Summary
Background
PLC-β signaling is generally thought to be mediated by allosteric activation by G proteins and Ca2+. While availability of the PIP2 substrate is limiting in some cases, its production has not been shown to be independently regulated as a signaling mechanism. WNK1 protein kinase is known to regulate ion homeostasis and cause hypertension when expression is increased by gene mutations. However, its signaling functions remain largely elusive.
Results
Using diacylglycerol-stimulated TRPC6 and inositol trisphosphate-mediated Ca2+ transients as cellular biosensors, we show that WNK1 stimulates PLC-β signaling in cells by promoting the synthesis of PIP2 via stimulation of phosphatidylinositol 4-kinase IIIα. WNK1 kinase activity is not required. Stimulation of PLC-β by WNK1 and by Gαq are synergistic; WNK1 activity is essential for regulation of PLC-β signaling by Gq-coupled receptors and basal input from Gq is necessary for WNK1 signaling via PLC-β. WNK1 further amplifies PLC-β signaling when it is phosphorylated by Akt kinase in response to insulin-like growth factor.
Conclusions
WNK1 is a novel regulator of PLC-β that acts by controlling substrate availability. WNK1 thereby coordinates signaling between G protein and Akt kinase pathways. Because PIP2 is itself a signaling molecule, regulation of PIP2 synthesis by WNK1 also allows the cell to initiate PLC signaling while independently controlling the effects of PIP2 on other targets. These findings describe a new signaling pathway for Akt-activating growth factors, a mechanism for G protein-growth factor crosstalk and a means to independently control PLC signaling and PIP2 availability.
Introduction
Mammalian WNK kinases are a family of four serine-threonine protein kinases with an atypical kinase active site [1–3]. Mutations in WNK1 and WNK4 cause the autosomal-dominant disease pseudohypoaldosteronism type 2 (PHA2), which is characterized by hypertension and hyperkalemia [2]. WNKs regulate various ion transporters through both kinase activity-dependent and -independent mechanisms [3, 4]. For example, WNK1 and 4 phosphorylate and activate Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress-responsive kinase-1 (OSR1), which in turn activate cation-chloride co-transporters NCC and NKCC [5–7]. Through kinase-independent events, WNK1 binds and somehow activates SGK kinase, causing it to activate the epithelial Na+ channel ENaC [8]; and WNK1 and 4 interact with the endocytic scaffold protein intersectin to enhance endocytosis of the renal K+ channel ROMK [9]. Dysregulation of ion transport in the kidney contributes to the hypertension and hyperkalemia of PHA2.
The functions of WNKs extend beyond regulation of ion homeostasis. WNK1 is ubiquitously expressed, and homozygous gene inactivation in mice causes cardiovascular developmental arrest and embryonic death [10]. WNK1 activates ERK5, and knockdown of WNK1 decreases activation of ERK5 by epidermal growth factor receptors [11]. WNK2 binds the small GTPases RhoA and Rac1. WNK2 knockdown decreases RhoA activation, but promotes Rac1 activation [12]. The Gq-coupled angiotensin II receptor regulates the activity of the NCC co-transporter through the WNK4 signaling pathway [13], indicating functional interactions between WNK and G protein signaling pathways.
Upstream regulation of the WNKs is incompletely understood, and it is likely that several distinct mechanisms of WNK activation are used in different cells [3, 4]. WNK1 is a substrate of Akt kinase [14, 15], which is a target of phosphatidylinositol 3-kinase (PI3K)-activating growth factors such as insulin and insulin-like growth factors (IGFs). Insulin and IGFs activate PI3K, and its PIP3 product recruits and activates Akt to phosphorylate WNK1 at threonine-58. It has been reported that Akt-catalyzed phosphorylation of WNK1 at T58 alters the ability of WNK1 to regulate membrane trafficking of ENaC and ROMK channels [8, 16].
Phospholipase C-βs (PLC-βs) are important effectors of G protein-coupled receptor (GPCR)-mediated signaling pathways [17, 18]. They hydrolyze PIP2, itself a signaling molecule, to form two other second messengers, diacylglycerol (DAG) and inositol-1,4,5-trisphosphate (IP3). PLC-βs are stimulated by Gα subunits of the Gq family, by Gβγ subunits of the Gi’s and, in the case of PLC-β2, by Rac [17–19]. The concentration of PIP2 in the plasma membrane is limiting for PLC-β activity, and re-synthesis of the substrate initiated by phosphatidylinositol-4-kinase (PI4K) after depletion is important for sustained PLC-β signaling [20, 21]. This dependence on substrate concentration agrees with in vitro studies showing that PLC activity increases linearly with the concentration of PIP2 regardless of stimulation by G proteins or Ca2+ [22, 23]. However, it is not known whether PIP2 production can be independently regulated as a signaling mechanism and, if so, by what physiological stimulus.
We show here that WNK1 stimulates PLC-β signaling by promoting the synthesis of PIP2 via stimulation of PI4KIIIα. Stimulation of PLC-β by WNK1 and by Gαq-GTP is thus synergistic, and WNK1 activity is essential for regulation of PLC-β signaling by Gq-coupled receptors. Phosphorylation of WNK1 by the PI3K-Akt kinase cascade enhances the ability of WNK1 to synergistically regulate PLC-β signaling via synthesis of PIP2. Regulated control of substrate availability represents a novel mechanism for regulation of PLC-β activity and for crosstalk between Gq-coupled receptors and receptors that activate the Akt kinase pathway.
Results
WNK1 Activates TRPC6 Channels
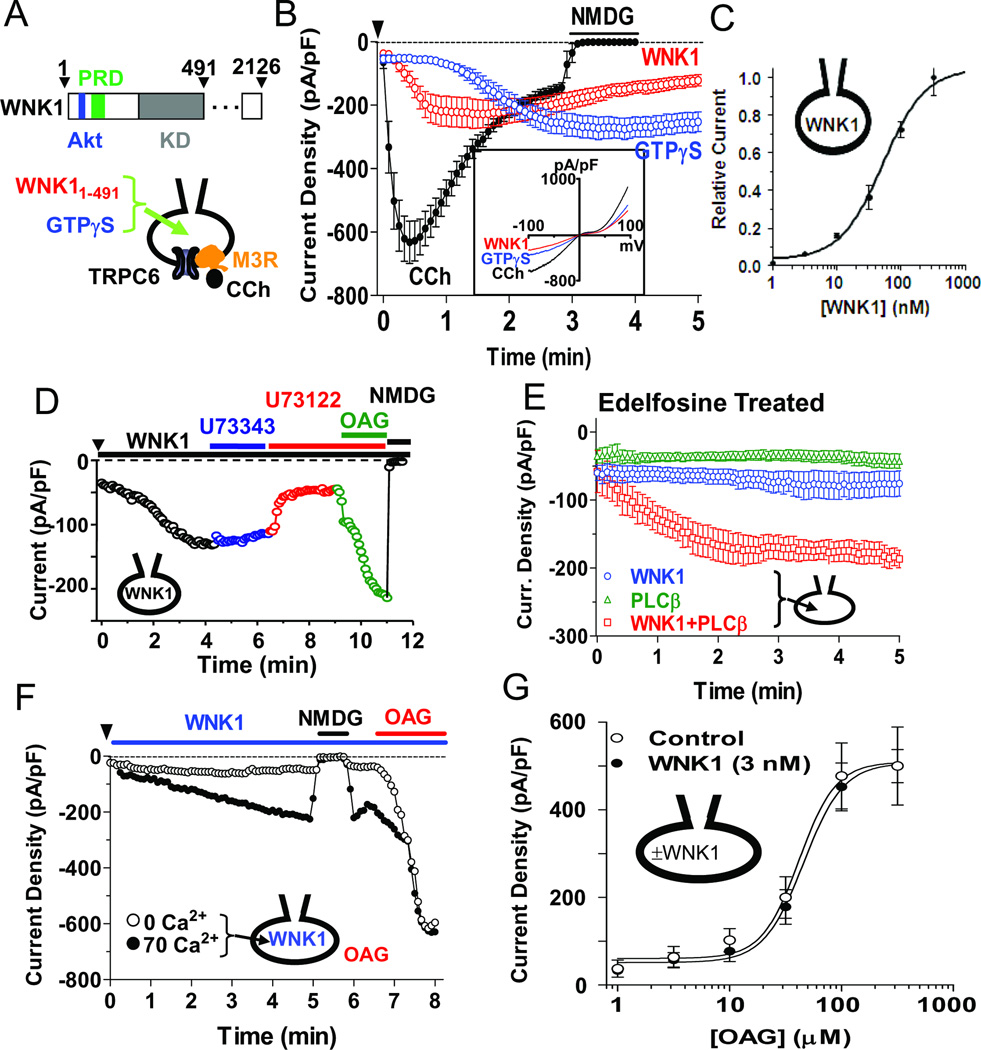
The TRPC6 channel is directly activated by DAG, a product of the PLC-catalyzed hydrolysis of PIP2 [24]. Inspired by a serendipitous observation that WNK1 knockdown altered Gq signaling, we used TRPC6 as a biosensor to examine the role of WNK1 in PLC-β signaling in native cell membranes. Full-length WNK1 is difficult to purify, and we tested the effect of WNK1 on TRPC6 by intracellular application of a purified fragment of rat WNK1 consisting of residues 1–491 (WNK11–491), which contains the Akt-phosphorylation site, the protein kinase domain and a N-terminal proline-rich domain (Figure 1A). As shown for HEK293 cells in Figure 1B, intracellular WNK11–491 increased TRPC6 current density, peaking in these cells at around 1–2 min and lasting for > 5 min. The half-maximal concentration of WNK11–491 for activation of TRPC6 was ~50 nM (Figure 1C). WNK11–491 also activates TRPC6 in membranes of HeLa cells (see Figure 6) and mouse embryo fibroblasts (see Figures 2C and 2D). TRPC6 was also activated by GTPγS, consistent with Gq-PLC-β signaling to produce DAG, and by the muscarinic agonist carbachol in cells that express the Gq-coupled M3 muscarinic cholinergic receptor (Figure 1B).
Figure 1.
Activation of TRPC6 by WNK1 requires PLC activity and is independent of WNK1 kinase activity. (A) Top: Domain structure of rat WNK1. Akt, its phosphorylation site; PRD, proline-rich domain; KD, kinase domain. Bottom: whole-cell patch-clamp recording of TRPC6. CCh: carbachol (100 µM); M3R: M3 muscarinic cholinergic receptor. Intracellular free [Ca2+] was 70 nM except as indicated in panel F. (B) Activation of TRPC6 by CCh via M3R, GTPγS or WNK11–491. HEK cells were transfected with TRPC6 with or without M3R. Data points (circles and error bars) are means ± SEM of inward current density (pA/pF at −100 mV; n = 5 for each). At the end of each recording, extracellular Na+ was replaced by the TRPC6-impermeable cation N-methyl-D-glucamine (NMDG) to gauge the background current. Inset shows current-voltage (I–V) relationships of activated TRPC6 currents. (C) Concentration-response curve for activation of TRPC6 by WNK11–491. (D) Effect of PLC inhibitor U73122 or inactive analog U73343 (2.5 µM each) on WNK11–491 activation of TRPC6. Inward current at −100 mV from a representative experiment. Similar results were observed in 6 independent experiments. (E) Cells were preincubated with 15 µM edelfosine for 1 hr and washed before recording. TRPC6 currents in response to 100 nM WNK11–491, 5 nM PLC-β1 or both were measured. (F) TRPC6 currents in response to WNK11–491 under 0 or 70 nM intracellular calcium. OAG was applied as indicated. (G) TRPC6 currents were measured in cells with or without intracellular application of 3 nM WNK11–491. OAG was applied to the extracellular solution at indicated concentrations. Although this concentration of WNK11–491 by itself does not activate TRPC6, it potentiated M3R activation of the channel (see Figure 3B). (see also Figure S1).
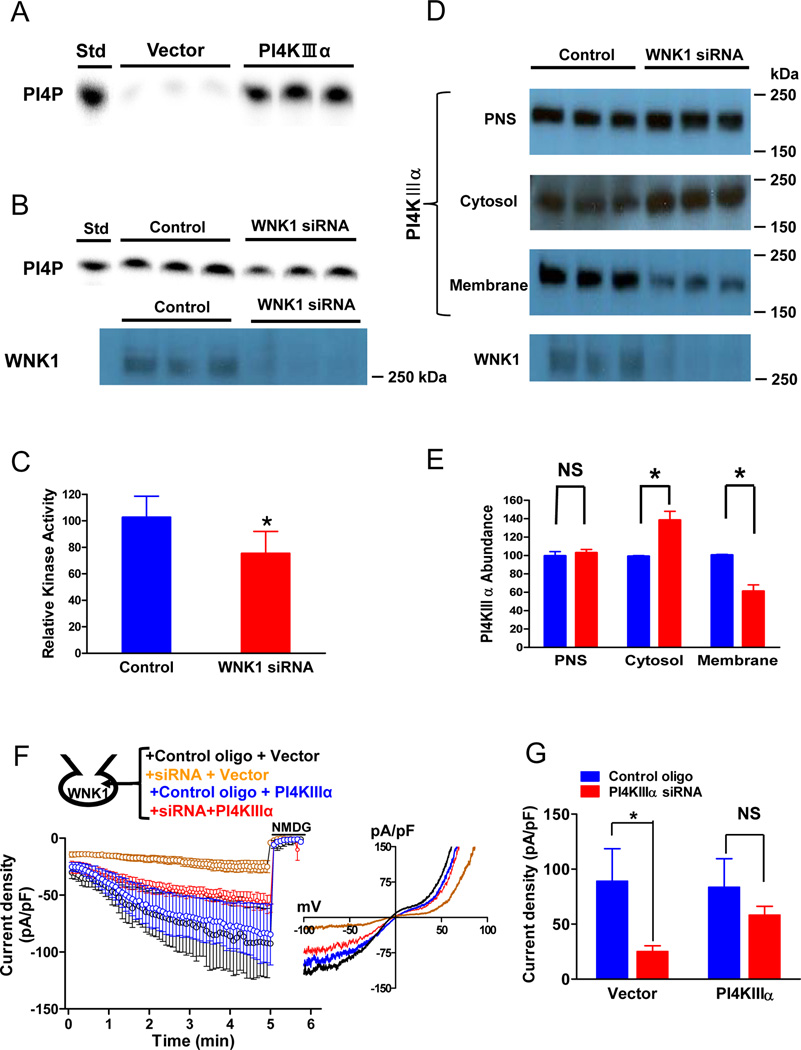
Figure 6.
Role of WNK1 in the production of PI4P by expressed PI4KIIIα. (A) Chromatogram showing PI4K activity in membranes prepared from HeLa cells transfected with vector or cDNA for PI4KIIIα. Std: PI4P standard. (B) Chromatogram showing PI4P production by membrane-associated recombinant PI4KIIIα from cells that were co-transfected with control oligonucleotide or WNK1 siRNA. Relative knockdown of WNK1 is shown in the Western blot. (C) Mean ± SEM of PI4P production from 5 separate experiments, each with triplicate samples, as shown in panel B. *, p < 0.01 control versus knockdown. (D) Abundance of PI4KIIIα in the post-nuclear supernatant (PNS), cytosol, and membrane fractions from control and WNK1 siRNA-transfected HeLa cells. A western blot at bottom shows relative WNK1 knockdown. (E) Mean ± SEM (5 separate experiments) of PI4KIIIα abundance in the post-nuclear supernatant (PNS), cytosol, and membrane fraction in control (blue) and WNK1 knockdown cell (red), shown relative to the control. *, p < 0.05 between indicated. (F) Whole-cell TRPC6 currents stimulated by WNK11–491 in cells co-transfected with control oligonucleotide or siRNA for human PI4KIIIα, with either empty vector or plasmid that encodes rat PI4KIIIα. (G) Peak WNK1-induced inward current density from panel F. *, p < 0.01 between indicated.
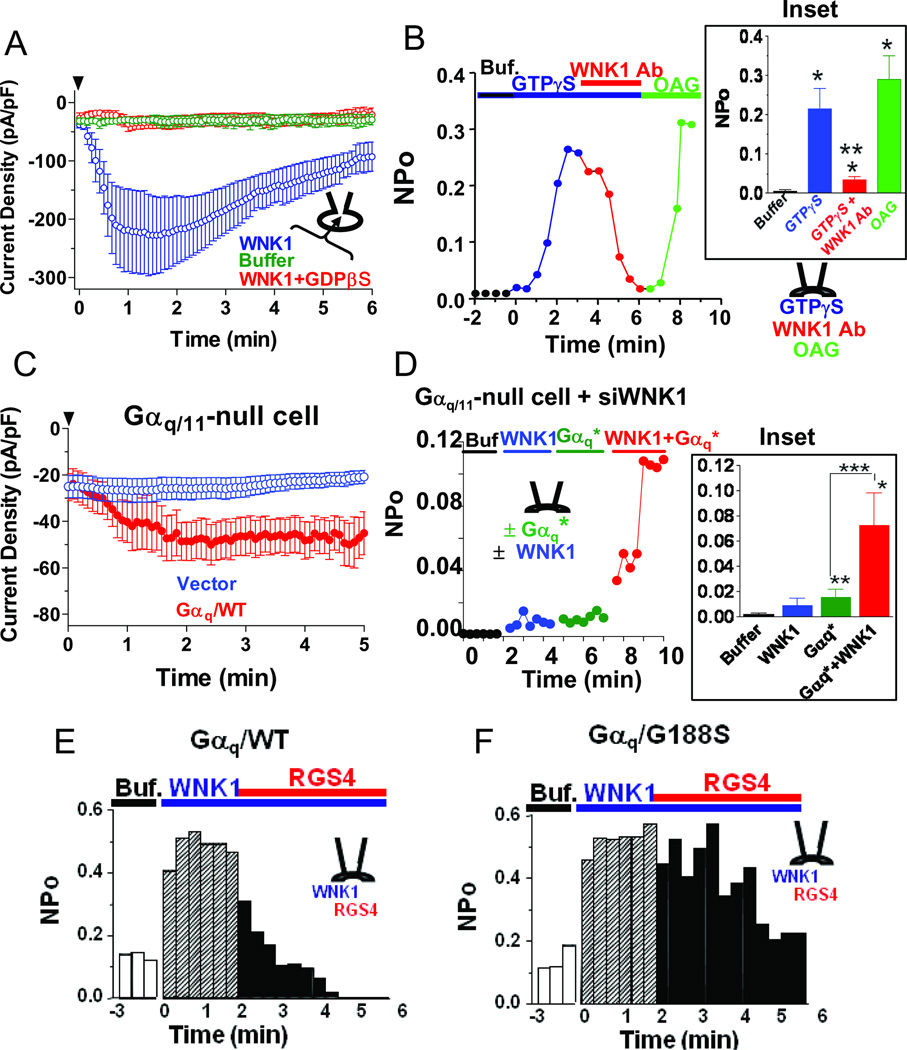
Figure 2.
Role of Gq in the activation of TRPC6 by WNK1. (A) GDPβS (0.2 mM) inhibits WNK1 activation of TRPC6. (B) Anti-WNK1 antibody inhibits TRPC6 activated by GTPγS. TRPC6 single channel activity was recorded from inside-out patches from TRPC6-expressing HEK cells. Buffer, GTPγS, anti-WNK1 Ab and/or OAG was applied to the cytoplasmic face as indicated. Each data point is NPo (number × open probability) averaged over 30 s. Inset shows mean ± SEM of peak open probability. *, p < 0.01 vs “buffer”. **, p < 0.01 “GTPγS” vs “GTPγS+anti-WNK1 antibody”. (C) Whole-cell WNK1-activated TRPC6 currents were measured in Gαq/11-null fibroblasts transfected with wild type Gαq (Gαq/WT) or empty vector. (D) Single channel activity was measured in inside-out patches from TRPC6-expressing Gαq/11-null fibroblasts that have endogenous WNK1 depleted by siRNA. Data show responses to GTPγS-preactivated Gαq (“Gαq*”) and/or WNK1 applied to the cytoplasmic face. Holding membrane potential is −80 mV. Inset shows mean ± SEM of peak open probability. *, p < 0.01 vs “buffer”. **, p < 0.05 vs “buffer”. ***, p < 0.02 between indicated groups. (E, F) Single channel activity was recorded in inside-out patches from HEK cells expressing both TRPC6 and wild type Gαq (Gαq/WT) (panel E) or G188S-mutant Gαq (Gαq/G188S) (panel F). Buffer, WNK11–491 and/or RGS4 (0.5 µM) was applied to the cytoplasmic face as indicated. NPo values were averaged every 20 s. There was a 2 min time gap between application of buffer and WNK1. (see also Figure S2).
Application of WNK11–491 to the cytoplasmic face of detached plasma membranes also activated TRPC6 single channel activity in membranes of cells that express TRPC6 (Figure S1A) but not in membranes from control cells. Thus, the cellular components required for activation of TRPC6 by WNK1 are contained in or are bound to the excised plasma membrane patch. Stimulation of TRPC6 was selectively blocked by antibody against amino acid residues 160–176 of WNK1 (Figure S1B). Purified WNK11–491 carrying a kinase-dead K233M mutation [1, 9] retained full ability to activate TRPC6 (Figure S1C), indicating that kinase activity of WNK1 is dispensable.
Activation of TRPC6 by WNK1 Requires PLC Activity
Several experimental results argue that the activation of TRPC6 by WNK1 is mediated by PLC-β-catalyzed production of the known TRPC6 activator DAG. First, activation of TRPC6 by WNK11–491 was blocked by the PLC inhibitors U73122 (Figure 1D) and edelfosine [25] (Figure 1E). Application of 1-oleoyl-2-acetyl glycerol (OAG), a soluble and membrane-permeant DAG, restored TRPC6 activity in the continued presence of U73122, which excludes effects of U73122 on the channel itself or on other components upstream of PLC. Second, because edelfosine acts slowly [25], we could rescue WNK1 signaling by addition of purified PLC-β1 after endogenous PLC was inhibited (Figure 1E). After edelfosine pretreatment, application of both purified PLC-β1 and WNK11–491 caused robust activation of TRPC6. Application of PLC-β1 without WNK1 had no effect, indicating that both WNK1 and PLC-β1 are required for activation of TRPC6. Third, PLC activity is dependent on cytosolic Ca2+ [17, 18], and removal of Ca2+ blocked TRPC6 stimulation by WNK11–491 but not direct stimulation by OAG (Figure 1F). Based only on the data above, WNK11–491 might activate TRPC6 by protecting DAG from hydrolysis by DAG lipase or phosphorylation by DAG kinase. However WNK11–491 did not alter the potency with which OAG activates TRPC6 (Figure 1G), indicating that its effect is at the level of PLC. Together, these results provide compelling evidence that WNK1 activates TRPC6 by stimulation of PLC to produce DAG, the proximal activator.
We also measured intracellular Ca2+ release triggered by IP3, another product of PIP2 hydrolysis, as a read-out for WNK1’s effects on PLC activity. Microinjection of anti-WNK1 antibody or knocking down endogenous WNK1 by small interfering RNA (siRNA) reduced the intracellular Ca2+ increases in response to carbachol (Figures S1D–F). Endogenous WNK1 is thus required for normal PLC-β signaling in response to stimulation of Gq.
WNK1 Works in Parallel With Gq to Synergistically Stimulate TRPC6
The PLC-βs are activated by Gαq [17, 18]. GDPβS, which blocks G protein activation, inhibited the stimulation of TRPC6 by WNK11–491 (Figure 2A). GTPγS stimulated the TRPC6 current without added WNK1, and stimulation was blocked by anti-WNK1 antibody (Figure 2B, Figures S2A and S2B). These data suggest both that some G protein is required for WNK1 stimulation of TRPC6 and that endogenous WNK1 is required for TRPC6 stimulation by a G protein. To determine whether WNK1 requires activation of PLC-β by Gq/11 in cells, we measured TRPC6 regulation by WNK1 in murine Gαq−/−-Gα11−/− embryonic fibroblasts [26]. In intact Gαq/11-null cells, WNK11–491 failed to activate TRPC6, and expression of wild type Gαq restored activation by WNK1 (Figure 2C). When the constitutively active Q209L-Gαq mutant was expressed, addition of WNK11–491 further activated TRPC6 (Figures S2C and S2D), which argues that WNK1 and Gαq act in parallel rather than in series to stimulate DAG synthesis by PLC-β.
When WNK1 was depleted in the Gαq/11-null cells by siRNA, neither WNK11–491 nor Gαq-GTPγS alone stimulated TRPC6 substantially, only simultaneous addition of both activators produced major TRPC6 opening (Figure 2D). Moreover, the Gq inhibitor RGS4 blocked activation of TRPC6 by WNK1 in cells and the N128G mutant of RGS4 [27] had no effect (Figures S2E and S2F). In detached patches, inhibition by RGS4 was reduced and delayed by expression of the RGS-insensitive G188S mutant of Gαq [28, 29] (Figures 2E and 2F). Thus, WNK1 and activated Gαq stimulate TRPC6 synergistically.
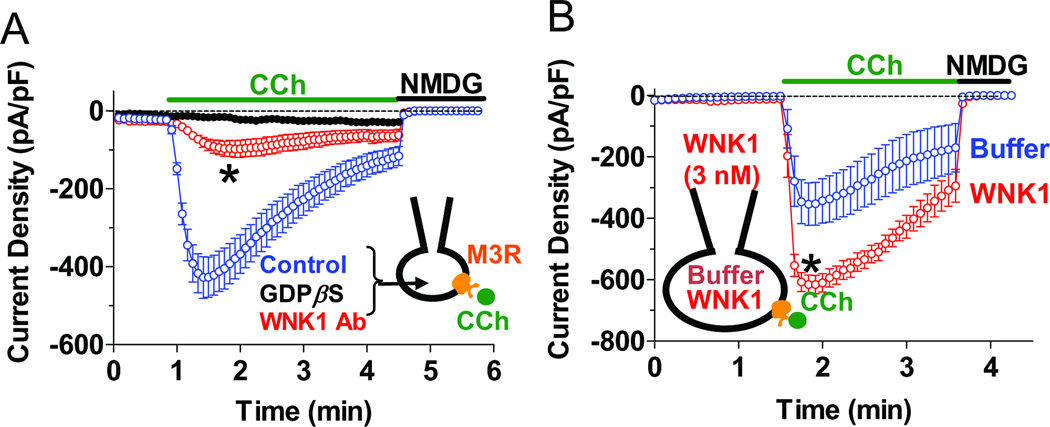
WNK1 Activity Is Necessary for Activation of TRPC6 by Gq-Coupled Receptors
Intracellular application of anti-WNK1 antibody markedly blunted the activation of TRPC6 by agonist through the expressed M3 muscarinic receptor (Figure 3A), the α1A-, α1B-adrenergic receptors and H1 histaminergic receptor in HEK cells (Figures S3A–C) or through endogenous muscarinic receptor in HeLa cells (Figure S3D). Conversely, increased WNK1 activity potentiated activation of TRPC6 by M3 receptor. Intracellular application of 3 nM WNK11–491, which is too low to activate TRPC6 by itself, approximately doubled the TRPC6 current elicited by M3 muscarinic stimulation (Figure 3B). Thus, some level of endogenous WNK1 activity is necessary for full Gq-PLC-β signaling and additional WNK1 potentiates Gq signaling in cells.
Figure 3.
Role of WNK1 in activation of TRPC6 by Gq-coupled receptor. (A) Whole-cell inward TRPC6 current density in response to CCh with anti-WNK1 Ab, GDPβS or control buffer applied via patch pipettes. *, p < 0.01, WNK1-Ab vs control. (B) Whole-cell CCh-induced TRPC6 currents were recorded in the presence of control buffer or WNK11–491 (3 nM), a concentration that does not appreciably stimulate TRPC6 by itself. *, p < 0.01, WNK1 vs buffer. (see also Figure S3).
Anti-WNK1 antibody did not significantly inhibit activation of TRPC6 by the Gi-coupled M2 muscarinic receptor (Figure S3E). However, Gi is a less effective activator of PLC-βs [17, 18], and TRPC6 activation by the M2 receptor is much smaller than that by the Gq-coupled receptors (Figure S3E vs Figure 3A). This apparent selectivity of anti-WNK1 antibody for Gq-coupled receptors suggests that WNK1 is needed to support the higher flux of the Gq-PLC-β pathway but is not detectably required for Gi-PLC-β signaling.
While these data are consistent with the stimulation of PLC-β activity by WNK1, we did not find any effect of purified WNK11–491 on the activity of purified PLC-β1, 2 or 3; its regulation by Gαq or Gβγ; or regulation of Gq by M1 muscarinic receptors (Figure S4).
Activation of TRPC6 by WNK1: PI4K-Mediated PIP2 Synthesis
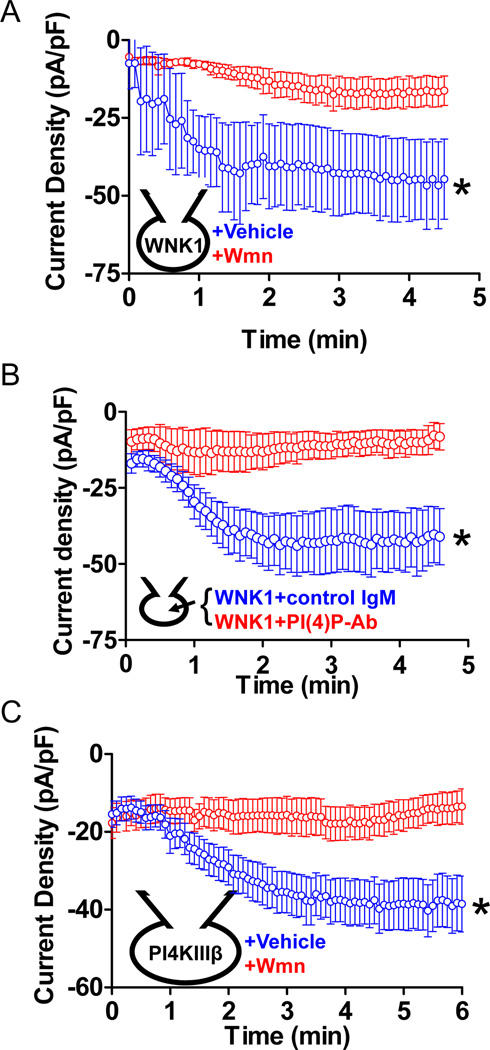
The interdependence and mutual potentiation of TRPC6 activation by the Gαq and WNK1 pathways suggests that they each increase the activity of PLC-β by distinct mechanisms. Gαq allosterically stimulates the catalytic activity of PLC-β. We therefore asked whether WNK1 acts by increasing the concentration of the PIP2 substrate. Generation of phosphatidylinositol 4-P (PI4P) by PI4K is believed to be the first step in the synthesis of PIP2 [30]. Pretreatment of cells with the PI4K inhibitor wortmannin inhibited activation of TRPC6 by WNK11–491 (Figure 4A) as did simultaneous application of an anti-PI4P antibody [31] (Figure 4B). Last, intracellular application of PI4KIIIβ activated TRPC6 even in the absence of added WNK1, and pretreatment with wortmannin prevented activation (Figure 4C). PI4P synthesis is thus involved in the stimulation of TRPC6 by WNK1, and WNK1 can be mimicked by addition of a PI4K.
Figure 4.
Role of PI4K in the activation of TRPC6 by WNK1. (A) Effect of wortmannin (Wmn) on whole-cell TRPC6 currents activated by WNK11–491. Cells were pretreated with 10 µM wortmannin or vehicle (DMSO) for 10 min before recording. *, p < 0.01, vehicle vs wortmannin. (B) Whole-cell TRPC6 currents in response to WNK11–491 co-applied with monoclonal anti-PI4P antibody or control mouse IgM. *, p < 0.01, control IgM vs PI(4)PAb. (C) Whole-cell TRPC6 current in response to intracellular delivery of 100 nM PI4KIIIβ preincubated with or without 10 µM wortmannin for 10 min. *, p < 0.01, vehicle vs wortmannin. Note that both type IIIα and IIIβ PI4K can generate PI4P for synthesis of PIP2 in the membrane. Due to difficulties in purification of PI4KIIIα, we used only PI4KIIIβ for experiments here. In all panels, data point is mean ± SEM of inward current density, n= 5–9 for each condition. (see also Figure S4).
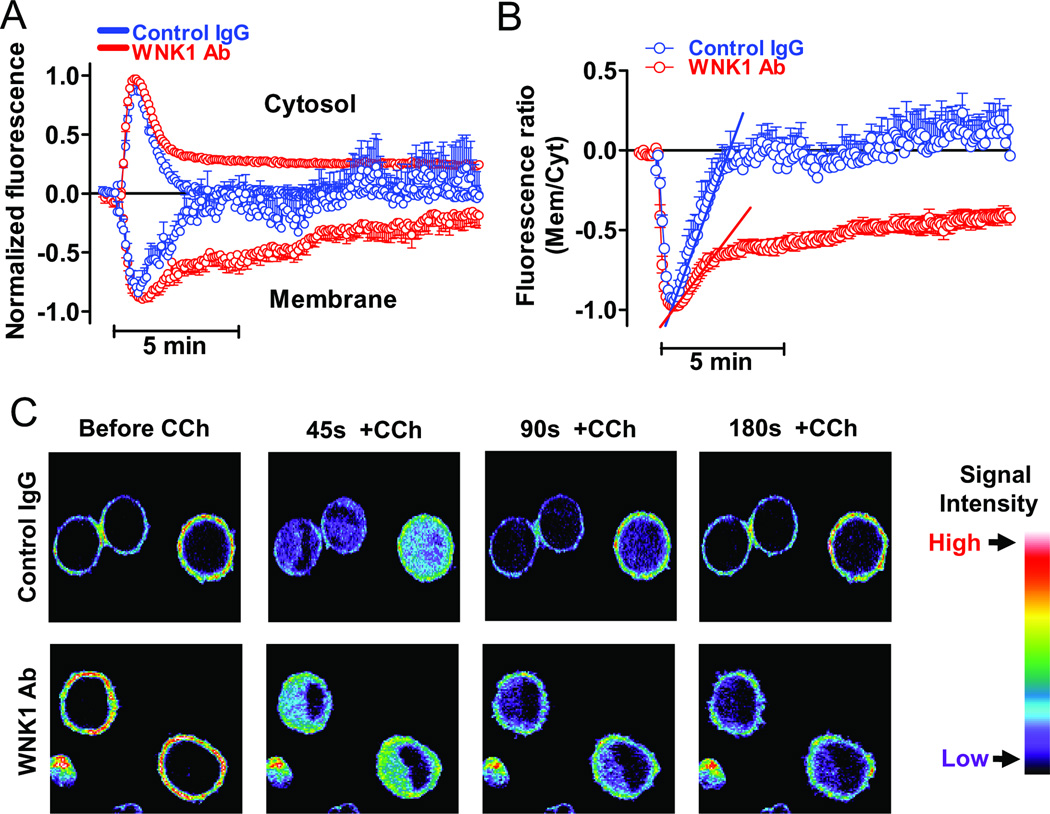
We used the GFP-tagged PH domain of PLC-δ1 and confocal imaging to examine the role of WNK1 in the degradation and re-synthesis of PIP2 in the plasma membrane. The PLC-δ1-PH domain binds both PIP2 and IP3 with relatively high affinity and specificity [32–34]. In cells that express M3 receptor, addition of carbachol decreased GFP-PLC-δ1-PH fluorescence at the plasma membrane, indicating reduced PIP2, and increased fluorescence in the cytosol, indicating increased IP3 (Figure 5). Both effects peak ~45 sec after carbachol addition and then decline to pre-stimulus levels, reflecting PLC-β-catalyzed hydrolysis of PIP2 and its subsequent re-synthesis.
Figure 5.
Anti-WNK1 antibody delays and inhibits plasma membrane PIP2 re-synthesis following receptor-stimulated hydrolysis. HeLa cells transfected with M3R and GFP-tagged PLC-δ1-PH domain were microinjected with control IgG or anti-WNK1 antibody and stimulated by 1 µM CCh. (A) Changes in fluorescence intensity at the plasma membrane (decrease) and cytosol (increase) in response to carbachol were normalized by setting maximal change to 1.0. Data are means ± SEM of 18 and 20 cells (7 separate experiments) that were microinjected with control rabbit IgG or anti-WNK1 antibody, respectively. (B) Ratio of fluorescence intensity in the plasma membrane to that in cytosol from panel A. Lines indicate slopes of the initial recovery rates (0.032 ± 0.002 and 0.014 ± 0.002 for control IgG and anti-WNK1 antibody, respectively; p<0.01). (C) Representative confocal images (obtained across the middle of cells) from one of 7 experiments showing fluorescence at the plasma membrane and cytosol before addition of CCh and at 45 s (peak), 90 s and 180 s after addition of CCh. Signal intensity is shown by pseudo-color.
Microinjection of anti-WNK1 antibody both delayed and decreased the recovery of GFP-PLC-δ1-PH fluorescence at the plasma membrane after treatment with carbachol. Instead of the monophasic return to baseline in <3 min observed in control cells, cells treated with anti-WNK1 antibody displayed biphasic recovery of plasma membrane fluorescence that was only ~50% complete after 15 min. The return of cytosolic fluorescence to baseline was also biphasic, with a greatly prolonged and incomplete second phase. These data indicate that anti-WNK1 antibody substantially inhibits re-synthesis of PIP2 after a burst of PLC activity. Taken together with the effects of the anti-PI4P antibody, they indicate that WNK1 is necessary to maintain cellular PIP2 synthesis.
WNK1 Potentiates PI4P Synthesis by PI4KIIIα
The PI4KIIIα isozyme is believed to be primarily responsible for re-synthesis of PIP2 in the plasma membrane during receptor stimulation of PLC [30]. To determine whether WNK1 regulates PI4KIIIα, we studied effects of manipulating WNK1 activity in cells that express recombinant PI4KIIIα, where this isoform is the predominant PI4K activity (~95%) in a light membrane fraction, which includes plasma membranes (Figure 6A). Knockdown of WNK1 by siRNA decreased both PI4K activity and the amount of immunoreactive PI4KIIIα in the light membrane fraction by 35–40% (Figures 6B–E) and increased the abundance of PI4KIIIα in cytosol (Figures 6D and 6E). WNK1 knockdown did not alter the amount of PI4KIIIα in the total post-nuclear supernatant (PNS). These data suggest that WNK1 recruits PI4KIIIα from cytosol to the light membrane fraction, presumably the plasma membrane. Recruitment may be indirect, because we did not detect physical association between PI4KIIIα and purified WNK11–491 in pull-down assays.
WNK1 failed to activate TRPC6 in PI4KIIIα-knockdown cells (Figures 6F and 6G) and expression of PI4KIIIα in the knockdown cells rescued activation by WNK1. These results are consistent with the idea that WNK1 increases PIP2 synthesis by recruitment of PI4KIIIα to its site of action, and that the consequently increased availability of PIP2 synergistically enhances Gq-coupled receptor signaling through PLC-β.
IGF1 Potentiates Gq-PLC-β Signaling via PI3K and Akt-Mediated Phosphorylation of WNK1
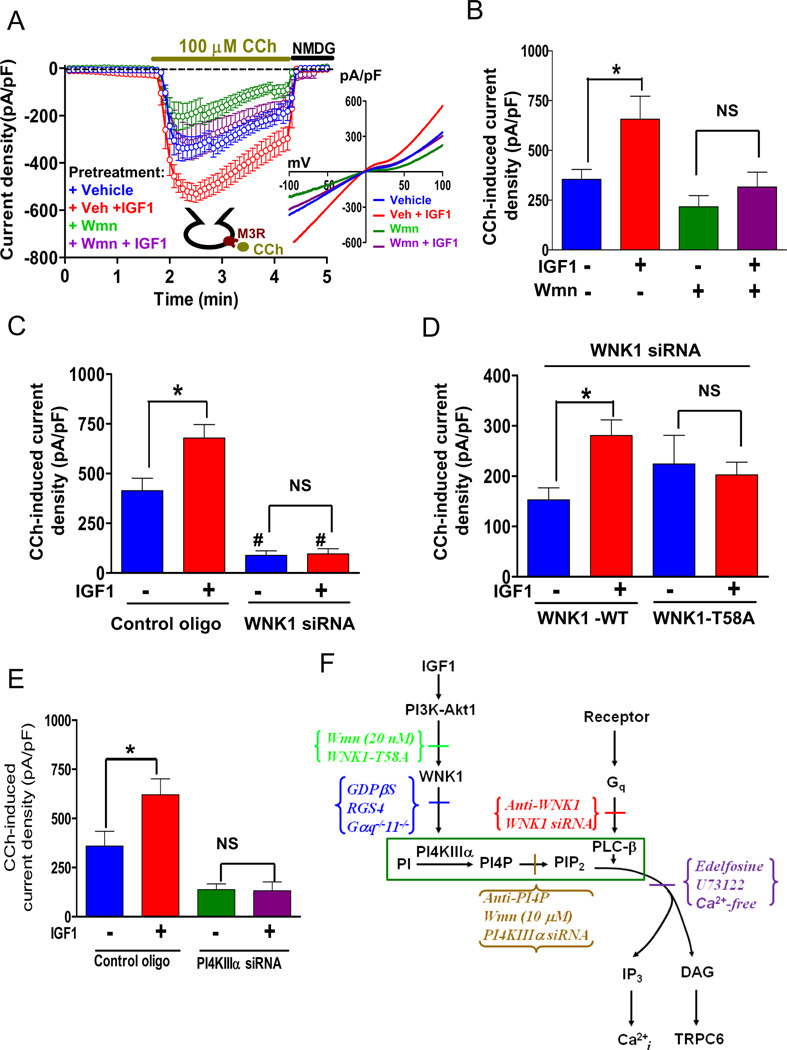
The physiological role of WNK1 in the regulation of Gq-PLC-β signaling was further supported by the finding that IGF1, a known upstream activator of WNK1, enhances the effect of WNK1. Pretreatment with IGF1 in serum-starved cells potentiated the activation of TRPC6 by either carbachol (Figures 7A and 7B) or GTPγS (Figures S5A and S5B), although IGF1 did not alter basal TRPC6 current. Lack of effect on the basal current is probably due to a low basal output from Gq-PLC-β in serum-starved cells, and IGF1 activated TRPC6 in these cells when pre-stimulated by GTPγS (Figure S5C). IGF1 also enhanced carbachol-induced intracellular Ca2+ transients (Figure S5D), consistent with a final common site of action at PLC-β. IGF1 did not increase the cell-surface abundance of TRPC6 (Figure S5E).
Figure 7.
IGF1 enhances Gq-coupled receptor activation of TRPC6 via WNK1. (A) Cells co-expressing TRPC6 and M3R were serum-starved for 18 h and incubated for 10 min with 100 ng/ml IGF1, 20 nM wortmannin, both or neither before ruptured whole-cell recording. Carbachol was applied to the bath where shown. (B) Peak CCh-induced current density from panel A. *, p < 0.01 between indicated groups. (C) Peak CCh-induced inward current density in cells transfected with control oligonucleotides or WNK1 siRNA and with or without IGF1 pretreatment. *, p < 0.01 between indicated groups. #, p < 0.01 vs cells transfected with control oligo and without IGF1 pretreatment. (D) Peak CCh-induced inward current density in cells transfected with WNK1 siRNA and cDNA for either wild type WNK1 or T58A mutant WNK1, and with or without IGF1 pretreatment. *, p < 0.01 between indicated. (E) Peak CCh-activated TRPC6 currents in cells transfected with control oligonucleotides or PI4KIIIα siRNA and with or without IGF1 pretreatment. *, p < 0.01 between indicated. (F) Interaction of WNK1, Gq-PLC-β and PI4KIIIα signaling pathways. Components of the pathways are shown in black. Inhibitors are color-coded per their biochemical sites of action and placed where their inhibitory effects on cellular signaling were noted in the experiments. Pathways are indicated by black arrows. The experimental results indicate that the point at which Gq and WNK1 interacts is at the level of PLC activity (marked by dark green box). (see also Figure S5).
Several observations argue that IGF1 potentiates GPCR-Gq signaling through a PI3K-Akt-WNK1-PI4KIIIα sequence. First, wortmannin, at a low concentration selective for PI3K (20 nM), blocked the effect of IGF1 on Gq signaling. Knockdown of WNK1 also blocked the ability of IGF1 to potentiate carbachol’s effect (Figure 7C). Expression of wild type WNK1, but not the T58A mutant WNK1, restored the ability of IGF1 to enhance carbachol activation of TRPC6 in WNK1-knockdown cells (Figure 7D), indicating that the synergistic effect of IGF1 and M3 muscarinic stimulation depends on Akt-mediated phosphorylation of WNK1 at T58. Note that both wild type WNK1 and the T58A mutant can support carbachol activation of TRPC6 in WNK1-knockdown cells. Thus, T58-phosphorylation is not required for WNK1 amplification of Gq-PLC-β signaling, but is indispensable for its potentiation by IGF1.
Knockdown of PI4KIIIα also prevented enhancement of carbachol activation of TRPC6 and intracellular Ca2+ transients by IGF1 (Figure 7E, Figure S5F). These results argue that PI3K-dependent activation of Akt by IGF1 leads to phosphorylation of WNK1 and enhances the ability of WNK1 to synergistically regulate PLC-β signaling by promoting PIP2 synthesis.
Discussion
Our data establish three new and distinct but inter-related signaling mechanisms: the mutual dependence and synergistic interaction between the Gq-PLC-β and Akt-WNK1 signaling pathways; the regulation of PLC-β activity by modulating the availability of its substrate; and the regulation of PI4KIIIα by WNK1 to control the concentration of PIP2-derived second messengers.
As summarized in Figure 7F, blocking WNK1 activity markedly inhibits PLC-β signaling by Gq-coupled receptors and, conversely, inhibiting Gq blocks PLC-β signaling by WNK1. Inhibition could be exerted by several approaches and at several steps in each pathway, both in cells and in detached membrane patches. In each case, replenishment of the inhibited or depleted species, or stimulation of a downstream component, restored normal activities of both WNK1 and Gq-PLC-β. Importantly, WNK1 can elicit PLC signals without added GPCR agonist, and WNK1 synergistically amplifies the stimulation of PLC-β by Gq.
In contrast to G proteins, WNK1 increases PLC-β activity by increasing the supply of the PIP2 substrate. Because the concentration of PIP2 in the plasma membrane is below Km [22, 23], PLC-β displays first-order substrate kinetics and increase in available PIP2 linearly amplifies PLC-β output. Note that WNK1 only modulates Gq signaling via PLC-β. Other outputs, such as stimulation of a rho GEF [36], are not involved. WNK1 thus alters the spectrum of Gq outputs as well as its amplitude.
WNK1 promotes synthesis of PIP2 by somehow activating PI4KIIIα (Figures 6 and 7F). PI4KIIIα is important for the re-synthesis of PIP2 following hydrolysis by receptor-stimulated PLC [30], but how the activity of PI4KIIIα is regulated is unknown. The amount of PI4KIIIα is decreased in membranes from WNK1 knockdown cells, suggesting that regulation of PI4KIIIα by WNK1 may involve its redistribution between plasma membrane and intracellular pools. However, WNK1 must do more than just recruit PI4KIIIα to the plasma membrane because it remains necessary for PLC-β signaling in detached patches. We have no evidence that WNK1 either binds or phosphorylates PI4KIIIα, and additional factors may be involved.
Regardless, our results indicate that the activity of PI4KIIIα is regulated and that its regulation by WNK1 is critical for PLC-β signaling by Gq-coupled receptors. The basal activity of WNK1 provides adequate tonic stimulation of PI4KIIIα to provide PIP2 for normal PLC-β signaling. However, additional WNK1 markedly amplifies signaling by Gq-coupled receptor even at a concentration that does not by itself activate TRPC6 (Figure 3B). In addition, PI3K-activating growth factors such as IGF1 activate WNK1 to further potentiate Gq-PLC-β signaling, probably via Akt-mediated phosphorylation of WNK1 at T58 (Figure 7). These results suggest that WNK1 is an important physiological mediator of the Akt kinase signaling pathway and coordinates Akt signaling with the Gq-PLC-β signaling pathway.
This action of WNK1 represents the first known instance of acute control of PIP2 availability as a mechanism of regulation of cellular DAG and IP3 concentrations without concurrent stimulation of the intrinsic activity of PLC. Application of WNK1 alone stimulates the production of DAG measured using the TRPC6 biosensor by an amount comparable to that initiated by GTPγS (Figure 1B). Some low output of PLC-β, evidently dependent on basal input from Gαq, is adequate to support this WNK1 signaling. By promoting PIP2 synthesis, WNK1 can also acutely modulate the amplitude of receptor-stimulated PLC-β signaling and/or its duration. Inhibition of endogenous WNK1 by anti-WNK1 antibody blocks both the DAG and IP3 limbs of PLC-β signaling stimulated by multiple Gq-coupled receptors by ≥50% (Figure 3A and Figures S1D–F and S3). Acute stimulation of WNK1 activity by IGF1 potentiates PLC-β signaling by ~2-fold (Figures 7A and 7B). WNK1 thus controls a major mode of desensitization of Gq signaling in addition to modulating acute responses. PIP2 itself is a signaling molecule that regulates many plasma membrane targets [37]. Increasing PIP2 synthesis by WNK1 is thus an important mechanism for increasing cellular DAG and IP3 concentrations without causing untoward effects of decreases in membrane PIP2 on other targets.
PLC-β enzymes function as co-incidence detectors that synergistically integrate multiple upstream inputs including Gαq, Gβγ, Ca2+, and Rac [35, 38, 39]. These mechanisms of synergism are results of combined super-additive stimulation of the catalytic activity of PLC-β. In contrast, synergism between WNK1 and Gαq depends on the ability of WNK1 to enhance substrate supply for PLC-β. This system is analogous to the interaction between PLCs and inositol phosphate kinases in which PLC provides the IP3 starting material that the cell-type-specific kinases then phosphorylate to the appropriate inositol polyphosphates [40].
The interactive control of PLC-β by WNK and Gq may have important implications in the pathogenesis of hypertension in patients with PHA2 that is caused by mutations of WNK1 that increase WNK1 expression [2]. Gq/11-mediated PLC-β signaling plays a central role in the regulation of vascular tone by virtually all vasoactive hormones [41], and potentiation of PLC-β signaling by WNK1 would promote vasoconstriction and cause hypertension. The role of WNK1 in the regulation of vascular tone is supported by the report that mice with heterozygous inactivation of Wnk1 display reduced blood pressure without renal Na+ wasting and hypovolemia [42]. Finally, the similarity between the phenotypes of WNK1-knockout and Gαq/Gα11-knockout mice (both lethal at embryonic day ~10.5, with cardiac defects) supports the idea that interaction between the WNK1 and Gαq/11 pathways is important for embryonic development [10, 26].
In conclusion, WNK1 potentiates Gq-PLC-β signaling by stimulating the activity of PI4KIIIα to increase synthesis of PIP2. Phosphorylation of WNK1 by Akt kinase increases its activity in this pathway, and allows tyrosine kinase receptors or other activators of the Akt kinase cascade to regulate G protein-mediated PLC-β signaling. The extent and physiological role of WNK1 in the regulation of PLC-β signaling will probably differ among specialized cells. Our understanding of these interactions should expand as we learn more ways in which WNK1 activity is regulated.
Experimental Procedures
Detailed experimental procedures are in Supplementary Information. Human embryonic kidney (HEK)-293, HeLa, and mouse embryonic fibroblasts were cultured and co-transfected with cDNAs for TRPC6-Flag with or without human M3 muscarinic receptor [43]. Ruptured whole-cell, cell-attached, and inside-out excised patch-clamp recording were performed as described [9, 43]. Intracellular Ca2+ transients were examined using ratiometric fluorescent Ca2+ images of fura-2-loaded cells. PIP2 and IP3 were localized according to fluorescence of a GFP-tagged PLC-δ1-PH domain by confocal microscopy [34]. PLC activity was measured by monitoring hydrolysis of [3H]PIP2 on large unilamellar phospholipid vesicles [22, 35]. PI4KIIIα activity from HeLa cells was measured by phosphorylation of phosphatidylinositol (PI) in PI/Triton X-100-mixed micelles [44].
Supplementary Material
Acknowledgements
This study was supported by the National Institutes of Health (GM30355 to E.M.R. and DK59530, DK85726 to C.L.H), the Welch Foundation (I-0982 to E.M.R.) and Genzyme, Inc. (GRIP grant to C.L.H.). We are particularly indebted to Barbara Barylko and Joseph Albanesi for purification of PI4KIIIβ and for continuing help and advice. We thank Melanie Cobb for anti-WNK1 antibody, Tamas Balla for GFP-PLC-δ1-PH, Melvin Simon for Gαq/11-null mouse fibroblasts, and Anna Dugas for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J. Biol. Chem. 2000;275:16795–16801. doi: 10.1074/jbc.275.22.16795. [DOI] [PubMed] [Google Scholar]
- 2.Wilson FH, Disse-Nicodème S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 3.McCormick JA, Ellison DH. The WNKs: atypical protein kinases with pleiotropic actions. Physiol. Rev. 2011;91:177–219. doi: 10.1152/physrev.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahle KT, Ring AM, Lifton RP. Molecular physiology of the WNK kinases. Annu. Rev. Physiol. 2008;70:329–355. doi: 10.1146/annurev.physiol.70.113006.100651. [DOI] [PubMed] [Google Scholar]
- 5.Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem. J. 2005;391:17–24. doi: 10.1042/BJ20051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moriguchi T, Urushiyama S, Hisamoto N, lemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J. Biol. Chem. 2005;280:42685–42693. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]
- 7.Anselmo AN, Earnest S, Chen W, Juang YC, Kim SC, Zhao Y, Cobb MH. WNK1 and OSR1 regulate the Na+, K+, 2Cl− cotransporter in HeLa cells. Proc. Natl. Acad. Sci. USA. 2006;103:10883–10888. doi: 10.1073/pnas.0604607103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu B, Stippec S, Lazrak A, Huang CL, Cobb MH. WNK1 activates SGK1 by a PI-3 kinase-dependent and non-catalytic mechanism. J. Biol. Chem. 2005;280:34218–34223. doi: 10.1074/jbc.M505735200. [DOI] [PubMed] [Google Scholar]
- 9.He G, Wang HR, Huang SK, Huang CL. Intersectin links WNK kinases to endocytosis of ROMK. J. Clin. Invest. 2007;117:1078–1087. doi: 10.1172/JCI30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie J, Wu T, Xu K, Huang IK, Cleaver D, Huang C-L. Endothelial expression of WNK1 is essential for angiogenesis and cardiovascular development in mice. Am. J. Pathology. 2009;175:1315–1327. doi: 10.2353/ajpath.2009.090094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu B, Stippec S, Lenertz L, Lee BH, Zhang W, Lee YK, Cobb MH. WNK1 activates ERK5 by an MEKK2/3-dependent mechanism. J. Biol. Chem. 2004;279:7826–7831. doi: 10.1074/jbc.M313465200. [DOI] [PubMed] [Google Scholar]
- 12.Moniz S, Matos P, Jordan P. WNK2 modulates MEK1 activity through the Rho GTPase pathway. Cell Signal. 2008;20:1762–1768. doi: 10.1016/j.cellsig.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 13.San-Cristobal P, Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH, Chari D, Kahle KT, Leng Q, Bobadilla NA, et al. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc. Natl. Acad. Sci. USA. 2009;106:4384–4389. doi: 10.1073/pnas.0813238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitari AC, Deak M, Collins BJ, Morrice N, Prescott AR, Phelan A, Humphreys S, Alessi DR. WNK1, the kinase mutated in an inherited high-blood-pressure syndrome, is a novel PKB (protein kinase B)/Akt substrate. Biochem. J. 2004;378:257–268. doi: 10.1042/BJ20031692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang ZY, Zhou QL, Holik J, Patel S, Leszyk J, Coleman K, Chouinard M, Czech MP. Identification of WNK1 as a substrate of Akt/protein kinase B and a negative regulator of insulin-stimulated mitogenesis in 3T3-L1 cells. J. Biol. Chem. 2005;280:21622–21628. doi: 10.1074/jbc.M414464200. [DOI] [PubMed] [Google Scholar]
- 16.Cheng CJ, Huang CL. Activation of phosphatidylinositol 3-kinase stimulates endocytosis of ROMK channel via Akt1/SGK1-dependent phosphorylation of WNK1. J. Am. Soc. Nephrol. 2011;22:460–471. doi: 10.1681/ASN.2010060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol. Rev. 2000;80:1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- 18.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hicks SN, Jezyk MR, Gershburg S, Seifert JP, Harden TK, Sondek J. General and versatile autoinhibition of PLC isozymes. Mol Cell. 2008;31:383–394. doi: 10.1016/j.molcel.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Creba JA, Downes CP, Hawkins PT, Brewster G, Michell RH, Kirk CJ. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem. J. 1983;212:733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakanishi S, Catt KJ, Balla T. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositol phospholipids. Proc. Natl. Acad. Sci. USA. 1995;92:5317–5321. doi: 10.1073/pnas.92.12.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biddlecome GH, Berstein G, Ross EM. Regulation of phospholipase C-β1 by Gq and m1 muscarinic cholinergic receptor. Steady-state balance of receptor-mediated activation and GTPase-activating protein-promoted deactivation. J. Biol. Chem. 1996;271:7999–8007. doi: 10.1074/jbc.271.14.7999. [DOI] [PubMed] [Google Scholar]
- 23.Feng J, Roberts MF, Drin G, Scarlata S. Dissection of the steps of phospholipase C-β2 activity that are enhanced by Gβγ subunits. Biochemistry. 2005;44:2577–2584. doi: 10.1021/bi0482607. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 25.Horowitz LF, Hirdes W, Suh BC, Hilgemann DW, Mackie K, Hille B. Phospholipase C in living cells: activation, inhibition, Ca2+ requirement, and regulation of M current. J. Gen. Physiol. 2005;126:243–262. doi: 10.1085/jgp.200509309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Offermanns S, Simon MI. Genetic analysis of mammalian G-protein signalling. Oncogene. 1998;17:1375–1381. doi: 10.1038/sj.onc.1202173. [DOI] [PubMed] [Google Scholar]
- 27.Posner BA, Mukhopadhyay S, Tesmer JJ, Gilman AG, Ross EM. Modulation of the affinity and selectivity of RGS protein interaction with Gα subunits by a conserved asparagine/serine residue. Biochemistry. 1999;38:7773–7779. doi: 10.1021/bi9906367. [DOI] [PubMed] [Google Scholar]
- 28.Lan KL, Sarvazyan NA, Taussig R, Mackenzie RG, DiBello PR, Dohlman HG, Neubig RR. A point mutation in Gαo and Gαi1 blocks interaction with regulator of G protein signaling proteins. J. Biol. Chem. 1998;273:12794–12797. doi: 10.1074/jbc.273.21.12794. [DOI] [PubMed] [Google Scholar]
- 29.Tu Y, Woodson J, Ross EM. Binding of regulator of G protein signaling (RGS) proteins to phospholipid bilayers. Contribution of location and/or orientation to GTPase-activating protein activity. J. Biol. Chem. 2001;276:20160–20166. doi: 10.1074/jbc.M101599200. [DOI] [PubMed] [Google Scholar]
- 30.Balla A, Balla T. Phosphatidylinositol 4-kinases: old enzymes with emerging functions. Trends Cell Biol. 2006;16:351–361. doi: 10.1016/j.tcb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Wang YJ, Wang J, Sun HQ, Martinez M, Sun YX, Macia E, Kirchhausen T, Albanesi JP, Roth MG, Yin HL. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell. 2003;114:299–310. doi: 10.1016/s0092-8674(03)00603-2. [DOI] [PubMed] [Google Scholar]
- 32.Lemmon MA, Ferguson KM, O'Brien R, Sigler PB, Schlessinger J. Specific and high-affinity binding of inositol phosphates to an isolated pleckstrin homology domain. Proc. Natl. Acad. Sci. USA. 1995;92:10472–10476. doi: 10.1073/pnas.92.23.10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stauffer TP, Ahn S, Meyer T. Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr. Biol. 1998;8:343–346. doi: 10.1016/s0960-9822(98)70135-6. [DOI] [PubMed] [Google Scholar]
- 34.Balla T, Várnai P. Visualization of cellular phosphoinositide pools with GFP-fused protein-domains. Curr. Protoc. Cell Biol. 2009 doi: 10.1002/0471143030.cb2404s42. Chapter 24:Unit 24.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Philip F, Kadamur G, González Silos R, Woodson J, Ross EM. Synergistic activation of phospholipase C-β3 by Gαq and Gβγ describes a simple two-state coincidence detector. Curr. Biol. 2010;20:1327–1335. doi: 10.1016/j.cub.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lutz S, Freichel-Blomquist A, Yang Y, Rümenapp U, Jakobs KH, Schmidt M, Wieland T. The guanine nucleotide exchange factor p63RhoGEF, a specific link between Gq/11-coupled receptor Signaling and RhoA. J. Biol. Chem. 2005;280:11134–11139. doi: 10.1074/jbc.M411322200. [DOI] [PubMed] [Google Scholar]
- 37.McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 38.Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M. Phospholipase Cβ serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–268. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Jezyk MR, Snyder JT, Gershberg S, Worthylake DK, Harden TK, Sondek J. Crystal structure of Rac1 bound to its effector phospholipase C-β2. Nature Struct. Mol. Biol. 2006;13:1135–1140. doi: 10.1038/nsmb1175. [DOI] [PubMed] [Google Scholar]
- 40.Otto JC, Kelly P, Chiou ST, York JD. Alterations in an inositol phosphate code through synergistic activation of a G protein and inositol phosphate kinases. Proc. Natl. Acad. Sci. USA. 2007;104:15653–15658. doi: 10.1073/pnas.0705729104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Docherty JR. Subtypes of functional α1-adrenoceptor. Cell Mol. Life Sci. 2010;67:405–417. doi: 10.1007/s00018-009-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zambrowicz BP, Abuin A, Ramirez-Solis R, Richter LJ, Piggott J, BeltrandelRio H, Buxton EC, Edwards J, Finch RA, Friddle CJ, et al. Wnk1 kinase deficiency lowers blood pressure in mice: a gene-trap screen to identify potential targets for therapeutic intervention. Proc. Natl. Acad. Sci. USA. 2003;100:14109–14114. doi: 10.1073/pnas.2336103100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nature Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barylko B, Mao YS, Wlodarski P, Jung G, Binns DD, Sun HQ, Yin HL, Albanesi JP. Palmitoylation controls the catalytic activity and subcellular distribution of phosphatidylinositol 4-kinase IIα. J. Biol. Chem. 2009;284:9994–10003. doi: 10.1074/jbc.M900724200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.