baseline suggests the absence of selection bias.
It was not surprising that only two-thirds of the HCC diagnoses were microscopically confirmed because diagnosis of HCC can be established with high positive predictive value by diagnostic imaging in combination with serum alpha-fetoprotein determination, without biopsy, if certain criteria are met (18). Although our measurement of HCV infection may have yielded false positives, which may result in incomplete control of confounding by HCV infection, other cohort studies have used similar methods to classify HCV infection (19, 20). Another limitation, faced by most epidemiologic studies, was our inability to assess duration of HIV, HCV, and HBV infection. However, it is possible that that FIB-4 acts in part as a surrogate for duration of infection. Obesity has been emerging as a potential risk factor for HCC (21, 22); however, due to a large proportion of missing data on body mass index (BMI), we did not include BMI as a covariate in the analysis.
In conclusion, advanced fibrosis places patients at elevated risk for HCC. We have shown that FIB-4, a marker of advanced fibrosis that is easily calculated from routine, non-invasive laboratory tests, is a useful tool for HCC risk stratification among HIV-infected persons. Future studies should investigate the relationship between FIB-4 trajectory and HCC, as well as FIB-4 as a risk factor for HCC among persons not infected with HIV.
Acknowledgments
We are grateful to the Veterans Affairs Central Cancer Registry (VACCR) for linking the VACS Virtual Cohort with the VACCR database and providing us with a dataset containing the linked records. This study would not have been possible without the VACCR’s generous assistance.
Grant Support
This work was supported by National Institutes of Health: National Institute on Alcohol Abuse and Alcoholism (U01-AA13566); National Institute on Aging (R01-AG029154); National Heart, Lung and Blood Institute (R01-HL095136, R01-HL090342, RCI-HL100347); National Institute of Allergy and Infectious Diseases (U01-AI069918, U01-AI069918); National Institute of Mental Health (P30-MH062294); and the Veterans Health Administration Office of Research and Development (VA REA 08-266) and Office of Academic Affiliations (Medical Informatics Fellowship).
Footnotes
Conflicts of interest: not applicable
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: National Cancer Institute; 2011. [Google Scholar]
- 3.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–91. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.London WT, McGlynn KA. Liver Cancer. In: Scottenfeld D, Fraumeni JF, editors. Cancer Epidemiology and Prevention. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 5.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterol. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 6.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatol. 2006;43:1317–25. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 7.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44:S6–9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, et al. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J Stud Alcohol. 2002;63:179–86. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- 9.Clifford GM, Rickenbach M, Polesel J, Dal Maso L, Steffen I, Ledergerber B, et al. Influence of HIV-related immunodeficiency on the risk of hepatocellular carcinoma. AIDS. 2008;22:2135–41. doi: 10.1097/QAD.0b013e32831103ad. [DOI] [PubMed] [Google Scholar]
- 10.Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S, Justice AC. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44:S25–30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 11.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al., editors. International Classification of Diseases for Oncology. Geneva: World Health Organization; 2000. [Google Scholar]
- 12.Ahmad W, Ijaz B, Javed FT, Gull S, Kausar H, Sarwar MT, et al. A comparison of four fibrosis indexes in chronic HCV: development of new fibrosis-cirrhosis index (FCI) BMC Gastroenterol. 11:44. doi: 10.1186/1471-230X-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1998;93:44–8. doi: 10.1111/j.1572-0241.1998.044_c.x. [DOI] [PubMed] [Google Scholar]
- 14.Villano SA, Vlahov D, Nelson KE, Cohn S, Thomas DL. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatol. 1999;29:908–14. doi: 10.1002/hep.510290311. [DOI] [PubMed] [Google Scholar]
- 15.Butt AA, McGinnis K, Rodriguez-Barradas MC, Crystal S, Simberkoff M, Goetz MB, Leaf D, Justice AC. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23:1227–34. doi: 10.1097/QAD.0b013e32832bd7af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Masi S, Tosti ME, Mele A. Screening for hepatocellular carcinoma. Dig Liver Dis. 2005;37:260–8. doi: 10.1016/j.dld.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Yang HI, Yuen MF, Chan L, Han KH, Chen PJ, Kim DY, et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol. 12:568–574. doi: 10.1016/S1470-2045(11)70077-8. [DOI] [PubMed] [Google Scholar]
- 18.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatol. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 19.Rockstroh JK, Mocroft A, Soriano V, Tural C, Losso MH, Horban A, et al. Influence of hepatitis C virus infection on HIV-1 disease progression and response to highly active antiretroviral therapy. J Infect Dis. 2005;192:992–1002. doi: 10.1086/432762. [DOI] [PubMed] [Google Scholar]
- 20.Spradling PR, Richardson JT, Buchacz K, Moorman AC, Finelli L, Bell BP, et al. Trends in hepatitis C virus infection among patients in the HIV Outpatient Study, 1996–2007. J Acquir Immune Defic Syndr. 53:388–96. doi: 10.1097/QAI.0b013e3181b67527. [DOI] [PubMed] [Google Scholar]
- 21.Polesel J, Zucchetto A, Montella M, Dal Maso L, Crispo A, La Vecchia C, et al. The impact of obesity and diabetes mellitus on the risk of hepatocellular carcinoma. Ann Oncol. 2009;20:353–7. doi: 10.1093/annonc/mdn565. [DOI] [PubMed] [Google Scholar]
- 22.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]