Abstract
The dose-limiting side effect of the anti-neoplastic agent, paclitaxel, is a chronic distal symmetrical peripheral neuropathy that produces sensory dysfunction (hypoesthesia and neuropathic pain) but little or no distal motor dysfunction. Similar peripheral neuropathies are seen with chemotherapeutics in the vinca alkaloid, platinum-complex, and proteasome inhibitor classes. Studies in rats suggest that the cause is a mitotoxic effect on axonal mitochondria. If so, then the absence of motor dysfunction may be due to mitotoxicity that affects sensory axons but spares motor axons. To investigate this, paclitaxel exposure levels in the dorsal root, ventral root, dorsal root ganglion, peripheral nerve, and spinal cord were measured, and the ultrastructure and the respiratory function of mitochondria in dorsal roots and ventral roots were compared. Sensory and motor axons in the roots and nerve had comparably low exposure to paclitaxel and exposure in the spinal cord was negligible. However, sensory neurons in the dorsal root ganglion had a very high and remarkably persistent (up to10 days or more after the last injection) exposure to paclitaxel. Paclitaxel evoked a significant increase in the incidence of swollen and vacuolated mitochondria in the myelinated and unmyelinated sensory axons of the dorsal root (as seen previously in the peripheral nerve) but not in the motor axons of the ventral root. Stimulated mitochondrial respiration in the dorsal root was significantly depressed in paclitaxel-treated animals examined 2-4 weeks after the last injection, whereas respiration in the ventral root was normal. We conclude that the absence of motor dysfunction in paclitaxel-evoked peripheral neuropathy may be due to the absence of a mitotoxic effect in motor neuron axons, while the sensory dysfunction may be due to a mitotoxic effect resulting from the primary afferent neuron's cell body being exposed to high and persistent levels of paclitaxel.
1. Introduction
The chemotherapeutic agent, paclitaxel, produces a distal, symmetrical, peripheral neuropathy characterized by sensory dysfunction (hypoesthesia and neuropathic pain) in the feet or in the hands and feet; chronic distal motor dysfunction in the same distribution is absent or rare (Lipton et al. 1989; Rowinsky et al. 1993; Forsyth et al., 1997; Quasthoff & Hartung, 2002; Dougherty et al., 2004; Windebank and Grisold, 2008; Park et al., 2011). The reason why paclitaxel affects sensory neurons but not motor neurons is unknown.
Rats treated with paclitaxel (four injections of 2 mg/kg on alternate days) develop a painful peripheral neuropathy like that seen clinically. Mechano-allodynia, mechano-hyperalgesia, and cold-allodynia (but no change in sensitivity to heat) first appear and reach peak severity with a distinct delay of 10 days or more (Flatters and Bennett, 2004, 2006; Bennett et al., 2011), similar to the “coasting” effect seen in patients where the neuropathy continues to worsen, or appears for the first time, during the interval between treatment cycles (van den Bent et al., 1997; Quastoff and Hartung, 2002). These animals have no degeneration of sensory neurons in the dorsal root ganglia (DRG) or of peripheral nerve axons, but do have partial degeneration of their intraepidermal sensory terminal arbors (Flatters and Bennett, 2006; Siau et al., 2006; Jin et al., 2008; Xiao et al., 2009). The degeneration appears after about the same delay that is seen for the pain symptoms (Bennett et al., 2011; Boyette-Davis et al., 2011).
Paclitaxel has a toxic effect on the mitochondria in axons in the peripheral nerve. The effect is manifest by a structural change -- an abnormal incidence of axonal mitochondria that are swollen and vacuolated, and by functional deficits -- impaired Complex I-mediated and Complex II-mediated respiration and ATP production (Flatters and Bennett, 2006; Flatters et al., 2006; Jin et al., 2008; Xiao et al., 2009; Zheng et al., 2011). However, this evidence comes from studies of mixed nerves and so it is impossible to say whether the affected mitochondria are in sensory or motor axons.
We have hypothesized that the symptoms of paclitaxel-evoked peripheral neuropathy are all consequences of an axonal energy deficit due to the mitotoxicity (Flatters and Bennett, 2006; Flatters et al., 2006; Siau and Bennett, 2006; Jin et al., 2008; Xiao and Bennett, 2008; Xiao et al., 2009; Bennett et al., 2011; Zheng et al., 2011). If the hypothesis is correct, then the absence of motor deficits implies that paclitaxel has little or no effect on mitochondria in motor axons. To investigate this prediction, we first measured the paclitaxel exposure of the sensory neurons in the DRG, the sensory axons in the dorsal root (DR), and the motor neurons in the spinal cord and their axons in the ventral root (VR). We then compared mitochondrial structure and function in DR vs. VR axons using electron microscopy and an assay of mitochondrial respiration.
2. Experimental Procedures
The experimental protocols were approved by the Animal Care and Use Committee of the Faculty of Medicine, McGill University, in accordance with the regulations of the Canadian Council on Animal Care and the ethical guidelines of the Canadian Institutes of Health Research, the National Institutes of Health (USA), and the International Association for the Study of Pain (Zimmermann, 1983).
2.1. Animals
Adult male Sprague-Dawley rats (200-300g, Harlan Inc., Indianapolis, IN; Frederick, MD breeding colony) were housed on sawdust bedding in plastic cages. Artificial lighting was provided on a fixed 12 hour light-dark cycle with food and water available ad libitum.
2.2. Paclitaxel
Paclitaxel (Taxol®; Bristol-Myers-Squibb, 6mg/ml) was diluted with saline to a concentration of 2 mg/ml and injected IP. Rats received our standard dosing regimen (Flatters and Bennett, 2004, 2006; Polomano et al., 2001): 2 mg/kg on four alternate days (days 0 (D0), D2, D4 and D6); total dose: 8 mg/kg). Control animals were injected with 1 ml/kg of the vehicle on the same schedule.
2.3. Paclitaxel measurement
Control rats (n = 4) and paclitaxel-treated rats (n = 6/group) were sacrificed on D7 (24h after the last paclitaxel injection) and on D16 (10 days after the last injection) via a sodium pentobarbital overdose (150 mg/kg, IP). Blood was obtained via cardiac puncture and plasma was obtained via centrifugation. The liver and brain (transected rostrally at the level of the olfactory bulbs and caudally at the level of the superior colliculi, and with the cerebellum removed) were harvested. Laminectomies exposed the L4-L6 DRG and spinal cord segments. The L4-L6 DR and VR were carefully dissected from the level of the DRG to their respective spinal cord segments (identified via their dorsal root entry zones), excised, and pooled bilaterally (i.e., 6 dorsal and 6 ventral roots per animal). The L4-L6 DRGs were isolated, excised (with care taken to exclude that portion of the VR that lies beneath the ganglion), and pooled. The L4-L5 spinal cord segments were excised en bloc. The sciatic nerves were excised bilaterally from the sciatic notch to the popliteal fossa and pooled. All samples were immediately frozen on dry ice and kept at -80° C until assayed.
Paclitaxel content was quantified with liquid chromatography combined with tandem mass spectrometry (LC-MS/MS). Tissue samples were homogenized in buffer (1/10 w/w, or in a minimal volume of 500 μl), solubilized with methanol and protein removed with acetonitrile. Samples were separated on a reversed phase LC-column (Polaris C18 3 μm, 50×4.6 mm; Varian, Palo Alto, CA). Mobile phases consisted of 0.1 % formic acid (solvent A) and acetonitrile (solvent B). Chromatographic separation was obtained by gradient elution: 70%/30% (A/B) to start and 5%/95% at completion; 3 min duration; 1.2 ml/min flow rate. Tandem MS analysis was carried out on an API-4000 MS/MS (Applied Biosystems, Toronto, ON) which was coupled to the HPLC-system (Agilent Technologies; Palo Alto, CA). The MS/MS was operated in the positive ion mode using electrospray ionization (TurboIonSpray interface) and was optimized for the quantification of paclitaxel (multiple reaction monitoring (MRM) transition: m/z 854.3 > 569). The quantification limit (QL) was 0.5 ng/ml for plasma and 5.0 ng/g for tissue. The accuracy (intra-batch accuracy from independent samples containing known concentrations) was between 80% and 120% of the nominal value over the entire range for plasma and tissue samples.
2.4. Paclitaxel's effects on motor function
Motor function has not been formally assessed with the paclitaxel treatment protocol used here. To remedy this, we examined paclitaxel's effects in the roto-rod test and assessed the status of motor nerve conduction.
Vehicle-treated and paclitaxel-treated rats (n = 8/group) were given three training trials on the roto-rod (7 cm diameter rod; 20 rpm) during the week before the first paclitaxel injection. Each trial lasted about 5 min and consisted of replacing the animal when it fell off the rod until the animal had achieved a criterion performance of 60 sec on the rod without falling. In order to confirm that the motor nerve conduction velocities (MNCV) data were from animals that had the paclitaxel-evoked sensory abnormalities, baseline responses to 4 g and 15 g von Frey hair (VFH) stimuli were obtained prior to and after treatment. On days D28 (30 days after the last roto-rod training session and 22 days after the last injection of paclitaxel) the behavioral pain assays were repeated and this was followed by a trial of roto-rod performance. On the following days, MNCV was assessed in the same animals. For comparison to the MNCV, we also assessed the status of conduction in the sural nerve, which in the rat is a nearly pure sensory nerve (Swett et al., 1986, 1991).
Roto-rod performance was assessed with a single trial with a 120 sec cut-off. VFH testing followed methods described elsewhere (Flatters and Bennett, 2004). An increased response frequency to the normally innocuous 4 g stimulus is indicative of mechano-allodynia; increased response frequency to the slightly painful 15 g stimulus indicates mechano-hyperalgesia.
For MNCV assessment, the rat was anesthetized with sodium pentobarbital (50 mg/kg, IP) for cannulation of the trachea and then placed on a ventilator. Respiration was adjusted to maintain expired CO2 in the normal range and anesthesia was maintained with isoflurane (1.0%). The flexor hallucis brevis muscle was exposed via midline incision in the ventral hind paw. The MNCV was recorded via an electrode inserted into the muscle following stimulation of the muscle's innervation, first at the level of the sciatic notch (S1) and then at the ankle (S2; i.e., the tibial nerve at the level of the medial malleolus). Stimulation was via percutaneous bipolar needle electrodes with 0.1 msec duration pulses at 1.0 Hz and intensity set at 25% above the level that evoked the maximal amplitude potential. The conduction velocity was determined by subtracting the latency to onset of the potential from S2 from that of S1 and measuring the distance between the cathodes of the two stimulating electrodes. Sensory nerve conduction was assessed by stimulating suralis at the level of the ankle (0.1 msec pulses, 1.0 Hz, intensity at 25% above the level that evoked the maximal amplitude potential) and recording from the distal end of the transected nerve in the popliteal fossa. We measured the sensory nerve conduction velocity (SNCV) to the onset of the potential, the velocity to the peak of the potential, the duration of the potential, and the potential's peak amplitude.
2.5. Electron microscopy
Vehicle-treated and paclitaxel-treated rats (n = 4/group) were sacrificed on D27, the approximate time of onset of peak neuropathic pain severity, the time of near maximal degeneration of intraepidermal nerve fibers, and a time at which functional mitochondrial deficits are demonstrable in peripheral nerve axons (Flatters and Bennett, 2004, 2006; Jin et al., 2008; Xiao et al., 2009; Bennett et al., 2011; Zheng et al., 2011). Following the induction of deep anesthesia via a sodium pentobarbital overdose (150 mg/kg; IP), the animals were perfused transcardially with a vascular rinse (0.1M PBS containing 0.05% sodium bicarbonate and 0.1% sodium nitrite) followed by freshly prepared mixture of 2% paraformaldehyde and 2% glutaraldehyde in 0.1M PBS, pH 7.4.
The lumbosacral vertebral column was excised and post-fixed for 3 h in the fixative described above. L4-L5 DR and VR samples were obtained as described above. A 0.5 cm long section of the saphenous nerve was excised from the middle of the lower leg and post-fixed for 3 h. The saphenous nerve was selected for the sake of comparison to prior studies of paclitaxel-evoked and vincristine-evoked painful peripheral neuropathies (Tanner et al., 1998; Topp et al., 2000; Flatters and Bennett, 2006; Jin et al., 2008; Xiao et al., 2009). Tissues were placed in 10% sucrose in 0.1 M PBS and kept at 4° C for 12 h, then incubated in 1% osmium tetroxide in 0.1 M PB, pH 7.4, at 4° C for 2 h, dehydrated in ascending concentrations of alcohol and propylene oxide at room temperature, and embedded in Epon. Ultrathin sections were acquired with a microtome using a diamond knife, collected on Formvar-coated grids, and counterstained with lead citrate and uranyl acetate. Grids were observed in a Philips EM410 electron microscope operated at 80 kV. Photomicrographs were taken and analyzed using a Megaview II CCD camera and AnalySIS 5.0 software (both from Soft Imaging System Corp., Lakewood, CO).
Random samples of mitochondria in axons (A-fibers and C-fibers) and Schwann cells (myelinating and non-myelinating) were obtained in the following way. Starting at the 7 o'clock position on the section and moving horizontally, every third field was photographed (10,200 X) until the edge of the section was reached. The section was then moved two fields vertically and the horizontal scan was resumed as before. For the VR, the scanning procedure was continued until at least 60 A-fibers and 60 myelinating Schwann cells were photographed. For the DR and nerve, the scan continued until at least 60 A-fibers, 60 myelinating Schwann cells, and 60 C-fibers were photographed. Remak bundles usually appear as several adjacent groups of C-fibers embedded in Schwann cell processes that are separated by extracellular space. Although it is very probable that such groups are formed by processes from the same Schwann cell, this can not be established without reconstructing serial sections, and it is thus difficult to enumerate the number of non-myelinating Schwann cells in a section. Therefore, to sample mitochondria in non-myelinating Schwann cells in the DR and nerve we continued the horizontal scanning procedure until we had photographed a sufficient number of Remak bundles to count at least 50 mitochondria.
All of the mitochondria in the axoplasm of each axon and in the cytoplasm of each myelinating and non-myelinating (i.e., Remak bundle) Schwann cell were counted. As in previous studies (Flatters and Bennett, 2006; Jin et al., 2008; Xiao et al., 2009), normal mitochondria were identified as circular or oval profiles with double membranes that had at least one axis of at least 165 nm in length. The minimal length requirement eliminated profiles that are due to sections that pass through the end of the mitochondrion, where the status of the cristae would be difficult to ascertain. Mitochondria were counted as atypical if they had at least a 2-fold increase in diameter and/or if 50% or more of the interior was electron-lucent.
2.6. Mitochondrial respiration assay
Vehicle-treated and paclitaxel-treated rats (n = 8/group) were sacrificed 21-30 days after the last injection. A pair of rats, one control and one paclitaxel-treated, was examined on each day with the order of testing counterbalanced. The rats were deeply anesthetized (2% isoflurane) and their L4-L6 DR and VR were harvested and placed in ice-cold mitochondrial respiration medium (MiR05; see below); the animal was then euthanized with a sodium pentobarbital overdose. Each root was minced into segments about 1 mm long and each segment was teased into filaments using #5 watchmaker's forceps. This is the same preparation that was used to assess mitochondrial function in peripheral nerve samples (Zheng et al., 2011).
The DR and VR samples were then transferred to separate recording chambers of a high-resolution respirometer (Oxygraph 2K; Oroboros Instruments; Innsbruck, Austria) and the chamber sealed. The recording chambers were maintained at 37° C and contained 2 ml of respiration medium that had been equilibrated for 30 min at in air such that a stable O2 concentration was obtained. Stable O2 consumption rates were present within 5 min of adding the preparation and sealing the chamber. The MiR05 respiration medium contained: 0.5 mM EGTA, 3.0 mM MgCl2, 60 mM K-lactobionate, 20 mM taurine, 10 mM KH2PO4, 110 mM sucrose, 0.1%; BSA, and 20 mM HEPES (Gnaiger et al., 2000).
The rate of O2 consumption was determined before and after the addition of a mixture of substrates for Complex I (NADH:ubiquinone oxidoreductase) and Complex II (succinate dehydrogenase) respiration (5.0 mM glutamate and 2.5 mM maleate, and 5.0 mM succinate, respectively), and then after stimulating respiration via the addition of ADP (1.0 mM). Pilot studies showed that this amount of ADP produces maximum stimulation of the oxidative phosphorylation system. O2 consumption was recorded when the response to each addition had reached a stable plateau.
O2 consumption rates were normalized with respect to the activity levels of the mitochondrion-specific enzyme, citrate synthase. Immediately after the respiration assay, the sample was removed from the recording chamber and stored at -80° C until enzyme activity was determined. For citrate synthase determination, the sample was homogenized in 0.8 ml lysis medium (CelLytic TM; Sigma, St. Louis, MO), centrifuged at 12,000 g for 12 min at 4° C, and the supernatant used to assay enzymatic activity according to the manufacturer's protocol (Citrate Synthase Assay Kit; Sigma, St. Louis, MO). Results were expressed as units of citrate synthase (CSU) activity: 10-7 mol/min/mg protein.
3. Results
3.1. Paclitaxel tissue levels
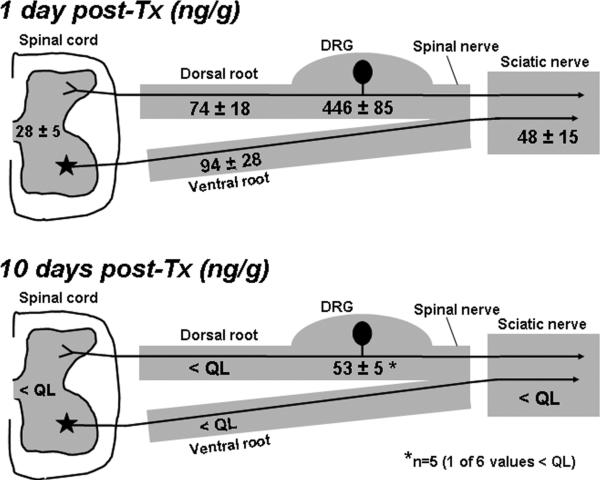
On D7 (24 h after the last injection), paclitaxel was clearly concentrated in the DRG (446 ± 85 ng/g) (Fig. 1). Lower levels were found in the DR (74 ± 18 ng/g), VR (94 ± 28 ng/g), and sciatic nerve (48 ± 15 ng/g). The amount of paclitaxel in brain was near the limit of quantification (8.1 ± 2.5 ng/g). The amount in spinal cord was greater but still quite low (28 ± 5 ng/g). The amount of paclitaxel in plasma was 9.4 ± 2.8 ng/ml. A very high paclitaxel level was present in the liver (744 ± 420 ng/g).
Fig. 1.
Paclitaxel levels one day after the treatment (Tx) protocol (D7) and 10 days post-treatment (D16) in L4-L5 spinal cord segments, L4-L6 dorsal roots (DR), L4-L6 ventral roots (VR), L4-L6 dorsal root ganglia (DRG), and sciatic nerves; (ng/g; mean ± SEM; n = 6/group). < QL: below the level of quantification (5.0 ng/g). * mean of 5 of 6 rats (1 <QL).
Ten days after the last injection (D16), paclitaxel was below the limit of quantification in DR, VR, sciatic nerve, spinal cord, brain, and plasma. In the DRG, 1 of 6 rats was below the limit of quantification; the average for the remaining 5 rats was 53 ± 5 ng/g. Levels in liver were below the limit of quantification in 3 of the 6 rats and averaged 8.0 ± 1.5 ng/g in the others.
3.2. Paclitaxel's effects on motor function
As shown in Table 1, paclitaxel treatment had no significant effect on roto-rod performance or MNCV. In these same animals, paclitaxel treatment produced the expected statistically significant mechano-allodynia and mechano-hyperalgesia, while the vehicle-treated control group had no significant change in response to the 4 g and 15 g VFH stimuli.
Table 1.
Paclitaxel effects on sensorimotor function.
| Control | Paclitaxel | |
|---|---|---|
| Motor: | ||
| Roto-rod (sec) | 119.4 ± 0.6 | 117.9 ± 1.9 |
| MNCV (m/s) | 46.95 ± 2.23 | 47.50 ± 5.74 |
| Sensory: | ||
| Mechano-allodynia: baseline → post-paclitaxel response freq. | 5.0 ± 1.3 → 6.3 ± 1.8 | 5.0 ± 1.4 → 52.5 ± 3.1* |
| Mechano-hyperalgesia: baseline → post-paclitaxel response freq. | 12.5 ± 2.0 → 16.3 ± 3.2 | 14.3 ± 2.1 → 73.8 ± 5.0* |
| SNCV | Onset: 43.12 ± 1.2 m/sec Peak: 32.39 ± 0.9 m/sec Duration: 0.61 ± 0.0 msec Amplitude: 3.46 ± 0.2 mV |
Onset: 43.28 ± 2.1 m/sec Peak: 31.45 ± 1.0 m/sec Duration: 0.66 ± 0.0 msec Amplitude: 3.01 ± 0.2 mV |
All values are mean ± SEM for the same groups of rats (n = 8/group). MNCV: motor nerve conduction velocity; SNCV: sensory nerve conduction velocity.
p < 0.001 vs. own baseline (t-tests).
Paclitaxel treatment had no significant effect on any of the parameters of sensory nerve conduction (Table 1). This is consistent with the absence of paclitaxel-evoked degeneration of peripheral nerve axons (Flatters and Bennett, 2006; Bennett et al., 2011).
3.3. Effects of paclitaxel on axonal mitochondrial ultrastructure
Paclitaxel caused a statistically significant increase in the incidence of swollen and vacuolated mitochondria in A-fiber and C-fiber axons in the DR and saphenous nerve, but not in the VR's A-fiber motor axons. The swollen and vacuolated mitochondria in the roots appeared identical (Fig. 2) to those previously described in peripheral nerve (Flatters and Bennett, 2006).
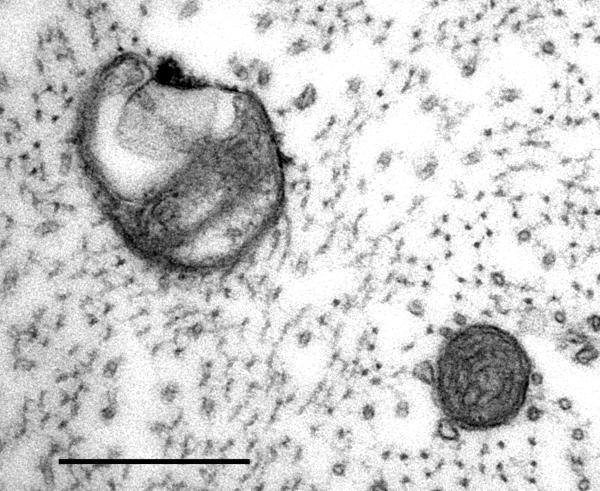
Fig. 2.
Axoplasmic mitochondria in a dorsal root A-fiber of a paclitaxel-treated rat. The mitochondrion on the upper left is swollen and its cristae have collapsed into one pole, leaving a large vacuole at the other pole. Normal mitochondria (lower right) have smaller diameters, intact cristae, intact double membranes, and no vacuoles. 53,000X; scale bar: 0.5 μm.
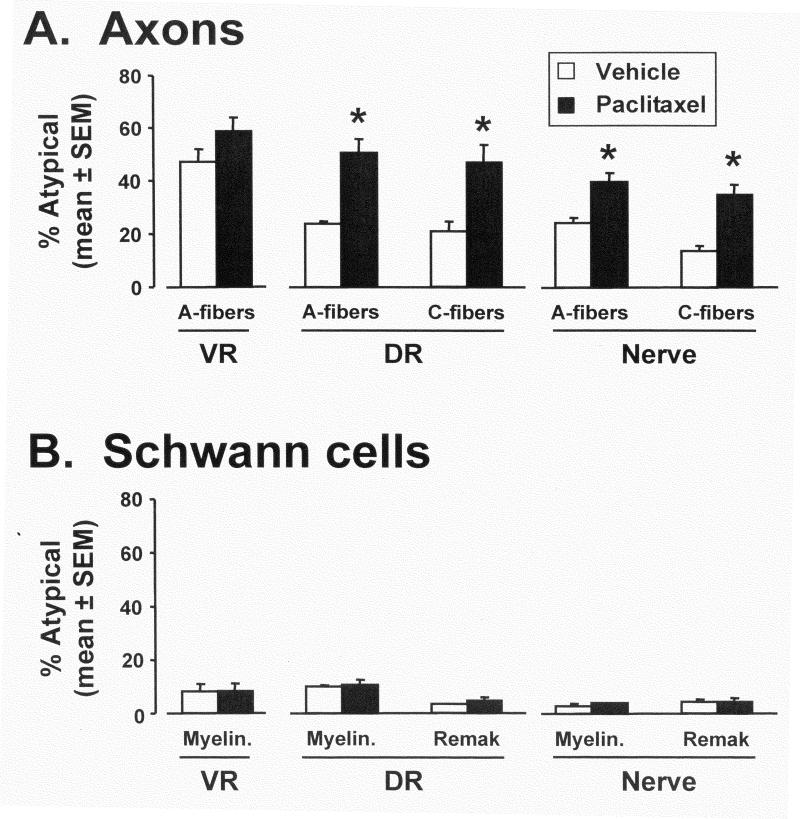
A-fiber sensory axons in the DR of the control group contained (mean ± SEM) 23.7 ± 0.1% (total number of mitochondria examined: n = 878) swollen and vacuolated mitochondria vs. 50.4 ± 5.3% (n = 1113) in the paclitaxel-treated group (Fig. 3A). This is a statistically significant mean increase of 112.7%. C-fiber sensory axons in the DR of the control group contained 21.0 ± 3.7% (n = 249) swollen and vacuolated mitochondria vs. 46.7 ± 6.4% (n = 271) in the paclitaxel-treated group. This is a statistically significant mean increase of 122.4%.
Fig. 3.
A. Incidence (mean ± SEM) of atypical (swollen and vacuolated) mitochondria in A-fiber motor axons in the ventral root (VR), myelinated A-fiber and unmyelinated C-fiber sensory axons in the dorsal root (DR), and in the saphenous nerve in vehicle-treated and paclitaxel-treated rats. B. Incidence (mean ± SEM) of swollen and vacuolated mitochondria in the myelinating Schwann cells in the ventral root (VR), myelinating and non-myelinating (Remak bundle) Schwann cells in the dorsal root (DR), and myelinating and non-myelinating Schwann cells in the saphenous nerve in vehicle-treated and paclitaxel-treated rats. * p < 0.05 control vs. paclitaxel-treated (Bonferroni-corrected t-tests).
For the A-fiber motor neuron axons in the VR, the incidence of swollen and vacuolated mitochondria in the control group was 47.1 ± 4.8% (n = 2066) vs. 58.5 ± 5.3% (n = 2123) in the paclitaxel-treated group. This is a statistically non-significant difference of 24.2%.
The results for the peripheral nerve were similar to those from the DR. For the peripheral nerve A-fiber axons: control group: 30.5 ± 4.6% (n = 1320) vs. paclitaxel group: 49.0 ± 2.2% (n = 1734); a statistically significant mean increase of 60.7%. For the peripheral nerve C-fiber axons: control group: 19.5 ± 3.8% (n = 378) vs. paclitaxel group: 41.3 ± 5.0% (n = 343); a statistically significant mean increase of 111.8%.
3.4. Effects of paclitaxel on the structure of Schwann cell mitochondria
Paclitaxel had no significant effect on the incidence of swollen and vacuolated mitochondria in myelinating Schwann cells in the DR, VR and peripheral nerve, or in the non-myelinating Schwann cells in the DR and nerve (Fig. 3B).
The incidence of swollen and vacuolated mitochondria in myelinating Schwann cells in the control group's DR was 10.1 ± 0.6% (n = 886) vs. 10.7 ± 1.7% (n = 975) in the paclitaxel-treated group. For the non-myelinating Schwann cells in the DR, the control group incidence was 3.3 ± 0.2 % (n = 242) vs. 4.2 ± 2.1% (n = 254) in the paclitaxel-treated group. The incidence of swollen and vacuolated mitochondria in the myelinating Schwann cells that surround the motor axons in the VR was 8.1 ± 2.8% (n = 1834) in the control group vs. 8.1 ± 2.7% (n = 1826) in the paclitaxel-treated group. For the myelinating Schwann cells in the saphenous nerve, the control group incidence was 6.0 ± 0.9% (n = 917) vs. 7.5 ± 1.6% (n = 980) in the paclitaxel-treated group. For the non-myelinating Schwann cells in the saphenous nerve, the control group incidence was 2.0 ± 1.0% (n = 245) vs. 2.4 ± 0.5% (n = 246) in the paclitaxel-treated group. None of the between-group differences are statistically significant.
3.5. Respiration in dorsal vs. ventral roots
Initial O2 consumption rates for the DR of vehicle-treated and paclitaxel-treated rats were very low and not significantly different (mean ± SEM; pmol O2/s/CSU): 12.0 ± 0.8 vs. 12.7 ± 2.4, respectively. The same was true for the VR: 4.7 ± 1.1 vs. 5.8 ± 1.4, respectively.
The addition of substrates for Complex I and II increased O2 consumption in the DR and VR from both vehicle-treated and paclitaxel-treated rats; the between-group differences were not significantly different: DR: 44.2 ± 1.9 vs. 48.5 ± 4.5; VR: 31.9 ± 2.3 vs. 31.1 ± 4.4.
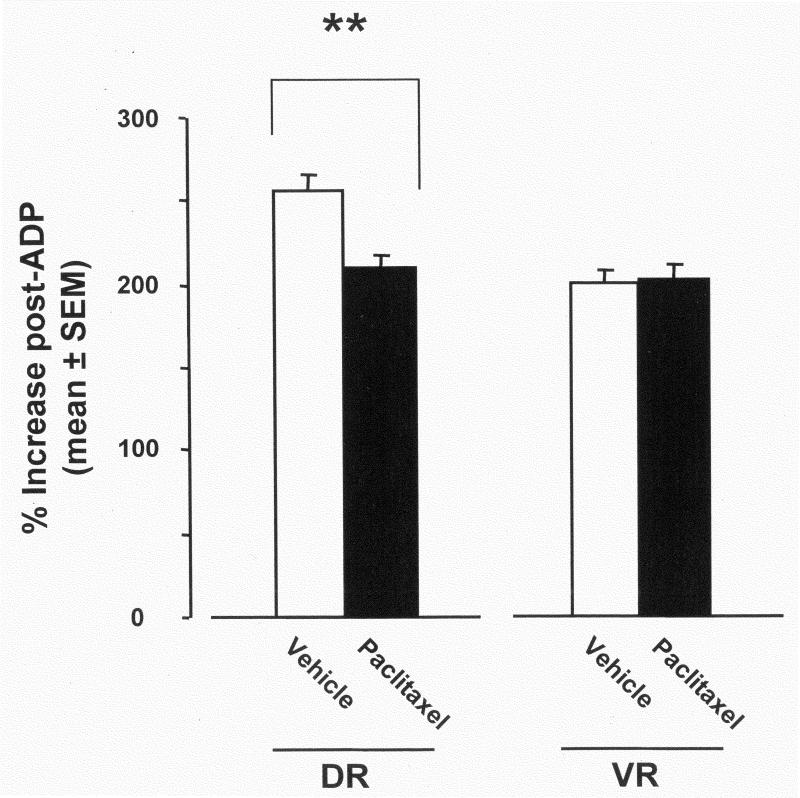
Stimulation of respiratory complex activity by the addition of ADP evoked the expected increases in O2 consumption rates in both vehicle-treated and paclitaxel-treated groups: DR: 113.1 ± 6.5 vs. 100.5 ± 8.0; VR: 64.2 ± 6.4 vs. 64.0 ± 10.3, respectively. The percent increase (relative to the non-stimulated rate) (Fig. 4) in the DR of the vehicle-treated controls (236 ± 0.1%) was significantly greater (52%) than in the paclitaxel-treated group (184 ± 0.1%). There was no significant difference between the percent increases seen in the VR (control group: 199 ± 0.01% vs. paclitaxel group: 201 ± 0.1%).
Fig. 4.
Percent increases in respiration rates after stimulation of the oxidative phosphorylation system with ADP in the DR and VR of vehicle-treated and paclitaxel-treated rats. ** p < 0.01; t-test.
4. Discussion
4.1. Paclitaxel distribution
Paclitaxel was greatly concentrated in the DRG one day after the last injection (D7). At this time, paclitaxel was also present in the DR, VR, and sciatic nerve, where the levels were similar to one another but 5-9 times lower than in the DRG. Our DRG and peripheral nerve results are consistent with a report from Cavaletti et al. (2000) who sampled one day after the last injection of a paclitaxel dosing protocol (5 mg/kg/day X 5, IV) that yields hypoesthesia, rather than the allodynia and hyperalgesia seen with our dosing protocol.
The large amount of paclitaxel found in the DRG on D7 is at least partly due to the presence of the fenestrated capillaries that are found in the dorsal, cell-rich region of the DRG (Olsson, 1990; Jimenez-Andrade et al., 2008). Fenestrated capillaries are not present in the DR, VR and peripheral nerve (Olsson, 1990; Pettersson et al., 1990); nevertheless, paclitaxel was present in each of these regions on the day after the last injection. It is known that the blood-nerve-barrier is functionally distinct from the blood-brain-barrier. Paclitaxel probably passes into the DR, VR, and nerve via pinocytotic trafficking across capillary endothelial cells (Olsson, 1990; Pettersson et al., 1990). A barely detectable level of paclitaxel was found in the brain on D7. As there is no evidence suggesting that paclitaxel crosses the blood-brain-barrier, this trace amount is almost certainly due to passage through the circumventricular organs. On D7, paclitaxel was found in the lumbar spinal cord; the level was very low but still distinctly greater than the trace found in brain. The blood-spinal cord-barrier is believed to be identical to its brain counterpart and so permeation via the spinal cord vasculature is unlikely. The paclitaxel in the spinal cord may arrive via axonal transport.
The noteworthy result of this experiment is that our treatment protocol yields a very high level of paclitaxel in the DRG and that this is remarkably persistent, with measurable amounts present on D16, 10 days after the last injection. We found no evidence that paclitaxel persists in DR, VR, or peripheral nerve; but, of course, we can not exclude the possibility that there is persistence at a level that is below our limit of quantification but nevertheless significant for the pathology.
The persistence of paclitaxel within the DRG is likely to be related to its binding to β-tubulin. A fraction of paclitaxel bound to β-tubulin would be expected to become sequestered into microtubules (Kingston et al., 2005). It may be of particular importance that at least some paclitaxel will become bound to the β-tubulin moiety that is associated with the voltage dependent anion channel (VDAC) (Carrè et al., 2002; Kidd et al., 2002). VDAC is a component of the mitochondrial permeability transition pore and paclitaxel bound to β-tubulin is known to alter VDAC function (Rostovtseva et al., 2008). It is possible that binding to other proteins also contributes to the persistence, e.g., bcl-2 (Ferlini et al., 2009; Rodi et al., 1999).
4.2. No paclitaxel effect on indices of motor function
The treatment protocol used here had no effect on MNCV and no effect on roto-rod performance in rats with established sensory dysfunction. We conclude that our protocol produces a purely sensory peripheral neuropathy. In the clinic, paclitaxel produces an acute pain state characterized by myalgia and arthralgia in the proximal joints and muscles (Loprinzi et al., 2011). However, clinical experience shows that paclitaxel rarely, if ever, causes chronic motor dysfunction with the bilaterally symmetrical distribution in the feet, or the hands and feet, which characterizes the sensory symptoms (Agosto et al., 2008).
4.3. Differential effects on the structure of mitochondria in sensory and motor axons
Paclitaxel caused significant increases in the incidence of swollen and vacuolated mitochondria in sensory axons in the DR, but not in motor axons in the VR. The differential effect was apparent even when the comparison was restricted to the DR's myelinated fibers (Fig. 3A). The increases of swollen and vacuolated mitochondria in DR A-fiber and C-fiber axons were comparable to those seen in the saphenous nerve (Flatters and Bennett, 2006; Jin et al., 2008; Xiao et al., 2009).
It is noteworthy that paclitaxel had no effect on the structure of the mitochondria in Schwann cells in the DR, VR and nerve, even though the Schwann cells must be exposed to the same amounts of paclitaxel as the axons that they surround. This indicates that the degree of exposure to paclitaxel is not the only factor accounting for mitotoxicity; cell-type specific factors must also make an important contribution.
Swollen and vacuolated mitochondria are found in peripheral nerve axons from normal rats (Flatters and Bennett, 2006; Jin et al., 2008) and here we found the same in the DR and VR axons of normal rats. The reason for the presence of such mitochondria in normal axons is not understood, although there is evidence showing that swelling and vacuolation may be associated with the degree of mitochondrial activity at the time of exposure to aldehydes (Brewer and Lynch, 1986). The rarity of swollen and vacuolated mitochondria in Schwann cells shows that their presence is not a simple artifact of inadequate fixation. The incidence in the control group's myelinated VR axons was particularly high, being about twice that found in the control group's myelinated DR axons. This suggests that under normal conditions there may be a distinct functional difference in the mitochondria in motor and sensory myelinated axons.
4.4. Differential effects on the function of mitochondria in sensory and motor axons
The physiological significance of the paclitaxel-evoked increase in the incidence of swollen and vacuolated mitochondria is ambiguous, especially given their presence in normal axons. It is thus important that we found a significant deficit in mitochondrial function in the DR but not in the VR of paclitaxel-treated rats.
Paclitaxel-evoked deficits in Complex I-mediated and in Complex II-mediated respiration (measured separately) have been noted in a sciatic nerve preparation (Zheng et al., 2011). In the present experiments, Complex I and Complex II activities were assessed together via the simultaneous addition of their substrates. Initial respiration rates (before substrate addition) were very low in DR and VR and similar in both groups. This is not surprising because the medium contains no substrates for respiration; any activity that is present must be due to endogenous stores. The addition of substrates evoked similar increases in respiration rates in both the DR and VR of both groups. The absence of a significant between-group difference after the addition of substrates is also not surprising because this condition represents baseline energy demand. Functional deficits are expected to be most apparent when a system is activated and operating in the upper part of its physiological range. Mitochondrial respiration is activated when energy demand increases, and this is signaled via ADP levels. Thus, respiration in all samples was greatly increased after the addition of ADP.
The important finding here is that the magnitude of the increase evoked by ADP-stimulation was significantly less than normal in the DR, but not in the VR, after paclitaxel treatment. It is noteworthy that the deficit in DR respiration was seen 2-4 weeks after dosing, when paclitaxel could not be detected in the DR. It is thus clear that paclitaxel causes persistent mitochondrial dysfunction and that this is likely to result in a persistent energy deficiency.
4.5. Why sensory and not motor?
It is clear that primary afferent sensory neurons and motor neurons receive very different exposures to paclitaxel and it is difficult to escape the conclusion that this is why the drug produces a sensory but not a motor neuropathy. However, differential exposure is probably not the whole story. First, despite the large and persistent exposure in the DRG, there is no evidence of degeneration of DRG neurons with the treatment protocol used here (Flatters and Bennett, 2006). Studies that used doses of paclitaxel higher than those used here have reported various pathological changes in the DRG, but none of these studies has reported DRG cell degeneration (Cavaletti et al., 1995, 1997, 2000; Authier et al., 2000; Persohn et al., 2005; Jimenez-Andrade et al., 2006; Jamieson et al., 2007; Peters et al., 2007a,b). The only paclitaxel-evoked lesion that has been found with the treatment protocol used here is very far from the DRG - degeneration at the distal-most tip of the sensory axon, i.e., the intraepidermal nerve fiber (IENF) that forms the terminal receptor arbor (Siau et al., 2006; Jin et al., 2008; Xiao et al., 2009; Bennett et al., 2011; Boyette-Davis et al., 2011).
Second, significant IENF degeneration is not apparent until about 10 days after the last injection of paclitaxel and it takes another several days to peak. Thus, IENF degeneration occurs at a distinctly later time than the DRG exposure. The IENF degeneration and the pain appear to be linked. Both have about the same delays to onset and peak, and treatments that block the development of the pain also protect against the degeneration (Flatters et al., 2006; Jin et al., 2008; Xiao et al., 2009; Bennett et al., 2011; Boyette-Davis et al., 2011; Zheng et al., 2011). Exposure to a high level of paclitaxel in the DRG does not account for the delays to symptom onset and peak.
Lastly, the greater exposure of mitochondria in sensory neurons does not account for the distribution of symptoms. It seems safe to assume that the mitochondria in sensory neurons in all DRG receive the same high and persistent exposure to paclitaxel. But the sensory symptoms occur first and predominately in the dermatomes innervated by ganglia L4-L5 and C6-C8 (feet and hands). It is noteworthy that paclitaxel-evoked sensory dysfunction is not present in the thoracic region or in the face (the trigeminal ganglion also has fenestrated capillaries (Olsson, 1990)) and the innervation density of the face (especially the lips) is as high as or higher than that of the feet and hands).
4.6. Conclusions
We have found that a paclitaxel dosing protocol in the rat that produces a painful peripheral neuropathy is associated with a mitotoxic effect in primary afferent sensory neuron axons but not in motor neuron axons. In addition, our data suggest that a high and persistent paclitaxel exposure to the sensory neuron's cell body in the DRG may contribute to the selective effect.
Chronic, distal, symmetrical, predominately sensory peripheral neuropathies are produced by other chemotherapeutics (Quasthoff and Hartung, 2002; Windebank and Grisold, 2008) and a mitotoxic effect that preferentially affects mitochondria in sensory neuron axons may be causative in all of these conditions. Determining whether this is true in patients will be difficult because the clinical situation is so complex: patients do not always receive identical treatment and they often have multiple potential causes of peripheral neuropathy and pain: e.g., tumor effects, prior or combination treatment with multiple neurotoxic agents, prior radiation therapy, co-morbidities like diabetes, etc. Additional work with animal models of these conditions may be necessary to answer this question.
Lehmann et al. (2011) have recently shown that there is an accumulation of mutations in mitochondrial DNA in distal peripheral nerve of man and monkey with immunodeficiency virus infection. They propose that this occurs during the long period of transit of mitochondria from the DRG to the distal axon (estimated to be two years or more for the human foot). This phenomenon is unlikely to play a role in the rat because the leg is too short, but clinically the idea is not incompatible with a drug-evoked mitotoxic effect that is relatively selective for sensory axons.
Highlights.
Mitotoxicity is believed to cause paclitaxel-evoked peripheral neuropathy
High and persistent levels of paclitaxel are found in the DRG
Paclitaxel evoked mitochondrial swelling and vacuolation only in sensory axons
Paclitaxel impaired mitochondrial respiration only in sensory axons
Mitotoxicity that is relatively selective for sensory axons may be common pathology
Acknowledgments
Author contributions: WHX performed the EM and electrophysiology studies, assisted in experimental design, and performed data analysis; GJB designed the experiments and wrote the paper; HZ performed the respiration assays; FYZ assisted with the EM quantification; RN and TFM performed and analyzed the LC-MS/MS data.
This work was supported by research grants to GJB from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, U.S.A. (R01-NS0522550), the Neuropathy Association, the Louise and Alan Edwards Foundation, and the Canada Research Chairs Program. All authors declare that they have no conflicts of interest with respect to this report.
Abbreviations
- ADP
adenosine diphosphate
- ATP
adenosine triphosphate
- DR
dorsal root
- DRG
dorsal root ganglion
- IENF
intraepidermal nerve fiber
- LC-MS/MS
liquid chromatography with tandem mass spectrometry
- MiR05
mitochondria respiration medium
- MNCV
motor nerve conduction velocity
- QL
quantification limit
- SNCV
sensory nerve conduction velocity
- VDAC
voltage-dependent anion channel
- VFH
von Frey hair
- VR
Ventral root
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Augusto C, Pietro M, Cinzia M, Sergio C, Sara C, Luca G, Scaioli V. Peripheral neuropathy due to paclitaxel: study of the temporal relationships between the therapeutic schedule and the clinical quantitative score (QST) and comparison with neurophysiological findings. J Neurooncol. 2008;86:89–99. doi: 10.1007/s11060-007-9438-8. [DOI] [PubMed] [Google Scholar]
- Authier N, Gilllet JP, Fialip J, Eschalier A, Coudore F. Description of a short-term Taxol-induced nociceptive neuropathy in rats. Brain Res. 2000;887:239–249. doi: 10.1016/s0006-8993(00)02910-3. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Liu GK, Xiao WH, Jin HW, Siau C. Terminal arbor degeneration (TAD): a novel lesion produced by the antineoplastic agent, paclitaxel. Eur J Neurosci. 2011;176:447–454. doi: 10.1111/j.1460-9568.2011.07652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyette-Davis J, Xin W, Zhang H, Dougherty PM. Intraepidermal nerve fiber loss corresponds to the development of Taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain. 2011;152:308–313. doi: 10.1016/j.pain.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer PA, Lynch K. Stimulation-associated changes in frog neuromuscular junctions. A quantitative ultrastructural comparison of rapid-frozen and chemically fixed nerve terminals. Neuroscience. 1986;17:881–895. doi: 10.1016/0306-4522(86)90052-7. [DOI] [PubMed] [Google Scholar]
- Carrè M, Andre N, Carles G, Borghi H, Brichese L, Briand C, Braguer D. Tubulin is an inherent component of mitochondrial membranes that interacts with the voltage-dependent anion channel. J Biol Chem. 2002;277:33664–33669. doi: 10.1074/jbc.M203834200. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Cavalletti E, Montaguti P, Oggioni N, De Negri O, Tredici G. Effect on the peripheral nervous system of the short-term intravenous administration of paclitaxel in the rat. Neurotoxicol. 1997;18:137–145. [PubMed] [Google Scholar]
- Cavaletti G, Cavalletti E, Oggioni N, Sottani C, Minoia C, D'Incalci M, Zucchetti M, Marmiroli P, Tredici G. Distribution of paclitaxel within the nervous system of the rat after repeated intravenous administration. Neurotoxicol. 2000;21:389–393. [PubMed] [Google Scholar]
- Cavaletti G, Tredici G, Braga M, Tazzari S. Experimental peripheral neuropathy induced in adult rats by repeated intraperitoneal administration of taxol. Exp Neurol. 1995;133:64–72. doi: 10.1006/exnr.1995.1008. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Cata JP, Cordella JV, Burton A, Weng HR. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109:132–142. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Cata JP, Burton AW, Vu K, Weng HR. Dysfunction in multiple primary afferent fiber subtypes revealed by quantitative sensory testing in patients with chronic vincristine-induced pain. J Pain Symp Manag. 2007;33:166–179. doi: 10.1016/j.jpainsymman.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Fazio R, Quattrini A, Bolognesi A, Bordogna G, Villa E, Previtali S, Canal N, Nemni R. Docetaxel neuropathy: a distal axonopathy. Acta Neuropath. 1999;98:651–653. doi: 10.1007/s004010051132. [DOI] [PubMed] [Google Scholar]
- Ferlini C, Cicchillitti L, Raspaglio G, Bartollino S, Cimitan S, Bertucci C, Mozzetti S, Gallo D, Persico M, Fattorusso C, Campiani G, Scambia G. Paclitaxel directly binds to Bcl-2 and functionally mimics activity of Nur77. Cancer Res. 2009;69:6906–6914. doi: 10.1158/0008-5472.CAN-09-0540. [DOI] [PubMed] [Google Scholar]
- Flatters SJL, Bennett GJ. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain. 2004;109:150–61. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Flatters SJL, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: Evidence for mitochondrial dysfunction. Pain. 2006;122:247–257. doi: 10.1016/j.pain.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatters SJL, Xiao WH, Bennett GJ. Acetyl-L-carnitine prevents and reduces paclitaxel-induced painful neuropathy. Neurosci Lett. 2006;397:219–223. doi: 10.1016/j.neulet.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth PA, Balmaceda C, Peterson K, Seidman AD, Brasher P, DeAngelis LM. Prospective study of paclitaxel-induced peripheral neuropathy with quantitative sensory testing. J Neurooncol. 1997;35:47–53. doi: 10.1023/a:1005805907311. [DOI] [PubMed] [Google Scholar]
- Gnaiger E, Kuznetsov AV, Schneeberger S, Seiler R, Brandacher G, Steurer W, Margreiter R. Mitochondria in the cold. In: Heldmaier G, Klingenspor M, editors. Life in the cold. Springer; Berlin: 2000. pp. 431–442. [Google Scholar]
- Jamieson SM, Liu JJ, Connor B, Dragunow M, McKeage MJ. Nucleolar enlargement, nuclear eccentricity and altered cell body immunostaining characteristics of large-sized sensory neurons following treatment of rats with paclitaxel. Neurotoxicol. 2007;28:1092–1098. doi: 10.1016/j.neuro.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Jimenez-Andrade JM, Herrera MB, Ghilardi JR, Vardanyan M, Melemedjian OK, Mantyh PW. Vascularization of the dorsal root ganglia and peripheral nerve of the mouse: implications for chemical-induced peripheral sensory neuropathies. Mol Pain. 2008;4:10. doi: 10.1186/1744-8069-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Andrade JM, Peters CM, Mejia NA, Ghilardi JR, Kuskowski MA, Mantyh PW. Sensory neurons and their supporting cells located in the trigeminal, thoracic and lumbar ganglia differentially express markers of injury following intravenous administration of paclitaxel in the rat. Neurosci Lett. 2006;405:62–67. doi: 10.1016/j.neulet.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Jin HW, Flatters SJL, Xiao WH, Mulhern HL, Bennett GJ. Prevention of paclitaxel-evoked painful peripheral neuropathy by acetyl-L-carnitine: Effects on axonal mitochondria, sensory nerve fiber terminal arbors, and cutaneous Langerhans cells. Exptl Neurol. 2008;210:229–237. doi: 10.1016/j.expneurol.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd JF, Pilkington MF, Schell MJ, Fogarty KE, Skepper JN, Taylor CW, Thorn P. Paclitaxel affects cytosolic calcium signals by opening the mitochondrial permeability transition pore. J Biol Chem. 2002;277:6504–6510. doi: 10.1074/jbc.M106802200. [DOI] [PubMed] [Google Scholar]
- Kingston DG, Bane S, Snyder JP. The taxol pharmacophore and the T-taxol bridging principle. Cell Cycle. 2005;4:279–289. [PubMed] [Google Scholar]
- Lehmann HC, Chen W, Borzan J, Mankowski JL, Höke A. Mitochondrial dysfunction in distal axons contributes to human immunodeficiency virus sensory neuropathy. Ann Neurol. 2011;69:100–110. doi: 10.1002/ana.22150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton RB, Apfel SC, Dutcher JP, Rosenberg R, Kaplan J, Berger A, Einzig AI, Wiernik P, Schaumburg HH. Taxol produces a predominantly sensory neuropathy. Neurology. 1989;39:368–373. doi: 10.1212/wnl.39.3.368. [DOI] [PubMed] [Google Scholar]
- Loprinzi CL, Reeves BN, Dakhil SR, Sloan JA, Wolf SL, Burger KN, Kamal A, Le-Lindqwister NA, Soori GS, Jaslowski AJ, Novotny PJ, Lachance DH. Natural history of paclitaxel-associated acute pain syndrome: Prospective cohort study NCCTG N08C1. J Clin Oncol. 2011;29:1472–1478. doi: 10.1200/JCO.2010.33.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson Y. Microenvironment of the peripheral nervous system under normal and pathological conditions. Crit Rev Neurobiol. 1990;5:265–311. [PubMed] [Google Scholar]
- Park SB, Lin CSY, Krishnan AV, Friedlander ML, Lewis CR, Kiernan MC. Early, progressive, and sustained dysfunction of sensory axons underlies paclitaxel-induced neuropathy. Muscle & Nerve. 2011;43:367–374. doi: 10.1002/mus.21874. [DOI] [PubMed] [Google Scholar]
- Persohn E, Canta A, Schoepfer S, Traebert M, Mueller L, Gilardini A, Galbiati S, Nicolini G, Scuteri A, Lanzani F, Giussani G, Cavaletti G. Morphological and morphometric analysis of paclitaxel and docetaxel-induced peripheral neuropathy in rats. Eur J Cancer. 2005;41:1460–1466. doi: 10.1016/j.ejca.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Peters CM, Jimenez-Andrade JM, Kuskowski MA, Ghilardi JR, Mantyh PW. An evolving cellular pathology occurs in dorsal root ganglia, peripheral nerve and spinal cord following intravenous administration of paclitaxel in the rat. Brain Res. 2007a;1168:46–59. doi: 10.1016/j.brainres.2007.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters CM, Jimenez-Andrade JM, Jonas BM, Sevcik MA, Koewler NJ, Ghilardi JR, Wong GY, Mantyh PW. Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exptl Neurol. 2007b;203:42–54. doi: 10.1016/j.expneurol.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Pettersson CAV, Sharma HS, Olsson Y. Vascular permeability of spinal nerve roots. Acta Neuropath. 1990;81:148–154. doi: 10.1007/BF00334503. [DOI] [PubMed] [Google Scholar]
- Polomano R, Clark U, Mannes AJ, Bennett GJ. A painful peripheral neuropathy in rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94:293–304. doi: 10.1016/S0304-3959(01)00363-3. [DOI] [PubMed] [Google Scholar]
- Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- Rodi DJ, Janes RW, Sanganee HJ, Holton RA, Wallace BA, Makowski L. Screening of a library of phage-displayed peptides identifies human bcl-2 as a taxol-binding protein. J Mol Biol. 1999;285:197–203. doi: 10.1006/jmbi.1998.2303. [DOI] [PubMed] [Google Scholar]
- Rostovtseva TK, Sheldon KL, Hassanzadeh E, Monge C, Saks V, Bezrukov SM, Sackett DL. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. PNAS. 2008;105:18746–18751. doi: 10.1073/pnas.0806303105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (Taxol). Semin Oncol. 1993;20:1–15. [PubMed] [Google Scholar]
- Siau C, Bennett GJ. Dysregulation of neuronal calcium homeostasis in chemotherapy-evoked painful peripheral neuropathy. Anesth Analg. 2006;102:1485–1490. doi: 10.1213/01.ane.0000204318.35194.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siau C, Xiao WH, Bennett GJ. Paclitaxel- and vincristine-evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Exptl Neurol. 2006;201:507–514. doi: 10.1016/j.expneurol.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swett JE, Torigoe Y, Elie VR, Bourassa CM, Miller PG. Sensory neurons of the rat sciatic nerve. Exptl Neurol. 1991;114:82–103. doi: 10.1016/0014-4886(91)90087-s. [DOI] [PubMed] [Google Scholar]
- Swett JE, Wikholm RP, Blanks RHI, Swett AL, Conley LC. Motoneurons of the rat sciatic nerve. Exptl Neurol. 1986;93:227–252. doi: 10.1016/0014-4886(86)90161-5. [DOI] [PubMed] [Google Scholar]
- Tanner KD, Levine JD, Topp KS. Microtubule disorientation and axonal swelling in unmyelinated sensory axons during vincristine-induced painful neuropathy in rat. J Comp Neurol. 1998;395:481–492. [PubMed] [Google Scholar]
- Topp KS, Tanner KD, Levine JD. Damage to the cytoskeleton of large diameter sensory neurons and myelinated axons in vincristine-induced painful peripheral neuropathy in the rat. J Comp Neurol. 2000;424:563–576. [PubMed] [Google Scholar]
- van den Bent MJ, van Raaij-van den Aarssen VJ, Verweij J, Doorn PA, Sillevis Smitt PA. Progression of paclitaxel-induced neuropathy following discontinuation of treatment. Muscle Nerve. 1997;20:750–752. doi: 10.1002/(sici)1097-4598(199706)20:6<750::aid-mus15>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13:27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- Xiao WH, Bennett GJ. Chemotherapy-evoked neuropathic pain: Abnormal spontaneous discharge in A-fiber and C-fiber primary afferent neurons and its suppression by acetyl-L-carnitine. Pain. 2008;135:262–270. doi: 10.1016/j.pain.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao WH, Zheng FY, Bennett GJ, Bordet T, Pruss RM. Olesoxime (cholest-4-en-3-one, oxime): Analgesic and neuroprotective effects in a rat model of painful peripheral neuropathy produced by the chemotherapeutic agent, paclitaxel. Pain. 2009;147:202–209. doi: 10.1016/j.pain.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Xiao WH, Bennett GJ. Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Exptl Neurol. 2011 doi: 10.1016/j.expneurol.2011.08.016. in press. ( http://dx.doi.org/10.1016/j.expneurol.2011.08.016) [DOI] [PMC free article] [PubMed]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]