Abstract
The impact of conductive hearing loss (CHL), the second most common form of hearing loss, on neuronal plasticity in the central auditory pathway is unknown. After short-term (1 day) monaural earplugging, the GluA3 subunits of the AMPA receptor (AMPAR) are upregulated at auditory nerve synapses on the projection neurons of the cochlear nucleus; glycine receptor α1 (GlyRα1) subunits are downregulated at inhibitory synapses in the same neuronal population. These data suggest that CHL affects receptor trafficking at synapses. We examined the impact of 7 days of CHL on the general expression of excitatory and inhibitory receptors by quantitative biochemistry and immunohistochemistry, using specific antibodies to detect AMPAR subunits (GluA1, GluA2, GluA2/3, and GluA4), GlyRα1, and the GABAA receptor subunit β2/3. Following monaural earplugging and an elevation of the hearing threshold by approximately 35 dB, the immunolabeling of the antibody for the GluA2/3 subunits but not the GluA2 subunit increased on bushy cells (BCs) and fusiform cells (FCs) of the ipsilateral ventral and dorsal cochlear nuclei. These same cell types showed a downregulation of the GlyRα1 subunit. Similar results were observed in the contralateral nuclei. The expression levels of GABAA β2/3 were unchanged. These findings suggest that, following longer periods of monaural conductive hearing loss, the synthesis and subsequent composition of specific glutamate and glycine receptors in projection neurons and their synapses are altered; these changes may contribute to abnormal auditory processing.
Keywords: ABRs, biochemistry, immunohistochemistry, earplugging, densitometry, GABAA β2/3
Introduction
Monaural conductive hearing loss (MCHL) reduces auditory nerve activity and affects sound processing along the central auditory pathway (Potash and Kelly, 1980). In addition, MCHL alters the metabolism and synthesis of proteins in central auditory system structures (Tucci et al., 1999; Hutson et al., 2007). The extent of such alterations depends on the duration, experimental procedure, and animal age (Tucci et al., 1999; Hutson et al., 2007).
Moreover, in response to decreased auditory nerve activity by MCHL (earplugging), homeostatic scaling mechanisms in the principal neurons of the cochlear nucleus cause compensatory changes in the surface expression of excitatory and inhibitory neurotransmitter receptors (Whiting et al., 2009). After 1 day of monaural earplugging (an approximately 20 dB reduction in sound), bushy cells (BCs) of the anteroventral cochlear nucleus (AVCN) and fusiform cells (FCs) of the dorsal cochlear nucleus (DCN) upregulate specific AMPA receptor (AMPAR) subunits and downregulate glycine receptor subunits. These findings suggest that short-term MCHL can alter the trafficking mechanisms of excitatory and inhibitory receptors to and from the synaptic plasma membrane.
Changes in receptor expression impact synapse strength (Savtchouk and Liu, 2011), as seen in the visual system in response to monocular deprivation or eyelid suture (Turrigiano and Nelson, 2004; Davis, 2006). Receptor trafficking in and out of synapses constitutes the molecular basis of the adaptive regulation of the density of receptors at synapses (Lévi et al., 2008). Neurotransmitter receptors are synthesized in the smooth endoplasmic reticulum (Malinow and Malenka, 2002; Derkach et al., 2007); this intracellular pool recycles continuously to and from the plasma and postsynaptic membranes, depending on the levels of synaptic activity (Malinow and Malenka, 2001; Passafaro et al., 2001; Park et al., 2004; Derkach et al., 2007). Therefore, we must assess whether longer periods of MCHL also lead to modifications in the synthesis of neurotransmitter receptors, because these alterations affect receptor trafficking at auditory nerve synapses. By examining the changes that occur in response to MCHL in the general pool (intracellular and at the plasma membrane) of excitatory and inhibitory receptors in cochlear nucleus neurons, we can assess the modifications in receptor synthesis and, consequently, the trafficking of receptor subunits at the synapse.
In this study, we assessed whether 1 week of MCHL (earplugging) affected the general expression of ionotropic glutamate (AMPA) and inhibitory receptors (GlyRα1 and GABAA β2/3 subunits) in the principal cells of the cochlear nucleus complex using biochemistry and immunohistochemistry. BCs and FCs of earplugged animals expressed more GluA3 AMPAR subunits than sham animals. This increase in GluA3 expression was accompanied by a decrease in GlyRα1 subunit levels.
Materials and Methods
Animals
Twenty-five P30 Sprague-Dawley rats were used. All experiments were performed per local and international guidelines on the ethical use of animals. All animals underwent auditory brainstem response (ABR) hearing tests and structural analysis. The animals were separated into a sham group (n = 9) and a monaural earplugged experimental group (n = 16). In the sham group, animals were subjected to the same surgical and anesthetic procedures for ABR testing as the earplugged animals but did not receive an earplug or stitches at the base of the pinna (see below). After sham or earplug surgery, animals were caged individually in adjacent cases so as to be exposed to the same acoustic environment. The handling and housing of the sham and earplugged animals was the same. No behavioral differences were observed between the 2 animal groups. The handling of the animals prior to and during the experiments was approved and supervised by the University of Connecticut and University of Pittsburgh IACUC per NIH guidelines.
The number of animals used and their suffering was minimized. Rats were anesthetized with isofluorane for ABR measurements, a mixture of ketamine (60 mg/kg) and xylazine (6.5 mg/kg) for insertion of the earplug and intracardiac perfusion, and CO2 for biochemical tests.
Monaural earplugging and ABRs
The earplugging protocol was similar to that of Whiting and colleagues (2009). Once anesthetized, the animals were placed on a warm blanket under a stereomicroscope. The skin was disinfected, and foam earplugs (Ear Classic, Aearo Company, Indianapolis, IN, USA) were cut to the appropriate size and placed in the right external canal in the experimental animals. ABR testing was conducted in a sound isolation cubicle (MED Associates Inc, St. Albans, VT, USA) using Tucker Davis Technology (TDT, Alachua, FL, USA) and a computer-based digital signal processing package and accompanying software (BioSig, Alachua, FL, USA).
Sound stimuli were presented through a calibrated TDT CF1 closed-field speaker, which was connected to a 2-mm diameter plastic probe that was inserted into the ear canal. Three subdermal electrodes were placed on the midline of the scalp at the vertex (+), the cheek toward the ear (−), and the animal's hind leg (ground). Threshold shifts were measured in the ipsi- and contralateral ears before, immediately after, and 1 week after the earplugging. ABR thresholds were obtained for clicks, and evoked potentials were averaged over 1024 sweeps. The waveforms were filtered using a 300–3000-Hz band-pass filter, and the stimulus levels were varied in 5-dB descending steps. The ABR threshold was defined as the lowest intensity at which reliable responses were recorded. The thresholds were confirmed by a second independent observer. The attenuation measured in each animal was averaged to determine the mean earplug attenuation. Average ABR thresholds were compared by a paired Student's t-test.
Antibodies
We used affinity-purified polyclonal primary antibodies against the following AMPA glutamate receptors: GluA1, GluA2/3, GluA4 (gifts from Dr. Robert Wenthold), and GluA2 (Chemicon/Millipore, Temecula, CA, USA). These antibodies were used at a concentration of 1.5 µg/ml each (Rubio et al., 2008; Whiting et al., 2009). We also used monoclonal antibodies against the glycine receptor α1 subunit (GlyRα1) (1:250; Alexis Biochemicals, San Diego, CA, USA) and GABAA β2/3 (1:200; De Blas et al., 1998; Miralles et al., 1999). To assess the specificity of the primary and secondary antibodies, sections were prepared in the absence the primary antibody during the incubation step or by preadsorption of the GluA2/3 and GluA2 antibodies with the corresponding peptides (Petralia et al., 1997; Rubio and Wenthold, 1997; Rubio and Wenthold, 1999, Matsui et al., 2005; Rubio, 2006). No immunostaining was observed on the sections after any control procedure.
Biochemical Analysis
Ten P30 Sprague-Dawley rats were used for the biochemical analysis (3 sham and 7 monaurally earplugged). After 1 week of earplugging, the animals were anesthetized with CO2 and decapitated. Brains were removed rapidly, and the entire cochlear nucleus of sham and earplugged animals (only ipsilateral to the earplug) and the cerebellum from sham rats were dissected in cold water with a protease inhibitor cocktail (Pierce, Thermo Fisher Scientific, Rockford, IL, USA). Tissues were homogenized in ddH2O, 10% sucrose, 50 mM Tris, and the protease inhibitor cocktail. Protein concentration was determined using a micro BCA protein assay (Pierce), and the samples were separated in an SDS gel as previously described (Rubio and Wenthold, 1999; Douyard et al., 2007). Affinity-purified antibodies against GluA2/3, GluA2, and GlyRα1 were used. The membranes were developed by chemiluminescence. The mean gray value (MGV) of each band was determined with ImageJ (version 1.43 u-Java 1.6.0_26; http://rsb.info.nih.gov/ij) and converted to a relative gray value (RGV) using the following equation: RGV = 100 − [(MGVband/MGVbackground) × 100] (see below). Samples were normalized to β-actin values. A Student's t-test was used to calculate the significance of the sample (P < 0.05) using Excel.
Immunohistochemistry
After 1 week of monaural earplugging and ABR testing, normal hearing (sham; n = 6) and experimental (n = 9) animals were transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Brains were dissected and postfixed in the same fixative for 1 hr at 4°C. Brainstems from sham animals were cut through the midline. To differentiate between the ipsilateral (plugged) and contralateral (unplugged) sides, a small hole was made on the left hemiside near the midline in the brainstems of earplugged animals.
Coronal vibratome sections were cut (approximately 40 µm thick) and subjected to immunohistochemistry. Half-brainstem sections from sham and whole brainstem sections from experimental animals were divided into 3 groups (each of which had 2 to 3 representative sections of the areas of interest). Sections from the control and earplugged animals were placed in the same container and exposed to the same conditions during the entire immunohistochemistry procedure, including the colorimetric development with 3,3’-diaminobenzidine (DAB).
Sections were blocked for 1 hr with 10% normal goat or horse serum in buffer and developed with DAB per a standard protocol (Rubio et al., 2008). Sections were mounted on glass slides, air-dried, and coverslipped for analysis on an Olympus BX51 microscope. Images of the normal hearing and earplugged AVCN and DCN were captured at 40× with a CCD camera and densitometrically analyzed using the Olympus MicroSuite Biological Suite (version 2.6, Melville, NY, USA). The image acquisition parameters were the same for the sham and earplugged sections. Grey values, which reflect immunostaining density, were measured over the regions of interest on the digitized images (see below). Specific immunostaining over an area of interest was defined by subtracting the background grey level value by a value obtained over a white matter area located near the region of interest in the same brainstem section (see below). Background values were not significantly different between groups (P > 0.05). Each of the micrographs of sham and earplugged animals was assigned a unique code for densitometric analysis by an independent observer.
Identification of cell types
The location within the cochlear nucleus, the shape and size of the cell bodies, and the proximal dendritic arborizations were used as criteria to identify bushy, fusiform, and cartwheel cells.
BCs in the AVCN
Sections were examined under a brightfield microscope, and only those that contained the AVCN (from caudal to rostral regions) were selected for densitometric analysis. BCs were located in the core region of the AVCN and were distinguished from multipolar stellate cells by the morphology of both their cell bodies and dendrites, following previously described criteria (Gómez-Nieto and Rubio, 2009). The BCs (globular and spherical) included in this study had round or ovoid cell bodies with a major diameter of approximately 20–25 µm. The nucleus was eccentric, located in the cell body, and unlabeled. Typically, 1 or 2 dendrites extended from opposite poles of the soma. Cell bodies with 3 or more primary dendrites were considered stellate cells and were not included in the analysis.
FCs and cartwheel cells (CwCs) in the DCN
The analyzed FCs were located in the FC layer and had a pyramidal or elongated cell body (approximately 25–30 µm major diameter and approximately 20 µm minor diameter) that was typically observed perpendicular to the pia. One or 2 apical and 1 basal dendritic tree extended from opposite poles of the cell body. The CwCs had medium sized (approximately 10 µm in diameter) cell bodies located in the molecular layer (Ryugo et al., 1995; Rubio et al., 2008).
Densitometry
For the densitometry analysis, we collected images from the caudal to the most rostral regions of the AVCN. The posteroventral cochlear nucleus was not included in the analysis. In the DCN, we selected sections in which we could clearly differentiate all layers of the nucleus. The most caudal sections of the DCN were not included in the analysis. The immunolabeling expression levels in normal hearing and earplugged animals were measured per Löhrke and Friauf (2002). Gray values of the digitized micrographs were analyzed with imaging software, wherein only the outlines of all identifiable (labeled) in-focus somata of the BCs of the AVCN (Figs. 3, 5; arrowhead) and the FCs and CwCs of the DCN (Figs. 4, 6, 7, 8; arrowhead and arrow, respectively) within a section were hand-drawn using the image software tools. Only those cell bodies with a clear and unlabeled nucleus were analyzed. The cell body outlines were considered to be regions of interest (a total of 100–313 somata per cell type, antibody, and animal). The outlined regions of interest for the corresponding cell types were compared between the antibodies used and were found to be similar in size (P > 0.5). Other neuronal cell bodies, glial cells, and out-of-focus structures (presumably labeled neurons) were excluded from the sample. The background of each section was measured by outlining an area in the nearby tissue in which the stain was very weak. The tonotopic axes of the ventral and dorsal cochlear nucleus were not considered in the analysis.
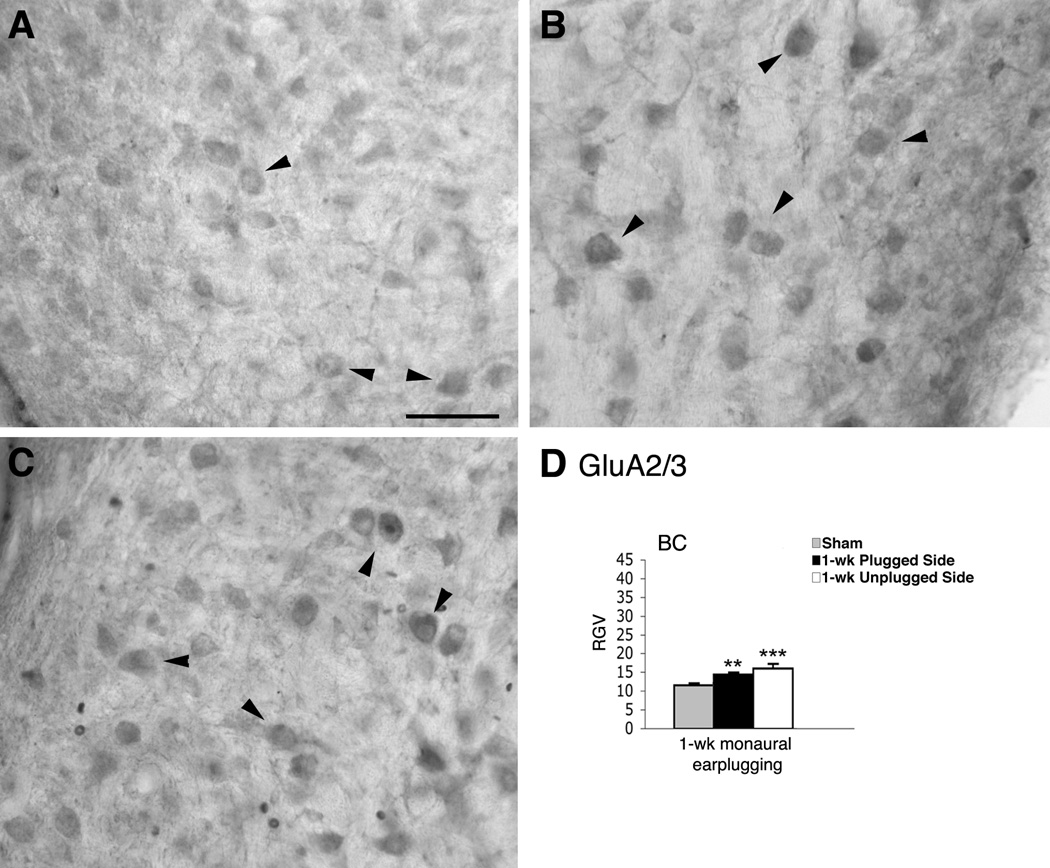
Figure 3.
Micrographs show immunoreaction for GluA2/3 in the AVCN of normal hearing (A) and monaural earplugged animals (B: ipsilateral plugged side; C: contralateral unplugged side). In A–C arrowheads = bushy cells. Scale bar : 50 µm. D, histogram of the RGVsoma values.
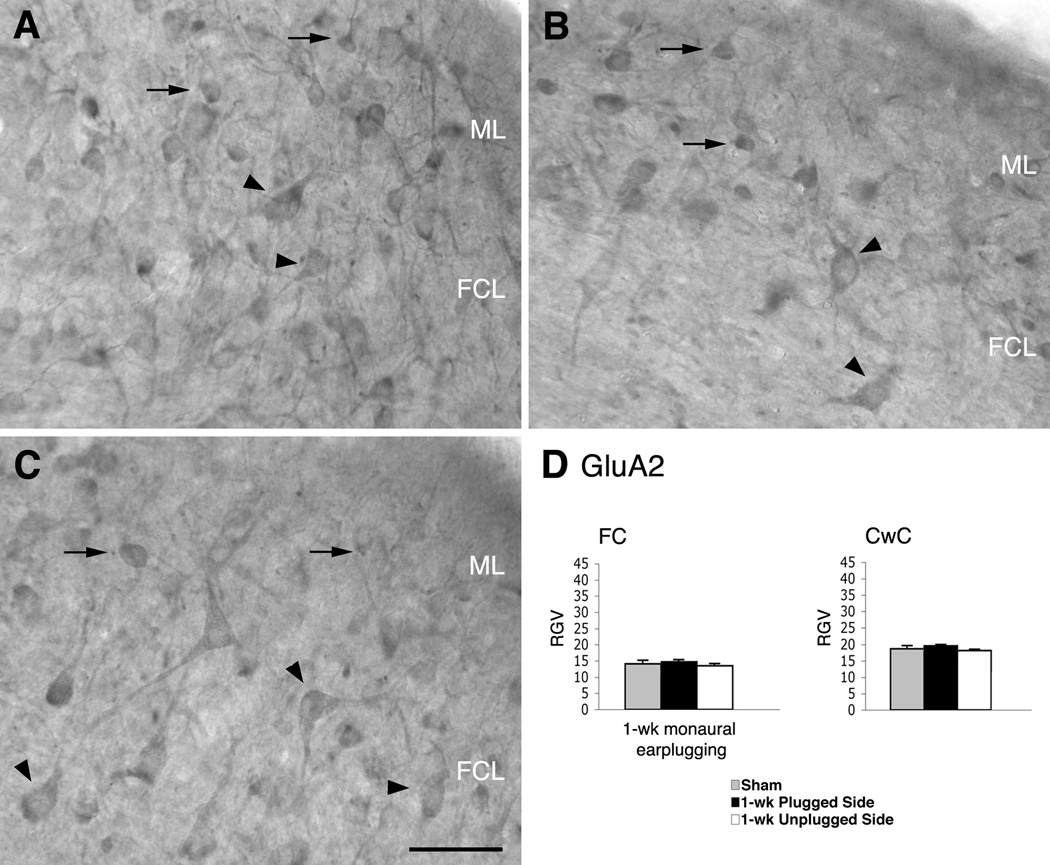
Figure 5.
Micrographs show immunoreaction for GluA2 in the DCN of normal hearing (A) and monaural earplugged animals (B: ipsilateral plugged side; C: contralateral unplugged side). In A–C arrowheads = fusiform cells; arrows = cartwheel cells. ML: molecular layer; FCL: fusiform cell layer; DL: deep layer. Scale bar : 50 µm. D, histogram of the RGVsoma values.
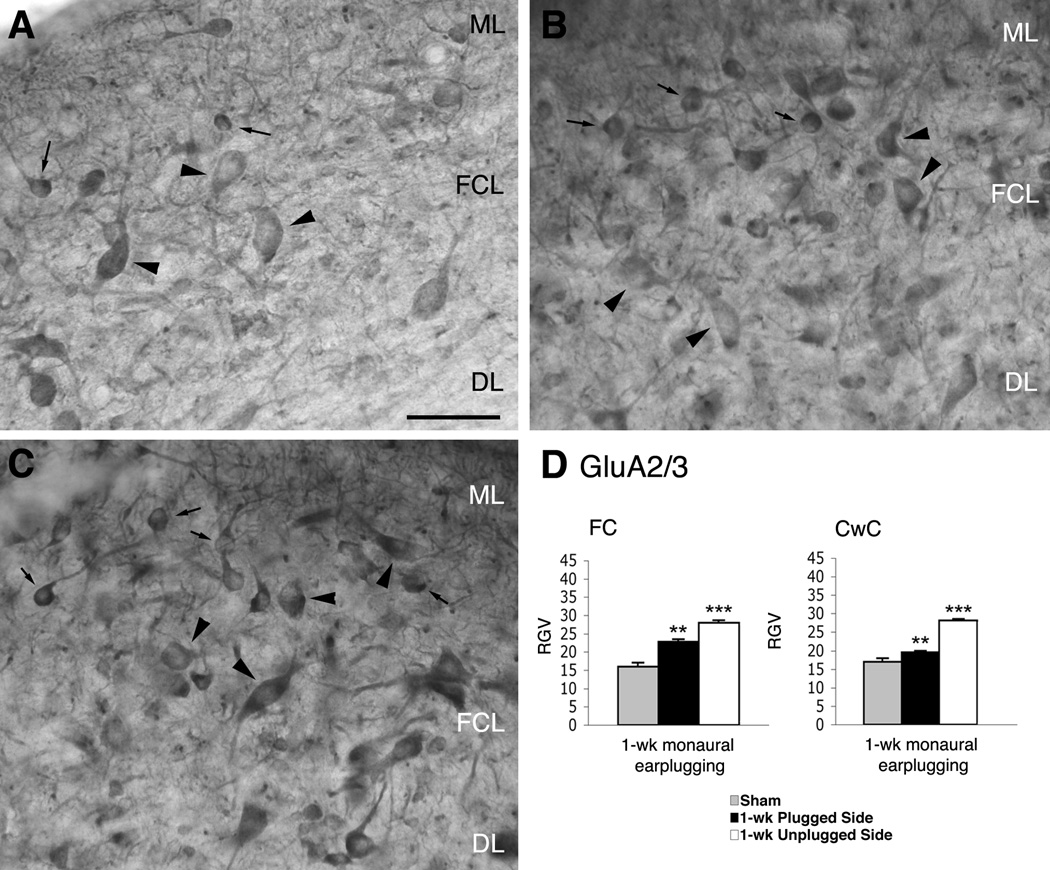
Figure 4.
Micrographs show immunoreaction for GluA2/3 in the DCN of normal hearing (A) and monaural earplugged animals (B: ipsilateral plugged side; C: contralateral unplugged side). In A–C arrowheads = fusiform cells; arrows = cartwheel cells. ML: molecular layer; FCL: fusiform cell layer; DL: deep layer. Scale bar : 50 µm. D, histogram of the RGVsoma values.
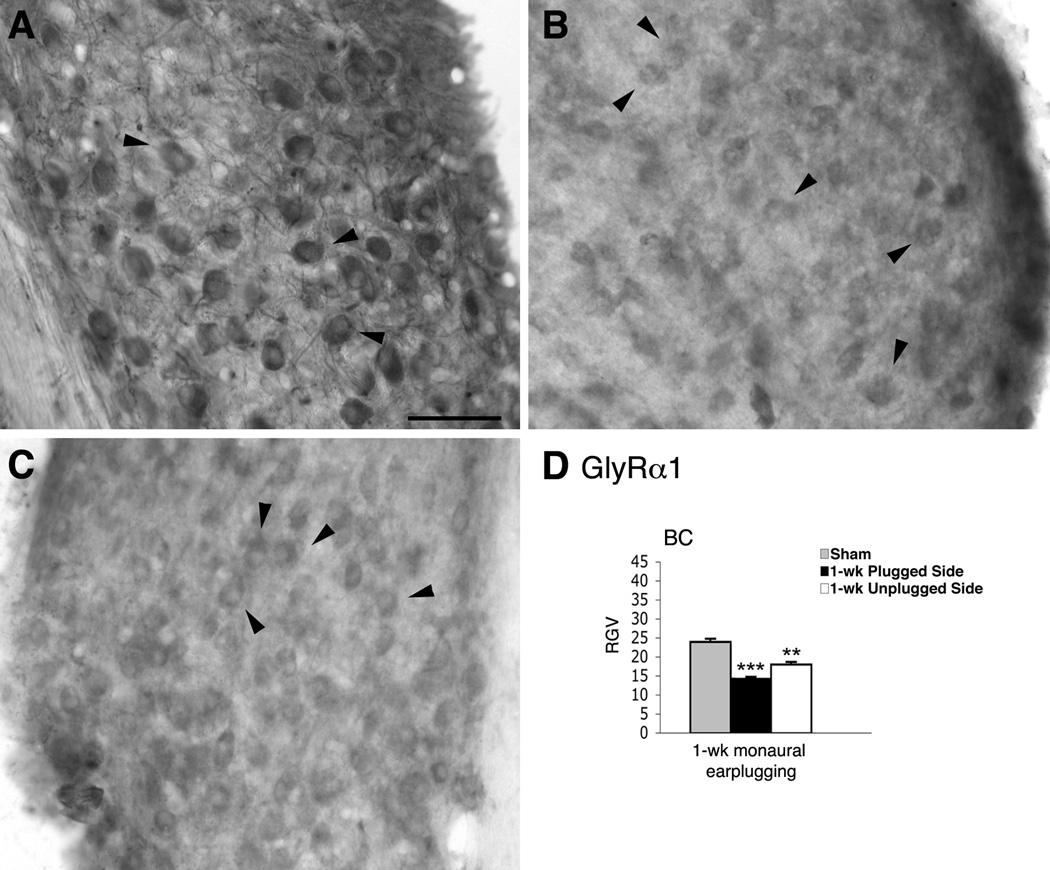
Figure 6.
Micrographs show immunoreaction for GlyRα1 in the AVCN of normal hearing (A) and monaural earplugged animals (B: ipsilateral plugged side; C: contralateral unplugged side). In A–C arrowheads = bushy cells. Scale bar : 50 µm. D, histogram of the RGVsoma values.
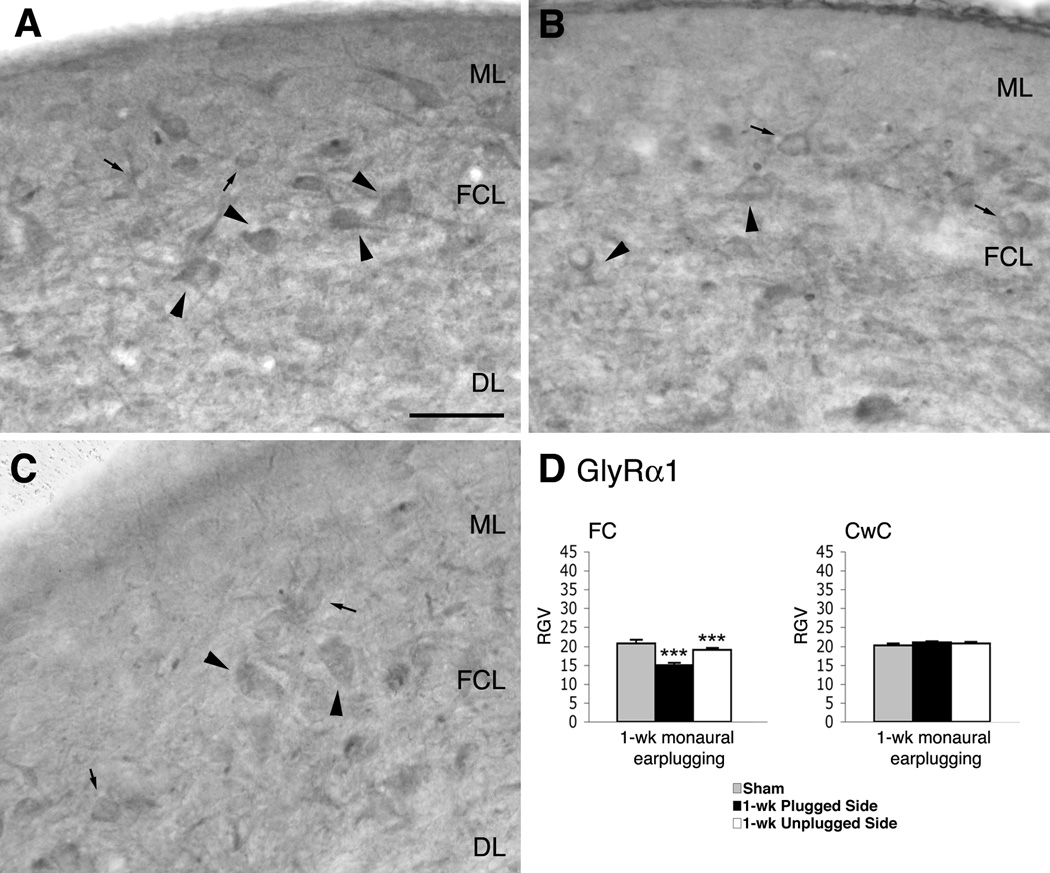
Figure 7.
Micrographs show immunoreaction for GlyRα1 in the DCN of normal hearing (A) and monaural earplugged animals (B: ipsilateral plugged side; C: contralateral unplugged side). In A–C arrowheads = putative fusiform cells; arrows = putative cartwheel cells. ML: molecular layer; FCL: fusiform cell layer; DL: deep layer. Scale bar: 50 µm. D, histogram of the RGVsoma values.
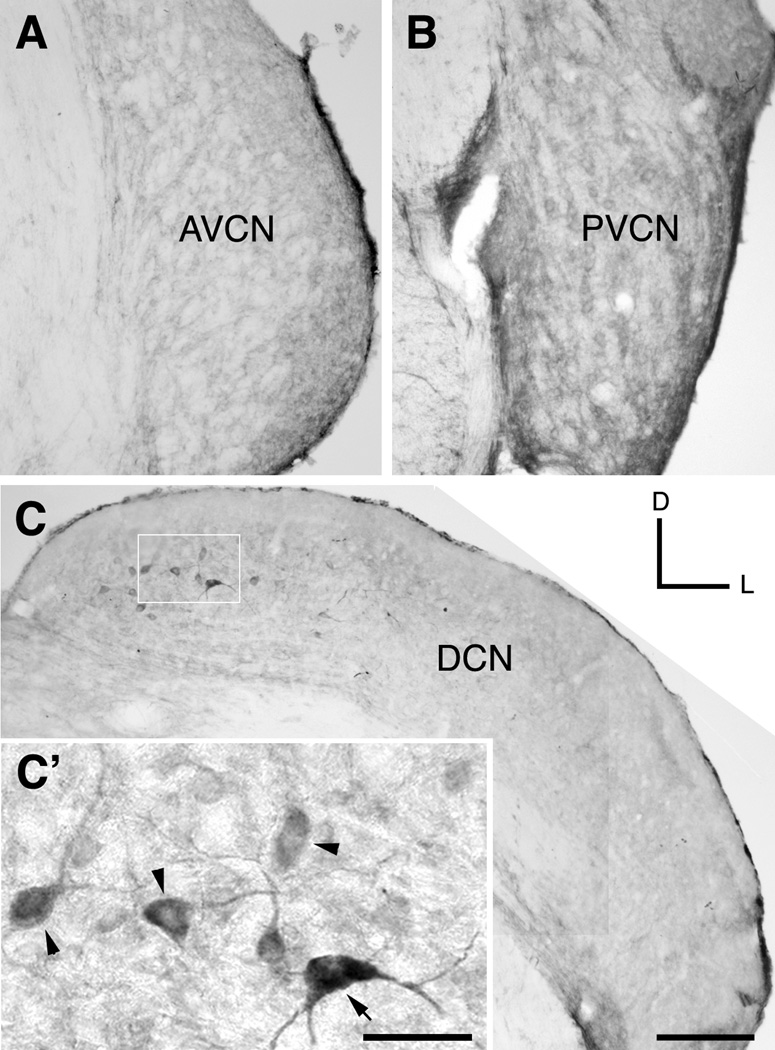
Figure 8.
Micrographs show immunoreaction for GABAA β2/3 in the VCN (A–B) and DCN (C–C’) of normal hearing animals. Scale bars AVCN: anteroventral cochlear nucleus; PVCN: posteroventral cochlear nucleus; DCN: dorsal cochlear nucleus.; D: dorsal; L: lateral Arrowheads: fusiform cells; arrow: multipolar cells. Scale bars, A–C: 200 µm; C’: 50 µm.
Because the digitized micrographs were taken at 8 bits of resolution, the pixels could have absolute gray values (AGVs) between 0 (black) and 255 (white). For each section of the AVCN and DCN analyzed, we calculated the mean AGV from the total number of corresponding pixels (AGVsoma and AGVbackground). These AGVs represent an objective measurement of the gray values. In contrast, the perception of gray values by eye is subjective and can lead to incorrect interpretations.
When AGVs are used to quantify the intensity of epitope-specific labeling (i.e., the signal) within a section, nonspecific labeling (i.e., noise) can occur. To compensate for the noise, we determined AGVbackground and calculated the signal-to-noise ratio, i.e., the relative gray value (RGV), per the following equation (Löhrke and Friauf, 2002): RGVsoma = 100 − [(AGVsoma/AGVbackground) × 100].
For each soma, the RGV (RGVsoma) was calculated from its AGV (AGVsoma) and the AGV of the background (AGVbackground). The equation above has several implications. First, if the signal of a soma has the same intensity as the background (AGVsoma = AGVbackground), when there is no epitope-specific labeling, the RGV will be zero. Second, negative RGVs can occur if the labeling of the somata is weaker than that of the background. Third, darkly stained somata (equivalent to a low AGVsoma) in sections with weak backgrounds (equivalent to a high AGVbackground) will generate a high RGV. Finally, an AGVsoma of zero (black) corresponds to an RGVsoma of 100 (which never occurred in our samples). We considered a soma to be immunoreactive if its RGV was greater than zero.
No significant difference in the RGVs was found between the animals in the control group (ANOVA, P > 0.05). There was also no significant difference in the RGVs between the animals in the experimental group (ANOVA, P > 0.05). Therefore, we were able to used pooled results to compare the sham group to the experimental group. The mean (± standard error of the mean, SEM) of all of the RGVsoma was calculated to obtain an average value for each section (RGVsomata; Table 1). RGVsomata values were ultimately used to objectively compare the immunoreactivity of a given antibody between sections of sham and earplugged animals. A Student's t-test was used to calculate the significance of the sample (P < 0.05) using Excel.
Table 1.
RGV values of AMPAR and GlyRα1 in FCs, CwCs and BCs in response to 1-week monaural CHL.
| AVCN-BC | Normal Hearing (±SEM) |
1-wk monaural earplugging Ipsilateral side (±SEM) |
1-wk monaural earplugging Contralateral unplugged side (±SEM) |
|---|---|---|---|
| AMPA | |||
| GluA2/3 | 12.0 ± 0.6 | 14.3 ± 0.4 | 16.0 ± 1.1 |
| GluA2 | 9.0 ± 0.4 | 11.0 ± 0.8 | 11.5 ± 0.6 |
| GluA4 | 9.2 ± 0.4 | 9.8 ± 0.5 | ----- |
| GlyRα1 | 23.0 ± 0.8 | 14.2 ± 0.5 | 18.0 ± 0.6 |
| DCN-FC | |||
| AMPA | |||
| GluA2/3 | 16.0 ± 1.0 | 22.8 ± 0.6 | 28.0 ± 0.7 |
| GluA2 | 14.3 ± 1.3 | 14.9 ± 0.5 | 13.0 ± 0.6 |
| GlyRα1 | 20.8 ± 0.9 | 15.8 ± 0.6 | 19.0 ± 0.6 |
| GABAAβ2/3 | 26.2 ± 1.2 | 27.3 ± 1.6 | 27.2 ± 1.2 |
| DCN-CwC | |||
| AMPA | |||
| GluA1 | 16.0 ± 1.8 | 14.0 ± 0.8 | 14.1 ± 1.0 |
| GluA2/3 | 17.0 ± 0.9 | 19.5 ± 0.5 | 28.1 ± 0.1 |
| GluA2 | 19.2 ± 0.8 | 17.9 ± 0.4 | 18.6 ± 0.4 |
| GlyRα1 | 20.2 ± 0.5 | 21.0 ± 0.2 | 20.7 ± 0.3 |
RGVsoma values of BCs of the AVCN, and FCs and CwCs of the DCN of normal hearing and earplugged animals. SEM: standard error of the mean.
As a positive control for immunostaining, we measured the gray level intensity for GluA2/3 and GluA2 immunostaining in the cerebellar cortex (Purkinje cell somata of lobules I–II). The RGVsomata of the Purkinje cells were found to be similar in sham and earplugged animals (P > 0.5).
Results
ABRs
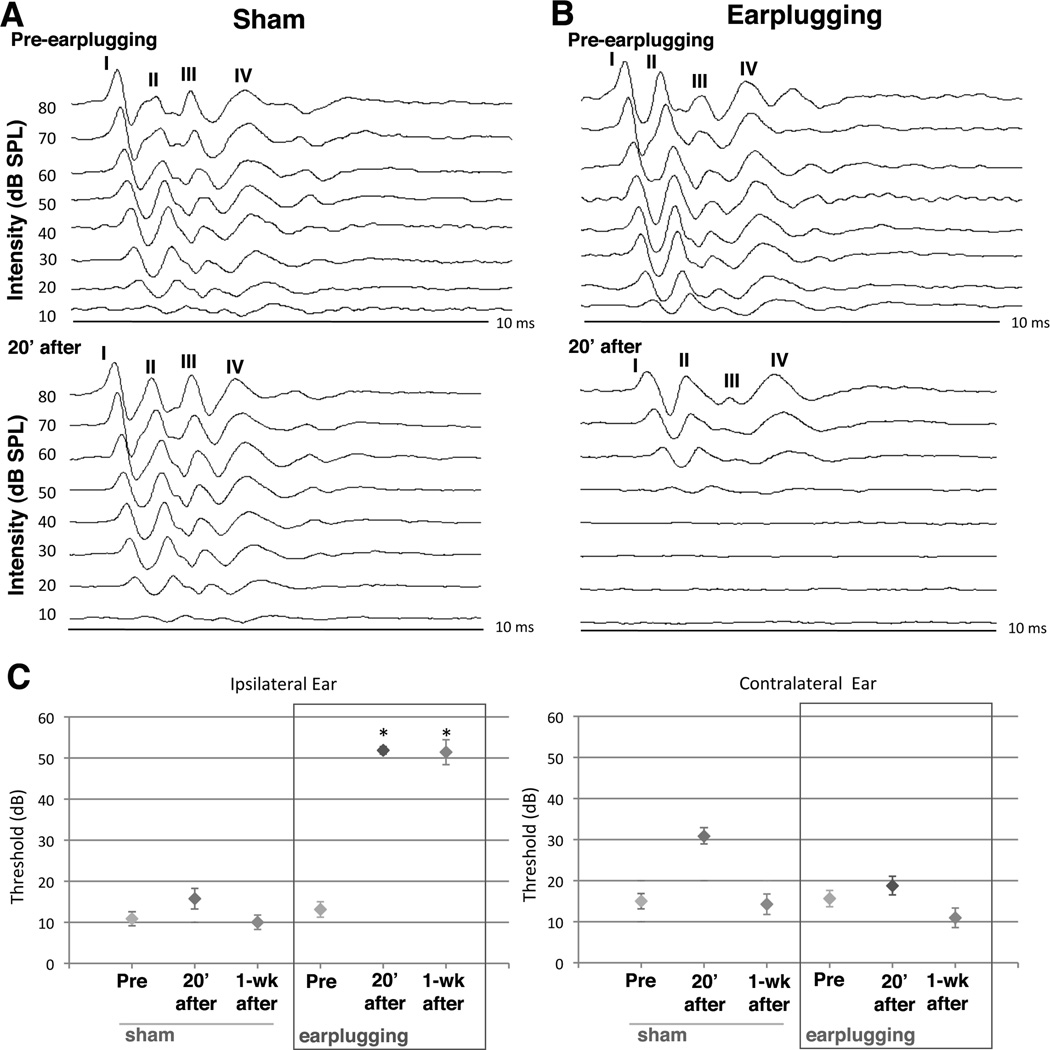
All animals in this study showed an acoustic startle reflex, suggesting that their auditory systems were intact. The sensitivity of the peripheral hearing of sham and monaurally earplugged rats was established using the ABR thresholds in response to clicks. Thresholds were measured prior to, 20 min after, and 1 week after earplugging.
As shown in the top of Figs. 1A–B, prior to the earplugging, sham and experimental rats exhibited characteristic ABR waveforms at sound pressure levels as low as 10 dB. Twenty min after earplugging, sham and monaural earplugging animals were retested (Figs. 1A–B, bottom). Upon insertion of the earplug, experimental animals showed an approximately 35 dB increase in ABR thresholds, a shift that was maintained for all 7 days of earplugging (Fig. 1C, ipsilateral). Twenty min after the sham treatment, the ABR thresholds in the control animals were the same as before the procedure (Fig. 1C, ipsilateral). There were no significant threshold shifts in the ABR thresholds of the contralateral ears for either sham or the earplugged animals (Fig. 1C).
Figure 1.
Monaural earplugging effects on ABR thresholds. A) Representative ipsilateral ABR recordings before (top) and 20 min after earplugging (bottom). Sham control rats exhibited the characteristic ABR waveform at sound pressure levels 10 dB before and after the procedure. B) For monaural earplugged rats, ipsilateral ABR threshold was elevated about 40 dB 20 minutes after earplugging. C) Mean ABR thresholds are shown for clicks from ipsilateral and contralateral ears. Monaural plugged ears (ipsilateral) showed significant threshold elevation 20 mins after and one week following earplugging (P<0.05, 6 shams, 7 earplugged animals). Error bars represent SEMs.
One week of monaural earplugging altered the expression of GluA2/3 but not GluA2 or GluA4 in the BCs of the AVCN and the FCs of the DCN
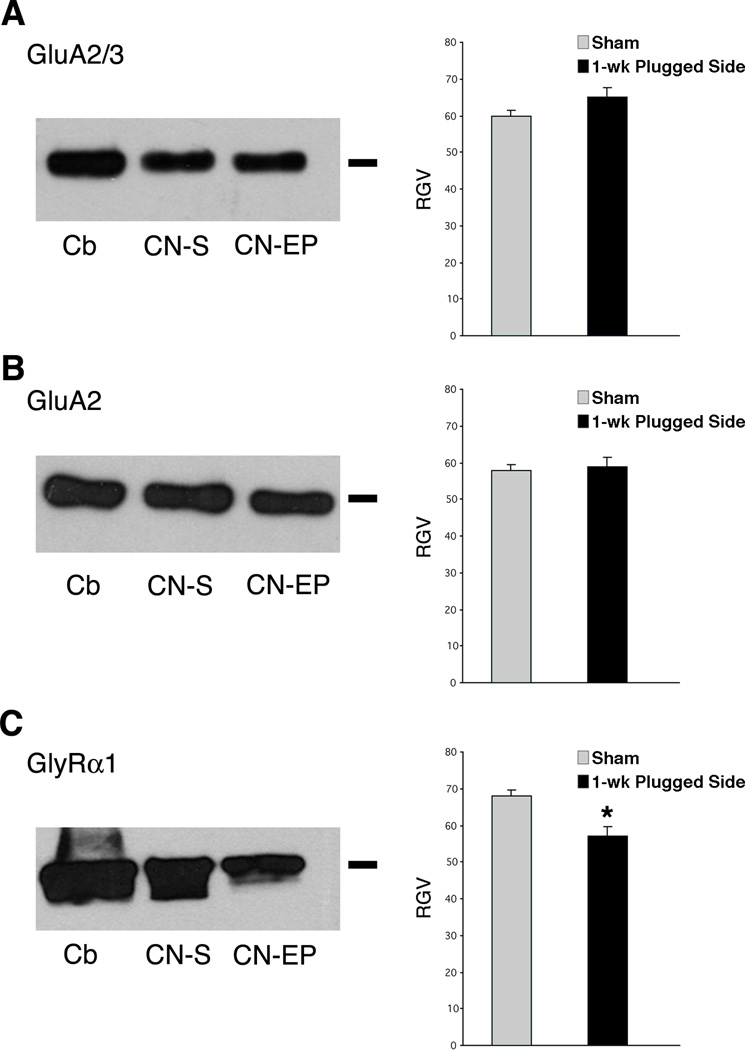
In western blot analysis, the antibodies for the GluA2/3 and GluA2 subunits labeled a single band that migrated to approximately 105 kD in homogenates of sham cerebellums and cochlear nuclei and earplugged cochlear nuclei (Figs. 2A–B). We calculated the RGVs for the bands of the sham and earplugged cochlear nuclei. The RGV for GluA2/3 in the earplugged group was 8.8% higher than the RGV in the control group, but this difference was not statistically significant (P > 0.05). The RGVs for GluA2 were similar between sham and earplugged animals (P > 0.05) (Fig. 2B).
Figure 2.
Western blots of cochlear nucleus homogenates for GluA2/3 (A), GluA2 (B) and GlyRα1 (C) of normal hearing and plugged side. The bar on the right sight points out to the molecular weight of the proteins ~ 108 kD for GluA2/3 and GluA2 and ~ 48 kD for GlyRα1. Histograms show the gray value levels. (*P < 0.05).
We determined the changes in the expression levels of GluA2/3, GluA2, and GluA4 in BCs and FCs by immunohistochemistry in sham and 1-week earplugged animals. The immunostaining pattern of GluA2/3, GluA2, and GluA4 in the AVCN of sham sections was similar to what has been described (Petralia et al., 1996; 1997). Specifically, the antibodies against GluA2/3 and GluA4 labeled cell bodies and primary dendrites of BCs (Fig. 3A; data not shown), but the GluA2 immunoreactivity was weak (data not shown). In earplugged animals, BCs were labeled with GluA2/3, and the reaction product was more robust than in shams (Fig. 3B). Qualitatively, GluA2 labeling in the earplugged animals was light, as it was in the shams. The immunostaining for GluA4 was also similar to the shams. Densitometric analysis of BC cell bodies showed a significantly higher RGV for GluA2/3 but not for GluA2 in the earplugged animals compared to the shams (Table 1; Fig. 3D). Thus, the higher levels of GluA2/3 in the earplugged animals were related to an upregulation of the GluA3 subunit. The RGVsoma for GluA4 was found to be similar between earplugged animals and shams (Table 1).
In the DCN of sham animals, GluA2/3 and GluA2 immunostaining were observed in many types of neurons throughout the nucleus, as previously described (Petralia et al., 1996; 1997). The labeling for GluA4 was dispersed throughout the nucleus, and the neuronal cell bodies were not distinguishable from the immunostaining in the neuropil (data not shown; Rubio and Wenthold, 1997). FC cell bodies, identified by their typically elongated shape and their location within the FC layer, were positively immunostained for GluA2/3 (Fig. 4A). Generally, the staining extended along the primary apical and basal dendrites, which were perpendicular to the surface and in the deep regions of the nucleus. Similar immunostaining patterns were observed with the GluA2 subunit-specific antibody (Fig. 5A).
In sections from 1-week monaurally earplugged animals, the antibody for GluA2/3 labeled similar cells throughout the DCN as in shams. Cell bodies and dendrites of FCs were positive for GluA2/3, although the stain was darker than in shams (Fig. 4B). Qualitatively, GluA2 immunostaining in the DCN and the FCs was similar to that in shams (Figs. 5A–B). Densitometric analysis of FC cell bodies showed a significantly higher RGVsoma for GluA2/3 but not for GluA2 in the earplugged animals compared to the sham animals (Table 1, Figs. 4D, 5D). Thus, the higher levels of GluA2/3 were related to an upregulation of the GluA3 subunit. The immunostaining for GluA4 was similar in the DCN of earplugged and sham animals. The cell bodies of the FCs were not distinguishable from the rest of the immunostaining for GluA4; therefore, we could not calculate the RGVsoma for this subunit. Instead, we calculated the RGV for the entire DCN and found no differences between earplugged and sham animals (sham: 25.5 ± 2.76 SEM; earplugged: 24.0 ± 1.1 SEM).
BCs and FCs had lower expression of the GlyRα1 subunit in response to 1 week of monaural earplugging
In western blot analysis, the GlyRα1 subunit appeared as a band at approximately 48 kD in homogenates from sham (cerebellum and cochlear nucleus) and earplugged (cochlear nucleus) animals (Fig. 2C); the band in the earplugged animals was thinner than in the shams. We calculated the RGVs for the cochlear nucleus bands and observed a significantly lower (approximately 29.6%) expression of GlyRα1 in the earplugged cochlear nuclei than the sham cochlear nuclei (P < 0.05) (Fig. 2C).
We performed immunohistochemistry with a specific antibody for GlyRα1 to determine its distribution and expression levels in cochlear nuclei. Cell bodies and dendrites of BCs within the AVCN of shams were labeled (Fig. 6A). In earplugged animals, BCs were positive for GlyRα1, although the stain was lighter than in shams (Fig. 6B). The RGVsoma data from the BCs showed that GlyRα1 expression was significantly lower in earplugged animals than in shams (Table 1; Fig. 6D).
In the DCN of sham animals, GlyRα1 subunit immunostaining was observed in medium to large cell bodies (Fig. 7A). This subunit was also expressed along the primary dendrites and throughout the neuropil, as previously described (Wang et al., 2009). FCs were identified by the shape and location of the cell body within the nucleus, which stained positive for GlyRα1.
After 1 week of monaural earplugging, GlyRα1 immunostaining was lighter than in shams (Fig. 7B). The cell bodies of the FCs were identified, and the RGVsoma was significantly lower than in shams than in the earplugged animals (Table 1; Fig. 7D).
GABAA β2/3 immunolabeling in normal hearing and in response to 1 week of monaural earplugging
In the AVCN and the posteroventral cochlear nucleus of normal hearing animals, GABAA β2/3 staining was weak and did not appear to be expressed in the cell bodies (Figs. 8A–B). In contrast, robust neuronal labeling was observed in the medial (high frequency) regions of the DCN (Figs. 8C–C’). GABAA β2/3 was observed only in medium to large neurons and extended from the cell bodies to the primary dendrites. Some of these neurons were identified as FCs, whereas others were larger and lay deeper in the nucleus and were identified as multipolar cells. We obtained a similar pattern of immunostaining in parasagittal brainstem sections containing the DCN (unpublished data).
To determine the specificity of the immunolabeling, we examined the cerebellar cortex. As previously described (De Blas et al., 1998; Miralles et al., 1999), GABAA β2/3 staining was robust in the granular cell layer (data not shown).
Similar patterns of GABAA β2/3 immunostaining were observed in the 1-week earplugged animals and the shams, and the RGVsoma of FCs were not significantly different between these groups (Table 1).
CwCs of the DCN modified only the expression of GluA2/3 in response to 1 week of monaural earplugging
CwCs are the chief inhibitory interneurons in the superficial DCN and innervate the apical dendrites of FCs. In sham animals, CwCs were positive for GluA1 (data not shown), GluA2/3, GluA2, and GlyRα1 but not for GABAA β2/3 (Figs. 4, 5, 7, 8). Similar labeling was observed in 1-week monaurally earplugged animals, although the GluA2/3 immunoreaction was stronger than in shams. We calculated the RGVsoma of sham and monaurally earplugged animals for GluA1, GluA2/3, GluA2, and GlyRα1. Only GluA2/3 showed a higher expression in the earplugged animals than the shams; the values for GluA1, GluA2, and GlyRα1 were similar to those seen in the sham animals (Table 1; Figs. 4D, 5D, 7D). Thus, the increase in GluA2/3 was related to an upregulation of the GluA3 subunit.
BCs and FCs of the contralateral side modified the expression of GluA2/3 and GlyRa1, but not GluA2, GluA4 or GABAA β2/3 after 1 week of monaural earplugging
To determine whether the BCs and FCs contralateral to the earplugging responded to 1 week of MCHL, we analyzed the immunostaining patterns and expression levels of GluA2/3, GluA2, GlyRα1, and GABAA β2/3 (the latter only in FCs). Qualitatively, the labeling for all subunits was similar to that seen on the side ipsilateral to the earplug (Figs. 3–7; data not shown). In densitometric analysis of BCs and FCs, only the RGVsoma for GluA2/3 and GlyRα1 were significantly higher and lower, respectively (Table 1; Figs. 3–7) in earplugged animals than in sham animals. We also analyzed the GluA1, GluA2/3, GluA2, and GlyRα1 RGVsoma of CwCs. As observed on the ipsilateral side of the earplug, only the GluA2/3 RGVsoma was higher for these cells in earplugged animals than in the sham animals (Table 1; Figs. 4, 5, 7).
Discussion
In this study, we addressed whether the general expression of excitatory and inhibitory ionotropic receptors in the principal neurons of the AVCN and DCN was altered after 1 week of MCHL. BCs and FCs upregulated GluA3 and downregulated GlyRα1 expression on both the ipsilateral and contralateral sides of the earplug. These data suggest that 1 week of MCHL can modify the synthesis and trafficking of neurotransmitter receptors in cochlear nucleus principal neurons, which could affect auditory processing.
Technical considerations
We used the relative gray values obtained from computer-assisted image analysis to normalize the results following DAB immunostaining for GluA1-4 AMPAR, GlyRα1, and GABAA β2/3 receptor subunits in cochlear nucleus neurons following 1 week of monaural earplugging. This is an indirect method to detect the corresponding antigen in the sections and is not a direct measurement of antigen expression. This is because it is not possible to control of all the amplification steps in the staining procedure to obtain reliable information on the quantity of the antigen present (Leblond, 1979). In our study, to minimize the variability of the immunostaining amplification steps, we put brainstem sections from both sham and experimental animals in the same vials. In this way, the immunohistochemical procedure and the DAB development were performed simultaneously on tissue from the 2 groups. In addition, it has been shown that the analysis of immunocytochemical staining with computer-assisted image analysis, as performed in this study, can be a reliable and sensitive method to determine the relative levels of antigens in tissue sections and to detect discrete regional changes in specific proteins of interest (Huang et al., 1996).
We used additional procedures and analyses to support our results. We used densitometry of cochlear nucleus homogenates of sham and earplugged animals to determine changes in the expression levels of glutamate and glycine receptor subunits. These densitometric results support the data obtained with the immunostaining. We also used the cerebellar cortex as positive control. We found no differences in the expression levels of GluA2/3 and GluA2 subunits between shams and earplugged animals. Furthermore, 2 independent researchers performed the quantitative analysis of the DAB immunostaining in sham and earplugged brainstem sections and obtained similar results.
One week of MCHL altered the synthesis of GluA3 and GlyRα1
Neuronal networks must maintain a balance between excitation and inhibition for normal brain function. In response to a decrease in neuronal activity, neurons upregulate excitatory neurotransmitter receptors and downregulate inhibitory ones (O’Brien et al., 1998; Turrigiano and Nelson, 2004; Davis, 2006; Hou et al., 2008).
In the cochlear nucleus, we have shown that short-term MCHL (1 day) redistributes the synaptic expression of specific glutamate and glycine receptor subunits (Whiting et al., 2009). The GluA3 subunit of AMPARs was upregulated in auditory nerve synapses on BCs and FCs, while the GlyRα1 subunit was downregulated in inhibitory synapses on the same cell types. The replacement of these subunits at the synapse, from or toward the extrasynaptic or intracellular pools, suggests that the recycling of receptors in and out of the postsynaptic membrane can be modified relatively quickly with only an approximately 20–35-dB reduction in sound.
Changes in the subunit composition of AMPA and glycine receptors at the synapse can affect neuronal synaptic responses (Liu and Cull-Candy, 2000; Lévi et al., 2008; Savtchouk and Liu, 2011). If the GluA3 and GlyRα1 subunits regulate the balance of excitation and inhibition in cochlear nucleus principal neurons, we should expect similar changes in response to longer periods of MCHL. Moreover, under longer periods of MCHL, neurons will likely adapt by modifying the translation mechanisms of excitatory and inhibitory receptors, which will affect the general expression of GluA3 and GlyRα1 receptor subunits in the cell body.
Our study supports this model, because, in densitometry analysis, we observed that the expression of both the GluA3 and GlyRα1 subunits in BCs and FCs was altered (higher and lower, respectively) when an ear was plugged for 1 week. Whether long-term MCHL alters the subcellular synaptic expression of these subunits requires further examination. Nevertheless, changes in the neuronal cytoplasmic pool can affect receptors at the synapse (Malinow and Malenka, 2002; Shepherd and Huganir, 2007; Newpher and Ehlers, 2008).
After 1 week of monaural earplugging, we also observed an upregulation of the GluA2/3 subunits in CwCs, without a modification in the expression levels of GluA2. Therefore, synthesis of new GluA3 subunits might explain the upregulation seen when an antibody recognizing both the GluA2 and GluA3 subunits was used. Although CwCs respond to sound (Davis and Young, 1997; Portfors and Roberts, 2007), they do not receive direct auditory nerve inputs. These inhibitory interneurons receive glutamatergic innervation from the parallel fiber endings of granule cells, which convey information from a wide range of auditory and nonauditory sources. Granule cells preferentially receive somatosensory and vestibular inputs, auditory cortex and inferior colliculus descending inputs, and possibly type II auditory nerve fiber inputs (Itoh et al., 1987; Weinberg and Rustioni, 1987; Brown et al., 1988; Caicedo and Herbert, 1993; Weedman and Ryugo, 1996; Wright and Ryugo, 1996; Shore et al., 2003; Haenggeli et al., 2005; Zhou and Shore, 2004; Schofield and Coomes, 2005). Evidence suggests that CHL could affect descending auditory pathways (Xu et al., 2007). Thus, it is possible that the response we obtained in CwCs after 1 week of MCHL was related to a modification in the normal processing of descending inputs to cochlear nucleus neurons.
BCs and FCs contralateral to the earplug upregulated GluA3 and downregulated GlyRα1 in response to 1 week of MCHL
MCHL alters the cellular metabolism and protein synthesis in cells of the cochlear nucleus of the contralateral side (Tucci et al., 1999; Hutson et al., 2007). In this study, we observed that BCs and FCs on the side contralateral to the earplug altered their general expression of GluA3 (up) and GlyRα1 (down) in response to 1 week of MCHL. These neuronal responses could be attributed to altered firing rates of afferents to the contralateral side of the earplug.
The cochlear nucleus complex is the first site at which binaural information converges in the CNS (for review, see Cant and Benson, 2003). Communication between cochlear nuclei occurs through commissural projections (Alibardi, 2003; Shore et al., 2003) or via descending inputs from upper auditory nuclei (Sprangler et al., 1987; Shore et al., 1991). It appears that glutamatergic neurons in the ventral cochlear nucleus and collateral projections from cholinergic neurons mediate the excitation in response to contralateral sound (Bledsoe et al., 2009; Sumner et al., 2005). Alternatively, stimulation by contralateral sound suppresses auditory nerve activity through the olivocochlear bundle (Liberman and Brown, 1986; Warren and Liberman, 1989a, b; Darrow et al., 2006). Thus, it appears that MCHL creates an imbalance in the neuronal synaptic circuitry of the contralateral cochlear nucleus through 2 pathways, the commissural fibers and the auditory nerve of the unplugged ear.
More research is needed to determine the contribution of each pathway in normal hearing and in response to monaural hearing loss. However, our studies show that, independent of the duration (1 day or 1 week) or the procedure (monaural deafferentation or MCHL), BCs and FCs on the contralateral side have similar homeostatic responses to compensate for unilateral reductions in sound. The GluA3 subunit of the AMPAR is upregulated, whereas the GlyRα1 subunit is downregulated (Rubio, 2006; Whiting et al., 2009; present study).
Function of the GABAA β2/3 subunit in the cochlear nucleus
Glycine and GABA are the principal inhibitory neurotransmitters in the cochlear nucleus (Alibardi, 1998; Moore et al., 1996; Doucet et al., 1999; Davis and Young, 2000). The expression of glycine receptor subunits in normal hearing animals has been shown (Wang et al., 2009). Further, the expression of glycine receptors has been shown to be lower in the hearing impaired (Potashner et al., 2000; Whiting et al., 2009; Wang et al., 2009; the present study).
However, the expression and localization of ionotropic GABA (GABAA) receptor subunits in cochlear nuclei have not been well described. GABAA receptors are pentamers that assemble from at least 2 α and 2 β subunits and include a single γ2 or δ subunit (Luscher and Keller, 2004). GABAA receptors in the CNS mediate both fast synaptic (mediated by low-affinity receptors at the synapse) and tonic (mediated by high-affinity receptors at extrasynaptic sites) inhibition. Electrophysiological and anatomical evidence from the cerebellum suggests that phasic inhibitory currents are mediated by postsynaptic GABAA receptors (Farrant and Nuser, 2005) that contain the γ2 subunit in combination with diverse α and β subunits. In general, δ subunit-containing receptors are extrasynaptic, although not all extrasynaptic GABAA receptors contain δ subunits. In the cochlear nucleus, a study of GABAA receptor mRNA levels in normal hearing rats reported that BCs and FCs expressed similar subunits (α1, α3, α5, γ2L, and β3) but that α5 was not detected in FCs (Campos et al., 2001). Thus, both types of principal neurons in the cochlear nucleus could theoretically mediate both fast and tonic inhibition.
We examined the expression and distribution of most of these subunits in normal hearing rats (sham) and rats after 1 week of MCHL using proprietary and commercial antibodies. We observed positive and consistent neuronal cell body staining for the GABAA β2/3 subunits in both sham and earplugged animals (Fig. 8; unpublished data). Notably, only FCs and large neurons in the deep layers of the medial (high-frequency region) DCN were labeled. The expression levels of GABAA β2/3 were unchanged in response to 1 week of MCHL. In another study, Dong and colleagues (2009) found a decrease in the mRNA expression of the α1 subunit in the guinea pig cochlear nucleus ipsilateral to a partial peripheral deafferentation (Dong et al., 2009).
In the absence of data on the expression of other GABAA receptor subunits at the protein level, it would be premature to speculate on the subunit composition of the channel and its function in cochlear nucleus neurons. Furthermore, more targeted studies are needed to determine whether cochlear nucleus neurons modify GABAA receptors in response to hearing loss.
GluA3 and GlyRα1 appear to be important receptor subunits in response to hearing loss
MCHL is not the only hearing deficit that alters the balance between excitation and inhibition along the auditory pathway. Studies on sensorineural deafferentation and hair cell damage after exposure to noise or chemical ototoxicity have reported changes in the expression of glutamate and glycine receptor types in auditory central structures (Milbrandt et al., 2000; Potashner et al., 2000; Vale and Sanes, 2002; Rubio, 2006; Argence et al., 2008; Whiting et al., 2009). Recent evidence indicates that hyperactivity in the DCN is a potential cause of tinnitus following acoustic overexposure. It is thought that chronic tinnitus develops from a net loss of glycinergic or GABAergic inhibition in the DCN and higher auditory structures (Brozoski et al., 2002, 2007; Bauer et al., 2008; Middleton et al., 2011; Wang et al., 2009).
Whether MCHL leads to hyperactivity in the cochlear nucleus should be investigated. However, our data on 1-day and 1-week MCHL suggest that, in conjunction with decreased glycinergic innervation, the increased synthesis and recomposition of synaptic AMPARs may contribute to hyperactivity in the dorsal and ventral cochlear nuclei.
Acknowledgments
We gratefully acknowledge to Dr. David Ryugo and our colleagues in the lab for comments and critically reading the manuscript. We thank Robert Wenthold for providing us with the antibodies against AMPARs. The authors thank NIH/NIDCD RO1 DC006881.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alibardi L. Ultrastructural and immunocytochemical characterization of neurons in the rat ventral cochlear nucleus projecting to the inferior colliculus. Ann Anat. 1998;180:415–426. doi: 10.1016/S0940-9602(98)80102-7. [DOI] [PubMed] [Google Scholar]
- Alibardi L. Ultrastructural distribution of glycinergic and GABAergic neurons and axon terminals in the rat dorsal cochlear nucleus, with emphasis on granule cell areas. J Anat. 2003;203:31–56. doi: 10.1046/j.1469-7580.2003.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argence M, Vassias I, Kerhuel L, Vidal PP, de Waele C. Stimulation by cochlear implant in unilaterally deaf rats reverses the decrease of inhibitory transmission in the inferior colliculus. Eur J Neurosci. 2008;28:1589–1602. doi: 10.1111/j.1460-9568.2008.06454.x. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008;86:2564–2578. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Peden DR, Rosahl TW, Wafford KA, Lambert JJ. Extrasynaptic GABA(A) receptors of thalamocortical neurons: a molecular target for hypnotics. J Neurosci. 2005;25:11513–11520. doi: 10.1523/JNEUROSCI.2679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe SC, Jr, Koehler S, Tucci DL, Zhou J, Le Prell C, Shore SE. Ventral cochlear nucleus responses to contralateral sound are mediated by commissural and olivocochlear pathways. J Neurophysiol. 2009;102:886–900. doi: 10.1152/jn.91003.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M, Berglund A, Kiang N, Ryugo D. Central trajectories of type II spiral ganglion neurons. J Comp Neurol. 1988;278:581–590. doi: 10.1002/cne.902780409. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22:2383–2390. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Ciobanu L, Bauer CA. Central neural activity in rats with tinnitus evaluated with manganese-enhanced magnetic resonance imaging (MEMRI) Hear Res. 2007;228:168–179. doi: 10.1016/j.heares.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Caicedo A, Herbert H. Topography of descending projections from the inferior colliculus to auditory brainstem nuclei in the rat. J Comp Neurol. 1993;328:377–392. doi: 10.1002/cne.903280305. [DOI] [PubMed] [Google Scholar]
- Campos ML, De Cabo C, Wisden W, Jiuz JM, Merlo D. Expression of GABAA receptor subunits in rat brainstem auditory pathways: cochlear nuclei, superios olivary complex and nucleus of the lateral lemniscus. Neuroscience. 2001;102:625–638. doi: 10.1016/s0306-4522(00)00525-x. [DOI] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res Bull. 2003;60:457–474. doi: 10.1016/s0361-9230(03)00050-9. Review. [DOI] [PubMed] [Google Scholar]
- Darrow KN, Maison SF, Liberman MC. Cochlear efferent feedback balances interaural sensitivity. Nat Neurosci. 2006;9:1474–1476. doi: 10.1038/nn1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. Review. [DOI] [PubMed] [Google Scholar]
- Davis KA, Young ED. Pharmacological evidence of inhibitory and disinhibitory neuronal circuits in dorsal cochlear nucleus. J Neurophysiol. 2000;83:926–940. doi: 10.1152/jn.2000.83.2.926. [DOI] [PubMed] [Google Scholar]
- De Blas AL, Vitorica J, Friedrich P. Localization of the GABAA receptor in the rat brain with a monoclonal antibody to the 57,000 Mr peptide of the GABAA receptor/benzodiazepine receptor/Cl- channel complex. J Neurosci. 1988;8:602–614. doi: 10.1523/JNEUROSCI.08-02-00602.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KA, Young ED. Granule cell activation of complex-spiking neurons in dorsal cochlear nucleus. J Neurosci. 1997;17:6798–6806. doi: 10.1523/JNEUROSCI.17-17-06798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- Dong S, Mulders WHAM, Rodger Jm, Robertson D. Changes in neuronal activity and gene expression in guinea-pig auditory brainstem after unilateral partial hearing loss. Neuroscience. 2009;159:1164–1174. doi: 10.1016/j.neuroscience.2009.01.043. [DOI] [PubMed] [Google Scholar]
- Doucet JR, Ross AT, Gillespie MB, Ryugo DK. Glycine immunoreactivity of multipolar neurons in the ventral cochlear nucleus which project to the dorsal cochlear nucleus. J Comp Neuro. 1999;408:515–531. [PubMed] [Google Scholar]
- Douyard J, Shen L, Huganir RL, Rubio ME. Differential neuronal and glial expression of GluR1 AMPA receptor subunit and the scaffolding proteins SAP97 and 4.1N during rat cerebellar development. J Comp Neurol. 2007;502:141–256. doi: 10.1002/cne.21294. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Flores-Otero J, Davis RL. Synaptic proteins are tonotopically graded in postnatal and adult type I and type II spiral ganglion neurons. J Comp Neurol. 2011;519:1455–1475. doi: 10.1002/cne.22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazula VR, Strumbos JG, Mei X, Chen H, Rahner C, Kaczmarek LK. Localization of Kv1.3 channels in presynaptic terminals of brainstem auditory neurons. J Comp Neurol. 2010;518:3205–3220. doi: 10.1002/cne.22393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenggeli CA, Pongstaporn T, Doucet JR, Ryugo DK. Projections from the spinal trigeminal nucleus to the cochlear nucleus in the rat. J Comp Neurol. 2005;484:191–205. doi: 10.1002/cne.20466. [DOI] [PubMed] [Google Scholar]
- Holt AG, Asako M, Lomax CA, MacDonald JW, Tong L, Lomax MI, Altschuler RA. Deafness-related plasticity in the inferior colliculus: gene expression profiling following removal of peripheral activity. J Neurochem. 2005;93:1069–1086. doi: 10.1111/j.1471-4159.2005.03090.x. [DOI] [PubMed] [Google Scholar]
- Hou Q, Zhang D, Jarzylo L, Huganir RL, Man HY. Homeostatic regulation of AMPA receptor expression at single hippocampal synapses. Proc Natl Acad Sci U S A. 2008;105:775–780. doi: 10.1073/pnas.0706447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Chen S, Tietz Immunocytochemical detection of regional protein changes in rat sections using computer-assisted image analysis. J Histochem Cythochem. 1996;44:981–987. doi: 10.1177/44.9.8773563. [DOI] [PubMed] [Google Scholar]
- Hutson KA, Durham D, Tucci DL. Consequences of unilateral conductive hearing loss: Time dependent regulation of protein synthesis in auditory brainstem nuclei. Hear Res. 2007;233:124–134. doi: 10.1016/j.heares.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Itoh K, Kamiya H, Mitani A, Yasui Y, Takada M, Mizuno N. Direct projections from the dorsal column nuclei and the spinal trigeminal nuclei to the cochlear nuclei in the cat. Brain Res. 1987;400:145–250. doi: 10.1016/0006-8993(87)90662-7. [DOI] [PubMed] [Google Scholar]
- Kasugai Y, Swinny JD, Roberts JD, Dalezios Y, Fukazawa Y, Werner Sieghart W, Shigemoto R, Somogy P. Quantitative localisation of synaptic and extrasynaptic GABAA receptor subunits on hippocampal pyramidal cells by freeze-fracture replica immunolabelling. Eur J Neurosci. 2010;32:1868–1888. doi: 10.1111/j.1460-9568.2010.07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond CP. Quantification of immunohistochemical reactions by radiography in section exposed to 125I-linked antibodies. J Histochem Cytochem. 1979;27:1516–1517. doi: 10.1177/27.11.390042. [DOI] [PubMed] [Google Scholar]
- Lévi S, Schweizer C, Bannai H, Pascual O, Charrier C, Triller A. Homeostatic regulation of synaptic GlyR numbers driven by lateral diffusion. Neuron. 2008;59:261–273. doi: 10.1016/j.neuron.2008.05.030. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Brown MC. Physiology and anatomy of single olivocochlear neurons in the cat. Hear Res. 1986;24:17–36. doi: 10.1016/0378-5955(86)90003-1. [DOI] [PubMed] [Google Scholar]
- Liu SQ, Cull-Candy SG. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature. 2000;405:454–458. doi: 10.1038/35013064. [DOI] [PubMed] [Google Scholar]
- Löhrke S, Friauf E. Developmental distribution of the glutamate receptor subunits KA2, GluR6/7, and delta 1/2 in the rat medial nucleus of the trapezoid body. A quantitative image analysis. Cell Tissue Res. 2002;308:19–33. doi: 10.1007/s00441-002-0533-z. [DOI] [PubMed] [Google Scholar]
- Lüscher B, Keller CA. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol Ther. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. Review. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Matsui CE, Jahr, Rubio ME. High concentration rapid transient of glutamate mediates neuron-glia communication via ectopic release. J Neurosci. 2005;25:7538–7547. doi: 10.1523/JNEUROSCI.1927-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton JW, Kiritani T, Pedersen C, Turner JG, Shepherd GM, Tzounopoulos T. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proc Natl Acad Sci U S A. 2011;108:7601–7606. doi: 10.1073/pnas.1100223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt JC, Holder TM, Wilson MC, Salvi RJ, Caspary DM. GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hear Res. 2000;147:251–260. doi: 10.1016/s0378-5955(00)00135-0. [DOI] [PubMed] [Google Scholar]
- Miralles CP, Li M, Mehta AK, Khan ZU, De Blas AL. Immunocytochemical localization of the beta(3) subunit of the gamma-aminobutyric acid(A) receptor in the rat brain. J Comp Neurol. 1999;413:535–548. [PubMed] [Google Scholar]
- Moore JK, Osen KK, Storm-Mathisen J, Ottersen OP. γ-Aminobutyric acid and glycine in the baboon cochlear nuclei: an immunoctyochemical colocalization study with reference to interspecies differences in inhibitory systems. J. Comp. Neurol. 1996;369:497–519. doi: 10.1002/(SICI)1096-9861(19960610)369:4<497::AID-CNE2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GC, Huganir RL. Activity-dependent modulation of AMPA receptor accumulation. Neuron. 21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycing endosome supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- Osen KK, Ottersen OP, Storm-Mathisen J. Colocalization of glycinelike and GABA-like immunoreactivities: a semiquantitative study of individual neurons in the dorsal cochlear nucleus. In: Ottersen OP, Storm-Mathisen J, editors. Glycine neurotransmission. Chichester, UK: John Wiley & Sons; 1990. pp. 417–451. [Google Scholar]
- Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Zhao HM, Wenthold RJ. Ionotropic and metabotropic glutamate receptors show unique postsynaptic, presynaptic, and glial localizations in the dorsal cochlear nucleus. J Comp Neurol. 1996;372:356–383. doi: 10.1002/(SICI)1096-9861(19960826)372:3<356::AID-CNE3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Mayat E, Wenthold RJ. Glutamate receptor subunit 2-selective antibody shows a differential distribution of calcium-impermeable AMPA receptors among populations of neurons. J Comp Neurol. 1997;385:456–476. doi: 10.1002/(sici)1096-9861(19970901)385:3<456::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Portfors CV, Roberts PD. Temporal and frequency characteristics of cartwheel cells in the dorsal cochlear nucleus. J Neurophysiol. 2007;98:744–756. doi: 10.1152/jn.01356.2006. [DOI] [PubMed] [Google Scholar]
- Potash M, Kelly J. Development of directional responses to sounds in the rat (Rattus norvegicus) J Comp Physiol Psychol. 1980;94:864–877. doi: 10.1037/h0077819. [DOI] [PubMed] [Google Scholar]
- Potashner SJ, Suneja SK, Benson CG. Altered glycinergic synaptic activities in guinea pig brain stem auditory nuclei after unilateral cochlear ablation. Hear Res. 2000;147:125–136. doi: 10.1016/s0378-5955(00)00126-x. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Wenthold RJ. Glutamate receptors are selectively targeted to postsynaptic sites in neurons. Neuron. 1997;18:939–950. doi: 10.1016/s0896-6273(00)80333-5. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Wenthold RJ. Calnexin and the immunoglobulin binding protein (BiP) coimmunoprecipitate with AMPA receptors. J Neurochem. 1999;73:942–948. doi: 10.1046/j.1471-4159.1999.0730942.x. [DOI] [PubMed] [Google Scholar]
- Rubio ME. Redistribution of synaptic AMPA receptors at glutamatergic synapses in the dorsal cochlear nucleus as an early response to cochlear ablation in rats. Hear Res. 2006;216–217:154–167. doi: 10.1016/j.heares.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Gudsnuk KA, Smith Y, Ryugo DK. Revealing the molecular layer of the primate dorsal cochlear nucleus. Neuroscience. 2008;154:99–113. doi: 10.1016/j.neuroscience.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryugo DK, Pongstaporn T, Wright DD, Sharp AH. Inositol1,4,5-triphosphate receptors: immunocytochemical localization in the dorsal cochlear nucleus. J Comp Neurol. 1995;358:102–118. doi: 10.1002/cne.903580107. [DOI] [PubMed] [Google Scholar]
- Savtchouk I, Liu SJ. Remodeling of synaptic AMPA receptor subtype alters the probability and pattern of action potential firing. J Neurosci. 2011;31:501–511. doi: 10.1523/JNEUROSCI.2608-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield BR, Coomes DL. Auditory cortical projections to the cochlear nucleus in guinea pigs. Hear Res. 2005;199:89–102. doi: 10.1016/j.heares.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Shore SE, Helfert RH, Bledsoe SC, Jr, Altschuler RA, Godfrey DA. Descending projections to the dorsal and ventral divisions of the cochlear nucleus in guinea pig. Hear Res. 1991;52:255–268. doi: 10.1016/0378-5955(91)90205-n. [DOI] [PubMed] [Google Scholar]
- Shore SE, Sumner CJ, Bledsoe SC, Lu J. Effects of contralateral sound stimulation on unit activity of ventral cochlear nucleus neurons. Exp Brain Res. 2003;153:427–435. doi: 10.1007/s00221-003-1610-6. [DOI] [PubMed] [Google Scholar]
- Spangler KM, Cant NB, Henkel CK, Farley GR, Warr WB. Descending projections from the superior olivary complex to the cochlear nucleus of the cat. J Comp Neurol. 1987;259:452–465. doi: 10.1002/cne.902590311. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Takagi H, Richards JG, Mohler H. Subcellular localization of benzodiazepine/ GABAA receptors in the cerebellum of rat, cat, and monkey using monoclonal antibodies. J Neurosci. 1989;9:2197–2209. doi: 10.1523/JNEUROSCI.09-06-02197.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner CJ, Tucci DL, Shore SE. Responses of the ventral cochlear nucleus to contralateral sound following conductive hearing loss. J Neurophysiol. 2005;94:4234–4243. doi: 10.1152/jn.00401.2005. [DOI] [PubMed] [Google Scholar]
- Tucci DL, Cant NB, Durham D. Conductive hearing loss results in a decrease in central auditory system activity in the young gerbil. Laryngoscope. 1999;109:1359–1371. doi: 10.1097/00005537-199909000-00001. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. Review. [DOI] [PubMed] [Google Scholar]
- Vale C, Sanes DH. The effect of bilateral deafness on excitatory and inhibitory synaptic strength in the inferior colliculus. Eur J Neurosci. 2002;16:2394–2404. doi: 10.1046/j.1460-9568.2002.02302.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience. 2009;164:747–759. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren EH, 3rd, Liberman MC. Effects of contralateral sound on auditory-nerve responses. I. Contributions of cochlear efferents. Hear Res. 1989a;37:89–104. doi: 10.1016/0378-5955(89)90032-4. [DOI] [PubMed] [Google Scholar]
- Warren EH, 3rd, Liberman MC. Effects of contralateral sound on auditory-nerve responses. II. Dependence on stimulus variables. Hear Res. 1989b;37:105–121. doi: 10.1016/0378-5955(89)90033-6. [DOI] [PubMed] [Google Scholar]
- Weedman DL, Ryugo DK. Pyramidal cells in primary auditory cortex project to cochlear nucleus in rat. Brain Res. 1996;706:97–102. doi: 10.1016/0006-8993(95)01201-x. [DOI] [PubMed] [Google Scholar]
- Weinberg RJ, Rustioni A. A cuneocochlear pathway in the rat. Neuroscience. 1987;20:209–219. doi: 10.1016/0306-4522(87)90013-3. [DOI] [PubMed] [Google Scholar]
- Whiting B, Moiseff A, Rubio ME. Cochlear nucleus neurons redistribute synaptic AMPA and glycine receptors in response to monaural conductive hearing loss. Neuroscience. 2009;163:1264–1276. doi: 10.1016/j.neuroscience.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DD, Ryugo DK. Mossy fiber projections from the cuneate nucleus to the cochlear nucleus in the rat. J Comp Neurol. 1996;365:159–172. doi: 10.1002/(SICI)1096-9861(19960129)365:1<159::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Xu H, Kotak VC, Sanes DH. Conductive hearing loss disrupts synaptic and spike adaptation in developing auditory cortex. J Neurosci. 2007;27:9417–9426. doi: 10.1523/JNEUROSCI.1992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Shore S. Projections from the trigeminal nuclear complex to the cochlear nuclei: a retrograde and anterograde tracing study in the guinea pig. J Neurosci Res. 2004;78:901–907. doi: 10.1002/jnr.20343. [DOI] [PubMed] [Google Scholar]