Abstract
We recently reported that daily dietary supplementation with 100 μg selenium (a dose exceeding a rat’s nutritional requirement by about 33-fold) initiated immediately after total-body irradiation (TBI) and maintained for 21 weeks mitigates radiation nephropathy in a rat model as indicated by blood urea nitrogen (BUN) levels and histopathological criteria (Radiat Res. 2009; 17:368–73). In this follow-up study, we explored the risks and benefits of delaying the onset of supplementation, shortening periods of supplementation, and escalating selenium supplementation beyond 100 μg/day. Supplementation with 200 μg selenium/day (as selenite or seleno-L-methionine) substantially improved the mitigation of radiation nephropathy by lowering BUN levels at 4 months after TBI from 115 to as low as 34 mg/dl and by proportionally lowering the incidence of histopathological abnormalities. Shortening the period of supplementation to 3 or 2 months did not compromise efficacy. Delaying the onset of supplementation for 1 week reduced but did not abrogate the mitigation of radiation nephropathy. Supplementation with 300 μg/day mitigated radiation nephropathy less effectively than 200 μg and was poorly tolerated. Rats that had been given 10 Gy of TBI were less tolerant of high-dose selenium than nonirradiated rats. This reduced tolerance of high-dose selenium would need to be taken into consideration when selenium is used for the mitigation of radiation injury in victims of nuclear accidents or acts of radiological terrorism. The high dose requirements, the pronounced threshold effect, and the superior performance of selenite suggest that the mitigation of radiation nephropathy involves mechanisms that go beyond the induction of selenoproteins.
INTRODUCTION
Medical countermeasures for nuclear accidents or acts of radiological terrorism require agents that are safe and easy to administer, do not compromise the performance of first responders, and are effective when given after radiation exposure. Agents that remain effective even when administered with a delay of several days are most desirable, because delays have to be expected in the delivery of disaster relief.
We recently reported that dietary supplementation with high-dose selenium in the form of sodium selenite or seleno-L-methionine initiated immediately after total-body irradiation (TBI; 10 Gy, single dose) and maintained for 21 weeks reduces radiation injury to kidneys in a rat model (1). Irradiated rats on selenium-supplemented drinking water had lower blood urea nitrogen (BUN) levels and showed fewer histopathological abnormalities than age-matched irradiated rats on standard drinking water. In the original study, selenium supplementation was used at doses of 50 or 100 μg/day, was initiated 2–4 h after radiation exposure, and was maintained for the duration of the experiment (about 21 weeks). Of the tested doses, only the higher one (100 μg/day; a 33-fold excess over the rat’s nutritional requirement for selenium) had a mitigating effect on radiation nephropathy. Both selenium species, sodium selenite and seleno-L-methionine, appeared to be about equally effective and equally well tolerated by the rats. Doses in excess of 100 μg/day were tried but were rejected by the rats.
The present report is a follow-up to the original pilot study. It explores whether rats can be trained to accept drinking water designed to deliver in excess of 100 μg selenium per day, whether such dose escalations improve the mitigating effect of selenium on radiation nephropathy, and whether periods of selenium supplementation can be shortened or initiated with a 1-week delay without compromising mitigation.
In the context of this paper, the term “mitigation” refers to interventions that are initiated after radiation exposure but before the onset of clinical symptoms (2). “Prevention” and “prophylaxis” refer to interventions that are initiated before or during radiation exposure, and “treatment” refers to interventions that are initiated after the onset of clinical symptoms.
MATERIALS AND METHODS
WAG/Rij/Cmcr rats were bred and housed in a moderate-security barrier inside the central animal facility of the Medical College of Wisconsin. Animals were free of Mycoplasma pulmonis, Pseudomonas and common rat viruses. Chow (8604 Teklad, Harlan Laboratories, Madison, WI) and drinking water were provided ad libitum. Drinking water was dispensed from bottles rather than a centralized watering system to facilitate the daily monitoring of water consumption. The selenium content of the rat chow was 0.33 mg/kg and thus provided about 6.6 μg of dietary selenium per rat per day (corresponding to about 220% of a rat’s RDA for selenium). The animal facility is fully accredited by the American Association for the Accreditation of Laboratory Animal Care, and all animal experiments were conducted under protocols approved by the Institutional Animal Care and Use Committee.
Male rats (7–9 weeks old) with a body weight of 176 ± 2 g (arithmetic mean ± SE) at the time of irradiation were given 10 Gy (single fraction) of total-body irradiation (TBI) delivered by a X-Rad 320 orthovoltage X-ray machine (Precision X-Ray, North Branford, CT) at a rate of 1.73 Gy/min. The machine was operated at 320 kVp with a half-value layer (HVL) of 1.4 mm copper. For additional dosimetry details, see Cohen et al. (3). Since the LD50 radiation dose for WAG/Rji/Cmcr rats is 6.3 Gy (at 30 days) without a bone marrow transplant and 13.4 Gy (at 7 days) with a bone marrow transplant, all irradiated rats were given syngeneic bone marrow transplants to rescue the animals from otherwise lethal damage to the hematopoietic system (4, 5). Bone marrow was obtained from CO2-asphyxiated syngeneic donor rats. Femurs were stripped of associated soft tissue, and bone marrow plugs were expelled with minimal essential medium supplemented with 10% fetal bovine serum using a 6-ml syringe with an 18-gauge needle. Each femur was flushed two or three times, and the bone marrow was dispersed into single cells. Approximately 6 × 107 nucleated marrow cells were injected via tail vein into each irradiated rat in 0.3–0.4 ml of culture medium (4, 5). Unless indicated otherwise, rats were randomized to standard or selenium-supplemented drinking water 2–4 h after TBI, and selenium supplementation was maintained for 4 months. Rats were housed in groups of 3 (sometimes including one group of 4 or 2 when the total number of animals in a treatment or control arm was not a multiple of 3) identically treated animals for the duration of the experiment unless members of a group died or had to be euthanized before the scheduled completion of the experiment.
Selenium Supplements
Sodium selenite was purchased from Sigma (St. Louis, MO) and seleno-L-methionine from Sigma or Acros Organics (Geel, Belgium). Supplements were dissolved in distilled water and sterilized by filtration through 0.2-μm membrane filters. Dose calculations were based on the assumption that the average fluid intake was 20 ml per day. To facilitate comparisons between different selenium species, this paper expresses all selenium doses as amounts of atomic selenium. Thus, when the target dose was 200 μg of (atomic) selenium per day, the concentrations of sodium selenite (formula weight: 174.94) and selenomethionine (formula weight: 196.11) in supplemented drinking water were 21.9 μg/ml and 24.8 μg/ml, respectively.
Rats readily accepted supplemented drinking water designed to deliver 100 μg selenium/day. Training was required to get them accustomed to drinking water delivering higher doses. To this end, the daily dose of selenium delivered via drinking water was increased in 50-μg increments every second day until the target dose was reached. During training, the balance of the target dose was delivered by oral gavage (1 dose per day in a volume of ≤1 ml). In other words, if the target dose was 200 μg/day, rats received 100 μg via drinking water and 100 μg by gavage on days 1 and 2, 150 μg via drinking water and 50 μg by gavage on days 3 and 4, and 200 μg via drinking water on day 5 and subsequent days. The fluid intake of individual rats may have varied somewhat as a function of the rat’s rank within the social hierarchy of caged animals, the stage of their hematopoietic recovery after TBI, or the presence/severity of radiation nephropathy.
Histopathological Analyses
Unless indicated otherwise, 4 months after TBI, kidneys were harvested from irradiated rats and age-matched controls, fixed in zinc-formalin, and embedded in paraffin. Four-micrometer sections were prepared and stained with hematoxylin and eosin. Slides were examined with a light microscope at total magnifications of 200× and 400×. The quantitative scoring of histopathological changes was done in a masked fashion by one of the coauthors (EPC) as described previously (1, 6). In brief, each specimen was assigned a total numerical score between 0 and 10 based on the cumulative scores of four types of histopathological changes according to the scheme shown in Table 1.
TABLE 1.
Histological Scoring System
| Abnormality | Score |
|---|---|
| Cysts | |
| none | 0 |
| microscopic | 1 |
| macroscopic | 2 |
| Sclerosed glomeruli (20 glomeruli analyzed per slide) | |
| none | 0 |
| 1–2 | 1 |
| 3–4 | 2 |
| ≥5 | 3 |
| Interstitial fibrosis | |
| none | 0 |
| scattered | 1 |
| diffuse | 2 |
| Glomerular mesangiolysis | |
| absent | 0 |
| variably present | 1 |
| present in most glomeruli | 2 |
| present in all glomeruli | 3 |
Chemical and Biochemical Analyses
Blood was obtained from the retro-orbital venous plexus under flurane anesthesia and assayed for blood urea nitrogen (BUN) levels by the enzymatic colorimetric Bertholet method using a commercial assay kit (Sterling Diagnostics, Sterling Heights, MI) according to the manufacturer’s instructions. Serum selenium levels were determined by inductively coupled plasma-mass spectroscopy (ICP-MS; Huffman Laboratories, Golden, CO) after refluxing with nitric and perchloric acid. Serum alanine aminotransferase (ALT) and serum aspartate aminotransferase (AST) were assayed by Dynacare Laboratories (Milwaukee, WI).
Statistical Analyses
Statistical analyses were performed using the StatView (Abacus Concepts, Berkeley, CA) and Prism (GraphPad, La Jolla, CA) software packages.
RESULTS
Training of Rats
To determine whether rats could be trained to accept drinking water designed to deliver in excess of 100 μg selenium per day, 4 nonirradiated rats were placed on drinking water that was supplemented with selenium in the form of sodium selenite (2 rats) or seleno-L-methionine (2 rats). The starting dose was 100 μg selenium/day. Selenium doses were increased in 50-μg increments every second day until a daily dose of 500 μg (upper limit imposed by animal use protocol) was reached. The rats were subsequently maintained on 500 μg selenium/day for the remainder of the study. One rat on selenite-supplemented drinking water was euthanized after 49 days of supplementation because it suddenly refused food and water and stopped grooming itself. The remaining 3 rats remained alive and healthy until the experiment was terminated after 240 days of selenium supplementation.
Kidney Function
BUN levels in nonirradiated age-matched controls remained in the range of 20–24 mg/dl for the duration of the experiment (Fig. 1a, b). Irradiated rats on standard drinking water showed moderately elevated BUN levels 2 months after TBI. Four months after TBI, they were in severe renal failure (BUN ≥120 mg/dl) or were approaching severe renal failure (Fig. 1a, b). Supplementation of drinking water with selenium at 150 or 200 μg/day reduced BUN levels in irradiated rats. At 2 months after TBI, all irradiated rats on selenium-supplemented drinking water had significantly lower BUN values than irradiated rats on standard drinking water (P ≤ 0.05, Games-Howell ANOVA post hoc test). Even the small dose escalation from 150 to 200 μg/day resulted in a significant additional reduction of BUN values, suggesting that we were operating in a steep range of the dose–effect curve. At 4 months after TBI, with the exception of irradiated rats on 150 μg selenium/day, all irradiated rats on selenium-supplemented drinking water had significantly lower BUN levels than irradiated rats on standard drinking water (P ≤ 0.05, Games-Howell ANOVA post hoc test). In fact, BUN levels in irradiated rats on selenite-supplemented drinking water (200 μg selenium/day) were not significantly different from the BUN levels of normal controls.
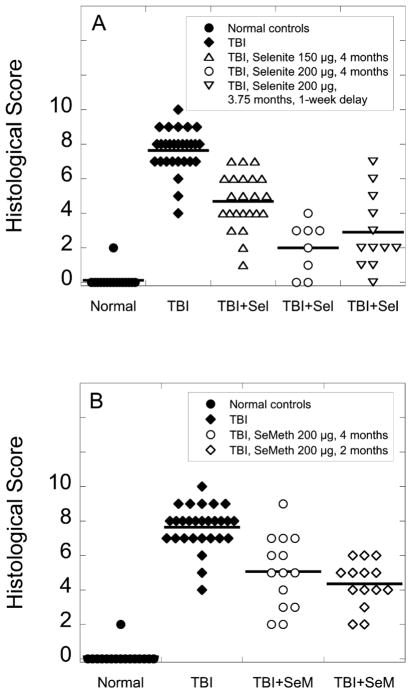
FIG. 1.
Blood urea nitrogen (BUN) levels in age-matched nonirradiated control rats on standard drinking water (panels A and B), irradiated (10 Gy, TBI) rats on standard drinking water (panels A and B), and irradiated (10 Gy, TBI) rats on drinking water that was supplemented with sodium selenite (150 or 200 μg selenium/day; panel A) or seleno-L-methionine (200 μg selenium/day; panel B). Supplementation was initiated 2–4 h or 1 week after TBI and was maintained for 2–4 months. Data are geometric means ± SE and are expressed as geometric rather than arithmetic means because physiological parameters in groups showing abnormal kidney function are log-normally distributed (6). Sample sizes were as follows. Normal controls: 15 at 2 and 4 months, 7 at 5 months, 3 at 6 months. TBI: 24 at 2 and 4 months. TBI, selenite, 150 μg: 11 at 2 and 4 months. TBI, selenite, 200 μg, 4 months: 6 at 2 months, 4 at 4 months. TBI, selenite, 200 μg, 3 months: 4 at 2, 4, 5, and 6 months. TBI, selenite, 200 μg, 3.75 months: 6 at 2 and 4 months. TBI, seleno-L-methionine, 200 μg, 4 months: 14 at 2 and 4 months, 7 at 5 months, 6 at 6 months. TBI, seleno-L-methionine, 200 μg, 2 months: 7 at 2 and 4 months. Most sample sizes dropped off after 4 months because animals were euthanized at 4 months to harvest tissue for histological analyses.
When used at 200 μg selenium/day, sodium selenite (Fig. 1a) was superior to seleno-L-methionine (Fig. 1b). For example, after 4 months of supplementation, sodium selenite had reduced BUN levels from 115 ± 16 mg/dl (geometric mean ± SE) to 34 ± 5 mg/dl whereas seleno-L-methionine had reduced them to 68 ± 4 mg/dl (significant difference, P ≤ 0.05, Games-Howell ANOVA post hoc test).
Shortening the duration of selenium supplementation to 3 months (sodium selenite; Fig. 1a) or 2 months (seleno-L-methionine; Fig. 1b) did not compromise the mitigating effect on radiation nephropathy. Periods of supplementation of less than 2 months were not tried. Initiating selenite supplementation with a 1-week delay reduced but did not abrogate mitigation (Fig. 1a). Taken together, these results suggested that the window during which radiation nephropathy is amenable to mitigation by selenium is relatively short and begins soon after radiation exposure.
Doses in excess of 200 μg/day were not pursued in detail because pilot experiments showed that increasing supplementation to 300 μg selenium/day was counterproductive in the sense that 4 months after TBI, irradiated rats on 300 μg selenium/day had substantially higher BUN values (106 ±40 mg/dl in the selenite group and 100 ±22 mg/dl in the seleno-L-methionine group) than irradiated rats on 200 μg selenium/day. Furthermore, several rats (see details below) had to be euthanized early during the course of supplementation because they refused food and water and stopped grooming themselves.
Kidney Histology
Semiquantitative histopathological analyses of kidney sections prepared 4 months after TBI confirmed all trends revealed by BUN monitoring (Fig. 2a, b). They confirmed the beneficial effect of escalating the selenium dose from 100 to 200 μg/day, and they confirmed that selenium in the form of selenite was more effective than selenium in the form of seleno-L-methionine. The coefficient of correlation for BUN values and histology scores was 0.83 (P < 0.0001, Spearman).
FIG. 2.
Distribution of histological scores for kidneys from age-matched nonirradiated control rats on standard drinking water (panels A and B), irradiated (10 Gy, TBI) rats on standard drinking water (panels A and B), irradiated (10 Gy, TBI) rats on drinking water that was supplemented with sodium selenite (Sel, 150 or 200 μg Se/day; panel A) or seleno-L-methionine (SeM, 200 μg Se/day; panel B). Supplementation was initiated 2–4 h after TBI and was maintained for 2–4 months as indicated. Horizontal bars indicate arithmetic means. Sample sizes were 16 for normal controls, 28 for irradiated rats on standard drinking water, 23 for irradiated rats on selenite (150 μg, 4 months), 8 for irradiated rats on selenite (200 μg, 4 months), 12 for irradiated rats on selenite (200 μg, 3.75 months) and 14 for irradiated rats on seleno-L-methionine (200 μg, 2 or 4 months). All irradiated animals on selenium-supplemented drinking water had lower histology scores than irradiated rats on standard drinking water. All reductions of scores were statistically significant (P < 0.05, Dunn’s multiple comparison test) with the exception of the scores for irradiated rats on seleno-L-methionine-supplemented drinking water.
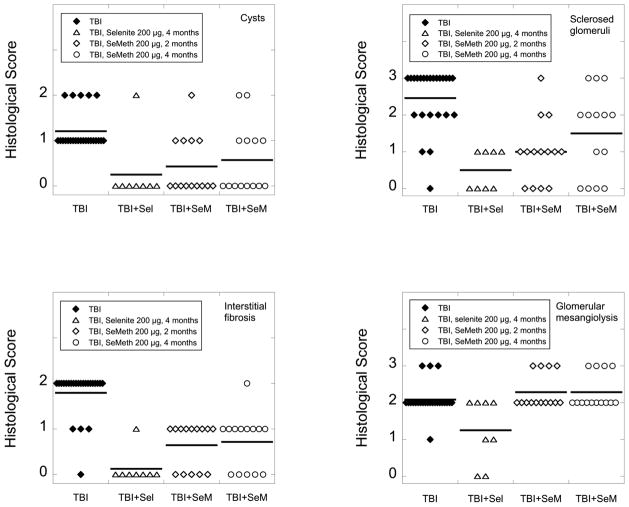
Each of the four histopathological abnormalities was mitigated more effectively by selenite than by seleno-L-methionine (Fig. 3). Direct comparisons between the mitigation of individual abnormalities are, of course, somewhat problematic because a different set of criteria is used to quantify each abnormality. However, it is worth noting that only 1 of 8 irradiated animals on selenite-supplemented drinking water showed evidence of cysts or interstitial fibrosis, whereas kidneys from the remaining 7 animals were scored as “normal” as far as cysts and interstitial fibrosis were concerned. By contrast, 6 of 8 irradiated animals continued to show evidence of glomerular mesangiolysis despite identical levels of selenium supplementation. This suggested that radiation-induced glomerular mesangiolysis was less amenable to mitigation by selenite than radiation-induced cysts and interstitial fibrosis. Furthermore, under the chosen experimental conditions, only selenite had a mitigating effect on glomerular mesangiolysis (Fig. 3). Seleno-L-methionine was ineffective.
FIG. 3.
Effect of high-dose selenium on the incidence of cysts, sclerosed glomeruli, interstitial fibrosis, and glomerular mesangiolysis in the kidneys of irradiated (10 Gy, TBI) rats. The histological scoring was performed 4 months after TBI as described in the Materials and Methods section. Horizontal bars indicate arithmetic means of histological scores. Sample sizes were 24 for irradiated rats on standard drinking water, 8 for irradiated rats on selenite (Sel), and 14 for irradiated rats on seleno-L-methionine (SeM). Selenite reduced the incidence of all four types of histopathological abnormalities and reduced them more effectively than seleno-L-methionine. Seleno-L-methionine mitigated only cysts, sclerosed glomeruli and interstitial fibrosis. It had no effect on glomerular mesangiolysis.
Serum Selenium Levels
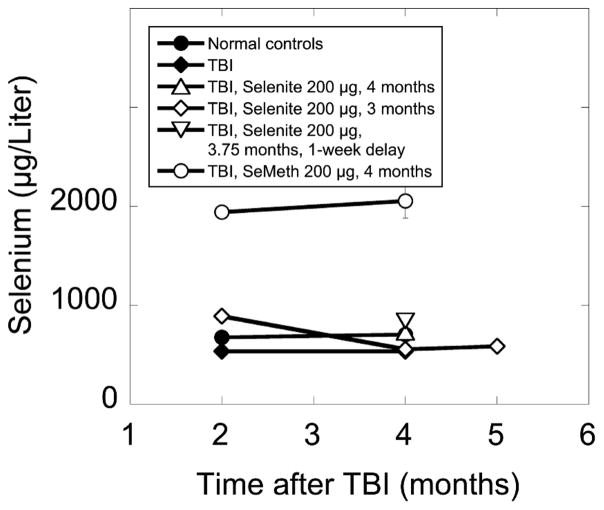
Blood and serum selenium levels do not accurately predict tissue selenium levels, but they often offer the only practical way to monitor short-term clinical applications of selenium-based interventions. Serum selenium levels were therefore determined in randomly selected animals at 2, 4 and 5 months after TBI (Fig. 4). In normal controls, which had a daily selenium intake of about 6.6 μg, average serum selenium levels were 676 ± 24 and 704 ± 23 μg/liter (arithmetic means ± SE) at 2 and 4 months after TBI, respectively. In irradiated animals on standard drinking water, serum selenium levels were significantly lower (P ≤ 0.05, Games-Howell ANOVA post hoc test) with 534 ± 9 and 536 ± 22 μg/liter at 2 and 4 months after TBI, respectively. There was no trend toward a normalization of serum selenium levels in irradiated animals during the period of observation (Fig. 4).
FIG. 4.
Serum selenium levels in age-matched nonirradiated control rats on standard drinking water, irradiated (10 Gy, TBI) rats on standard drinking water, and irradiated (10 Gy, TBI) rats on drinking water that was supplemented with sodium selenite or seleno-L-methionine (200 μg selenium/day). Supplementation was initiated 2–4 h or 1 week after TBI and was maintained for 2–4 months. Four-month data points for irradiated animals on selenite show arithmetic means of 2 samples ± SE. All other data points show arithmetic means of 5 samples ± SE. Most SE are smaller than the data symbols.
Supplementing the drinking water of irradiated rats with selenium (200 μg/day) in the form of sodium selenite increased the dietary selenium supply about 31-fold but increased serum selenium levels by only 51% (Fig. 4). This modest increase was expected, because selenium excretion is increased under conditions of high intake (7, 8). When the same amount of supplemental selenium was administered as seleno-L-methionine, serum selenium levels in irradiated rats increased 273% (Fig. 4), an increase consistent with the greater bioavailability of seleno-L-methionine and the non-specific incorporation of seleno-L-methionine into proteins in place of L-methionine. When supplementation was discontinued, serum selenium levels rapidly returned to the subnormal levels seen in irradiated rats on standard drinking water (Fig. 4).
Toxicity
None of the rats on 200 or 300 μg selenium/day showed “classic” signs of selenium toxicity such as diarrhea, hair loss or neurological symptoms. However, 5 of 20 rats receiving 200 μg selenium/day as sodium selenite had to be euthanized under the approved animal use protocol after 10–117 days (median: 32 days) of supplementation because they suddenly refused food and water and stopped grooming themselves. One of 21 rats receiving 200 μg selenium/day as seleno-L-methionine had to be euthanized for the same reason after 146 days of supplementation. The terminal BUN test of the latter animal showed a BUN level of 141 mg/dl whereas other (healthy) animals of the same group had 5-month BUN levels between 51 and 94 mg/dl (median: 86 mg/dl). When the blood sample of the euthanized rat was centrifuged in preparation for the BUN test, the volume of packed red cells was much smaller than expected, and the color of the serum fraction was orange/red rather than pale yellow, suggesting hemolysis. Furthermore, the rat’s liver and kidneys appeared pale on gross examination. Taken together, this indicated that the rat had hemolytic anemia.
Of 7 irradiated rats receiving 300 μg selenium/day as sodium selenite, 4 had to be euthanized after 17–21 days (median: 19 days) of supplementation because of inadequate water and food intake. One of the 4 rats was given to a pathology laboratory (Charles River, Wilmington, MA) for a comprehensive necropsy. The necropsy showed no evidence of toxicity or infection. The only abnormal findings were dehydration, bone marrow and lymphoid depletion consistent with recent TBI, and minimal neutrophilic and eosinophilic mucosal infiltrates in the stomach. No gastric ulcers were noted by gross or microscopic examination. Lung, heart, liver and kidneys were within normal limits.
Of the 7 irradiated rats receiving 300 μg selenium/day as seleno-L-methionine, one was found dead after 97 days of supplementation, and 4 had to be euthanized because they refused food and water and stopped grooming themselves after 110–173 days (median: 132 days) of supplementation. Three of these 4 rats were in severe renal failure with BUN values in excess of 120 mg/dl at the time of euthanasia. One additional rat on the 300 μg selenium/day (as seleno-L-methionine) regimen had to be euthanized after 86 days of supplementation because BUN levels had reached 117 mg/dl after 2 months of supplementation and 197 mg/dl 2 weeks later. The volume of packed red cells in the terminal blood sample of the latter animal was much smaller than expected, indicating that the animal was severely anemic.
One small group of nonirradiated rats was kept on 200 μg selenium/day as selenite (5 animals) or seleno-L-methionine (6 animals) for 225 days without incident. No animals died or had to be euthanized, and all animals maintained normal BUN levels for the duration of the experiment.
Two of 26 irradiated rats on standard drinking water suddenly refused food and water and had to be euthanized 132 day after TBI while the analysis of their 4-month BUN tests was in progress. Both animals turned out to be in severe renal failure with BUN values of 220 and 249 mg/dl, respectively.
While renal failure and anemia offered plausible explanations for a rat’s refusal of food and water, refusals during the first few weeks of supplementation were more difficult to explain. We considered the possibility of a selenium dosing error and therefore submitted aliquots of the suspect supplemented drinking water to an analytical laboratory for quantitative selenium analyses. All samples turned out to contain the correct concentration of selenium.
Since the liver is a major site of selenium metabolism and therefore a potential target of selenium toxicity, selected animals were tested for serum transaminase levels 4 months after TBI. Irradiated rats on standard drinking water showed the expected (9–11) drop of ALT levels from 39.4 ± 3.5 (units/liter) to 9.6 ±2.2 and of AST levels from 127.8 ± 17.8 to 67.0 ± 4.0 (arithmetic means ± SE) (Fig. 5) as well as a reduction of the ALT/AST ratio (10) from 0.31 to 0.14. Supplementation with seleno-L-methionine (200 μg selenium/day) had no significant effect on ALT (8.3 ± 2.4) or AST (55.3 ± 5.5) levels and left the ALT/AST ratio virtually unchanged at 0.15. Supplementation with selenite (200 μg selenium/day), however, raised ALT and AST levels to 26.5 ± 1.3 and 79.5 ± 4.1, respectively, and restored a normal ALT/AST ratio of 0.33. Serum ALT levels in irradiated rats on selenite-supplemented drinking water were significantly higher than ALT levels in irradiated rats on standard drinking water and were not significantly different from ALT levels in normal controls (P ≤ 0.05, Games-Howell ANOVA post hoc test). Taken together these data indicate that neither selenite nor seleno-L-methionine exacerbated radiation-induced liver damage and that one of the supplements – selenite – had a mitigating effect on radiation-induced liver damage.
FIG. 5.
Serum alanine aminotransferase (panel A) and aspartate aminotransferase (panel B) levels in age-matched nonirradiated control rats on standard drinking water, irradiated (10 Gy, TBI) rats on standard drinking water, and irradiated (10 Gy, TBI) rats on drinking water that was supplemented with sodium selenite (Sel) or seleno-L-methionine (SeM) (200 μg selenium/day). Samples were taken 4 months after TBI. Horizontal bars indicate arithmetic means of 4–7 samples.
Body weights were not monitored during this study. However, another study that examined the effects of long-term supplementation with selenium (200 μg/day) in locally (whole-brain) irradiated rats of the same strain has shown that rats on selenite or seleno-L-methionine gained 37% or 10% less weight, respectively, than rats on standard drinking water (Sieber F, Muir SA, unpublished). The study was not designed to determine the effects of selenium supplementation on hematopoietic reconstitution after TBI. However, no animals were lost to infections or hemorrhages, and at 2 months, all surviving animals appeared to have normal hematocrits, including those that later developed anemia. This argued against selenium supplementation having major adverse effects on engraftment.
DISCUSSION
This project was part of a larger effort to develop medical countermeasures against the type of radiation injury that might be sustained during a nuclear accident or an act of radiological terrorism. This explains why selenium was used in a mitigating rather than a prophylactic mode and why radiation was administered as a single dose rather than the fractionated doses typically used in radiation therapy.
The pathobiology of late normal-tissue effects of radiation is not well understood. If one accepts the premise that late effects may be at least in part the result of chronic oxidative stress and/or chronic inflammation (12), selenium has considerable appeal as a potential mitigating agent, because this trace mineral has anti-inflammatory activity and also plays a key role in selenoprotein-mediated antioxidant defenses (13). Furthermore, selenium has already proven beneficial in the treatment of severe burns and trauma (14, 15), which makes it an attractive candidate for the mitigation/treatment of the type of combined injuries that might be expected in nuclear accidents or acts of radiological terrorism.
The results of this study show that escalating dietary selenium supplementation from the previously used 100 μg/day to 200 μg/day substantially improves the mitigating effect of selenium on radiation nephropathy as indicated by improved kidney function and a lower incidence of histopathological abnormalities. The mechanism by which selenium mitigates radiation nephropathy is not yet understood. Selenium is an integral part of selenoproteins, several of which are glutathione peroxidases or thioredoxin reductases that play key roles in antioxidant defense mechanisms (16). However, several lines of evidence suggest that the mitigation of radiation nephropathy we observed in this study involved processes that went beyond the induction of selenoproteins and beyond the reduction of oxidative stress.
For example, a recent review by Sunde (17) shows that to the extent that rat selenoproteins have been characterized, a dietary selenium content of about 0.1 μg/g is sufficient to maximally induce enzymatic activities as well as mRNA levels. The selenium content of the chow used for our study was 0.33 μg/g. Therefore, antioxidant selenoproteins were most likely maximally induced in our rats even before the introduction of selenium-supplemented drinking water. It is possible, of course, that among the selenoproteins that have not yet been fully characterized, there are antioxidant selenoproteins with higher selenium requirements. However, it is unlikely that their dietary selenium intake requirements would exceed those of known selenoproteins by 2 orders of magnitude.
Subnormal serum selenium levels have been reported in patients who receive TBI as part of the conditioning regimen for hematopoietic stem cell transplants, and the argument has been made that patients who undergo TBI may have higher selenium intake requirements than healthy individuals (18). There is also an ongoing debate whether low serum selenium levels after radiation therapy are indeed the result of radiation injury or if they are a consequence of prior chemotherapy, pre-existing nutritional deficiencies, or the presence of a tumor (18–20). Our study clearly shows that TBI (in the absence of a tumor, pre-existing nutritional deficiencies, or prior chemotherapy) is sufficient to cause a long-lasting reduction of serum selenium levels. However, the reduction is modest and does not offer a plausible explanation for the large doses of selenium required to successfully mitigate radiation nephropathy.
Our experiments were not designed to distinguish between subnormal serum selenium levels as a result of radiation damage to major sites of selenium metabolism (e.g. liver and kidney) and subnormal selenium levels as a result of reduced selenium uptake. As judged by histological criteria, the gastrointestinal tract of the rat recovers quickly after 10 Gy TBI (1). However, normal histology does not rule out radiation-induced functional impairments that interfere with the absorption of dietary selenium. The fact that irradiated rats tend to drink more and eat less than nonirradiated controls may also have contributed to lower serum selenium levels after TBI.
Both seleno-L-methionine and sodium selenite can induce selenoproteins, but the chemical and physiological characteristics of the two selenium species are quite different [reviewed in ref. (21)]. Selenite is absorbed by passive diffusion, is quite reactive, can act as an oxidizing agent (thereby potentially exacerbating oxidative stress), and engages in redox cycling and redox signaling. Seleno-L-methionine, on the other hand, is absorbed by active transport, is more bioavailable than selenite (which gives seleno-L-methionine an advantage with regard to the induction of selenoproteins), and has antioxidant properties. Seleno-L-methionine does not engage in redox cycling, but one of its metabolites –methylselenol – does. Thus, if the mitigation of radiation nephropathy in our model had been a matter of reducing oxidative stress either by scavenging radicals or by inducing antioxidant selenoproteins, supplementation with seleno-L-methionine should have been more effective than supplementation with selenite. If, on the other hand, the mitigation of radiation injury involved redox signaling, selenite would be expected to be more effective. It is possible that the complete lack of a mitigating effect of seleno-L-methionine (but not selenite) on radiation-induced glomerular mesangiolysis reflects a qualitative difference between the mitigating mechanisms of the two selenium species. However, other explanations cannot be ruled out at this point.
Selenium doses used for this study were higher than those used typically for the correction of nutritional deficiencies or the prophylaxis against radiation injury (22–24). They were in a range known to cause some chronic toxicity in rats. While we did not notice classic signs of selenium toxicity such as hair loss, diarrhea or neurological problems, several animals had to be euthanized because they had stopped feeding and grooming themselves. When this happened after prolonged periods of supplementation, the affected animals usually had very poor kidney function and sometimes also severe anemia. Anemia is common in advanced radiation nephropathy (25), and hemolytic anemia is one of the known manifestations of chronic selenium toxicity (26). Both renal failure and anemia can cause lethargy and loss of appetite, which may explain why the animals refused food and water. Refusals of food and water during the early phases of supplementation were more difficult to explain, because these animals appeared to be healthy. However, it is interesting to note in this context that in one trial of high-dose selenium in cattle, one of 17 steers refused selenium-supplemented corn after 11 days of supplementation but then completed the trial with selenium supplied in capsule form for the balance of the 120-day trial (27). This suggests that individual animals may occasionally refuse high-dose selenium supplements because they object to the taste of the supplement rather than because of a major selenium-induced health problem.
In our study, sudden refusal of food and water was most often encountered in animals that received both TBI and high-dose selenium supplementation. It was only rarely seen in animals that had been exposed to radiation but were maintained on standard drinking water, and it was never observed in nonirradiated animals on selenium-supplemented (200 μg/day) drinking water. Furthermore, in an ongoing study of selenium supplementation in rats that were exposed to localized radiation (10 Gy, single dose, whole-brain), no animals have died or had to be euthanized despite being on selenium-supplemented (200 μg/day) drinking water for 15–18 months. Taken together, this suggests that animals that were exposed to 10 Gy TBI are less tolerant of high doses of selenium than nonirradiated animals, possibly because of radiation-induced injury to major sites of selenium metabolism such as kidney and/or liver. This reduced tolerance would need to be taken into consideration when high-dose selenium is used for the mitigation of radiation injuries in victims of nuclear accidents or acts of radiological terrorism. Shortening the period of supplementation is one possible way to manage the risk of high-dose selenium interventions. Periodic monitoring of kidney and liver functions as well as hematocrits should also help guard against potentially serious side effects of high-dose selenium interventions.
While this study was designed to develop agents for the mitigation of radiation injuries sustained during nuclear accidents or acts of radiological terrorism, it may also have implications for cancer patients who sustain normal tissue injury as a result of radiation therapy.
Acknowledgments
This project was supported by a pilot grant from the Center for Medical Countermeasures Against Radiological Terrorism (U19-AI067734) and the MACC Fund. We thank Dr. Wolfgang H. H. Günther for stimulating discussions.
References
- 1.Sieber F, Muir SA, Cohen EP, North PE, Fish BL, Irving AA, et al. High-dose selenium for the mitigation of radiation injury: a pilot study in a rat model. Radiat Res. 2009;171:368–73. doi: 10.1667/0033-7587-171.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone HB, Moulder JE, Coleman CN, Ang KK, Anscher MS, Barcellos-Hoff MH, et al. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI workshop, December 3–4, 2003. Radiat Res. 2004;162:711–28. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 3.Cohen EP, Fish BL, Sharma M, Li XA, Moulder JE. Role of angiotensin II type-2 receptor in radiation nephropathy. Trans Res. 2007;150:106–15. doi: 10.1016/j.trsl.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrams RA, Buck P, Fish BL, Moulder JE. Relationship of cell dose and freezing rate to reconstitute potency in a hematopoietic reconstitution model. Transplantation. 1986;43:581–3. [PubMed] [Google Scholar]
- 5.Moulder JE, Fish BL. Late toxicity of total body irradiation with bone marrow transplantation in a rat model. Int J Radiat Oncol Biol Phys. 1989;16:1501–9. doi: 10.1016/0360-3016(89)90955-3. [DOI] [PubMed] [Google Scholar]
- 6.Cohen EP, Fish BL, Moulder JE. The renin-angiotensin system in experimental radiation nephropathy. J Lab Clin Med. 2002;139:251–7. doi: 10.1067/mlc.2002.122279. [DOI] [PubMed] [Google Scholar]
- 7.McAdam PA, Levander OA. Chronic toxicity and retention of dietary selenium fed to rats as D- or L-selenomethionine, selenite, or selenate. Nutr Res. 1987;7:601–10. [Google Scholar]
- 8.Whanger P, Vendeland S, Park YC, Xia Y. Metabolism of subtoxic levels of selenium in animals and humans. Ann Clin Lab Sci. 1996;26:99–113. [PubMed] [Google Scholar]
- 9.Moulder JE, Fish BL, Holcenberg JS, Sun GX. Hepatic function and drug pharmacokinetics after total body irradiation plus bone marrow transplant. Int J Radiat Oncol Biol Phys. 1990;19:1389–96. doi: 10.1016/0360-3016(90)90349-o. [DOI] [PubMed] [Google Scholar]
- 10.Geraci JP, Mariano MS. Radiation hepatology of the rat: parenchymal and nonparenchymal cell injury. Radiat Res. 1993;136:205–13. [PubMed] [Google Scholar]
- 11.Baker JE, Fish BL, Su J, Haworth ST, Strande JL, Komorowski RA, et al. 10 Gy total body irradiation increases risk of coronary sclerosis, degeneration of heart structure and function in a rat model. Int J Radiat Biol. 2009;85:1089–100. doi: 10.3109/09553000903264473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao W, Robbins ME. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem. 2009;16:130–43. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]
- 13.Hatfield DL, Berry MJ, Gladyshev VN, editors. Selenium. its molecular biology and role in human health. New York: Springer; 2006. [Google Scholar]
- 14.Gärtner R, Albrich W, Angstwurm MW. The effect of a selenium supplementation on the outcome of patients with severe systemic inflammation, burn and trauma. Biofactors. 2001;14:199–204. doi: 10.1002/biof.5520140125. [DOI] [PubMed] [Google Scholar]
- 15.Angstwurm MW, Engelmann L, Zimmermann T, Lehmann C, Spes CH, Abel P, et al. Selenium in intensive care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit Care Med. 2007;35:118–26. doi: 10.1097/01.CCM.0000251124.83436.0E. [DOI] [PubMed] [Google Scholar]
- 16.Gladyshev VN. Selenoproteins and selenoproteomes. In: Hatfield DL, Berry MJ, Gladyshev VN, editors. Selenium. its molecular biology and role in human health. New York: Springer; 2006. pp. 99–110. [Google Scholar]
- 17.Sunde RA. Regulation of glutathione peroxidase-1 expression. In: Hatfield DL, Berry MJ, Gladyshev VN, editors. Selenium. its molecular biology and role in human health. New York: Springer; 2006. pp. 149–160. [Google Scholar]
- 18.Kauf E, Fuchs D, Winnefeld K, Herrmann J, Zintl F. Blood selenium after conditioning and in the course of bone marrow transplantation of children with malignant diseases. Med Klin. 1997;92 (Suppl 3):46–7. [PubMed] [Google Scholar]
- 19.Hadjibabaie M, Iravani M, Shamshiri AR, Zaker Z, Mousavi A, Alimoghaddam K, et al. The prevalence of low selenium levels in adult patients undergoing bone marrow transplantation: a brief communication. Nutr Cancer. 2008;60:837–9. doi: 10.1080/01635580802196107. [DOI] [PubMed] [Google Scholar]
- 20.Fraunholz I, Eberlein K, Schopohl B, Böttcher HD, Rödel C. Selenium levels during the course of radiotherapy. No influence of irradiation on blood selenium concentration. Strahlenther Onkol. 2008;184:411–5. doi: 10.1007/s00066-008-1867-6. [DOI] [PubMed] [Google Scholar]
- 21.Surai PF. Selenium in nutrition and health. Nottingham: Nottingham University Press; 2006. [Google Scholar]
- 22.Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1–20. doi: 10.1016/s0300-483x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 23.Gehrisch A, Dörr W. Effects of systemic or topical administration of sodium selenite on early radiation effects in mouse oral mucosa. Strahlenther Onkol. 2007;183:36–42. doi: 10.1007/s00066-007-1594-4. [DOI] [PubMed] [Google Scholar]
- 24.Muecke R, Schomburg L, Glatzel M, Berndt-Skorka R, Baaske D, Reichl B, et al. Multicenter, phase 3 trial comparing selenium supplementation with observation in gynecologic radiation oncology. Int J Radiat Oncol Biol Phys. 2010;78:828–35. doi: 10.1016/j.ijrobp.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Cohen EP, Robbins ME. Radiation nephropathy. Semin Nephrol. 2003;23:486–99. doi: 10.1016/s0270-9295(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 26.Halverson AW, Tsay DT, Triebwasser KC, Whitehead EI. Development of hemolytic anemia in rats fed selenite. Toxicol Appl Pharmacol. 1970;17:151–9. doi: 10.1016/0041-008x(70)90139-0. [DOI] [PubMed] [Google Scholar]
- 27.O’Toole D, Raisbeck MF. Pathology of experimentally induced chronic selenosis (alkali disease) in yearling cattle. J Vet Diagn Invest. 1995;7:364–73. doi: 10.1177/104063879500700312. [DOI] [PubMed] [Google Scholar]