Abstract
Approximately 170 million inhabitants of the American continent live at risk of malaria transmission. Although the continent’s contribution to the global malaria burden is small, at least 1 to 1.2 million malaria cases are reported annually. Sixty per cent of the malaria cases occur in Brazil and the other 40% are distributed in 20 other countries of Central and South America. Plasmodium vivax is the predominant species (74.2 %) followed by P. falciparum (25.7 %) and P. malariae (0.1%), and no less than 10 Anopheles species have been identified as primary or secondary malaria vectors. Rapid deforestation and agricultural practices are directly related to increases in Anopheles species diversity and abundance, as well as in the number of malaria cases. Additionally, climate changes profoundly affect malaria transmission and are responsible for malaria epidemics in some regions of South America. Parasite drug resistance is increasing, but due to bio-geographic barriers there is extraordinary genetic differentiation of parasites with limited dispersion. Although the clinical spectrum ranges from uncomplicated to severe malaria cases, due to the generally low to middle transmission intensity, features such as severe anemia, cerebral malaria and other complications appear to be less frequent than in other endemic regions and asymptomatic infections are a common feature. Although the National Malaria Control Programs (NMCP) of different countries differ in their control activities these are all directed to reduce morbidity and mortality by using strategies like health promotion, vector control and impregnate bed nets among others. Recently, international initiatives such as the Malaria Control Program in Andean-country Border Regions (PAMAFRO) (implemented by the Andean Organism for Health (ORAS) and sponsored by The Global Fund to Fight AIDS, Tuberculosis and Malaria (GFATM)) and The Amazon Network for the Surveillance of Antimalarial Drug Resistance (RAVREDA) (sponsored by the Pan American Health Organization/World Health Organization (PAHO/WHO) and several other partners), have made great investments for malaria control in the region. We describe here the current status of malaria in a non-Amazonian region comprising several countries of South and Central America participating in the Centro Latino Americano de Investigación en Malaria (CLAIM), an International Center of Excellence for Malaria Research (ICEMR) sponsored by the National Institutes of Health’s (NIH) National Institute of Allergy and Infectious Diseases (NIAID).
Keywords: malaria, Plasmodium falciparum, Plasmodium vivax, malaria elimination, epidemiology, Latin America
1. Current malaria problem in non-Amazonian regions of Latin America
1.1 Changing epidemiology of Plasmodium falciparum and P. vivax
1.1.1 General picture in the American Continent
According to recent estimates, approximately 170 million people live at risk of Plasmodium vivax and P. falciparum transmission in 21 countries of Latin America (LA) and the Caribbean (Figure 1) (Guerra et al., 2008; Guerra et al., 2010). Approximately 60% of the malaria cases in the Americas are reported from Brazil, with an incidence almost exclusively restricted to the Amazon Region, whereas the other 40% of the cases are reported from Colombia (14.2%), Peru (8.8%), Venezuela (5.4%), Bolivia (1.9%) and Ecuador (1.1%), as well as from the Caribbean, mainly Haiti (2.8%), and some Central American countries, Guatemala (3.8%), Panama (0.4%) and Honduras (1.5%). A limited number of cases (0.3%) are also reported from Mexico (PAHO and WHO, 2007a). The contribution of LA to the global malaria burden is small, with an estimated <1% (~3 million cases) of the total world malaria cases occurring in LA in 2007 (Hay et al., 2010).
Figure 1. Distribution of Plasmodium vivax (a) and P. falciparum malaria (b) in the study areas of CLAIM and neighboring countries.

Risk is stratified according to API into stable transmission (dark grey areas; API ≥0.1 per 1,000 per annum), unstable transmission (medium grey areas; API <0.1 per 1,000 per annum) and malaria free (light grey areas; API =0) (Guerra et al., 2008; Guerra et al., 2010).
About 90% of these malaria cases originate in the Amazon basin shared by Bolivia, Brazil, Colombia, Ecuador, French Guiana, Guyana, Peru, Suriname and Venezuela (PAHO, 2006) whereas the other 10% is contributed by non-Amazon regions, mainly the Andean region (2%) and Central America (4.1%) (Figure 1).
In terms of malaria species, 74.2% of infections are caused by P. vivax and 25.7% by P. falciparum, with an estimated mortality of 1%. Less than 0.1% of the cases reported are caused by P. malariae in scattered foci in different countries (Sina, 2002; WHO, 2009). Additionally, in some areas of the Amazon forest, parasites containing gene sequences corresponding to the simian parasite P. simiovale have been described (de Arruda et al., 1998; Kremsner et al., 1992; Qari et al., 1993; Rosenberg et al., 1989). Figure 2 shows the evolution of malaria in the Americas in terms of parasite species predominance.
Figure 2. Malaria transmission in the Americas.
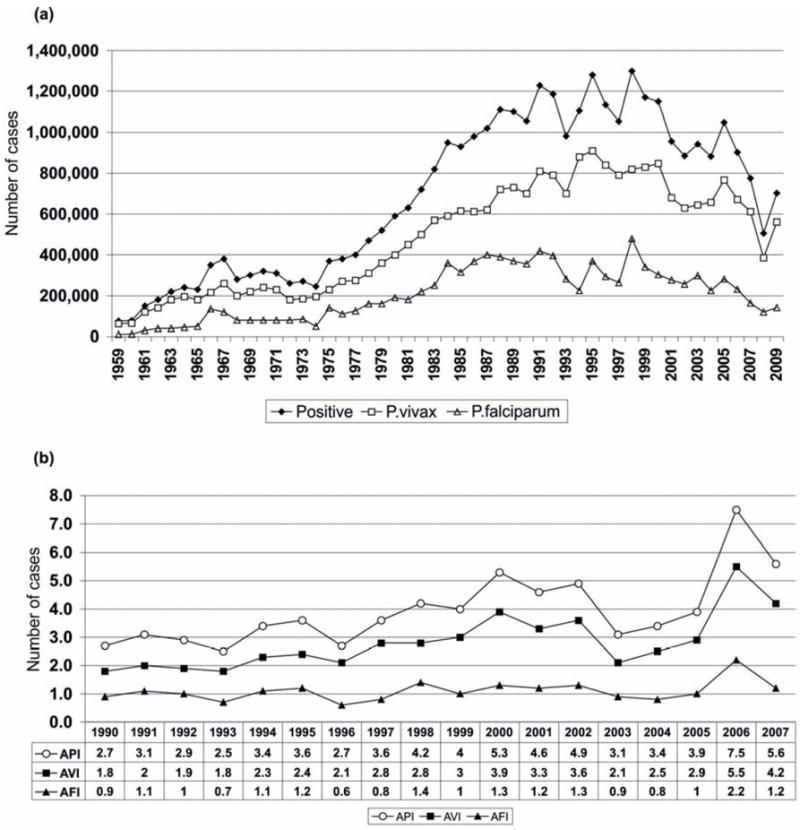
(a) Evolution of malaria cases between 1959 and 2009 (Carter, 2009; Najera and Zaim, 2003). (b) Annual Parasite Index (API), Annual Index of P. vivax (AVI) and Annual Index of P. falciparum (AFI), 1990-2007(PAHO and WHO, 2008b).
Despite the great regional efforts to control malaria and the evident success of the Global Malaria Eradication Program (GMEP) in eliminating malaria or significantly reducing its transmission in numerous areas of the continent by the1960s, (Gusmao, 1999), malaria transmission maintained an increasing trend in recent decades, with periodical epidemic peaks in some areas likely associated with the climatic changes introduced by the warm phase of ENSO (Mantilla et al., 2009). However, there has been a significant decrease in incidence in the last five years (PAHO and WHO, 2008a).
In an effort to better understanding of the epidemiology of malaria in non Amazonian settings, four countries of the region: Colombia, Guatemala, Panama and Peru have been initially selected to conduct a study in the context of the project Centro Latinoamericano de Investigación en Malaria (CLAIM), to assess the diversity of parasite populations related to the epidemiology, vectors and clinical findings of malaria. The aim is to establish a scientific framework that supports the development of new intervention strategies for malaria elimination in these Latin American countries.
1.1.2 Malaria transmission in non-Amazonian regions
Malaria in non-Amazonian regions of LA is found mainly along the coastal regions and lowland Andean valleys as well as in meso-America, from Mexico to Panama (PAHO and WHO, 2008a). We describe here the current status of malaria in four countries of South and Central America participating in the CLAIM.
Guatemala has an estimated population of 14.4 million inhabitants and an area of 109,117 km2; 80% of its territory is considered to support malaria transmission. In 2008, a total of 7,198 malaria cases were reported, caused almost exclusively by P. vivax with less than 1% caused by P. falciparum. The annual parasite incidence (API) in 2008 for P. vivax was 23/1,000 person-years (p-y) and the API for P. falciparum was 0.9/1,000 p-y. Malaria is mainly transmitted in rural areas at altitudes <1,500 m and there is year-round transmission with a peak during the long rainy season. However, in areas such as Quiché, Petén, Alta Verapaz and Fray Bartolome there are two peaks of higher transmission. Three important malaria transmission foci have been identified. One persists along the Pacific coast (Escuintla, Suchitepequez, San Marcos and Quetzaltenango) due to human migration associated with agricultural activities that contribute to the proliferation of mosquito breeding grounds. There is a second focus in Baja Verapaz, located in the middle east of Guatemala and a third in the northern departments of Peten, Izabal and Alta Verapaz . In 2010, 21.47% of malaria cases (n=1,546) were from Alta Verapaz. In the last two years malaria transmission has been reduced by about 95% in the most endemic areas using a combination of rapid diagnosis, prompt treatment, and the distribution and use of insecticide impregnated bed nets (Sanchez, 2010).
Panama has an estimated population of 3.4 million inhabitants and an area of 77,381 km2. Malaria transmission in currently occurring in the provinces of Chiriqui and Bocas del Toro on the Panamanian border with Costa Rica, where P. vivax is the most prevalent parasite species. The province of Darien on the border with Colombia, East Panama, specifically the Chepo area and Comarca Kuna Yala, is also P. vivax malaria endemic with some peaks of P. falciparum during the year. However, according to 2006 WHO reports Darien reported 193 malaria cases, being the third leading province in the country with the majority of cases due to P. falciparum in this year. Chloroquine resistance has been reported, with cases likely coming from Colombia (Samudio, 2005). After more than three decades of control, malaria reemerged as a major public health problem and an epidemic peak (5,095 malaria cases: 82.7% P. vivax and 17.3% P. falciparum) was observed in 2004. However, the overall number of malaria cases has dropped with 744 cases reported in 2008 and mainly due to P. vivax (95%) (PAHO and WHO, 2008a). Chepo and the Comarca Kuna Yala, on the border with Colombia have been selected as field sites for CLAIM activities.
Colombia has a population of 45 million and an area of 1.15 million km2 with approximately 60% of the latter suitable for malaria transmission. However, 80% of the malaria transmission is concentrated in five of the 32 states of the country: Antioquia, Cauca, Chocó, Cordoba and Valle. Between 2003 and 2007, the average annual number of malaria cases reported was close to 120,000 (INS, 2008; WHO, 2008) representing an annual incidence of 2.6/1,000 with a predominance of P. vivax cases (80%) (INS, 2008). The Pacific coastal region, representing only about 5% of the whole country population, accounts for about 30% of the total malaria cases, most of them (80%) produced by P. falciparum. The population of this region is composed mainly of Afro-descendents with a high prevalence of Duffy negative (Fy-) blood group, and this Fy- P.vivax refractory population could as in the case of Haiti, bias the prevalence towards P.falciparum.
A region comprising Urabá, Sinu and Bajo Cauca located in the northwestern region of the country where Antioquia and Córdoba states account for ~ 45% of the total cases of the country. Cordoba, Tierralta, Montelíbano and Puerto Libertador contribute with 22.4% of malaria cases, and the remaining cases occur in Antioquia (The Bagre, Cáceres and Zaragoza). The Orinoquía region, Guaviare and Meta states account for 15% of malaria cases and the Amazon region of Colombia, with about 10% of the total malaria cases. The last two regions do not contribute much to the overall malaria burden of the country due to its limited population. Tumaco (Nariño state) and Buenaventura (Valle state) on the Pacific coast and Tierralta (Cordoba state) are the most representative sites with high intensity of transmission and therefore have been selected as field sites for CLAIM activities (Figure 3).
Figure 3. Map of malaria risk in Colombia.

Malaria transmission is concentrated in five of the 32 states of the country located in four regions: Uraba, Sinu and Bajo Cauca region, Orinoquia region, Amazon region and Pacific coast region.
Peru has an estimated population of 30 million inhabitants living in an area of 1.28 million km2. The Amazonian region is the most malaria endemic, with almost 80% of the total malaria cases in the country reported in this region in 2009 (Chapilliquen et al., 2009). The Northern coast region, which includes Piura and Tumbes departments, represents the second most important focus of malaria transmission. In 1998, this region experienced a large malaria epidemic with over 100,000 malaria cases (70% of them caused by P. falciparum) linked to ENSO. There has been a significant decreasing trend in malaria incidence in this region with 4,153 cases reported in 2009, all caused by P. vivax (Chapilliquen et al., 2009). This region is characterized by the presence of coastal valleys with significant human migration driven by labor-intense agricultural activities (MINSA, 1999). The area is characterized by a winter season (April-November) with cloudy and cool days, and a hot and rainy summer (December-March), this seasonal pattern is periodically altered by ‘El Niño’ phenomenon, with torrential rains and strong winds that lead to flooding and landslides (Roberts et al., 1997). The most affected communities live in precarious houses and work in rice fields that favor mosquito breeding. Piura department has been selected as a field site for CLAIM activities.
Other countries in the region that are currently envisaged as potential participants of CLAIM include Honduras, Ecuador, Haiti, and Dominican Republic. Brief summaries of the malaria situation in these countries are presented as follows:
In Honduras, with an area of 112,492 km2 and 8,202,681 inhabitants, a total of 8,225 malaria cases were reported in 2008, with >90% of them caused by P.vivax (PAHO and WHO, 2008a). Malaria transmission is mainly due to population movements particularly to the Department of Gracias a Dios, an area ecologically favorable for An. albimanus breeding.
Ecuador has an estimated population of 14 million inhabitants living in an area of 283,561 km2. In 2009, about 5,000 malaria cases were reported in the country, with >90% caused by P. vivax (SNEM, personal communication April 2009). An almost equal number of malaria cases were reported from Coastal (48%) and Amazonian provinces (45%). Lowland endemic areas of some Andean provinces contribute with the difference.
In 2009, Haiti and Dominican Republic together reported 38,614 malaria cases. P. falciparum is the predominant species (>90%).
1.2 Transmission by vector populations
1.2.1. Malaria vectors in LA
In LA, around 90 Anopheles species have been described and some of them, i.e. An. darlingi, An. nuneztovari, An. pseudopunctipennis, An. albimanus and An. aquasalis are considered malaria vectors of regional-scale importance (Herrera et al., 1987; Rubio-Palis and Zimmerman, 1997; Sinka et al.,2010; Zimmerman, 1992). Other species such as An. albitarsis s.l., An.punctimacula, An. vestitipennis, An. trinkae, and species of subgenus Kerteszia such as An. neivai s.l., An. bellator and An. cruzii s.l. are considered secondary vectors (Hayes et al., 1987; Rubio-Palis and Zimmerman, 1997; Zimmerman, 1992). However, some other, widely distributed in the region, have been incriminated as local malaria vectors or found positive for the presence of either P. falciparum or P. vivax circumsporozoite protein, and their role in malaria transmission is still unknown. Such is the case An. oswaldoi (Hayes et al., 1987), An. rangeli, An. oswaldoi B (Quinones et al., 2006; Ruiz et al., 2005), An benarrochi (Flores-Mendoza et al., 2004), An.neomaculipalpus (Herrera et al., 1987; Moreno et al., 2005), An. marajoara (Conn et al., 2002) An. triannulatus (de Arruda et al., 1986).
In LA, the presence of species complexes (Harbach, 2004; Silva-Do-Nascimento et al., 2006) and lack of information on vector competence, particularly for species from the non-amazon region, are the major obstacles to identify the role of the different anopheline species in malaria transmission and to determine strategies for malaria control in the region (Gonzalez-Ceron et al., 2007; Gonzalez-Ceron et al., 1999; Vaughan et al., 1994). The diversity of anopheline mosquito fauna in LA is being addressed by the Mosquito Barcoding Initiative (MBI) as a basis for improved species recognition and associated vector incrimination studies directed towards control (Cywinska et al., 2006). CLAIM will combine MBI with integrated morphological and molecular studies, basic studies on the biology, behavior and ecology of the different species present in the endemic region under study in order to clarify both their taxonomy and vector role status.
Fundamental relationships between the environment and Anopheles vector ecology and behavior are central to the design and implementation of vector control strategies (Najera and Zaim, 2003). However, in many malaria endemic areas, there are gaps in information on the distribution, behavior, species composition, and how human activity, climate change, and environmental changes impact vectors populations (PAHO and WHO, 2008a). In LA, changes in land use are directly related to increases in anopheline species diversity, increases in vector abundance, and increases in the number of malaria cases (Conn et al., 2002; Singer and de Castro, 2006; Vittor et al., 2006). Each Anopheles species occupies a unique ecological niche, and operates at a different level of vector potential. Thus, information on how environmental changes affect vector abundance and species succession is vital for planning and evaluating vector control interventions (Najera and Zaim, 2003).
1.2.2. Vector control in the LA region
After extensive use of DDT during and after the GMEP, its use was banned from most of the countries around 1992. Thereafter, pyrethroids were mainly used for indoor residual spraying (IRS), but the coverage was limited to prioritized areas. With limited resources, the malaria control programs could not afford to maintain the required coverage and therefore priority was given to case detection and treatment rather than to vector control (Chareonviriyaphap et al., 1997).
Current methods for vector control in countries of the LA region are based primarily on the use of long-lasting insecticide treated nets (LLINs) and indoor residual spraying (IRS). In contrast to many African countries where LLINs have been tested extensively (Beach et al., 1993; Hawley et al., 2003; Lindsay et al., 1993; Magesa et al., 1991; Quinones et al., 1998; Robert and Carnavale, 1991; Thomson et al., 1995) comparatively less information exists in the LA region, although extensive distribution of LLINs has been promoted in the region with support from international organizations during the past 10 years. Little information is available on the impact treated nets have had on suppressing malaria vector populations, reducing levels of malaria parasite transmission, and reducing malaria incidence and malaria-related morbidity and mortality in LA.
One of the major threats for malaria vector control is the development of insecticide resistance. In LA, An. albimanus has shown variable levels of insecticide resistance along its distribution range (Brogdon et al., 1999; Dzul et al., 2007; Feachem et al., 2009). In addition, in recent years, insecticide resistance by An. darlingi (Fonseca-Gonzalez et al., 2009b) and An. nuneztovari (Fonseca-Gonzalez et al., 2009a) has also been reported, which stresses the necessity to develop local and systematic evaluation.
Integrated vector management (IVM) has been slowly introduced for malaria control in LA (Feachem et al., 2009; PAHO, 2006) but it has been insufficiently evaluated in the region. However it is clear that when IVM strategies have been comprehensively implemented in African countries they have successfully controlled malaria transmission (Beier et al., 2008).
The countries of this ICEMR network (Colombia, Guatemala, Panama, and Peru) have decentralized NMCPs that operate at the municipality level. Major problems limiting the effectiveness of vector control in these countries include: 1) a lack of fundamental baseline data on the bionomics and vector potential of dominant vector species needed to guide operational malaria vector control programs, 2) insufficient information on how changing patterns of malaria epidemiology in human populations are related to fundamental changes in vector populations and their transmission potential due to anthropogenic environmental changes, 3) a limited set of tools to control malaria vectors and uncertainties of how vector species are adapting to control measures through behavioral changes and the development of insecticide resistance, and 4) a lack of evidence-based field research and rigorous evaluation strategies to guide the development and implementation of effective and environmentally sound IVM components of NMCPs. These 4 key factors present serious obstacles that must be overcome for the countries in this ICEMR region to achieve their goals of effective, sustainable malaria control and eventual elimination.
1.3 Parasite populations
There is substantial spatial and temporal heterogeneity of malaria parasite populations in relation to infections caused by each parasite, in addition to the limited information about their genetic diversity. Indeed, in LA malarial parasites in LA exhibit strong geographic differentiation (Cornejo and Escalante, 2006; Joy et al., 2003; Tanabe et al., 2010). The Andean ridge, for instance, separates endemic areas on the Pacific Coast from those in the Amazon and Orinoco basins (Cortese et al., 2002; McCollum et al., 2007). This has resulted in the isolation of parasite populations, which may have consequences for malaria control strategies as is the case with the limited dispersion of mutations associated with anti-malarial drug resistance in P. falciparum populations (Corredor et al., 2010; Cortese et al., 2002). Resistant mutations that are common in the Amazon and Orinoco basins (Bacon et al., 2009) have not been introduced to the Pacific Coast. In addition, the low transmission characteristic of this region (Hay et al., 2009) generates strong linkage disequilibrium due to inbreeding, resulting in clonal population structures (Griffing et al., 2010; McCollum et al., 2007; Urdaneta et al., 2001). The temporal and spatial stability of such clonal lineages in P. falciparum have been poorly documented and their public health consequences need to be evaluated. As an example, clonal lineages resistant to sulfadoxine-pyrimethamine and chloroquine appear to be stable in some areas even when there is no longer drug pressure (Griffing et al., 2010; McCollum et al., 2007). Little information is available on P. vivax, but it appears that its populations in Latin America harbor higher levels of genetic diversity, at least in microsatellite markers (Van den Eede et al.). Despite these complexities, the consequences of the diverse P. vivax and P. falciparum population structures on the epidemiology of malaria remain poorly understood.
1.4 Clinical malaria and pathogenesis
Malaria has a broad clinical spectrum that ranges from asymptomatic infections to severe and complicated multi-systemic failure and death. The classical triad of chills, headache and high fever (39-41°C) varies according to the Plasmodium species, resulting in either the tertian (48 hours: P. falciparum, P. vivax, P. ovale) or the quartan (72 hours: P. malariae) malaria cycles. P. vivax produces dormant liver forms, or hypnozoites, which can cause relapses up to several months or even years after primary infection (Anstey et al., 2009). Despite the prevailing dogma that P. vivax rarely causes severe disease, recent studies in Asia showed that 21-27% of patients with severe malaria had infections by P. vivax only, and presented an overall mortality between 0.8 – 1.6%, with infants being the most affected group (Kochar et al., 2009; Price et al., 2009). The major manifestations included severe anemia and respiratory distress particularly in populations with limited access to healthcare, high prevalence of co-morbidity and presence of parasite drug-resistance (Baird, 2009; Kochar et al., 2009; Price et al., 2009; Sarkar et al., 2010).
The clinical spectrum of malaria in LA does not appear to differ from that in other areas with low to middle transmission intensities, but there is a noticeable scarcity of reports on the clinical manifestations of malaria in this region. Although severe and complicated cases caused by P. falciparum and P. vivax species have been reported (Andrade et al., 2010b; Echeverri et al., 2003; Marcano et al., 2004) their prevalence seems to be significantly lower than in malaria endemic areas of Africa and Asia. This pattern might be explained by the better access to healthcare in LA, a generally higher socio-economic level and a relatively lower parasite multidrug resistance (MDR) (WHO, 2009). It has been observed that the occurrence of severe cases is highly influenced by MDR (Alexandre et al., 2010; Orjuela-Sanchez et al., 2009; Ramos Junior et al., 2010; Soto et al., 2001) and in some areas of LA both P. falciparum and P. vivax are still highly susceptible to chloroquine (Calzada et al., 2008). However, it is also likely that, at least in the case of P. vivax, the number of severe and complicated cases is significantly underestimated due to limitations in malaria diagnosis. (Alexandre et al., 2010; Andrade et al., 2010b; Lacerda et al., 2008; Siqueira et al., 2010). The increasing use of PCR diagnostic techniques has resulted in more frequent reporting of severe and complicated cases caused by P. vivax (Andrade et al., 2010a; Genton et al., 2008; Kochar et al., 2010; Mueller et al., 2009).
It has been suggested that exposure to both parasite species in mixed infections also increases the severity of the disease, although some studies suggest the opposite because of the development of species cross protection (Chuangchaiya et al., 2010; Sutton et al., 2009; Whitehorn et al., 2010). Diagnoses of P. falciparum and P. vivax mixed infection may be difficult as the correct identification of ring forms on Giemsa-stained thick blood smears is not always possible. Epidemiological surveys in Thailand and Laos detected <1-2% mixed infections when using microscopy compared to 55 to 65% when PCR techniques were used (Mayxay et al., 2004). Because there is very limited information about the prevalence of mixed infections in the non-Amazonian regions of LA as well as on the role of both parasite species on the clinical profile and disease severity of mixed infections, CLAIM will focus efforts its assessment in the countries under study. In Colombia, between 1 to 2% of all malaria cases are reportedly caused by simultaneous P. falciparum and P. vivax infection (Cucunuba et al., 2008). In Panama, Honduras and Guatemala mixed infections appear to account for less than 1% and no mixed infections have been reported outside the Amazonian region in Peru (MINSA, 2009; PAHO and WHO, 2007b). It is likely that studies using PCR would detect higher prevalence of mixed infections. Defining the prevalence of mono versus mixed species infections is not only clinically relevant but has also significant epidemiological importance given the presence of P. falciparum chloroquine resistance in areas where P. vivax is still completely susceptibility to this antimalarial. This represents one of the most important aspects to be studied by CLAIM.
It has been demonstrated that individuals permanently exposed to malaria infection by a given species develop a degree of immunity. Although it does not provide complete protection, this immunity, or premunition, modulates the severity of the disease induced by that Plasmodium species (O’Meara et al., 2008). In highly endemic areas of Africa and Papua New Guinea (PNG), children are sufficiently exposed to malaria to develop significant clinical immunity by the age of 5 years. However, residents in endemic areas of Latin America are less exposed to infection and can develop from acute and severe disease to milder and more chronic infections at all ages; adult men are particularly at risk of suffering malaria in endemic areas of LA, due to occupational exposure in this region (Feachem et al., 2009; Shekalaghe et al., 2009).
Because of unstable transmission of both P. vivax and P. falciparum, it is difficult to assess the influence of one parasite species on further or simultaneous infections caused by the other. However, the differences in transmission intensity may explain the differences in clinical manifestations to those reported in Africa. For example, both P. falciparum and P. vivax can cause severe anemia (Rodriguez-Morales et al., 2007), but this appears to be less common in LA than in Africa and other highly endemic settings (Caicedo et al., 2010). In P. falciparum infections, severe anemia is commonly associated with other severe clinical manifestations such as cerebral malaria, hypoglycemia, metabolic acidosis and respiratory distress. This clinical picture is rarely seen in P. vivax infections (Anstey et al., 2009; Bardaji et al., 2008), where severe infections in children may be accompanied by anemia associated with jaundice and acute renal failure, and in some cases associated with G6PD deficiency (Alexandre et al., 2010; Ernandez et al., 2009), but seldom with cerebral malaria (Ernandez et al., 2009; Ozen et al., 2006; Valecha et al., 1992). These different clinical presentations of vivax malaria infection are strongly associated to activation of pro-inflammatory responses and cytokines imbalance (Andrade et al., 2010b).
Malnutrition and worm infections that frequently co-exist as confounding factors in the development of anemia have been poorly studied (Scrimshaw et al., 1969). Paradoxically, a recent longitudinal study conducted in rural areas of the Brazilian Amazon found that intestinal helminthiasis provides protection against anaemia in children infected with P. vivax (Melo et al., 2010). Further studies are required to better assess this observation. The high prevalence of anemia in Africa is partly explained by the presence of malnutrition (Ehrhardt et al., 2006). In LA there is significantly less malnutrition and therefore the role of nutritional factors and worm infections in the epidemiology and clinical outcome of malaria in this region deserves further investigation (Melo et al., 2010).
One of the most critical issues in malaria morbidity and mortality is the occurrence of infections in non-immune children and in pregnant women. Physiologically, pregnancy is associated with a certain degree of immunosuppresion and therefore a higher risk of infections (Mor and Cardenas, 2010). In highly malaria endemic areas of Africa and PNG, both children and pregnant women are more susceptible to severe disease and death (Brabin et al., 1990; Kalilani et al., 2010).
In LA, particularly in some of the CLAIM countries, the incidence of malaria in pregnancy (MIP) is variable. In 2008, from the total number of malaria cases reported in Colombia and Ecuador, 2% corresponded to MIP, in Guatemala reported incidence 1%, 13% in Panama reported and no cases were reported in Peru (WHO, 2009). More than reflecting true differences in incidence of MIP these numbers might represent variations in the level or reporting between countries. A cross-sectional study in the municipality of Sifontes in Venezuela, conducted in 2005-2006, found an incidence of 27.4% of MIP associated mostly to P. vivax infection (87%). The most frequent clinical manifestations were fever, jaundice and severe anemia (Gomez et al., 2009). In Colombia, a recent study in 2,117 pregnant women living in Uraba (Antioquia state) reported a prevalence of ~10% of gestational malaria, 2.7% of congenital malaria and 11.7% of placental malaria, predominantly produced by P. vivax (76%) (Carmona-Fonseca and Maestre, 2009). Preliminary data from a study currently being conducted in Tierralta (Cordoba state), in a series of 1,728 pregnant women, report an incidence of 3.2% of MIP (Pregvax /CRESIB personal communication). In general, there is little information on the factors associated with MIP and congenital malaria in most regions of LA with high prevalence of P. vivax infection. The MIP and its impact on neonatal health and child development in the region will be subject of further research within the framework of CLAIM.
1.5 Asymptomatic infections
As people become repeatedly infected in malaria endemic areas, a great deal of clinical immunity is achieved, and individuals may develop a quasi-normal life despite the fact that they carry malaria parasites. This common feature in highly endemic areas appears to be the manifestation of effective anti-disease immunity (Karunaweera et al., 1998). Although these asymptomatic infections have not been extensively studied in LA (Alves et al., 2002; Coura et al., 2006) there is growing evidence that sub-clinical infections are more common in the region than previously thought (Cerutti et al., 2007; Cucunuba et al., 2008; Roshanravan et al., 2003). Unfortunately, most studies designed to identify asymptomatic infections have been carried out using microscopy in cross-sectional surveys with limited information about the host’s capacity to produce gametocytes. The presence of mature and functional gametocytes may represent a factor highly contributing to malaria transmission. An important question to be considered is whether asymptomatic individuals can become gametocyte carriers and how effectively can they contribute to the persistence of malaria transmission (Alves et al., 2005; Bousema et al., 2004; Cucunuba et al., 2008) and even more, if they could be more effective reservoirs of parasites for the mosquito vectors.
2.0 National Malaria Control Programs in LA
Similarly to what has occurred in other malaria endemic areas, LA has seen the widespread implementation of various malaria control strategies including the use of LLINs, IRS and artemisinin combination therapies (ACT). Such measures have decreased malaria transmission in several areas of LA (Bhattarai et al., 2007). These successes, together with a considerable increase in funds available for malaria control activities, have sparked renewed optimism for malaria elimination programmes especially in areas with seasonal or unstable transmission (Feachem et al., 2009; Hotez et al., 2008; Moonen et al.,; Roberts, 2010). The effectiveness of available control measures supports the notion that a successful control program requires coordinated and rigorous applications. Therefore, despite the progress seen, developing more effective strategies of integrated control remains a challenge (WHO, 2008). In many regions, control programs have failed due to resistance to insecticides and antimalarial treatments, deficit of efficient diagnostic tools, poverty in endemic populations, demographic pressure, human migration, and the lack of immunopreventive methods (WHO, 2008). The situation is worsened by poor integrated knowledge about biological, environmental, behavioral, and social factors affecting malaria transmission. Indeed, most of the malaria research has addressed the relative importance of these factors separately (Boisier et al., 2002; Mauny et al., 2004; Peterson et al., 2009; Trung et al., 2005). Such approaches do not offer appropriate information to design and implement integrated controls programs (Peterson et al., 2009). Efforts for developing integrated approaches are hampered by factors such as differences in the local epidemiology and health-seeking behaviour of the affected populations, differences in vector biology, and the uneven economical development that affects the quality of health services. The situation in LA is even more complex if we consider the predominance of P. vivax in the region (WHO, 2009). Although the prevalence of P. falciparum infection is lower in these P. vivax endemic regions (Guerra et al., 2008; Guerra et al., 2010; Gusmao, 1999; Singh et al., 2006) the communities are permanently exposed to both parasites species with an unknown number of mixed infections in which the two malaria parasites interact influencing disease outcome (Bruce et al., 2000; Genton et al., 2008; Snounou and White, 2004). Whether falciparum-vivax mixed infections are clinically important in the region requires further investigation, as in certain areas of LA where P. falciparum and P. vivax transmission appears to be temporally and/or spatially decoupled (Chowell et al., 2009). In general, regardless of the great regional importance of P. vivax, there is still limited epidemiologic and operational information aimed at assessing the disease burden for this species.
At the operational level, there are other interactions derived from the use of particular control measures. For example, it has been hypothesized that the frequency of mutations associated with chloroquine resistance in P. falciparum could be affected by the availability and use of this drug to treat P. vivax (Alexandre et al., 2010; Bacon et al., 2009; Huang et al., 1988). Thus, understanding how these two species interact in terms of clinical outcomes and effectiveness of control interventions is of great importance for NMCPs.
A key element for malaria control and elimination is reliable measures of malaria transmission intensity which is a crucial determinant of the burden of malaria disease (Greenwood, 2008; Reyburn et al., 2005)(Hay et al. 2009, Hay et al. 2010). Malaria transmission in LA is notably heterogeneous and different control measures may be better suited to different transmission intensities or ecological conditions. Even though parasite rate (PR) estimates, through microscopy or RDTs, are a quick method for assessing transmission intensity, these estimates are significantly influenced by sensitivity of the tests, antimalarial drug use and the timing of sample collection in areas of seasonal or unstable transmission. Moreover, despite the straightforward use of RDTs, these are yet to be adopted by most NMCPs in LA. Although, in most of the cases anti-malarial treatment is given once malaria is diagnosed, the accurate estimation of transmission is further challenged because the control programs in LA usually rely on passive surveillance of clinical cases ignoring asymptomatic infections that may highly contribute to maintaining malaria transmission.
LA presents a complex scenario regarding malaria vectors due mainly to the variability in anopheline species, and the lack of studies on their role in malaria transmission. Basic knowledge at the local level is essential for an approximation of which vector control strategies are required and the frequency of application. Vectors in LA do not have a high degree of late biting and indoor resting as compared to that in Africa. Furthermore, the type of housing differs. Therefore, to achieve a significant reduction in malaria transmission, IRS and even LLINs may need to be complemented with other strategies, which should be evidence-based; for example breeding site control has been used and promoted with some success in Central America (Grieco et al., 2005). Vector surveillance needs to include at the least: Anopheles species determination, biting and resting behavior, responses to insecticides in terms of level of resistance and avoidance behavior, and determination of the impact of control strategies on the malaria vector populations. Measures such as the entomological inoculation rate (EIR) provide guidance for assessing the intensity of transmission, and can be used to evaluate control strategies. However, few attempts have been made to estimate EIR in LA, mainly due to the low sporozoite rates prevailing.
3.0 External resources for Malaria Control
During the last year multilateral initiatives such as the GFATM awarded grants to 11 countries including a multi-country program for malaria control on Andean-country border areas (PAMAFRO). This community based approach has covered 23 Border States of Colombia, Ecuador, Peru and Venezuela (719 municipalities and more than 6,000 communities) with the goal of decreasing malaria incidence, as measured by the API, by 50% and overall mortality by 70%. Although PAMAFRO was expected to reach this goal by 2009, preliminary data by 2008 showed a decrease trend of malaria morbidity by 37.2% and reduced mortality of 30% and 14% in Colombia and Peru, respectively; no official data were reported from Venezuela and Ecuador (PAMAFRO, 2009). Simultaneously, RAVREDA was launched in 2001, financially supported by the US Agency for International Development (USAID), coordinated by PAHO and with technical support from Management Science for Health (MSH), Centers for Disease Control and Prevention (CDC) and United States Pharmacopea (USP). Within this framework, the participating countries have validated and adopted operational activities regarding malaria surveillance and control. Unfortunately, these two major initiatives did not include accurate evaluation strategies or operational research components. RAVREDA has maintained alliances with international and local organizations in these countries to achieve their goals, which included various components of the Regional Strategic Plan for Malaria in the Americas 2006-2010, lined up with national and global strategies and targets.
Currently, several malaria control programs are being initiated in some of the countries participating in CLAIM, sponsored by international funding agencies and the corresponding national governments. In Colombia, the GFATM is sponsoring a five-year project called “Use of epidemiological intelligence with social participation, to strengthen program management, improving access to diagnosis, treatment and implement effective interventions for prevention and control of malaria” (INTEMAL) directed to intensify malaria control in the five departments with highest malaria transmission (Cordoba, Antioquia, Choco, Valle and Cauca). INTEMAL is currently joining efforts with the NIAID sponsored CLAIM program in order to develop both an intense control program together with research in three main components: epidemiology, vectors and malaria immunopathology (CLAIM, 2010).
Moreover, the recently launched Mesoamerican Health Initiative 2015 (SM2015) is aimed at reducing critical health problems including malaria. This is a five–year initiative co-sponsored by the BMGF, the Instituto Carlos Slim de la Salud (ICSS), the Spanish Agency for International Cooperation (AECID), the Inter American Development Bank (IADB) and the Ministries of Health of Mesoamerican countries. The malaria operational strategy is planned as a proof-of-concept to evaluate whether malaria transmission could be eliminated in selected areas of the region, so as to provide evidence-based interventions.
4.0 Critical areas of concern for improving malaria control
Although malaria elimination appears feasible in LA, improving malaria control in low-endemic malaria areas of the region still represents a great challenge and requires creativity to design realistic and comprehensive plans to move from control to elimination. The increase in funding from external sources has allowed a scale-up of malaria control activities in many countries of the region, and although coverage with preventive measures and access to effective treatments still remain below expected levels, in countries such as Colombia, Peru, Guatemala and Panama there has been a notable decrease in mortality and morbidity (Carter, 2009). However, as low-endemic control is approached, operational challenges multiply, particularly regarding how to quantify the malaria problem and how to proceed with infected asymptomatic individuals. In preparation for elimination, the non-Amazonian LA areas must reinforce the NMCP and concentrate simultaneous efforts on:
Identifying and eliminating transmission foci through surveillance activities including both passive and active case detection methods in order to eliminate not only clinical cases but also asymptomatic infections, which represent parasite pools for continued transmission.
Diagnoses should be accompanied by effective antimalarial treatments, which present different challenges for P. vivax (the need for extended primaquine treatment) and P. falciparum (multidrug resistance).
Being a vector-borne disease, malaria control has to emphasize efforts on understanding vector biology.
Improving the understanding of P. vivax biology and epidemiology, which despite its great global significance, remains poorly understood (Guerra et al. 2010; Mueller et al. 2009). P. vivax is highly prevalent in most malaria endemic countries in LA and presents particular challenges for malaria control and elimination.
4.1 Malaria diagnoses
In the current epidemiological situation where numbers of malaria cases are decreasing in LA, there is a need for improving diagnostic methods, because at low endemicity lower parasite density infections are more common and sometimes asymptomatic likely generating better conditions for continued transmission. Although microscopy and RDTs are sufficiently sensitive to detect malaria parasites in symptomatic patients, both tests have limitations for detecting low parasite density infections (Coleman et al., 2002a; Coleman et al., 2002b). Additionally, quality assurance for microscopy is operationally challenging and labor intensive, whereas standardized protocols for quality assurance of RDTs, especially to confirm potentially large numbers of negative results, are not available. Although DNA PCR techniques are promising, they are unlikely to be available for broad-based field work in the near future (Moonen et al., 2010). Whereas prevalence is decreasing in most LA countries, the traditional approach of community surveys may become more difficult and less meaningful. Transition from control to elimination will require more active detection of cases.
Other critical issues include: 1) improving health seeking behavior to optimize the use of public and private health facilities. In endemic areas this may be a challenging goal as poor communities have become familiar with fever syndromes of different causes. Within the spectrum of economical constraints and subsistence difficulties, malaria represents only one more problem. A common attitude in these communities is to wait and see how fever evolves. 2) Decentralization of malaria diagnoses and treatment in remote areas is transferring this important component of the NMCP to private health providers with poor training on diagnoses and a significant proportion of the communities lacking adequate insurance coverage. 3) Even if sufficient capacity for public and private passive case detection was available, active case detection would require enormous efforts and easy-to-follow algorithms to ensure high testing rates (Moonen et al., 2010); 4) None of these factors would be easily approached without strong educational programs for both communities and decision-makers.
4.2 Availability of antimalarials and drug resistance
The problem of malaria multidrug resistance is less of a problem in LA compared to other malaria endemic regions (Bacon et al., 2009). However, there is concern about the pace of multidrug resistance dissemination in LA, even in areas outside the Amazon basin. Most emphasis on malaria treatment is focused on the asexual blood stages responsible for the clinical manifestations of the diseas, leaving aside the other parasite stages (Collins and Jeffery, 1996). Although P. falciparum malaria may be treated with about 12 different therapies (Baird, 2010), the only treatment available today for the dormant liver parasite stages of P. vivax infection is primaquine, which represents a serious risk for individuals with glucose-6-phosphate dehydrogenase (G6PD) deficiency (Wells and Poll, 2010). Despite the observed high prevalence of G6PD deficiency (3-15%) in endemic regions of LA (Carmona-Fonseca et al., 2008; Santana et al., 2009), and the extended therapeutic regimes (7-14 days) without any previous screening for G6PD deficiency in most countries, there is a low treatment compliance which generates the risk of drug resistance development and higher probability of relapses. In accordance with WHO recommendations (WHO, 2006), between 2002 and 2006 RAVREDA promoted the introduction of ACT for P. falciparum non-complicated malaria (WHO, 2009).
4.3 Understanding vector biology
The high diversity of Anopheles species in LA demands integrated mosquito taxonomic approaches for determining which of these species are acting as malaria vectors (Sinka et al. 2010). This information will allow the study of vector biology and ecology, particularly biting and resting behavior, distribution, seasonality and natural infectivity, to select appropriate control measures. Also, the response of each malaria vector species to control measures needs to be evaluated so that appropriate IVM strategies can be implemented in each area, taking into account the local epidemiology and vector ecology. After DDT was replaced by pyrethroids and organophosphates, insecticide resistance surveillance of the malaria vector species has been focused on research rather than practical vector control operations. Behavioral changes of the vectors due to the implementation of LLINs or IRS, such us irritability, exiting or feeding inhibition are largely unknown for LA malaria vector species.
4.4 Improving understanding of P. vivax biology and epidemiology
Although P. vivax is highly prevalent in LA, there is poor understanding of its local and regional epidemiology. Most countries in the area of influence of CLAIM have developed surveillance systems that are not optimally used for decision-making. P. vivax control urgently needs improved diagnostic tests, a robust point-of-care method for screening for G6PD deficiency and better drugs to radically eliminate P. vivax hypnozoites (Baird 2007; Baird 2009; Mueller et al. 2009).
5.0 Challenges and prospects for malaria elimination in the non-Amazonian region in LA
5.1 Global prospects for malaria elimination
Elimination of malaria transmission is a possible goal; since 1945, a total of 79 countries have successfully achieved elimination. Currently, 32 of the 99 malaria endemic countries worldwide are undertaking malaria elimination activities (Feachem et al., 2009).
During the last two years, the Bill and Melinda Gates Foundation (BMGF) has promoted the goal of eventual global eradication of human malaria (Feachem et al., 2009). As a first step, the BMGF has sponsored the establishment of a Malaria Eradication Research Agenda (malERA), with the overall purpose of developing a multidisciplinary global research and development (R&D) agenda that could be actionable by research and public health agencies and sponsors. A comprehensive R&D agenda is about to be published including subjects such as drugs, vaccines, vector control, modeling, monitoring, evaluation and surveillance, integration strategies and health systems, operational research and diagnostics (Alonso et al., 2010; TDR/WHO/MalERA, 2009).
5.2 Perspectives for pre-elimination in the non Amazonian malaria endemic areas of Latin America
As previously mentioned, there have been developments in malaria control in non-Amazonian malaria endemic regions of both South and Central America. The progress achieved together with current initiatives, create appropriate conditions to envision pre-elimination at least in geographically defined areas of Peru, Colombia and Ecuador, where PAMAFRO, and RAVREDA programs have made significant impacts by improving malaria control operations. In certain areas, malaria incidence has been reduced by 70% (e.g., Tumaco and Buenaventura in Colombia). The Colombian government is strongly committed to continue reducing malaria transmission in areas already intervened by the PAMAFRO program and INTEMAL has already started working jointly with CLAIM to develop an intensive and comprehensive control and research agenda in Colombia. The results of this combined control and research agenda is planned to be extended to the other countries currently participating in CLAIM (Peru, Panama and Guatemala) as well as in future partner countries such as Ecuador and Honduras. Moreover, with the leadership of Mexico and Spain, Central American countries that most likely have the greatest potential for malaria elimination are about to start an ambitious program for control of vector transmitted diseases. This program that will give priority to malaria and dengue is denominated Mesoamerican Health Initiative 2015 (SM2015) and will have the economical support from multiple funding agencies. CLAIM is joining the SM2015 efforts and will contribute to the research agenda of this regional elimination program. The major activities to be developed in the framework of these control/research partnership activities are:
Assess the socio-demographic epidemiology of malaria in different sites to establish risk factors as a basis for improving NMCPs interventions.
Describe the incidence and prevalence of symptomatic and asymptomatic malaria and the relationship of the latter with the continued transmission in areas of high to low malaria endemicity.
Characterize the immune response of individuals and relate this to resistance and mutations of the parasite relative to different treatment regimens.
Describe existing measures of control of the parasite and the vector in order to measure their impact when compared with other measures implemented by government agencies.
Address major gaps in understanding in the ecology, behavior, vector potential and control of Anopheles malaria vectors to guide the development and implementation of more effective IVM-based strategies of NMCPs.
Provide a better understanding of the immunopathogenesis of malaria in LA.
Prepare conditions for the assessment of both P. vivax and P. falciparum malaria vaccines currently under clinical development.
Highlights.
We describe the current status of malaria in four countries of South and Central America four (Guatemala, Panama, Colombia and Peru) currently participating in the Centro Latino Americano de Investigación en Malaria (CLAIM)
We provide information on the experiences in the implementation of various malaria control strategies and the effectiveness of current National Malaria Control Programs in Latin America (LA)
We identify critical areas of concern for improving malaria control in the non-Amazonian LA countries in preparation for it elimination
We present the major activities of the CLAIM for malaria pre-elimination in the non-Amazonian region of LA
Acknowledgments
This work has been supported by the US National Institute of Allergy and Infectious Diseases (NAID/NIH) (Grant number R01 HL086488-01) and the Colombian Research Council (Colciencias) (Grant number 409-2009) to Fundación Centro Internacional de Vacunas. We are grateful to the Malaria Atlas Project (MAP; www.map.ox.ac.uk) for providing bespoke maps for Figure 1 and to Lorena Meneses for editorial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexandre MA, Ferreira CO, Siqueira AM, Magalhaes BL, Mourao MP, Lacerda MV, Alecrim MG. Severe Plasmodium vivax malaria, Brazilian Amazon. Emerg Infect Dis. 2010;16:1611–1614. doi: 10.3201/eid1610.100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso PL, Brown G, Herrera-Arevalo M, Binka F, Chitnis C, Collins F, Doumbo O, Hall L, Levine M, Mendis K, Newman RD, Plowe C, Rodriguez MH, Sinden R, Slutsker L, Tanner M. A research agenda to underpin malaria erradication. PLoS Med. 2010 doi: 10.1371/journal.pmed.1000406. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves FP, Durlacher RR, Menezes MJ, Krieger H, Silva LH, Camargo EP. High prevalence of asymptomatic Plasmodium vivax and Plasmodium falciparum infections in native Amazonian populations. Am J Trop Med Hyg. 2002;66:641–648. doi: 10.4269/ajtmh.2002.66.641. [DOI] [PubMed] [Google Scholar]
- Alves FP, Gil LH, Marrelli MT, Ribolla PE, Camargo EP, Da Silva LH. Asymptomatic carriers of Plasmodium spp. as infection source for malaria vector mosquitoes in the Brazilian Amazon. J Med Entomol. 2005;42:777–779. doi: 10.1093/jmedent/42.5.777. [DOI] [PubMed] [Google Scholar]
- Andrade BB, Reis-Filho A, Barros AM, Souza-Neto SM, Nogueira LL, Fukutani KF, Camargo EP, Camargo LM, Barral A, Duarte A, Barral-Netto M. Towards a precise test for malaria diagnosis in the Brazilian Amazon: comparison among field microscopy, a rapid diagnostic test, nested PCR, and a computational expert system based on artificial neural networks. Malar J. 2010a;9:117. doi: 10.1186/1475-2875-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade BB, Reis-Filho A, Souza-Neto SM, Clarencio J, Camargo LM, Barral A, Barral-Netto M. Severe Plasmodium vivax malaria exhibits marked inflammatory imbalance. Malar J. 2010b;9:13. doi: 10.1186/1475-2875-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey NM, Russell B, Yeo TW, Price RN. The pathophysiology of vivax malaria. Trends Parasitol. 2009;25:220–227. doi: 10.1016/j.pt.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Bacon DJ, McCollum AM, Griffing SM, Salas C, Soberon V, Santolalla M, Haley R, Tsukayama P, Lucas C, Escalante AA, Udhayakumar V. Dynamics of malaria drug resistance patterns in the Amazon basin region following changes in Peruvian national treatment policy for uncomplicated malaria. Antimicrob Agents Chemother. 2009;53:2042–2051. doi: 10.1128/AAC.01677-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird JK. Resistance to therapies for infection by Plasmodium vivax. Clin Microbiol Rev. 2009;22:508–534. doi: 10.1128/CMR.00008-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird JK. Eliminating malaria--all of them. Lancet. 2010;376(9756):1883–1885. doi: 10.1016/S0140-6736(10)61494-8. [DOI] [PubMed] [Google Scholar]
- Bardaji A, Sigauque B, Bruni L, Romagosa C, Sanz S, Mabunda S, Mandomando I, Aponte J, Sevene E, Alonso PL, Menendez C. Clinical malaria in African pregnant women. Malar J. 2008;7:27. doi: 10.1186/1475-2875-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach RF, Ruebush TK, 2nd, Sexton JD, Bright PL, Hightower AW, Breman JG, Mount DL, Oloo AJ. Effectiveness of permethrin-impregnated bed nets and curtains for malaria control in a holoendemic area of western Kenya. Am J Trop Med Hyg. 1993;49:290–300. doi: 10.4269/ajtmh.1993.49.290. [DOI] [PubMed] [Google Scholar]
- Beier JC, Keating J, Githure JI, Macdonald MB, Impoinvil DE, Novak RJ. Integrated vector management for malaria control. Malar J. 2008;7(Suppl 1):S4. doi: 10.1186/1475-2875-7-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai A, Ali AS, Kachur SP, Martensson A, Abbas AK, Khatib R, Al-Mafazy AW, Ramsan M, Rotllant G, Gerstenmaier JF, Molteni F, Abdulla S, Montgomery SM, Kaneko A, Bjorkman A. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med. 2007;4:e309. doi: 10.1371/journal.pmed.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisier P, Jambou R, Raharimalala L, Roux J. Relationship between parasite density and fever risk in a community exposed to a low level of malaria transmission in Madagascar highlands. Am J Trop Med Hyg. 2002;67:137–140. doi: 10.4269/ajtmh.2002.67.137. [DOI] [PubMed] [Google Scholar]
- Bousema JT, Gouagna LC, Drakeley CJ, Meutstege AM, Okech BA, Akim IN, Beier JC, Githure JI, Sauerwein RW. Plasmodium falciparum gametocyte carriage in asymptomatic children in western Kenya. Malar J. 2004;3:18. doi: 10.1186/1475-2875-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabin BJ, Ginny M, Sapau J, Galme K, Paino J. Consequences of maternal anaemia on outcome of pregnancy in a malaria endemic area in Papua New Guinea. Ann Trop Med Parasitol. 1990;84:11–24. doi: 10.1080/00034983.1990.11812429. [DOI] [PubMed] [Google Scholar]
- Brogdon WG, McAllister JC, Corwin AM, Cordon-Rosales C. Independent selection of multiple mechanisms for pyrethroid resistance in Guatemalan Anopheles albimanus (Diptera: Culicidae) J Econ Entomol. 1999;92:298–302. doi: 10.1093/jee/92.2.298. [DOI] [PubMed] [Google Scholar]
- Bruce MC, Donnelly CA, Alpers MP, Galinski MR, Barnwell JW, Walliker D, Day KP. Cross-species interactions between malaria parasites in humans. Science. 2000;287:845–848. doi: 10.1126/science.287.5454.845. [DOI] [PubMed] [Google Scholar]
- Caicedo O, Villamor E, Forero Y, Ziade J, Perez P, Quinones F, Arevalo-Herrera M, Herrera S. Relation between vitamin B12 and folate status, and hemoglobin concentration and parasitemia during acute malaria infections in Colombia. Acta Trop. 2010;114:17–21. doi: 10.1016/j.actatropica.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzada JE, Samudio F, Bayard V, Obaldia N, 3rd, de Mosca IB, Pascale JM. Revising antimalarial drug policy in Central America: experience in Panama. Trans R Soc Trop Med Hyg. 2008;102:694–698. doi: 10.1016/j.trstmh.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Carmona-Fonseca J, Alvarez G, Rios A, Vasquez M. Deficiencia de glucosa-6-fosfato deshidrogenasa en hombres sanos y en pacientes malaricos; Turbo (Antioquia, Colombia) Rev Bras Epidemiol. 2008;11:252–265. [Google Scholar]
- Carmona-Fonseca J, Maestre A. Incidencia de las malarias gestacional, congenita y placentaria en Uraba, Antioquia, (Colombia), 2005-2007. Revista Colombiana de Obstetricia y Ginecologia. 2009;60:19–33. [Google Scholar]
- Carter K. Situacion de la Malaria en la Region de las Americas. PAHO/WHO. 2009 www.mex.ops-oms.org.
- Cerutti C, Jr, Boulos M, Coutinho AF, Hatab Mdo C, Falqueto A, Rezende HR, Duarte AM, Collins W, Malafronte RS. Epidemiologic aspects of the malaria transmission cycle in an area of very low incidence in Brazil. Malar J. 2007;6:33. doi: 10.1186/1475-2875-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapilliquen F Direccion General de Epidemiologia, Epidemiologia, R.N.d., Ministerio de Salud. Situacion de la malaria en Peru. Boletin Epidemiologico. 2009;18 [Google Scholar]
- Chareonviriyaphap T, Roberts DR, Andre RG, Harlan HJ, Manguin S, Bangs MJ. Pesticide avoidance behavior in Anopheles albimanus, a malaria vector in the Americas. J Am Mosq Control Assoc. 1997;13:171–183. [PubMed] [Google Scholar]
- Chowell G, Munayco CV, Escalante AA, McKenzie FE. The spatial and temporal patterns of falciparum and vivax malaria in Peru: 1994-2006. Malar J. 2009;8:142. doi: 10.1186/1475-2875-8-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuangchaiya S, Jangpatarapongsa K, Chootong P, Sirichaisinthop J, Sattabongkot J, Pattanapanyasat K, Chotivanich K, Troye-Blomberg M, Cui L, Udomsangpetch R. Immune response to Plasmodium vivax has a potential to reduce malaria severity. Clin Exp Immunol. 2010;160:233–239. doi: 10.1111/j.1365-2249.2009.04075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAIM. Centro Latino Americano de Investigacion en Malaria. 2010 www.caucaseco.org.
- Coleman RE, Maneechai N, Ponlawat A, Kumpitak C, Rachapaew N, Miller RS, Sattabongkot J. Short report: Failure of the OptiMAL rapid malaria test as a tool for the detection of asymptomatic malaria in an area of Thailand endemic for Plasmodium falciparum and P. vivax. Am J Trop Med Hyg. 2002a;67:563–565. doi: 10.4269/ajtmh.2002.67.563. [DOI] [PubMed] [Google Scholar]
- Coleman RE, Maneechai N, Rachapaew N, Kumpitak C, Soyseng V, Miller RS, Thimasarn K, Sattabongkot J. Field evaluation of the ICT Malaria Pf/Pv immunochromatographic test for the detection of asymptomatic malaria in a Plasmodium falciparum/vivax endemic area in Thailand. Am J Trop Med Hyg. 2002b;66:379–383. doi: 10.4269/ajtmh.2002.66.379. [DOI] [PubMed] [Google Scholar]
- Collins WE, Jeffery GM. Primaquine resistance in Plasmodium vivax. Am J Trop Med Hyg. 1996;55:243–249. doi: 10.4269/ajtmh.1996.55.243. [DOI] [PubMed] [Google Scholar]
- Conn JE, Wilkerson RC, Segura MN, de Souza RT, Schlichting CD, Wirtz RA, Povoa MM. Emergence of a new neotropical malaria vector facilitated by human migration and changes in land use. Am J Trop Med Hyg. 2002;66:18–22. doi: 10.4269/ajtmh.2002.66.18. [DOI] [PubMed] [Google Scholar]
- Cornejo OE, Escalante AA. The origin and age of Plasmodium vivax. Trends Parasitol. 2006;22:558–563. doi: 10.1016/j.pt.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corredor V, Murillo C, Echeverry DF, Benavides J, Pearce RJ, Roper C, Guerra AP, Osorio L. Origin and dissemination across the Colombian Andes mountain range of sulfadoxine-pyrimethamine resistance in Plasmodium falciparum. Antimicrob Agents Chemother. 2010;54:3121–3125. doi: 10.1128/AAC.00036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese JF, Caraballo A, Contreras CE, Plowe CV. Origin and dissemination of Plasmodium falciparum drug-resistance mutations in South America. J Infect Dis. 2002;186:999–1006. doi: 10.1086/342946. [DOI] [PubMed] [Google Scholar]
- Coura JR, Suarez-Mutis M, Ladeia-Andrade S. A new challenge for malaria control in Brazil: asymptomatic Plasmodium infection--a review. Mem Inst Oswaldo Cruz. 2006;101:229–237. doi: 10.1590/s0074-02762006000300001. [DOI] [PubMed] [Google Scholar]
- Cucunuba ZM, Guerra AP, Rahirant SJ, Rivera JA, Cortes LJ, Nicholls RS. Asymptomatic Plasmodium spp. infection in Tierralta. Colombia Mem Inst Oswaldo Cruz. 2008;103:668–673. doi: 10.1590/s0074-02762008000700007. [DOI] [PubMed] [Google Scholar]
- Cywinska A, Hunter FF, Hebert PD. Identifying Canadian mosquito species through DNA barcodes. Med Vet Entomol. 2006;20:413–424. doi: 10.1111/j.1365-2915.2006.00653.x. [DOI] [PubMed] [Google Scholar]
- de Arruda M, Carvalho MB, Nussenzweig RS, Maracic M, Ferreira AW, Cochrane AH. Potential vectors of malaria and their different susceptibility to Plasmodium falciparum and Plasmodium vivax in northern Brazil identified by immunoassay. Am J Trop Med Hyg. 1986;35:873–881. doi: 10.4269/ajtmh.1986.35.873. [DOI] [PubMed] [Google Scholar]
- de Arruda M, Souza RC, Veiga ME, Ferreira AF, Zimmerman RH. Prevalence of Plasmodium vivax variants VK247 and P. vivax-like human malaria: a retrospective study in indigenous Indian populations of the Amazon region of Brazil. Trans R Soc Trop Med Hyg. 1998;92:628. doi: 10.1016/s0035-9203(98)90788-x. [DOI] [PubMed] [Google Scholar]
- Dzul FA, Patricia Penilla R, Rodriguez AD. Susceptibility and insecticide resistance mechanisms in Anopheles albimanus from the southern Yucatan Peninsula, Mexico. Salud Publica Mex. 2007;49:302–311. doi: 10.1590/s0036-36342007000400010. [DOI] [PubMed] [Google Scholar]
- Echeverri M, Tobon A, Alvarez G, Carmona J, Blair S. Clinical and laboratory findings of Plasmodium vivax malaria in Colombia, 2001. Rev Inst Med Trop Sao Paulo. 2003;45:29–34. doi: 10.1590/s0036-46652003000100006. [DOI] [PubMed] [Google Scholar]
- Ehrhardt S, Burchard GD, Mantel C, Cramer JP, Kaiser S, Kubo M, Otchwemah RN, Bienzle U, Mockenhaupt FP. Malaria, anemia, and malnutrition in african children--defining intervention priorities. J Infect Dis. 2006;194:108–114. doi: 10.1086/504688. [DOI] [PubMed] [Google Scholar]
- Ernandez JA, Osorio L, Murillo O, Escobar H, Bustamante P, Agudelo H, Martinez LP, Olaya B, Castro G. Characterization of malaria mortality in Valle del Cauca, 2005-2006. Biomedica. 2009;29:582–590. [PubMed] [Google Scholar]
- Feachem RGA, Phillips AA, Targett GA. Shrinking the Malaria Map: A Prospectus on Malaria Elimination. The Global Health Group; San Francisco: 2009. [Google Scholar]
- Flores-Mendoza C, Fernandez R, Escobedo-Vargas KS, Vela-Perez Q, Schoeler GB. Natural Plasmodium infections in Anopheles darlingi and Anopheles benarrochi (Diptera: Culicidae) from eastern Peru. J Med Entomol. 2004;41:489–494. doi: 10.1603/0022-2585-41.3.489. [DOI] [PubMed] [Google Scholar]
- Fonseca-Gonzalez I, Cardenas R, Quinones ML, McAllister J, Brogdon WG. Pyrethroid and organophosphates resistance in Anopheles (N.) nuneztovari Gabaldon populations from malaria endemic areas in Colombia. Parasitol Res. 2009a;105:1399–1409. doi: 10.1007/s00436-009-1570-2. [DOI] [PubMed] [Google Scholar]
- Fonseca-Gonzalez I, Quinones ML, McAllister J, Brogdon WG. Mixed-function oxidases and esterases associated with cross-resistance between DDT and lambda-cyhalothrin in Anopheles darlingi Root 1926 populations from Colombia. Mem Inst Oswaldo Cruz. 2009b;104:18–26. doi: 10.1590/s0074-02762009000100003. [DOI] [PubMed] [Google Scholar]
- Genton B, D’Acremont V, Rare L, Baea K, Reeder JC, Alpers MP, Muller I. Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med. 2008;5:e127. doi: 10.1371/journal.pmed.0050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez E, Lopez E, Ache A. Malaria and pregnancy. San Isidro parish, municipality Sifontes, state of Bolivar, Venezuela, 2005-2006. Invest Clin. 2009;50:455–464. [PubMed] [Google Scholar]
- Gonzalez-Ceron L, Rodriguez MH, Chavez-Munguia B, Santillan F, Nettel JA, Hernandez-Avila JE. Plasmodium vivax: impaired escape of Vk210 phenotype ookinetes from the midgut blood bolus of Anopheles pseudopunctipennis. Exp Parasitol. 2007;115:59–67. doi: 10.1016/j.exppara.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Ceron L, Rodriguez MH, Nettel JC, Villarreal C, Kain KC, Hernandez JE. Differential susceptibilities of Anopheles albimanus and Anopheles pseudopunctipennis to infections with coindigenous Plasmodium vivax variants VK210 and VK247 in southern Mexico. Infect Immun. 1999;67:410–412. doi: 10.1128/iai.67.1.410-412.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BM. Control to elimination: implications for malaria research. Trends Parasitol. 2008;24:449–454. doi: 10.1016/j.pt.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Grieco JP, Achee NL, Roberts DR, Andre RG. Comparative susceptibility of three species of Anopheles from Belize, Central America, to Plasmodium falciparum (NF-54) J Am Mosq Control Assoc. 2005;21:279–290. doi: 10.2987/8756-971X(2005)21[279:CSOTSO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Griffing S, Syphard L, Sridaran S, McCollum AM, Mixson-Hayden T, Vinayak S, Villegas L, Barnwell JW, Escalante AA, Udhayakumar V. pfmdr1 Amplification and Fixation of pfcrt Chloroquine Resistance Alleles in Plasmodium falciparum in Venezuela. Antimicrob Agents Chemother. 2010:1572–1579. doi: 10.1128/AAC.01243-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra CA, Gikandi PW, Tatem AJ, Noor AM, Smith DL, Hay SI, Snow RW. The limits and intensity of Plasmodium falciparum transmission: implications for malaria control and elimination worldwide. PLoS Med. 2008;5:e38. doi: 10.1371/journal.pmed.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra CA, Howes RE, Patil AP, Gething PW, Van Boeckel TP, Temperley WH, Kabaria CW, Tatem AJ, Manh BH, Elyazar IR, Baird JK, Snow RW, Hay SI. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Dis. 2010;4:e774. doi: 10.1371/journal.pntd.0000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusmao R. Overview of malaria control in the Americas. Parassitologia. 1999;41:355–360. [PubMed] [Google Scholar]
- Harbach RE. The classification of genus Anopheles (Diptera: Culicidae): a working hypothesis of phylogenetic relationships. Bull Entomol Res. 2004;94:537–553. doi: 10.1079/ber2004321. [DOI] [PubMed] [Google Scholar]
- Hawley WA, Phillips-Howard PA, ter Kuile FO, Terlouw DJ, Vulule JM, Ombok M, Nahlen BL, Gimnig JE, Kariuki SK, Kolczak MS, Hightower AW. Community-wide effects of permethrin-treated bed nets on child mortality and malaria morbidity in western Kenya. Am J Trop Med Hyg. 2003;68:121–127. [PubMed] [Google Scholar]
- Hay SI, Guerra CA, Gething PW, Patil AP, Tatem AJ, Noor AM, Kabaria CW, Manh BH, Elyazar IR, Brooker S, Smith DL, Moyeed RA, Snow RW. A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med. 2009;6(3):e1000048. doi: 10.1371/journal.pmed.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay SI, Okiro EA, Gething PW, Patil AP, Tatem AJ, Guerra CA, Snow RW. Estimating the global clinical burden of Plasmodium falciparum malaria in 2007. PLoS Med. 2010;7:e1000290. doi: 10.1371/journal.pmed.1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J, Calderon G, Falcon R, Zambrano V. Newly incriminated anopheline vectors of human malaria parasites in Junin Department, Peru. J Am Mosq Control Assoc. 1987;3:418–422. [PubMed] [Google Scholar]
- Herrera S, Suarez M, Quinones M, Sanchez G, M Hd. Uso de la tecnica inmunoradiometrica IRMA para la identificacion de esporozoitos de Plaasmodium en Anopheles de Colombia. Colombia Medica. 1987;18:2–6. [Google Scholar]
- Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl Trop Dis. 2008;2:e300. doi: 10.1371/journal.pntd.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang OL, Ouyang WC, Zhou JX, Wu Z, Zhang KY, Huang JK, Cai XZ, Pang XJ, Fu SG, Wang XF, et al. Effectiveness of amodiaquine, sulfadoxine-pyrimethamine, and combinations of these drugs for treating chloroquine-resistant falciparum malaria in Hainan Island, China. Bull World Health Organ. 1988;66:353–358. [PMC free article] [PubMed] [Google Scholar]
- INS, Colombia, National Institute of Health. Monitoring System of Public Health. 2008 http//:www.ins.gov.com/?idcategoria=5951.
- Joy DA, Feng X, Mu J, Furuya T, Chotivanich K, Krettli AU, Ho M, Wang A, White NJ, Suh E, Beerli P, Su XZ. Early origin and recent expansion of Plasmodium falciparum. Science. 2003;300:318–321. doi: 10.1126/science.1081449. [DOI] [PubMed] [Google Scholar]
- Kalilani L, Mofolo I, Chaponda M, Rogerson SJ, Meshnick SR. The effect of timing and frequency of Plasmodium falciparum infection during pregnancy on the risk of low birth weight and maternal anemia. Trans R Soc Trop Med Hyg. 2010;104:416–422. doi: 10.1016/j.trstmh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunaweera ND, Carter R, Grau GE, Mendis KN. Demonstration of anti-disease immunity to Plasmodium vivax malaria in Sri Lanka using a quantitative method to assess clinical disease. Am J Trop Med Hyg. 1998;58:204–210. doi: 10.4269/ajtmh.1998.58.204. [DOI] [PubMed] [Google Scholar]
- Kochar DK, Das A, Kochar A, Middha S, Acharya J, Tanwar GS, Gupta A, Pakalapati D, Garg S, Saxena V, Subudhi AK, Boopathi PA, Sirohi P, Kochar SK. Thrombocytopenia in Plasmodium falciparum, Plasmodium vivax and mixed infection malaria: A study from Bikaner (Northwestern India) Platelets. 2010;21:623–627. doi: 10.3109/09537104.2010.505308. [DOI] [PubMed] [Google Scholar]
- Kochar DK, Das A, Kochar SK, Saxena V, Sirohi P, Garg S, Kochar A, Khatri MP, Gupta V. Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg. 2009;80:194–198. [PubMed] [Google Scholar]
- Kremsner PG, Neifer S, Zotter GM, Bienzle U, Rocha RM, Maracic M, Clavijo P, Nussenzweig RS, Cochrane AH. Prevalence and level of antibodies to the circumsporozoite proteins of human malaria parasites, including a variant of Plasmodium vivax, in the population of two epidemiologically distinct areas in the state of Acre, Brazil. Trans R Soc Trop Med Hyg. 1992;86:23–27. doi: 10.1016/0035-9203(92)90423-a. [DOI] [PubMed] [Google Scholar]
- Lacerda MV, Hipolito JR, Passos LN. Chronic Plasmodium vivax infection in a patient with splenomegaly and severe thrombocytopenia. Rev Soc Bras Med Trop. 2008;41:522–523. doi: 10.1590/s0037-86822008000500021. [DOI] [PubMed] [Google Scholar]
- Lindsay SW, Alonso PL, Armstrong Schellenberg JR, Hemingway J, Adiamah JH, Shenton FC, Jawara M, Greenwood BM. A malaria control trial using insecticide-treated bed nets and targeted chemoprophylaxis in a rural area of The Gambia, west Africa.7. Impact of permethrin-impregnated bed nets on malaria vectors. Trans R Soc Trop Med Hyg. 1993;87(Suppl 2):45–51. doi: 10.1016/0035-9203(93)90175-p. [DOI] [PubMed] [Google Scholar]
- Magesa SM, Wilkes TJ, Mnzava AE, Njunwa KJ, Myamba J, Kivuyo MD, Hill N, Lines JD, Curtis CF. Trial of pyrethroid impregnated bednets in an area of Tanzania holoendemic for malaria. Part 2. Effects on the malaria vector population. Acta Trop. 1991;49:97–108. doi: 10.1016/0001-706x(91)90057-q. [DOI] [PubMed] [Google Scholar]
- Mantilla G, Oliveros H, Barnston AG. The role of ENSO in understanding changes in Colombia’s annual malaria burden by region, 1960-2006. Malar J. 2009;8:6. doi: 10.1186/1475-2875-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcano TJ, Morgado A, Tosta CE, Coura JR. Cross-sectional study defines difference in malaria morbidity in two Yanomami communities on Amazonian boundary between Brazil and Venezuela. Mem Inst Oswaldo Cruz. 2004;99:369–376. doi: 10.1590/s0074-02762004000400005. [DOI] [PubMed] [Google Scholar]
- Mauny F, Viel JF, Handschumacher P, Sellin B. Multilevel modelling and malaria: a new method for an old disease. Int J Epidemiol. 2004;33:1337–1344. doi: 10.1093/ije/dyh274. [DOI] [PubMed] [Google Scholar]
- Mayxay M, Pukrittayakamee S, Newton PN, White NJ. Mixed-species malaria infections in humans. Trends Parasitol. 2004;20:233–240. doi: 10.1016/j.pt.2004.03.006. [DOI] [PubMed] [Google Scholar]
- McCollum AM, Mueller K, Villegas L, Udhayakumar V, Escalante AA. Common origin and fixation of Plasmodium falciparum dhfr and dhps mutations associated with sulfadoxine-pyrimethamine resistance in a low-transmission area in South America. Antimicrob Agents Chemother. 2007;51:2085–2091. doi: 10.1128/AAC.01228-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo GC, Reyes-Lecca RC, Vitor-Silva S, Monteiro WM, Martins M, Benzecry SG, Alecrim MG, Lacerda MV. Concurrent helminthic infection protects schoolchildren with Plasmodium vivax from anemia. PLoS One. 2010;5:e11206. doi: 10.1371/journal.pone.0011206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINSA, Peru, Ministry of Health. Economic impact of malaria in Peru. MINSA; 1999. http://www.minsa.gob.pe/portada/ [Google Scholar]
- MINSA, Peru, Ministry of Health. Health Information System. Peru: Ministry of Health; 2009. http://www.minsa.gob.pe/portada/ [Google Scholar]
- Moonen B, Cohen JM, Snow RW, Slutsker L, Drakeley C, Smith DL, Abeyasinghe RR, Rodriguez MH, Maharaj R, Tanner M, Targett G. Operational strategies to achieve and maintain malaria elimination. Lancet. 2010;376:1592–1603. doi: 10.1016/S0140-6736(10)61269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JE, Rubio-Palis Y, Paez E, Perez E, Sanchez V, Vaccari E. Anopheles (Anopheles) neomaculipalpus: a new malaria vector in the Amazon basin? Med Vet Entomol. 2005;19:329–332. doi: 10.1111/j.1365-2915.2005.00572.x. [DOI] [PubMed] [Google Scholar]
- Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, Alonso PL, del Portillo HA. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9:555–566. doi: 10.1016/S1473-3099(09)70177-X. [DOI] [PubMed] [Google Scholar]
- Najera JA, Zaim M. Malaria vector control decision making criteria and procedures for judicious use of insecticides. WHO/CDS/WHOPES/2002.5 Rev 1 2003 [Google Scholar]
- O’Meara WP, Mwangi TW, Williams TN, McKenzie FE, Snow RW, Marsh K. Relationship between exposure, clinical malaria, and age in an area of changing transmission intensity. Am J Trop Med Hyg. 2008;79:185–191. [PMC free article] [PubMed] [Google Scholar]
- Orjuela-Sanchez P, de Santana Filho FS, Machado-Lima A, Chehuan YF, Costa MR, Alecrim MG, del Portillo HA. Analysis of single-nucleotide polymorphisms in the crt-o and mdr1 genes of Plasmodium vivax among chloroquine-resistant isolates from the Brazilian Amazon region. Antimicrob Agents Chemother. 2009;53:3561–3564. doi: 10.1128/AAC.00004-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozen M, Gungor S, Atambay M, Daldal N. Cerebral malaria owing to Plasmodium vivax: case report. Ann Trop Paediatr. 2006;26:141–144. doi: 10.1179/146532806X107494. [DOI] [PubMed] [Google Scholar]
- PAHO. Programa Regional de Acción y Demostración de Alternativas Sostenibles para el Control de Vectores de la Malaria sin Uso de DDT en México y América Central, Mexico. 2006 http://www.sica.int/busqueda/busqueda_archivo.aspx?Archivo=info_20565_1_18012008.pdf.
- PAHO, WHO. 27th Pan American Sanitary Conference. Pan American Health Organization, World Health Organization; 2007a. http://www.paho.org/english/gov/csp/csp27index-e.htm. [Google Scholar]
- PAHO, WHO. Malaria in the Americas: time series epidemiological data from 200 to 2007. Pan American Health Organization, World Health Organization; 2007b. http://www.paho.org/Project.asp?SEL=TP&LNG=ENG&ID=79&PRGRP=docs_gen. [Google Scholar]
- PAHO, WHO. Informe de la situación del Paludismo en las Américas, 2008. Pan American Health Organization, World Health Organization; 2008a. http://new.paho.org/hq/index.php?option=com_content&task=view&id=2459&Itemid=2000&lang=es. [Google Scholar]
- PAHO, WHO. Integrated vector management: a comprehensive response to vector-borne diseases, 48th directing council 60th session of the regional committee. WHO; Washington, D.C.: 2008b. http://www.paho.org/English/GOV/CD/cd48-13-e.pdf. [Google Scholar]
- PAMAFRO. Reporte de Resultados Fase II, No. 8. 2009 www.orasconhu.org/documentos/1PAMAFRO.
- Peterson I, Borrell LN, El-Sadr W, Teklehaimanot A. Individual and household level factors associated with malaria incidence in a highland region of Ethiopia: a multilevel analysis. Am J Trop Med Hyg. 2009;80:103–111. [PubMed] [Google Scholar]
- Price RN, Douglas NM, Anstey NM. New developments in Plasmodium vivax malaria: severe disease and the rise of chloroquine resistance. Curr Opin Infect Dis. 2009;22:430–435. doi: 10.1097/QCO.0b013e32832f14c1. [DOI] [PubMed] [Google Scholar]
- Qari SH, Shi YP, Povoa MM, Alpers MP, Deloron P, Murphy GS, Harjosuwarno S, Lal AA. Global occurrence of Plasmodium vivax-like human malaria parasite. J Infect Dis. 1993;168:1485–1489. doi: 10.1093/infdis/168.6.1485. [DOI] [PubMed] [Google Scholar]
- Quinones ML, Lines J, Thomson MC, Jawara M, Greenwood BM. Permethrin-treated bed nets do not have a ‘mass-killing effect’ on village populations of Anopheles gambiae s.l. in The Gambia. Trans R Soc Trop Med Hyg. 1998;92:373–378. doi: 10.1016/s0035-9203(98)91053-7. [DOI] [PubMed] [Google Scholar]
- Quinones ML, Ruiz F, Calle DA, Harbach RE, Erazo HF, Linton YM. Incrimination of Anopheles (Nyssorhynchus) rangeli and An. (Nys.) oswaldoi as natural vectors of Plasmodium vivax in Southern Colombia. Mem Inst Oswaldo Cruz. 2006;101:617–623. doi: 10.1590/s0074-02762006000600007. [DOI] [PubMed] [Google Scholar]
- Ramos Junior WM, Sardinha JF, Costa MR, Santana MS, Alecrim MG, Lacerda MV. Clinical aspects of hemolysis in patients with P. vivax malaria treated with primaquine, in the Brazilian Amazon. Braz J Infect Dis. 2010;14:410–412. doi: 10.1590/s1413-86702010000400017. [DOI] [PubMed] [Google Scholar]
- Reyburn H, Mbatia R, Drakeley C, Bruce J, Carneiro I, Olomi R, Cox J, Nkya WM, Lemnge M, Greenwood BM, Riley EM. Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. Jama. 2005;293:1461–1470. doi: 10.1001/jama.293.12.1461. [DOI] [PubMed] [Google Scholar]
- Robert V, Carnavale P. Influence of deltamethrin treatment of bed nets on malaria transmission in the Kou valley. Burkina Faso, Geneva: 1991. pp. 735–740. [PMC free article] [PubMed] [Google Scholar]
- Roberts DR, Laughlin LL, Hsheih P, Legters LJ. DDT, global strategies, and a malaria control crisis in South America. Emerg Infect Dis. 1997;3:295–302. doi: 10.3201/eid0303.970305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L. Elimination meets reality in Hispaniola. Science. 2010;328:850–851. doi: 10.1126/science.328.5980.850. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Morales AJ, Sanchez E, Arria M, Vargas M, Piccolo C, Colina R, Franco-Paredes C. Haemoglobin and haematocrit: the threefold conversion is also non valid for assessing anaemia in Plasmodium vivax malaria-endemic settings. Malar J. 2007;6:166. doi: 10.1186/1475-2875-6-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R, Wirtz RA, Lanar DE, Sattabongkot J, Hall T, Waters AP, Prasittisuk C. Circumsporozoite protein heterogeneity in the human malaria parasite Plasmodium vivax. Science. 1989;245:973–976. doi: 10.1126/science.2672336. [DOI] [PubMed] [Google Scholar]
- Roshanravan B, Kari E, Gilman RH, Cabrera L, Lee E, Metcalfe J, Calderon M, Lescano AG, Montenegro SH, Calampa C, Vinetz JM. Endemic malaria in the Peruvian Amazon region of Iquitos. Am J Trop Med Hyg. 2003;69:45–52. [PubMed] [Google Scholar]
- Rubio-Palis Y, Zimmerman RH. Ecoregional classification of malaria vectors in the neotropics. J Med Entomol. 1997;34:499–510. doi: 10.1093/jmedent/34.5.499. [DOI] [PubMed] [Google Scholar]
- Ruiz F, Quinones ML, Erazo HF, Calle DA, Alzate JF, Linton YM. Molecular differentiation of Anopheles (Nyssorhynchus) benarrochi and An. (N.) oswaldoi from southern Colombia. Mem Inst Oswaldo Cruz. 2005;100:155–160. doi: 10.1590/s0074-02762005000200008. [DOI] [PubMed] [Google Scholar]
- Samudio F, Santamaría AM, Obaldía N, Pascale JM, Bayard V, Calzada JE. Short Report: Prevalence of Plasmodium falciparum mutations associated with antimalarial drug resistance during an epidemic in Kuna Yala, Panama, Central America. Am J Trop Med Hyg. 2005;73:839–841. [PubMed] [Google Scholar]
- Sanchez E. Reporte de casos de Malaria en Guatemala. Ministerio de Salud Publica y Asistencia Social; 2010. http://portal.mspas.gob.gt. [Google Scholar]
- Santana MS, de Lacerda MV, Barbosa MG, Alecrim WD, Alecrim MG. Glucose-6-phosphate dehydrogenase deficiency in an endemic area for malaria in Manaus: a cross-sectional survey in the Brazilian Amazon. PLoS One. 2009;4:e5259. doi: 10.1371/journal.pone.0005259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Saha K, Das CS. Three cases of ARDS: An emerging complication of Plasmodium vivax malaria. Lung India. 2010;27:154–157. doi: 10.4103/0970-2113.68323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrimshaw NS, Taylor CE, Gordon JE. Interactions of nutrition and infection. WHO Chron. 1969;23:369–374. [PubMed] [Google Scholar]
- Shekalaghe S, Alifrangis M, Mwanziva C, Enevold A, Mwakalinga S, Mkali H, Kavishe R, Manjurano A, Sauerwein R, Drakeley C, Bousema T. Low density parasitaemia, red blood cell polymorphisms and Plasmodium falciparum specific immune responses in a low endemic area in northern Tanzania. BMC Infect Dis. 2009;9:69. doi: 10.1186/1471-2334-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Do-Nascimento TF, Wilkerson RC, Lourenco-De-oliveira R, Monteiro FA. Molecular confirmation of the specific status of Anopheles halophylus (Diptera: Culicidae) and evidence of a new cryptic species within An. triannulatus in central Brazil. J Med Entomol. 2006;43:455–459. doi: 10.1603/0022-2585(2006)43[455:mcotss]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Sina B. Focus on Plasmodium vivax. Trends Parasitol. 2002;18:287–289. doi: 10.1016/s1471-4922(02)02329-2. 7. [DOI] [PubMed] [Google Scholar]; Singer B, de Castro MC. Enhancement and suppression of malaria in the Amazon. Am J Trop Med Hyg. 2006;74:1–2. [PubMed] [Google Scholar]
- Singh N, Chand SK, Mishra AK, Bharti PK, Singh MP, Ahluwalia TP, Dash AP. Epidemiology of malaria in an area of low transmission in central India. Am J Trop Med Hyg. 2006;75:812–816. [PubMed] [Google Scholar]
- Sinka ME, Rubio-Palis Y, Manguin S, Patil AP, Temperley WH, Gething PW, Van Boeckel T, Kabaria CW, Harbach RE, Hay SI. The dominant Anopheles vectors of human malaria in the Americas: occurrence data, distribution maps and bionomic precis. Parasit Vectors. 2010;3:72. doi: 10.1186/1756-3305-3-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira AM, Alexandre MA, Mourao MP, Santos VS, Nagahashi-Marie SK, Alecrim MG, Lacerda MV. Severe rhabdomyolysis caused by Plasmodium vivax malaria in the Brazilian Amazon. Am J Trop Med Hyg. 2010;83:271–273. doi: 10.4269/ajtmh.2010.10-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snounou G, White NJ. The co-existence of Plasmodium: sidelights from falciparum and vivax malaria in Thailand. Trends Parasitol. 2004;20:333–339. doi: 10.1016/j.pt.2004.05.004. 7. [DOI] [PubMed] [Google Scholar]; Soto J, Toledo J, Gutierrez P, Luzz M, Llinas N, Cedeno N, Dunne M, Berman J. Plasmodium vivax clinically resistant to chloroquine in Colombia. Am J Trop Med Hyg. 2001;65:90–93. doi: 10.4269/ajtmh.2001.65.90. [DOI] [PubMed] [Google Scholar]
- Sutton PL, Neyra V, Hernandez JN, Branch OH. Plasmodium falciparum and Plasmodium vivax infections in the Peruvian Amazon: propagation of complex, multiple allele-type infections without super-infection. Am J Trop Med Hyg. 2009;81:950–960. doi: 10.4269/ajtmh.2009.09-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K, Mita T, Jombart T, Eriksson A, Horibe S, Palacpac N, Ranford-Cartwright L, Sawai H, Sakihama N, Ohmae H, Nakamura M, Ferreira MU, Escalante AA, Prugnolle F, Bjorkman A, Farnert A, Kaneko A, Horii T, Manica A, Kishino H, Balloux F. Plasmodium falciparum accompanied the human expansion out of Africa. Curr Biol. 2010;20:1283–1289. doi: 10.1016/j.cub.2010.05.053. [DOI] [PubMed] [Google Scholar]
- TDR/WHO/MalERA. Monitoring and Evaluation, and Surveillance Meeting; January 26-27, 2009; Barcelona, Spain. 2009. http://maleratropikanet/ [Google Scholar]
- Thomson MC, Adiamah JH, Connor SJ, Jawara M, Bennett S, D’Alessandro U, Quinones M, Langerock P, Greenwood BM. Entomological evaluation of the Gambia’s National Impregnated Bednet Programme. Ann Trop Med Parasitol. 1995;89:229–241. doi: 10.1080/00034983.1995.11812948. [DOI] [PubMed] [Google Scholar]
- Trung HD, Bortel WV, Sochantha T, Keokenchanh K, Briet OJ, Coosemans M. Behavioural heterogeneity of Anopheles species in ecologically different localities in Southeast Asia: a challenge for vector control. Trop Med Int Health. 2005;10:251–262. doi: 10.1111/j.1365-3156.2004.01378.x. [DOI] [PubMed] [Google Scholar]
- Urdaneta L, Lal A, Barnabe C, Oury B, Goldman I, Ayala F, Tibayrenc M. Evidence for clonal propagation in natural isolates of Plasmodium falciparum from Venezuela. Proc Natl Acad Sci U S A. 2001;12:6725–6729. doi: 10.1073/pnas.111144998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valecha N, Bagga A, Chandra J, Sharma D. Cerebral symptoms with P. vivax malaria. Indian Pediatr. 1992;29:1176–1178. [PubMed] [Google Scholar]
- Van den Eede P, Van der Auwera G, Delgado C, Huyse T, Soto-Calle VE, Gamboa D, Grande T, Rodriguez H, Llanos A, Anne J, Erhart A, D’Alessandro U. Multilocus genotyping reveals high heterogeneity and strong local population structure of the Plasmodium vivax population in the Peruvian Amazon. Malar J. 9:151. doi: 10.1186/1475-2875-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan JA, Noden BH, Beier JC. Sporogonic development of cultured Plasmodium falciparum in six species of laboratory-reared Anopheles mosquitoes. Am J Trop Med Hyg. 1994;51:233–243. doi: 10.4269/ajtmh.1994.51.233. [DOI] [PubMed] [Google Scholar]
- Vittor AY, Gilman RH, Tielsch J, Glass G, Shields T, Lozano WS, Pinedo-Cancino V, Patz JA. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of Falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg. 2006;74:3–11. [PubMed] [Google Scholar]
- Wells TN, Poll EM. When is enough enough? The need for a robust pipeline of high-quality antimalarials. Discov Med. 2010;9:389–398. [PubMed] [Google Scholar]
- Whitehorn J, Coltart C, Manser D, Doherty T. A mixed malaria infection: is Plasmodium vivax good for you? Trans R Soc Trop Med Hyg. 2010;104:240–241. doi: 10.1016/j.trstmh.2009.08.004. [DOI] [PubMed] [Google Scholar]
- WHO. Guidelines for the treatment of malaria. World Health Organization; Geneve: 2006. http://www.who.int/malaria/publications/atoz/9789241547925/en/index.html. [Google Scholar]
- WHO. World Malaria report 2008. World Health Organization; Geneve: 2008. p. 215. http://www.who.int/malaria/publications/atoz/9789241563697/en/index.html. [Google Scholar]
- WHO. World Malaria report 2009. World Health Organization; Geneve: 2009. p. 40. http://whqlibdoc.who.int/publications/2009/9789241563901_eng.PDF. [Google Scholar]
- Zimmerman RH. Ecology of malaria vectors in the Americas and future direction. Mem Inst Oswaldo Cruz. 1992;87(Suppl 3):371–383. doi: 10.1590/s0074-02761992000700064. [DOI] [PubMed] [Google Scholar]


