Abstract
The plague bacterium Yersinia pestis has a number of well-described strategies to protect itself from both host cells and soluble factors. In an effort to identify additional anti-host factors, we employed a transposon site hybridization (TraSH)-based approach to screen 105 Y. pestis mutants in an in vitro infection system. In addition to loci encoding various components of the well-characterized type III secretion system (T3SS), our screen unambiguously identified ompA as a pro-survival gene. We go on to show that an engineered Y. pestis ΔompA strain, as well as a ΔompA strain of the closely related pathogen Y. pseudotuberculosis, have fully functioning T3SSs but are specifically defective in surviving within macrophages. Additionally, the Y. pestis ΔompA strain was outcompeted by the wild-type strain in a mouse co-infection assay. Unlike in other bacterial pathogens in which OmpA can promote adherence, invasion, or serum resistance, the OmpA of Y. pestis is restricted to enhancing intracellular survival. Our data show that OmpA of the pathogenic Yersinia is a virulence factor on par with the T3SS.
Keywords: Pathogenesis, Virulence, Yersinia pestis, OmpA, intracellular
1. Introduction
Gram-negative bacteria possess an outer membrane (OM) that is composed of LPS, phospholipids, and proteins. The best characterized of the latter is the E. coli OmpA which has become a popular model for studies on membrane protein structure and folding dynamics (Smith et al. 2007). A variety of studies have also shown that in pathogenic E. coli and Klebsiella pneumonia OmpA mediates a wide-range of activities including resistance to complement and antibacterial peptides, and invasion of and survival within eukaryotic cells (Weiser and Gotschlich 1991; Prasadarao et al. 1996; Fu et al. 2003; Llobet et al. 2009).
The plague bacterium Yersinia pestis expresses a number of virulence factors that enhances its survival and proliferation within the mammalian host including a type III secretion (T3S) system for protection against immune cells and the outer membrane protein Ail which confers protection against complement-mediated killing (Viboud and Bliska 2005; Bartra et al. 2008). Several loci have been shown to be critical for the intracellular survival of Y. pestis including ripA, mgtC, and ugd which encode putative acetyl CoA transferase, Mg2+ acquisition, and an intermediary product involved in promoting resistance to antimicrobial peptides, respectively (Pujol et al. 2005; Grabenstein et al. 2006). It is becoming increasingly clear that although Y. pestis possesses a potent ‘anti-phagocytosis’ activity (mediated by the T3SS), it has also evolved robust intracellular survival mechanisms as either a failsafe or alternative virulence strategy.
To identify Y. pestis genes that impact its interaction with macrophages, we screened a highly complex pool of Y. pestis transposon mutants in a cell culture infection model. This infection model has been previously shown to be sensitive to defects in the T3S system (Bartra et al. 2001). Here we report that, in addition to T3S-associated genes (as predicted from single mutant studies), our efforts revealed that OmpA is specifically required for the intracellular survival of Y. pestis and the related pathogen Y. pseudotuberculosis in macrophages.
2. Results and Discussion
2.1. Identifying OmpA as a pro-survival factor in a cell culture infection model
We used a transposon site hybridization (TraSH)-based approach (Sassetti et al. 2003) to identify Y. pestis genes that play a pro-survival function during infection. A culture containing approximately 90,000 unique Y. pestis transposon mutants were generated and used to infect cultured mouse macrophage-like RAW 264.7 cells (Fig.1). After a 14-hr infection period, the surviving cell-associated bacteria were collected and used for a second round of infection following which bacterial DNA was isolated and used to create probes that were applied to tiled microarrays. Mutants that survived and/or proliferated during the infection will generate positive signals in the corresponding spots of the array whereas those mutants that were eliminated during the infection will fail to show signals thus possibly indicating that the matching gene is important for survival during infection. DNA was isolated from a parallel culture of the library of Y. pestis mutants that was propagated in the absence of macrophages in order to control for genes important for normal growth. More details of the Y. pestis mutagenesis, infection, and data analysis are described in Methods and Fig. 1.
Fig. 1. Overview of TraSH-based approach for the identification of genes essential for infectivity of Yersinia pestis.
A library of Y. pestis mutants was used to infect murine RAW 264.7 macrophage-like cells. The mutants that survived infection were collected and genomic DNA was prepared. The DNA was digested with a restriction enzyme that cleaves once in the middle of Tn5, and the fragments were ligated to a linker DNA. Tn5-containing sequences were amplified by PCR using Tn5 and linker specific primers. Labeled RNA probes were made from the T7 promoters at the termini of Tn5, and were used to hybridize a custom genome tiling oligonucleotide microarray. Oligonucleotides are differentially hybridized depending on the abundance of the RNA probes corresponding to that region of the genome. Genes that are potentially important for infection can are indicated by their lower hybridization signals from the library survived infection compared to those from the library grown in tissue culture medium alone.
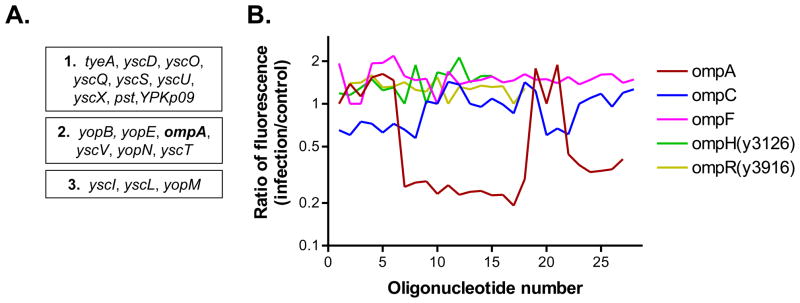
Using a high statistical cut-off, we identified 44 open reading frames (ORFs) that were specifically important for optimal infection. A number of these ORFs (17) are located on the extrachromosomal plasmids pCD1 and pPCP1 that encode the components of the T3SS and the pla protease, respectively, both of which have been shown to play central role in Y. pestis virulence (Viboud and Bliska 2005). Among the remaining genes, we were particularly interested that the ORF encoding the outer membrane protein A (ompA; y2735) was ranked among the various T3SS-encoding loci in terms of statistical significance (Fig.2A). The OmpA-encoding locus was the only representative among the numerous outer membrane proteins of Y. pestis that played a pro-survival function in the infection assay (Fig.2B).
Fig. 2. The Y. pestis ompA locus (y2735) encodes a pro-survival factor.
(A) The 17 infection-promoting loci encoded on the extracellular plasmids pCD1 and pPCP1, together with the ompA locus, that were identified in the TraSH screen were arranged into three groups according to their respective infection/control ratios: (1) those ORFs that had a ratio lower than 0.5; (2) those ORFs with a ratio of between 0.5 and 0.7; and (3) those ORFs with a ratio higher than 0.7 but significantly lower than that of non-essential genes. (B) Fluorescence associated with several omp or omp regulatory gene regions of the microarray. There is at least one probe specific for each of the indicated genes. The relative reduction of signal in the ompA sequences from the infection population indicates the relative underrepresentation of ompA(Tn+) mutants following infection compared to population propagated in the absence of macrophages.
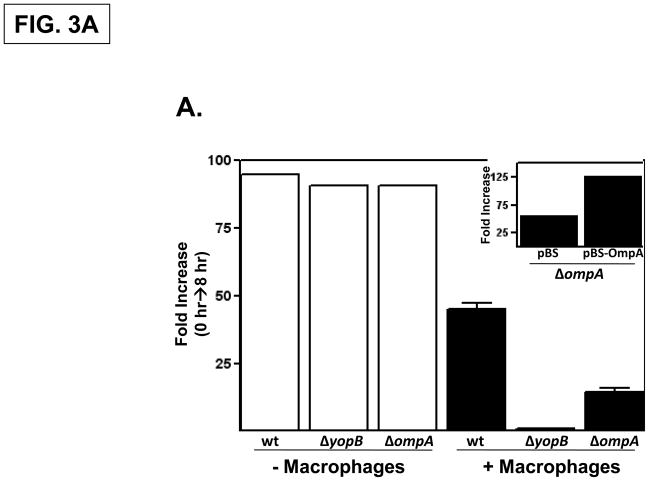
To confirm that OmpA is specifically required for Y. pestis to optimally infect macrophages, an ompA deletion strain was constructed as well as strains possessing deletions in ompC (y2966), ompF (y2759), ompN (y2983) and the previously described ail (y1324) (Bartra et al. 2008). These strains were individually tested in an infection assay similar to that used in the TraSH-based screen except that the ‘outputs’ (the surviving bacterial fraction) was assessed by viable plating. The infectivity of these mutant strains were compared to that of the parental Y. pestis KIM5 strain and to an isogenic strain lacking YopB, a T3SS translocon component. Similar to that described previously (Rosenzweig et al., 2005), there was an approximately 50-fold increase in the number of cell-associated wild-type Y. pestis during an 8-hour infection period whereas the number of cell-associated ΔyopB bacteria remained essentially unchanged during the infection period (Fig. 3A). The Y. pestis ΔompA strain exhibited an intermediate level of infectivity compared to the wild-type and ΔyopB strains (Fig. 3A) in contrast to the strains deleted for the ompC, ompF, ompN or ail genes which resembled the wild-type parental strain in this assay (data not shown). The proliferation of the Y. pestis wild-type, ΔyopB, and ΔompA strains in wells without macrophages was comparable indicating that under these conditions there is not a general growth defect (Fig. 3A). The expression of OmpA in trans in the ΔompA strain increased the infectivity compared to the ΔompA strain transformed with the vector control (Fig. 3A, inset). These data confirm the results of the TraSH-based screen indicating that OmpA promotes the survival of Y. pestis in the presence of macrophages.
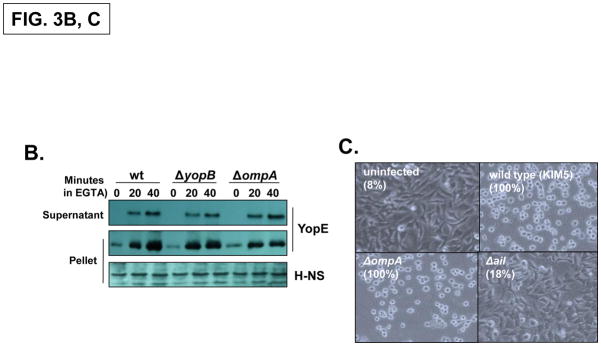
Fig. 3. The Y. pestis OmpA promotes infectivity independently of the T3SS.
(A) Empty wells (-macrophage) or wells containing RAW 264.7 cells were infected with Y. pestis KIM5, ΔyopB and ΔompA strains at a MOI of 1. Following a 30 minute attachment period, unattached bacteria were removed, and the number of cell-associated bacteria was determined by plating at the 0 hour and 8 hour timepoints. Three independent wells per strain were analyzed and the average fold-increase in the number of bacteria recovered for each strain over the 8 hour infection period is shown. (inset) The infectivity of the Y. pestis ΔompA strain was restored by a plasmid-encoded OmpA. (B) Yop secretion by the Y. pestis KIM5, ΔyopB and ΔompA strains. Strains were grown to mid-log phase and then EGTA was added to induce Yop secretion. YopE levels in both whole cells and supernatant fractions were determined by immunoblotting. H-NS levels were analyzed as a loading control. (C) HeLa cells were left uninfected or infected with wild-type Y. pestis KIM5, ΔompA or Δail strains at an MOI of 30 and photographed 2 hours after infection. Cell rounding (cytotoxicity) was quantified by counting all cells (N 200) in three different fields and the percentage is shown in parenthesis.
Previously we have shown that the macrophage infection assay used above is sensitive to defects in either the T3SS itself (e.g., YopB) or factors that affect T3SS functioning (e.g., RNase E) (Bartra et al. 2001; Yang et al. 2008). We therefore employed two different assays to determine whether the lack of OmpA in Y. pestis impacted T3SS activity. In a secretion assay in the absence of macrophages, the wild-type, ΔyopB, and ΔompA strains secreted the T3SS effector YopE in a similar manner (Fig. 3B). We also tested whether OmpA was required for the efficient delivery of Yop effectors into eukaryotic cells in an infection assay. Cells infected with the wild-type Y. pestis strain display a rounded morphology (‘cytotoxicity’) that is primarily due to the translocation of the YopE effector into the cytosol (Rosqvist et al. 1990). There were no detectable differences between the levels of cytotoxicity induced by the wild-type and ΔompA strains in contrast to a strain lacking Ail, an adhesion required for optimal Yop delivery (Fig. 3C; Tsang et al. 2010). These results indicate that the Y. pestis ΔompA strain possesses a functional T3SS that is able to efficiently secrete and translocate Yop proteins into eukaryotic cells. Consequently, the diminished growth of ΔompA strains in the presence of macrophages does not appear to be due to defects in T3SS function.
2.2. OmpA promotes the intracellular survival of Y. pestis and Y. pseudotuberculosis
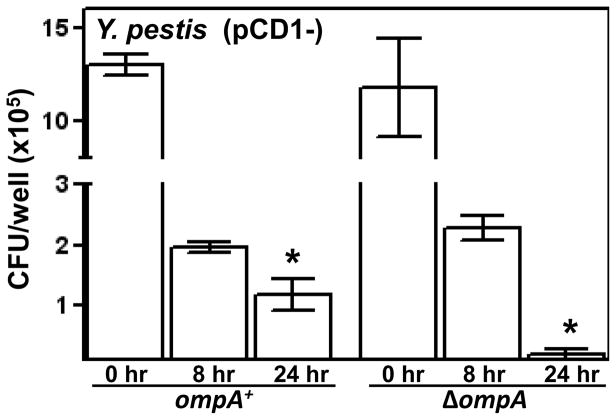
Previously it has been shown that Yersinia spp. including Y. pestis, are able to survive within macrophages (Finegold 1969; Une 1977; Tabrizi & Robins-Browne 1992; Yamamoto et al. 1996; Oyston et al. 2000). This characteristic of the yersiniae does not involve the T3SS (Zhang et al., 2008). We therefore determined whether the reduced infectivity of the ΔompA strain observed above could be, at least in part, accounted for by a reduction in intracellular survival. To promote the internalization of the Y. pestis ompA+ and ΔompA strains we used strains lacking the T3SS-encoding pCD1 virulence plasmid (ΔpCD1). The initial uptake of the Y. pestis ΔpCD1 ΔompA and the isogenic ompA+ strain into cultured macrophages were comparable; however, upon a longer infection period the survival of the ΔompA strain was significantly reduced compared to that of the ompA+ strain (Fig. 4). These data show that OmpA promotes the intracellular survival of Y. pestis.
Fig. 4. OmpA promotes intracellular survival of Y. pestis.
Wild-type and ΔompA strains of Y. pestis lacking the T3SS encoding virulence plasmid pCD1 were added to RAW 264.7 cells at a MOI of 10. Following a 45-min attachment/invasion period gentamicin was added to each well. After 0, 8 and 24 hrs of infection the number of cell-associated bacteria per well was determined by viable plating. One of three independent experiments is shown. (* P < 0.05)
The OmpA protein expressed by the enteropathogenic Y. pseudotuberculosis is identical to the OmpA of Y. pestis. We therefore generated a Y. pseudotuberculosis ΔompA deletion strain to investigate whether OmpA promoted the intracellular survival of Y. pseudotuberculosis. For these infection assays, we used the Y. pseudotuberculosis YPIII strain harboring the T3SS-encoding virulence plasmid pIB102 (Bolin et al. 1982). The initial uptake of the wild-type Y. pseudotuberculosis strain is much lower than that of the ΔyopB strain (Fig. 5); the enhanced uptake of the ΔyopB strain is characteristic of T3SS-defective strains and is due the anti-phagocytic activities of the Yop effectors (Hakansson et al., 1996). However, despite the differences in their uptake, the wild-type and ΔyopB strains displayed comparable levels of intracellular survival (Fig. 5) consistent with the studies of Y. pestis cited above showing that intracellular survival is independent of the T3SS. Even though the uptake of the Y. pseudotuberculosis ΔompA strain resembled that of the wild-type strain (which would be expected if the activities of their respective T3SSs were comparable), the intracellular survival of this strain is clearly reduced compared to the wild-type and ΔyopB strains (Fig. 5). These data indicate that OmpA in Y. pseudotuberculosis, like OmpA of Y. pestis, enhances intracellular survival within macrophages.
Fig. 5. OmpA promotes intracellular survival of Y. pseudotuberculosis.
Wild-type Y. pseudotuberculosis YPIII, ΔyopB and ΔompA strains were added to RAW 264.7 cells at a MOI of 10. After a 45-min attachment period excess bacteria were removed and gentamicin was added to half of the wells. ‘Uptake’ (open bars) was determined by dividing the number of CFUs recovered from the gentamicin-containing wells by the number of CFUs recovered from the untreated wells following an additional 1.5 hrs of infection. ‘Intracellular survival’ (closed bars) was determined by dividing the number of CFUs recovered from gentamicin-containing wells after 5 hrs of infection by the number of CFUs recovered from the gentamicin-containing wells after 1.5 hours of infection. One of three independent experiments is shown.
2.3. Comparable analysis of OmpA regulation and function in Y. pestis and E. coli
For a variety of reasons OmpA has been extensively studied in E. coli. For example, in E. coli it has been shown that OmpA confers resistance to serum factors (Weiser and Gotschlich 1991). However, we could not detect any differences between the Y. pestis wild-type and ΔompA strains using a serum killing assay which we have previously employed in our characterization of Ail (data not shown; Bartra et al. 2008). OmpA is a highly abundant outer membrane protein in E. coli that is preferentially expressed in growing cells and is readily visualized by Coomassie staining outer membrane preparations fractionated by SDS-PAGE (Smith et al. 2007). In contrast, by neither Coomassie nor silver staining could we detect differences between outer membrane preparations of wild-type and ΔompA strains of Y. pestis (data not shown). Therefore, OmpA in Y. pestis appears to be expressed at lower levels than in E. coli. However, despite these differences in expression levels, we found by Northern and Western analysis, that in Y. pestis, as has been shown in E. coli, the expression levels of ompA transcript and OmpA protein are higher in growing cells compared to stationary phase cells (data not shown).
A contributing factor to the growth phase regulation of OmpA expression in E. coli involves the negative regulator small RNA (sRNA) MicA, which is expressed at higher levels in stationary phase cells (Smith et al., 2007). The Y. pestis KIM5 genome harbors a sequence that resembles the sequence of the E. coli MicA. To determine whether Y. pestis expresses this MicA-like gene, we analyzed RNA from the wild-type strain as well as a strain in which this putative gene had been deleted (ΔmicA). We detected a transcript of the expected length in wild-type, but not from the ΔmicA strain (Fig. 6A). In E. coli the level of MicA transcripts are higher in strains lacking the ribonuclease polynucleotide phosphorylase (PNP) (Andrade & Arraiano, 2008). Similarly, in the Y. pestis Δpnp strain (Rosenzweig et al., 2005), we also observed higher levels of the MicA transcript (Fig. 6A). We also found that in Y. pestis, again, as has been described for E. coli, the levels of MicA are higher in stationary phase cells compared to log-phase cells (data not shown). These data indicate that Y. pestis expresses a functional MicA that likely contributes to the growth-phase regulation of OmpA expression.
Fig. 6. MicA expression in Y. pestis and functional consequences of its overexpression.
(A) Total RNA was isolated from the indicated Y. pestis strains and analyzed by Northen blot analysis using a MicA specific probe. (B) Either the empty vector or MicA-encoding plasmids were transformed into wild-type Y. pestis KIM5 and the levels of MicA transcript and OmpA protein levels in the resulting transformant strains were measured by primer extension and western analysis, respectively (α-HNS: loading control). (C) RAW 264.7 cells were infected with Y. pestis transformed with either empty vector or the MicA-encoding plasmids and analyzed as described in Fig. 1. One of three independent experiments is shown.
To test whether MicA overexpression would affect Y. pestis infection of macrophages, we cloned the MicA-encoding sequence in a high-copy plasmid. The strain carrying the MicA-encoding plasmid displayed increased levels of MicA transcripts and, as expected, reduced levels of OmpA protein (Fig. 6B). The MicA overexpressing strain also had a much reduced infectivity compared to the strain harboring the vector control plasmid (Fig. 6C). This effect was specific for MicA since overexpression of other sRNAs did not impact infectivity (data not shown). This experiment does not necessarily mean that MicA regulates OmpA levels during infection only that, like in E. coli, there is an inverse relationship between MicA and OmpA levels. Nonetheless, the phenotypic similarly between the MicA overexpression strain and the ΔompA mutant strain further indicates the importance of OmpA for Y. pestis infection.
2.4. Animal infections corroborate in vitro findings
In vivo competition assays provide a sensitive measure of the extent of virulence attenuation caused by a given mutation. A mouse BSL2 model of pneumonic plague (Galván et al. 2010) was used to compare the virulence of wild-type and ΔompA Y. pestis strains. C57BL/6 mice were inoculated intranasally with equal numbers of Y. pestis KIM5 (Pgm-) and an isogenic ompA deletion strain. Bacteria were recovered from the lungs, spleen and liver at 18 h, 36 h and 60 h post-infection. Analysis of the ratio of wild type CFUs to ΔompA CFUs recovered from the lungs, spleen and liver (Fig. 7) revealed that the ompA mutant was significantly out competed by the wild-type parent strain. These findings indicate that the ompA mutant is attenuated for virulence in the mouse BSL2 pneumonic plague model and is consistent with the in vitro-derived findings showing that OmpA is important for the intracellular survival of Y. pestis.
Fig. 7. Wild type Y. pestis out competes an isogenic ΔompA mutant in a mouse BSL2 model of pneumonic plague.
Y. pestis KIM5 and the isogenic ΔompA mutant were mixed 1:1 and inoculated intranasally into groups of five C57BL/6 mice. Bacteria were recovered from the lungs, liver and spleen at 18 h, 36 h and 60 h post-infection. The competitive index (CI) was calculated as the ratio of the CFU of wild type Y. pestis to the CFU of the ΔompA mutant recovered from the lungs, liver and spleen of each mouse. Statistically significant reductions in organ colonization by the mutant strain are labeled by asterisks (paired t-test, P < 0.05).
This is the first report of OmpA having a role in the pathogenesis of the plague bacterium Y. pestis. Our results fit with the developing theme that OmpA can potentially serve a variety of functions for Gram-negative pathogens. For Y. pestis (and Y. pseudotuberculosis) OmpA enhances intracellular survival (possibly by conferring resistance to antimicrobial peptides), but in contrast to other pathogens, serves no detectable role in adherence, invasion, or serum resistance.
3. Methods
3.1. Bacterial strains and in vitro infection assays
The parental Y. pestis KIM5-3001 and ΔyopB, Δpnp, and Δail strains have been described (Linder et al., 1990; Rosenzweig et al., 2005; Bartra et al., 2008). KIM5-3001 derivative strains were made with deletions in ompA (y2735; condons 14–340), ompC (y2966; codons 4–370), ompF (y2759; codons 1–326), and ompN (y2983; codons 8–335) using in vivo lambda red recombination (Datsenko and Wanner 2000). The parental Y. pseudotuberculosis YPIII/pIB102 strain (Bolin et al. 1982) was similarly deleted in ompA. For the TraSH-based analysis, about 1.5 × 107 murine macrophage-like RAW 264.7 were infected with the pooled Y. pestis transposon mutants (described below) at a MOI of 2 for 14 hrs. The infection assay with engineered strains of Y. pestis (Fig. 3) was performed as described (Rosenszweig et al., 2005). The invasion assay using Y. pestis (Fig. 4) involved infecting RAW 264.7 cells at a MOI of 10. After a 45 min attachment period, gentamicin was added at a concentration of 8 μg/ml which, after an additional hour, was replaced with media containing 4 μg/ml gentamicin. At the indicated times the infected cells were lysed with water which was plated for colony counts. For, the invasion assay using Y. pseudotuberculosis (Fig. 5) RAW 264.7 cells were infected with a MOI of 10 and 45 min later the media was removed and replaced by media containing 0.5 μg/ml gentamicin. At the indicated times infected cells were lysed with water and colony forming units determined by plating.
3.2. TraSH-based screening
A mutant library was generated by introducing a miniTn5 construct into Y. pestis KIM5 by electroporation. The miniTn5 was engineered to contain one outward facing T7 promoter at each end by joining a T7p-containing oligonucleotide to the EZ-Tn5™ <T7/KAN-2> DNA (Epicentre, Madison, WI) by PCR. The library of mutants was used in the infection experiment described above. The mutant bacteria that survived infection were collected to make genomic DNA. Labeled RNA probes were generated from gDNA samples and were used to hybridize a genome tiling microarray containing 40-mer oligonucleotides (Affimetrix, Santa Clara, CA), at the DNA microarray facility of Ocean Bridge Bioscience Inc., Palm Beach Gardens, FL. Standard hybridization, array scanning and data normalization procedures were used. In order to analyze gene-specific hybridization signals, we first identified those genes for which there is at least one probe that hybridizes only within the gene’s open reading frame. We have constructed a list of genes that are unimportant for infection by excluding the known important genes, and used this list as negative control for the identification of important genes. The raw data were pre-processed through median polishing of the LOESS normalized log2 fold changes. The pre-processed data were analyzed for each qualified gene using Welch’s t-test. Genes that are important for infection were identified if the gene’s specific signals are significantly lowered after infection as compared to unimportant genes control (p < 0.05).
3.3. Mice infection
The virulence defect of the ΔompA strain was studied in a co-infection experiment with the parental strain KIM5 in a mouse BSL-2 model of pneumonic plague (Galvan et al., 2010). Eight weeks old female C57BL/6 mice were purchased from the Jackson Laboratories. The mice were provided food and fresh water ad libitum during the experiments, which were performed according to the guidelines of the University of Pennsylvania Institutional Animal Care and Use Committee. Bacteria were grown in BHI at 26°C overnight, diluted 1:10 in fresh BHI containing 2.5 mM CaCl2, and cultured overnight at 37°C. The bacterial cells were pelleted by centrifugation, washed and re-suspended in PBS to A600 of 0.2 (approximately 2 × 107 CFU/ml). Equal volumes of the parental and ΔompA strain suspensions were mixed and 10-fold serially diluted in PBS to prepare the inoculum. Bacterial counts were determined on TB agar plates (Galvan et al., 2008). Mice were given three injection of iron dextran (Sigma; 4 mg each) intra-peritoneally at 24 h intervals. 3 h after the first injection with iron dextran, mice were anesthetized (ketamine/xylazin 100/10mg/kg body weight, i.p.) and infected by intra-nasal instillation with 25 μl of the bacterial inoculum containing 4 × 103 CFU of each strain. Lungs, livers and spleens were surgically removed at 18, 36 and 60 h post infection and homogenized in 5 ml sterile PBS by using a Stomacher Lab Blender (Seward Medical Limited). CFU/organ were determined by plating serial dilutions onto TB agar plates containing streptomycin or kanamycin. CFUs for the parental strain KIM5 were calculated by subtracting the ΔompA CFUs (kanamycin plates) from the total CFUs (streptomycin plates). Competitive index (C.I.) scores were calculated as the ratio of the KIM5 CFUs to the ΔompA CFUs. Statistical significance was determined by the paired t-test.
Acknowledgments
The gene screening work was supported by University of South Florida Center for Biological Defense, funded by U.S. Army Research, Development and Engineering Command (RDECOM), Contract W911SR-07-C-0084 (ZL). This work was also supported in part by Public Health Service grants AI076695 (DMS), AI50552 (GVP), and AI53459 (KS) from the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrade JM, Arraiano CM. PNPase is a key player in the regulation of small RNAs that control the expression of outer membrane proteins. RNA. 2008;14:543–551. doi: 10.1261/rna.683308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra S, Cherepanov P, Forsberg A, Schesser K. The Yersinia YopE and YopH type III effector proteins enhance bacterial proliferation following contact with eukaryotic cells. BMC Microbiol. 2001;1:e22. doi: 10.1186/1471-2180-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra SS, Styer KL, O’Bryant DM, Nilles ML, Hinnebusch BJ, Aballay A, Plano GV. Resistance of Yersinia pestis to complement-dependent killing is mediated by the Ail outer membrane protein. Infect Immun. 2008;76:612–622. doi: 10.1128/IAI.01125-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I, Norlander L, Wolf-Watz H. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect Immun. 1982;37:506–512. doi: 10.1128/iai.37.2.506-512.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold MJ. Pneumonic plague in monkeys. An electron microscopic study. Am J Pathol. 1969;54:167–185. [PMC free article] [PubMed] [Google Scholar]
- Fu H, Belaaouaj AA, Dahlgren C, Bylund J. Outer membrane protein A deficient Escherichia coli activates neutrophils to produce superoxide and shows increased susceptibility to antibacterial peptides. Microbes Infect. 2003;5:781–788. doi: 10.1016/s1286-4579(03)00145-x. [DOI] [PubMed] [Google Scholar]
- Galván EM, Nair MK, Chen H, Del Piero F, Schifferli DM. Biosafety level 2 model of pneumonic plague and protection studies with F1 and Psa. Infect Immun. 2010;78:3443–3453. doi: 10.1128/IAI.00382-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenstein JP, Fukuto HS, Palmer LE, Bliska JB. Characterization of phagosome trafficking and identification of PhoP-regulated genes important for survival of Yersinia pestis in macrophages. Infect Immun. 2006;74:3727–3741. doi: 10.1128/IAI.00255-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkansson S, Schesser K, Persson C, Galyov EE, Rosqvist R, Homblé F, Wolf-Watz H. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 1996;15:5812–5823. [PMC free article] [PubMed] [Google Scholar]
- Lindler LE, Klempner MS, Straley SC. Yersinia pestis pH 6 antigen: genetic, biochemical, and virulence characterization of a protein involved in the pathogenesis of bubonic plague. Infect Immun. 1990;58:2569–2577. doi: 10.1128/iai.58.8.2569-2577.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llobet E, March C, Giménez P, Bengoechea JA. Klebsiella pneumoniae OmpA confers resistance to antimicrobial peptides. Antimicrob Agents Chemother. 2009;53:298–302. doi: 10.1128/AAC.00657-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyston PC, Dorrell N, Williams K, Li SR, Green M, Titball RW, Wren BW. The response regulator PhoP is important for survival under conditions of macrophage-induced stress and virulence in Yersinia pestis. Infect Immun. 2000;68:3419–3425. doi: 10.1128/iai.68.6.3419-3425.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasadarao NV, Wass CA, Weiser JN, Stins MF, Huang SH, Kim KS. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect Immun. 1996;64:146–153. doi: 10.1128/iai.64.1.146-153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol C, Grabenstein JP, Perry RD, Bliska JB. Replication of Yersinia pestis in interferon gamma-activated macrophages requires ripA, a gene encoded in the pigmentation locus. Proc Natl Acad Sci U S A. 2005;102:12909–12914. doi: 10.1073/pnas.0502849102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig JA, Weltman G, Plano GV, Schesser K. Modulation of yersinia type three secretion system by the S1 domain of polynucleotide phosphorylase. J Biol Chem. 2005;280:156–163. doi: 10.1074/jbc.M405662200. [DOI] [PubMed] [Google Scholar]
- Rosqvist R, Forsberg A, Rimpiläinen M, Bergman T, Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990;4:657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol. 2003;48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- Smith SG, Mahon V, Lambert MA, Fagan RP. A molecular Swiss army knife: OmpA structure, function and expression. FEMS Microbiol Lett. 2007;273:1–11. doi: 10.1111/j.1574-6968.2007.00778.x. [DOI] [PubMed] [Google Scholar]
- Tabrizi SN, Robins-Browne RM. Influence of a 70 kilobase virulence plasmid on the ability of Yersinia enterocolitica to survive phagocytosis in vitro. Microb Pathog. 1992;13:171–179. doi: 10.1016/0882-4010(92)90018-j. [DOI] [PubMed] [Google Scholar]
- Tsang TM, Felek S, Krukonis ES. Ail binding to fibronectin facilitates Yersinia pestis binding to host cells and Yop delivery. Infect Immun. 2010;78:3358–3368. doi: 10.1128/IAI.00238-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Une T, Zen-Yoji H, Maruyama T, Yanagawa Y. Correlation between epithelial cell infectivity in vitro and O-antigen groups of Yersinia enterocolitica. Microbiol Immunol. 1977;21:727–729. doi: 10.1111/j.1348-0421.1977.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Viboud GI, Bliska JB. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu Rev Microbiol. 2005;59:69–89. doi: 10.1146/annurev.micro.59.030804.121320. [DOI] [PubMed] [Google Scholar]
- Weiser JN, Gotschlich EC. Outer membrane protein A (OmpA) contributes to serum resistance and pathogenicity of Escherichia coli K-1. Infect Immun. 1991;59:2252–2258. doi: 10.1128/iai.59.7.2252-2258.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Hanawa T, Ogata S, Kamiya S. Identification and characterization of the Yersinia enterocolitica gsrA gene, which protectively responds to intracellular stress induced by macrophage phagocytosis and to extracellular environmental stress. Infect Immun. 1996;64:2980–2987. doi: 10.1128/iai.64.8.2980-2987.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Jain C, Schesser K. RNase E regulates the Yersinia type 3 secretion system. J Bacteriol. 2008;190:3774–3778. doi: 10.1128/JB.00147-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Murtha J, Roberts MA, Siegel RM, Bliska JB. Type III secretion decreases bacterial and host survival following phagocytosis of Yersinia pseudotuberculosis by macrophages. Infect Immun. 2008;76:4299–4310. doi: 10.1128/IAI.00183-08. [DOI] [PMC free article] [PubMed] [Google Scholar]