Abstract
CD4 T cells are essential for immune control of gamma-(γ)-herpesvirus latency. We previously identified a murine MHC class II-restricted epitope in γHV68 glycoprotein 150 (gp15067–83IAb) that elicits CD4 T cells that are maintained throughout long-term infection. However, it is unknown if naïve cells can be recruited into the antiviral CD4 T cell pool during latency. Here we generate a mouse transgenic for a gp150-specific T cell receptor and show epitope-specific activation of transgenic CD4 T cells during acute and latent infections. Furthermore, although only dendritic cells can stimulate virus-specific CD8 T cells during latency, we show that both dendritic cells and B cells stimulate transgenic CD4 T cells. These studies demonstrate that naïve CD4 T cells specific for a viral glycoprotein can be stimulated throughout infection, even during quiescent latency, suggesting that CD4 T cell memory is maintained in part by the continual recruitment of naïve cells.
Introduction
CD4 T cells play an essential role in the control of persistent infections (1). In human gamma (γ)-herpesvirus infections, CD4 T cells can be specific for lytic or latent antigens, and disruption of CD4 T cell surveillance can lead to recrudescence and development of cancer (2–4). Virus reactivations and malignancies associated with the human γ-herpesviruses Epstein-Barr virus (EBV) and Kaposi’s sarcoma-associated herpesvirus (KSHV) are frequently found in HIV-infected patients with CD4 T cell deficiency (5). CD4 T cells are currently being targeted for therapies and vaccines for EBV-associated cancers (6, 7). However, how antiviral CD4 T cells are activated and maintained throughout persistent γ-herpesvirus infection is unclear.
In this study we analyze the activation and proliferation of virus-specific CD4 T cells using a tractable small animal model of γ-herpesvirus infection and immunity – infecting mice with murine γ-herpesvirus-68 (γHV68), a γ2-herpesvirus related to EBV and KSHV (8). Throughout γHV68 infection, antiviral CD4 T cells are maintained at high frequencies and have a high turnover rate (9, 10). The best characterized CD4 T cell epitope in C57BL/6 mice is derived from γHV68 glycoprotein 150 (gp15067–83IAb), a homolog of EBV gp350/220 and KSHV K8.1 (11). Here we report the generation of a T cell receptor (TCR) transgenic (tg) mouse with specificity for the γHV68 gp15067–83 epitope. Using this mouse, we demonstrate that gp150-specific CD4 T cells are activated and expand after γHV68 infection, traffic to multiple tissues, and can be stimulated during latency by both dendritic cells and B cells to proliferate in an antigen-specific manner, thereby suggesting that naïve CD4 T cells can be recruited into the ongoing immune response during quiescent latency.
Materials and Methods
Mice and viral infections
C57BL/6, B6.SJL-PtprcaPepcb/Boy (B6.SJL), Salmonella flagellin peptide (SM1427–441IAb) TCR tg (12), Mycobacterium tuberculosis early secreted antigenic target-6 (ESAT61–20IAb) TCR tg (13), and γHV68 gp15067–83IAb TCR tg mice were obtained from the Trudeau Institute animal facility and maintained under specific-pathogen free conditions. Mice were infected intranasally (i.n.) with 400 PFU of γHV68 (strain WUMS). All experiments were approved by the Institutional Animal Care and Use Committee of the Trudeau Institute. To generate the gp15067–83IAb TCR tg mouse, genes encoding the TCR Vα11 and Vβ12 chains were cloned from the gp15067–83-specific CD4 T cell hybridoma (clone 4211) (9) essentially as described using standard methods (13). Microinjection of C57BL/6 mouse embryos was performed by the Transgenic Mouse Facility at the University of Vermont (Burlington, VT).
Stimulations of transgenic CD4 T cells
In vitro stimulations were performed as described (13). For in vivo examination during lytic infection, 4 × 103 cells were injected intravenously (i.v.) into naïve congenic mice 24 hours before infection. Mediastinal lymph nodes (MLN), spleens, and lungs were examined for number and phenotype of transferred CD4 T cells at various times p.i. For examination during latency, 106 cells of each type were injected i.v. into congenic mice at various times p.i. Tissues were examined for phenotype of transferred CD4 T cells. To assess in vitro antigen presentation, CD19+ B cells or CD11c+ dendritic cells (DCs) were sorted from the spleens of mice 4 months p.i. CD4 T cells were enriched from naïve gp150 TCR tg mice by negative enrichment and labeled with CFSE (Molecular Probes). CD4 T cells were combined with B cells or DCs at varying effector to target (E:T) ratios. On day 3 of culture, cells were collected and analyzed by flow cytometry.
Results and Discussion
Generation of a gp150-specific TCR transgenic mouse strain
We previously identified an MHC class II-restricted epitope within γHV68 gp150 (gp15067–83IAb) that stimulates antiviral CD4 T cells to produce IFNγ (9, 10). To specifically monitor virus-specific CD4 T cells throughout infection, we generated a TCR tg mouse with specificity for gp15067–83IAb.
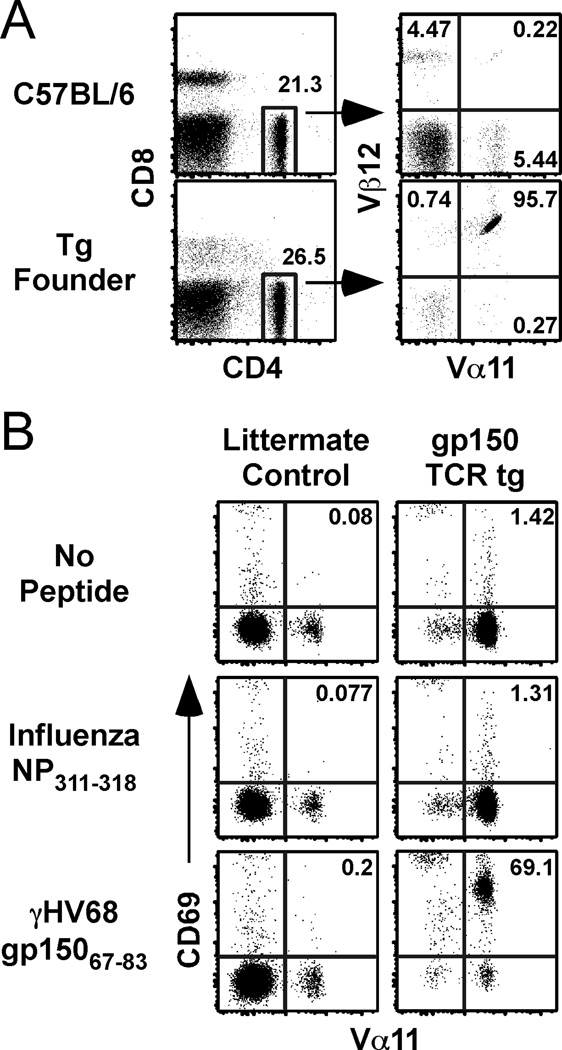
TCR genes were isolated from a T cell hybridoma that recognizes the gp15067–83 epitope (9, 10). The hybidoma expresses a TCR containing the Vα11 and Vβ12 TCR chains (data not shown). The TCRα and TCRβ genes were cloned into the human CD2 expression vector (14), and the construct was used to generate germ-line tg mice. C57BL/6 founder mice were identified based upon their expression of Vα11 and Vβ12 on the majority of CD4 T cells (Figure 1A). To confirm the specificity of the tg CD4 T cells for the gp15067–83IAb epitope, we measured the expression of an early activation marker, CD69, in vitro. As shown in Figure 1B, TCR tg CD4 T cells were activated in the presence of cognate peptide, but not an irrelevant control peptide (influenza NP311–325IAb), demonstrating that they exhibited the intended specificity.
Figure 1.
Generation of a gp15067–83/I-Ab-specific transgenic mouse. A, Splenocytes from a C57BL/6 littermate or the transgenic founder mouse were analyzed for expression of Vα11 and Vβ12. B, Splenocytes from transgenic or littlemate control mice were incubated for 24 h with congenic APCs plus influenza NP311–325, γHV68 gp15067–83, or no peptide control. The dot plots are gated on CD4 T cells and show Vα11 and CD69 expression after stimulation.
Activation of gp150-specific tg CD4 T cells during acute γHV68 infection
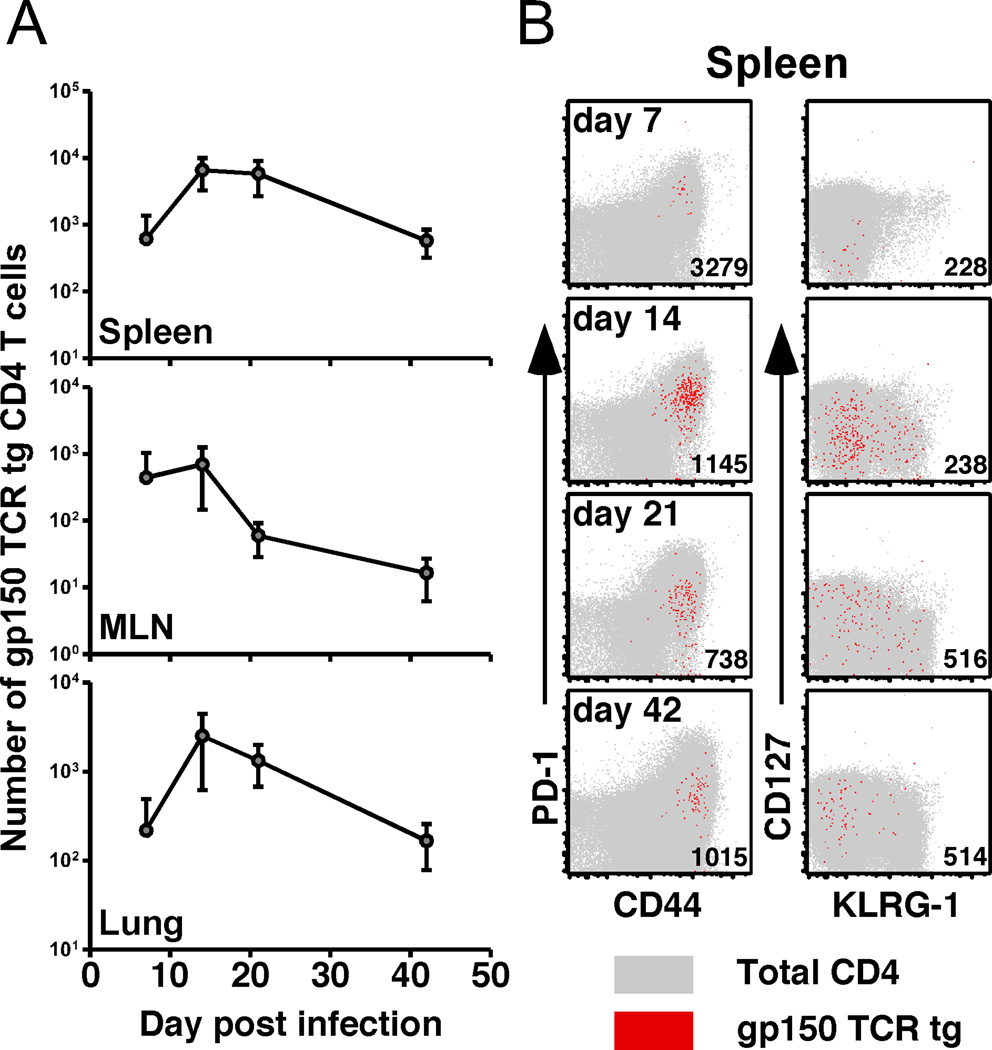
To analyze the ability of naïve CD4 T cells to respond to acute viral infection, we adoptively transferred 4000 gp150 TCR tg cells into congenic mice 1 day prior to γHV68 infection. Importantly, transferring 4000 tg CD4 T cells had no protective effect on either the initial lytic infection in the lung or the establishment of latency in the spleen (data not shown). As early as 7 days p.i., we could recover tg CD4 T cells from the spleens, MLN, and lung parenchyma (Figure 2A). These cells were highly activated, evidenced by expression of CD44 and PD-1, and a lack of CD127 expression in the spleen (Figure 2B). Over time, and consistent with the endogenous host CD4 T cells (shown in gray in Figure 2B), PD-1 levels decreased and CD127 expression increased on the donor tg cells. Unlike γHV68-specific CD8 T cells (15), we did not observe sustained expression of KLRG-1 on the tg CD4 T cells.
Figure 2.
CD4 T cell response kinetics after intranasal γHV68 infection. A, Number of gp150-specific TCR tg CD4 T cells recovered from spleens, MLN, and lungs at the indicated times (n=4–8/time point). B, Spleens were harvested at the indicated times. CD45.2+ gp150 TCR tg CD4 T cells from multiple mice are shown in red; the endogenous host CD45.1+ CD4 T cell population from a representative mouse is shown in gray. Numbers in the plots show average MFI for PD-1 (left column) or CD127 expression (right column) in the tg cells (n=4–8/time point, data are representative of 2 experiments).
Stimulation of gp150-specific transgenic CD4 T cells during latency
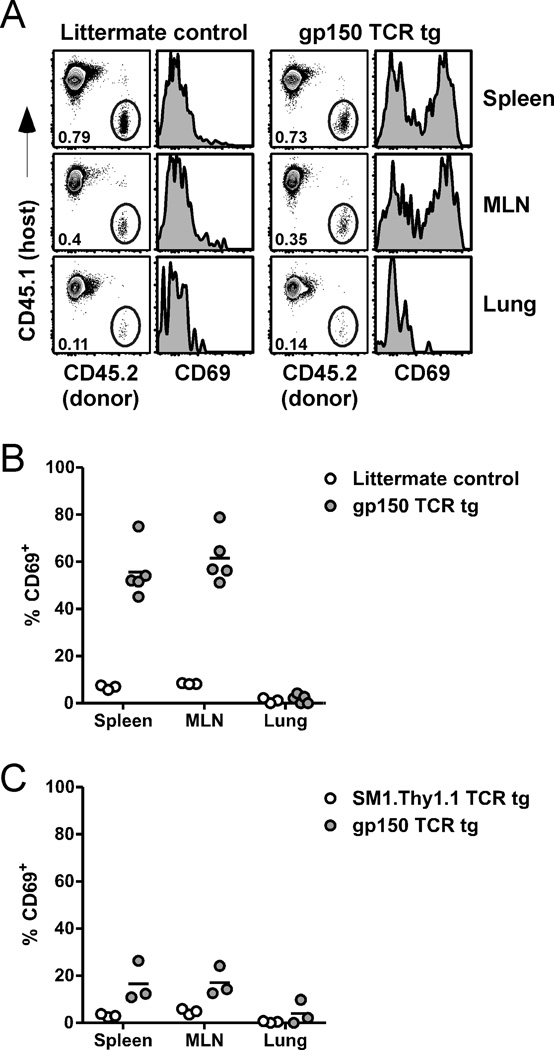
It has been shown previously that CD44hi CD4 T cells proliferate during γHV68 latency, as measured by incorporation of BrdU, and that treatment of latently-infected mice with a cell-cycle specific cytotoxic drug reduces gp15067–83-specific IFNγ production approximately 10-fold (10). These findings suggest that antiviral CD4 T cells are cycling continuously throughout the acute and latent infection, similar to observations with virus-specific CD8 T cells (16). The CD4 T cell response can be maintained either by proliferation of a self-renewing population or by the continual recruitment of naïve cells into the ongoing response (17). To determine whether naïve gp15067–83IAb-specific CD4 T cells can be activated and contribute to the ongoing immune response during γHV68 latency, we transferred CD4 T cells from uninfected gp150 TCR tg mice into latently-infected congenic mice and assessed their activation by measuring CD69 expression 18 hours later. When transferred into mice at 1 month p.i., an early stage of latency, we observed a robust increase in CD69 expression on gp150 TCR tg CD4 T cells, but not on cells from non-tg littermate control mice (Figure 3A,B). There was also a clear increase in CD69 expression on gp150 TCR tg CD4 cells transferred into latently-infected mice at 4 months p.i., although the percent of cells that expressed CD69 was reduced compared to the earlier time point (Figure 3C). Notably, at neither time did we observe activation of tg CD4 T cells recovered from the lungs of infected mice, suggesting the stimulatory environment in the lung is insufficient to activate naïve virus-specific CD4 T cells.
Figure 3.
Activation of naïve transgenic cells during latency. A, 18 h after transfer of cells into 1 month p.i. mice, spleens, MLN, and lungs from recipient mice were harvested. Dot plots show the percentage of CD4 T cells that are donor-derived. Histograms show the level of CD69 expression on the donor-derived cells. Data are representative of 3–5 mice. B, The percent of donor cells from panel A that are CD69+ is shown. C, In a separate experiment, CD45.2+ Thy1.2+ gp150 TCR tg cells and CD45.2+ Thy1.1+ SM1 TCR tg cells were co-transferred into CD45.1+ Thy1.2+ recipient mice 4 month p.i. 18 h later, spleens, MLN, and lungs were harvested and analyzed by flow cytometry. The percent of each donor population that is CD69+ is shown. All data are representative of at least 3 experiments.
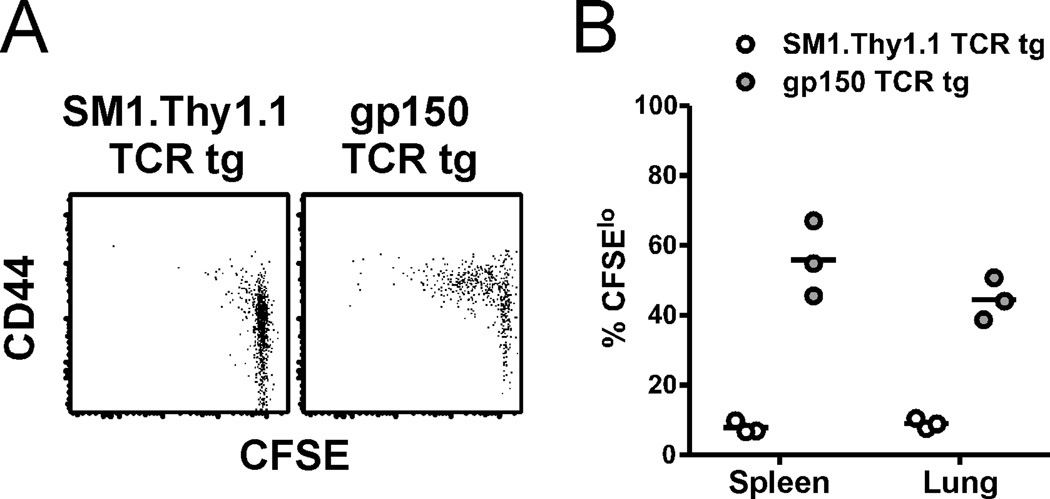
We next sought to measure whether naïve CD4 T cells activated during latency went on to proliferate. CFSE-labeled TCR tg CD4 T cells were transferred into B6.SJL mice that had been infected 2 months previously. We then assessed the donor T cells harvested from spleens or lungs for CD44 expression and CFSE dilution. At 7 days after transfer, we observed an increase in CD44 expression on, and CFSE dilution in, gp150 TCR tg CD4 T cells, but not co-transferred tg CD4 T cells of an irrelevant specificity, confirming that naïve virus-specific CD4 T cells could enter the antiviral immune response during latency (Figure 4A). However, even 14 days after transfer, not all the gp150-specific tg CD4 T cells had proliferated (Figure 4B), suggesting that naïve CD4 T cell recruitment during latency may be inefficient or occur infrequently. The apparent low-level recruitment may also be a consequence of the timing of gp15067–83IAb epitope expression – expression of the gp150 protein is associated with late, lytic infection (18) and it is not thought to be expressed during latency, so therefore, the gp15067–83IAb epitope may only be detectable by CD4 T cells during periodic episodes of virus reactivation. Consistent other persistent infection models (19, 20), our findings suggest a system in which antiviral T cells generated during acute γHV68 infection can be supplemented by recruitment of naïve cells during viral latency.
Figure 4.
Proliferation of naïve transgenic cells during latency. A, 7 d after transfer, spleens from recipient mice were harvested. Dot plots show the CFSE levels and CD44 expression on the indicated donor populations. B, 14 d after transfer, spleens and lungs were harvested and stained as in panel A. The percent of each donor population that exhibited CFSE dilution (CFSElo) is shown (n=3, representative of 2 experiments).
Both DCs and B cells from infected mice can stimulate naïve gp150-specific CD4 T cells
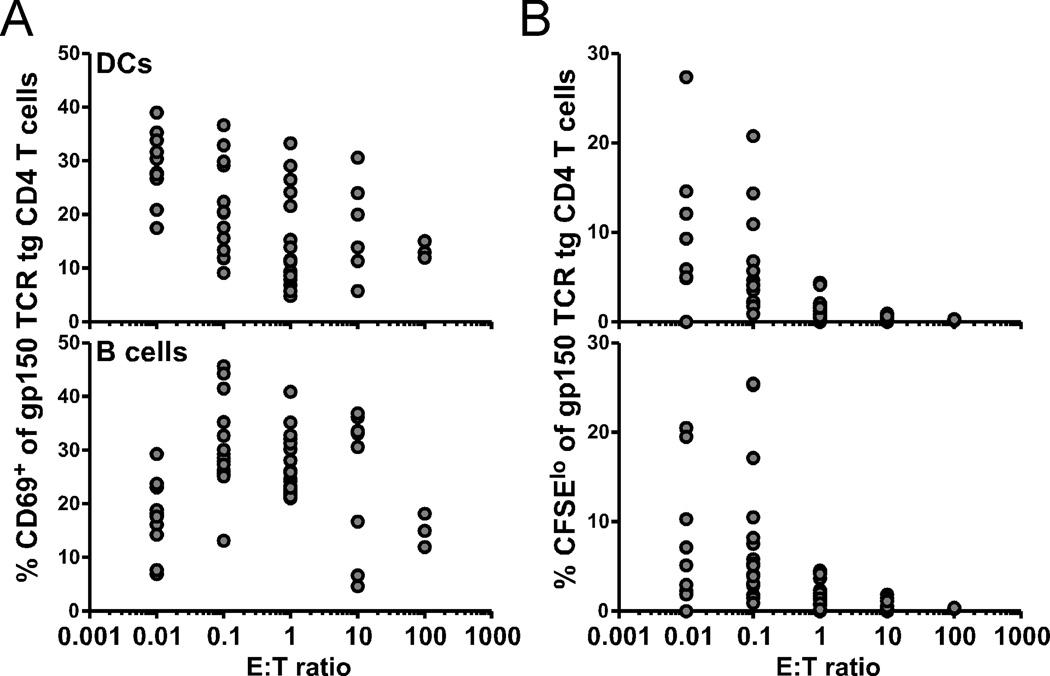
During γHV68 infection, it has been shown that CD8 T cells specific for a model lytic antigen and MHC class I-restricted γHV68-specific hybridomas are activated only by DCs (21, 22), even though B cells represent the major reservoir of latent virus (23). However, both DCs and B cells express MHC class II molecules, raising the possibility that both cell types could present antigens to CD4 T cells. To test this hypothesis, we sorted DCs and B cells from latently-infected mice and analyzed their ability to stimulate naïve gp150 TCR tg CD4 T cells. At an E:T ratio of 0.1, we observed a substantial increase of CD69 expression (Figure 5A) and CFSE dilution (Figure 5B) of the tg cells after stimulation by either DCs or B cells in vitro. We did not observe appreciable CD69 expression or proliferation (as measured by CFSE dilution) when naïve gp150 TCR tg CD4 T cells were stimulated by DCs or B cells from naïve mice (data not shown). Although the source of the antigenic signal in vivo is unclear, we conclusively show that both DCs and B cells isolated from the spleens of infected animals each retain the capacity to stimulate naïve CD4 T cells. This finding is in contrast to stimulation via MHC class I, which is limited to signals from the DC compartment (21, 22), and is consistent with data from EBV infections, where CD4 T cells can prevent lymphoproliferation by recognizing EBV antigens presented by B cells (24).
Figure 5.
Both DCs and B cells from infected mice can stimulate naïve CD4 T cells. DCs (top) and B cells (bottom) were sorted out of latently-infected B6.SJL mice 4 months p.i. and co-cultured with naïve CFSE-labeled gp150 TCR tg CD4 T cells at indicated E:T ratios. After 3 d, cultures were analyzed for CD69 expression (A) and dilution of CFSE (B) (n=3–10, representative of 2 experiments).
Given that CD4 T cells serve an important role in immunity to persistent γ-herpesvirus infections, it is essential to dissect the mechanisms of CD4 T cell generation and maintenance in order to better design antiviral therapies and vaccines. In the studies presented here, we describe the generation of a new CD4 TCR tg mouse that allowed us to answer three fundamental questions about CD4 T cell immunity during γHV68 infection – what are the kinetics of the CD4 T cell response to γHV68, can naïve CD4 T cells contribute to the ongoing immune response during latency, and what cell types can present antigens to antiviral CD4 T cells? First, we show that antiviral CD4 T cells specific for a late-lytic viral epitope were primed by 7 days p.i. and maintained for at least 42 days in multiple tissues. Second, we show that naïve antiviral CD4 T cells were continually recruited into the immune response in a tissue-specific manner during latent infection in vivo. Third, we demonstrate that both DCs and B cells from latently-infected mice stimulated antiviral CD4 T cells in vitro. These findings enhance our understanding of T cell immunity during γ-herpesvirus infections, providing key insight into the mechanisms by which the antiviral CD4 T cell response is activated and maintained during long-term infection.
Acknowledgments
We thank Pamela Eble for technical assistance, Sheri M. Eaton and Eric S. Huseby for technical advice, John W. Kappler and Philippa Marrack for generously providing essential reagents, and Jacob E. Kohlmeier and William W. Reiley for critical review of the manuscript.
Footnotes
This work was supported by NIH grants AI042927, AI082919, and CA148250 (to MAB), T32 AI049823 (to DLW), F32 AI084327 (to MLF), and funds from the Trudeau Institute.
References
- 1.Klenerman P, Hill A. T cells and viral persistence: lessons from diverse infections. Nat Immunol. 2005;6:873–879. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- 2.Robey RC, Lagos D, Gratrix F, Henderson S, Matthews NC, Vart RJ, Bower M, Boshoff C, Gotch FM. The CD8 and CD4 T-cell response against Kaposi's sarcoma-associated herpesvirus is skewed towards early and late lytic antigens. PLoS One. 2009;4:e5890. doi: 10.1371/journal.pone.0005890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munz C, Bickham KL, Subklewe M, Tsang ML, Chahroudi A, Kurilla MG, Zhang D, O'Donnell M, Steinman RM. Human CD4(+) T lymphocytes consistently respond to the latent Epstein- Barr virus nuclear antigen EBNA1. J. Exp. Med. 2000;191:1649–1660. doi: 10.1084/jem.191.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munz C, Moormann A. Immune escape by Epstein-Barr virus associated malignancies. Semin Cancer Biol. 2008;18:381–387. doi: 10.1016/j.semcancer.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.da Silva SR, de Oliveira DE. HIV, EBV and KSHV: viral cooperation in the pathogenesis of human malignancies. Cancer Lett. 2011;305:175–185. doi: 10.1016/j.canlet.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Taylor GS, Haigh TA, Gudgeon NH, Phelps RJ, Lee SP, Steven NM, Rickinson AB. Dual stimulation of Epstein-Barr Virus (EBV)-specific CD4+- and CD8+-T-cell responses by a chimeric antigen construct: potential therapeutic vaccine for EBV-positive nasopharyngeal carcinoma. J. Virol. 2004;78:768–778. doi: 10.1128/JVI.78.2.768-778.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merlo A, Turrini R, Bobisse S, Zamarchi R, Alaggio R, Dolcetti R, Mautner J, Zanovello P, Amadori A, Rosato A. Virus-specific cytotoxic CD4+ T cells for the treatment of EBV-related tumors. J. Immunol. 2010;184:5895–5902. doi: 10.4049/jimmunol.0902850. [DOI] [PubMed] [Google Scholar]
- 8.Virgin HW, Latreille P, Wamsley P, Hallsworth K, Weck KE, Dal Canto AJ, Speck SH. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, Usherwood EJ, Blackman MA, Woodland DL. T-Cell vaccination alters the course of murine herpesvirus 68 infection and the establishment of viral latency in mice. J. Virol. 1999;73:9849–9857. doi: 10.1128/jvi.73.12.9849-9857.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flano E, Woodland DL, Blackman MA, Doherty PC. Analysis of virus-specific CD4(+) T cells during long-term gammaherpesvirus infection. J. Virol. 2001;75:7744–7748. doi: 10.1128/JVI.75.16.7744-7748.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart JP, Janjua NJ, Pepper SD, Bennion G, Mackett M, Allen T, Nash AA, Arrand JR. Identification and characterization of murine gammaherpesvirus 68 gp150: a virion membrane glycoprotein. J. Virol. 1996;70:3528–3535. doi: 10.1093/benz/9780199773787.article.b00034574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McSorley SJ, Asch S, Costalonga M, Reinhardt RL, Jenkins MK. Tracking salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity. 2002;16:365–377. doi: 10.1016/s1074-7613(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 13.Reiley WW, Calayag MD, Wittmer ST, Huntington JL, Pearl JE, Fountain JJ, Martino CA, Roberts AD, Cooper AM, Winslow GM, Woodland DL. ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes. Proc. Nat. Acad. Sci. U.S.A. 2008;105:10961–10966. doi: 10.1073/pnas.0801496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhumabekov T, Corbella P, Tolaini M, Kioussis D. Improved version of a human CD2 minigene based vector for T cell-specific expression in transgenic mice. J Immunol Methods. 1995;185:133–140. doi: 10.1016/0022-1759(95)00124-s. [DOI] [PubMed] [Google Scholar]
- 15.Cush SS, Flano E. KLRG1+NKG2A+ CD8 T cells mediate protection and participate in memory responses during gamma-herpesvirus infection. J. Immunol. 2011;186:4051–4058. doi: 10.4049/jimmunol.1003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obar JJ, Crist SG, Leung EK, Usherwood EJ. IL-15-Independent Proliferative Renewal of Memory CD8+ T Cells in Latent Gammaherpesvirus Infection. J. Immunol. 2004;173:2705–2714. doi: 10.4049/jimmunol.173.4.2705. [DOI] [PubMed] [Google Scholar]
- 17.Lin E, Kemball CC, Hadley A, Wilson JJ, Hofstetter AR, Pack CD, Lukacher AE. Heterogeneity among viral antigen-specific CD4+ T cells and their de novo recruitment during persistent polyomavirus infection. J. Immunol. 2010;185:1692–1700. doi: 10.4049/jimmunol.0904210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Guzman D, Rickabaugh T, Wu TT, Brown H, Cole S, Song MJ, Tong L, Sun R. Transcription program of murine gammaherpesvirus 68. J. Virol. 2003;77:10488–10503. doi: 10.1128/JVI.77.19.10488-10503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snyder CM, Cho KS, Bonnett EL, van Dommelen S, Shellam GR, Hill AB. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29:650–659. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vezys V, Masopust D, Kemball CC, Barber DL, O'Mara LA, Larsen CP, Pearson TC, Ahmed R, Lukacher AE. Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J. Exp. Med. 2006;203:2263–2269. doi: 10.1084/jem.20060995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kupresanin F, Chow J, Mount A, Smith CM, Stevenson PG, Belz GT. Dendritic cells present lytic antigens and maintain function throughout persistent gamma-herpesvirus infection. J. Immunol. 2007;179:7506–7513. doi: 10.4049/jimmunol.179.11.7506. [DOI] [PubMed] [Google Scholar]
- 22.Cush SS, Anderson KM, Ravneberg DH, Weslow-Schmidt JL, Flano E. Memory generation and maintenance of CD8+ T cell function during viral persistence. J. Immunol. 2007;179:141–153. doi: 10.4049/jimmunol.179.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flano E, Husain SM, Sample JT, Woodland DL, Blackman MA. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 2000;165:1074–1081. doi: 10.4049/jimmunol.165.2.1074. [DOI] [PubMed] [Google Scholar]
- 24.Nikiforow S, Bottomly K, Miller G. CD4+ T-cell effectors inhibit Epstein-Barr virus-induced B-cell proliferation. J. Virol. 2001;75:3740–3752. doi: 10.1128/JVI.75.8.3740-3752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]