Abstract
Background
Periprosthetic infection in TKA is a devastating and challenging problem for both patients and surgeons. Two-stage exchange arthroplasty with an interval antibiotic spacer reportedly has the highest infection control rate. Studies comparing static spacers with articulating spacers have reported varying ROM after reimplant, which could be due to differences in articulating spacer technique.
Questions/purposes
We therefore determined whether one of three articulating spacer techniques was superior in terms of (1) infection control, (2) final ROM, and (3) cost.
Patients and Methods
We retrospectively reviewed 53 patients with infected TKAs who had two-stage exchange arthroplasty with one of three techniques with articulating spacers: autoclaving an original component (n = 15), a new femoral component (n = 16), and a silicone mold component (n = 22). We compared infection control, ROM, and cost. Minimum followup was 12 months (mean, 39 months; range, 12–105 months).
Results
We found no difference in infection control among the three techniques. Infection control was achieved in 13 of 15 (86.7%) autoclaved original component spacers at mean 73 months (range, 37–105 months), 15 of 16 (93.8%) new femoral component spacers at mean 19 months (range, 12–32 months), and 20 of 22 (90.9%) silicone mold component spacers at mean 32 months (range, 14–56 months). Mean final flexion was 95.7°, 98.3°, and 93.8°, respectively. Direct costs for all implants, molds, cement, and antibiotics were $932, $3589, and $3945, respectively.
Conclusions
We found comparable infection control and ROM for the three techniques. Direct cost was least for the autoclaved original component technique.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Periprosthetic infection (PPI) occurs in 1% to 2% of primary TKAs and 3% to 5% of revision TKAs [3] and remains a devastating and challenging problem for both patient and surgeon [2, 14, 21, 30]. Two-stage exchange arthroplasty with use of an interim antibiotic cement spacer is the accepted treatment in most circumstances with infection control rates ranging from 74.5% to 100% [23]. Both static and articulating antibiotic spacers have been used to maintain the joint space in distraction while providing high-dose local antibiotic delivery [4, 7, 18]. Articulating spacers preserve some knee motion between stages, reducing scarring and bone loss, with potential improvements in ROM, function, and second-stage exposure [10, 19, 22].
There are several approaches to creating articulating spacers. Hofmann et al. [16] described using the recycled autoclaved original femoral component (AOC) with a new tibial polyethylene insert for the articulation. These components were cemented into place but with the cement in a late doughy stage so they would not adhere well to the bone and be easy to remove at the time of a second-stage reimplantation. The same approach can be used with a new femoral component (NFC) and polyethylene insert. Cement-on-cement articulating spacers can also be used. These were initially hand-made [24], but subsequently silicone molds for intraoperatively fabricating cement components (silicon mold components [SMCs]) have been developed [9, 23]. Prefabricated cement spacers are also commercially available [27].
AOC spacers are inexpensive; however, there may be concerns due to the presence of a large surface that does not elute antibiotic or reuse of an implant designed for single use [28]. NFC spacers share the former concern, not the latter, but are more expensive. SMC spacers have a large surface area for antibiotic elution but have limited sizes and risk cement component fracture and cement debris [20].
We determined whether there would be a difference in (1) infection control, (2) ROM, and (3) cost among these three articulating spacer techniques.
Patients and Methods
We identified 74 patients undergoing treatment for infected TKA between January 2001 and June 2009 from our two institutional databases based on Current Procedural Terminology codes (27303, 27486, 27487, and 27488). Patients who underwent two-stage exchange arthroplasty with articulating spacers were considered for inclusion. We excluded a total of 21 patients who had previously failed a two-stage exchange arthroplasty (n = 2), who had undergone two-stage exchange with static spacers (n = 3), or who did not undergo the second-stage operation (n = 16). These exclusions left 53 patients with 53 knees: 38 men and 15 women with a mean age of 64 years. We used one of three techniques in all patients: (1) AOC and new polyethylene spacer (n = 15), (2) NFC and new polyethylene spacer (n = 16), and (3) SMCs (n = 22). The minimum followup was 12 months (mean, 39 months; range, 12–105 months). No patients in the study group were lost to followup. No patients were recalled specifically for this study; all data were obtained from medical records.
The three cohorts (AOC, NFC, and SMC) were comparable in terms of age, antibiotic-resistant microorganisms (Table 1), prior irrigation and débridement, comorbidities based on Charlson Comorbidity Index (CCI), duration between primary TKA and explants, and various intraoperative parameters (Table 2). There was a higher percentage of male patients in the AOC and NFC groups as expected in the Veterans Affairs hospital setting where these procedures took place.
Table 1.
Microorganisms identified
| Microorganism | Number of patients |
|---|---|
| Methicillin-resistant Staphylococcus aureus | 9 |
| Methicillin-resistant Staphylococcus epidermidis | 5 |
| Vancomycin-resistant Enterococcus | 1 |
| Methicillin-sensitive Staphylococcus aureus | 20 |
| Methicillin-sensitive Staphylococcus epidermidis | 2 |
| Diphtheroids | 2 |
| Escherichia coli | 1 |
| Propionibacterium acnes | 6 |
| Streptococcus bovis | 2 |
| Streptococcus viridans | 2 |
| α-Hemolytic Streptococcus | 1 |
| Group B Streptococcus | 1 |
| No growth | 1 |
Table 2.
Preoperative patient characteristics of the three groups
| Variable | AOC | NFC | SMC | p Value |
|---|---|---|---|---|
| Study population (number of patients) | 15 | 16 | 22 | |
| Mean age (years) | 67.3 | 63.6 | 61.1 | |
| Female (number) | 1 | 0 | 14 | |
| Male (number) | 14 | 11 | 8 | |
| Mean CCI score | 4.3 | 3.9 | 3.5 | 0.43 |
| Antibiotic-resistant microorganisms | 5 (33.3%) | 4 (25.0%) | 7 (31.8%) | 0.85 |
| Prior I&D (number of patients) | 7 | 8 | 4 | 0.07 |
| Mean primary TKA to explant duration (months) | 38.5 | 31.9 | 41.9 | 0.19 |
| Mean explant to reimplant duration (months) | 4.9 | 2.7 | 5.8 | 0.01 |
| Mean tourniquet time-reimplantation (minutes) | 135.1 | 134.3 | 132.5 | 0.99 |
| Mean operative time-explantation (minutes) | 152.4 | 132.2 | 141.6 | 0.18 |
AOC = autoclaved original component; NFC = new femoral component; SMC = silicon mold component; CCI = Charlson Comorbidity Index; I&D = irrigation and débridement.
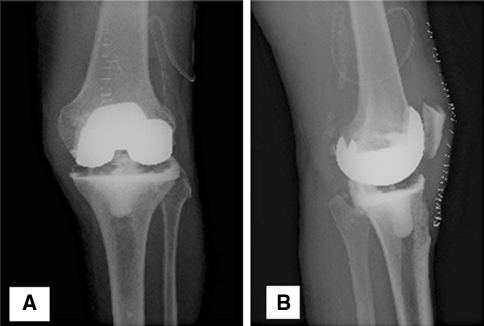
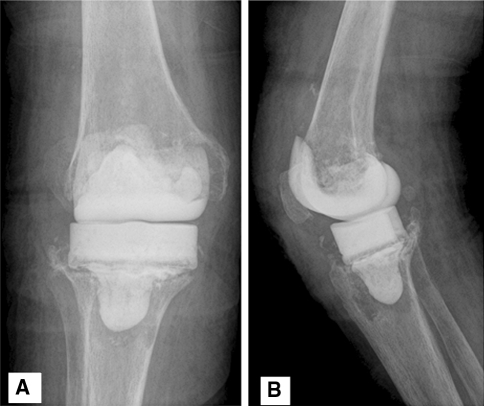
All patients underwent a two-stage exchange operation. The first-stage operation included complete removal of all components, periprosthetic membrane and cement, thorough débridement, copious irrigation, and placement of the articulating spacer by one of the three techniques. The choice of articulating spacer technique was made by the primary surgeon based on his/her customary practice. In the AOC technique, the explanted femoral component was cleaned of all debris, scrubbed with a Betadine® (Purdue Pharma LP, Stamford, CT) scrub brush, and then autoclaved for reimplantation with a new polyethylene spacer. The AOC technique was replaced with the NFC technique after 2006 due to institutional directives prohibiting reuse of an explanted component (Fig. 1). In the SMC technique, antibiotic cement was injected into silicone molds (StageOne™ Knee Cement Spacer Molds; Biomet, Inc, Warsaw, IN) intraoperatively to manufacture the femoral and tibial components (Fig. 2).
Fig. 1A–B.
(A) AP and (B) lateral knee radiographs demonstrate the NFC spacer technique.
Fig. 2A–B.
(A) AP and (B) lateral knee radiographs demonstrate the SMC spacer technique.
All techniques utilized three to four packs of bone cement with four 1.0-g doses of tobramycin powder and four 1.5-g doses of vancomycin powder. Cement baseplates and stems were formed around the polyethylene inserts in the AOC and NFC techniques and the cement was used in a doughy stage to limit cement intrusion and fixation to the bony surfaces. Intramedullary cement dowels or beads were used in all patients.
Postoperatively, patients were placed on at least 6 weeks of appropriate intravenous antibiotic therapy in consultation with infectious disease specialists. Gentle ROM and ambulation with a walker in a knee immobilizer were encouraged after the first-stage operation. Decision for the second-stage reimplantation was made after a minimum 2-week antibiotic-free interval when the clinical signs of infection had subsided and erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level had steadily trended toward normal for our laboratory.
The second-stage operation included explantation of the cement spacer, removal of all cement fragments, thorough débridement of the joint and the intramedullary canals, copious irrigation, and placement of the appropriate new total knee components with antibiotic-impregnated cement.
Postoperatively, patients received either a third-generation cephalosporin or vancomycin intravenously until the intraoperative cultures returned. Positive cultures were treated with further antibiotic treatment in consultation with an infectious disease consultant. Patients were mobilized after recovering from anesthesia and had daily inpatient supervised physical therapy. After discharge, patients had outpatient physical therapy for a variable length of time. Weightbearing restrictions were individualized based on the revision procedure and bone loss.
Patients were followed clinically at 2 weeks, 6 weeks, 3 months, and annually thereafter. Clinical examinations focused on wound healing, signs of infection, and active ROM. AP, patellar, and lateral knee radiographs were obtained at the 6-week, 3-month, and annual visits. ESR and CRP level were obtained at each visit starting at 6 weeks. Infection control was judged to be a joint with no clinical signs of infection, no progressive radiolucency on radiographs, and normal ESR and CRP level at latest followup without further surgical intervention. The conventional definition classifies patients with long-term antibiotic suppression after reimplantation as having infection control [1]. In this study, we assessed infection control with and without long-term antibiotic suppression after reimplantation.
We recorded demographic data, underlying diagnosis, prior surgeries on the knee including irrigation and débridement with or without polyethylene insert exchange, and comorbidities as defined by the CCI [13]. We also documented causative microorganism, antibiotic usage before and after surgeries, intraoperative details of the spacer techniques, and followup data regarding further surgical intervention.
We calculated descriptive statistics, including frequency, means, SDs, and ranges for all continuous data. Normally distributed continuous variables were compared by one-way ANOVA test and continuous variables not normally distributed were compared by Kruskal-Wallis nonparametric one-way ANOVA of ranks. Categorical variables were compared by chi square test. In our study, because there were multiple comparison procedures such as pairwise rank-sum and pairwise t tests where the overall error was not controlled, the significance level was “Bonferroni-corrected” by dividing 0.05 by the number of comparisons (n = 3), ie, 0.0167. We performed a univariable Cox regression analysis to determine the relationship between treatment failure and patient age, sex, comorbidities including CCI score, antibiotic resistance of the microorganism, spacer type, index TKA-explantation interval, explantation-reimplantation interval, and intraoperative parameters. Kaplan-Meier survival analysis was performed for the three spacer groups using further surgical intervention to control infection or long-term antibiotic suppression after the reimplantation as the end point. In cases with long-term antibiotic suppression after reimplantation, the date of reimplantation was considered as the date of failure. We used the log-rank and Wilcoxon test to compare survival of the three spacer groups. Analysis was performed using SAS® Software for Windows® Version 9.1.3 (SAS Institute, Inc, Cary, NC).
Results
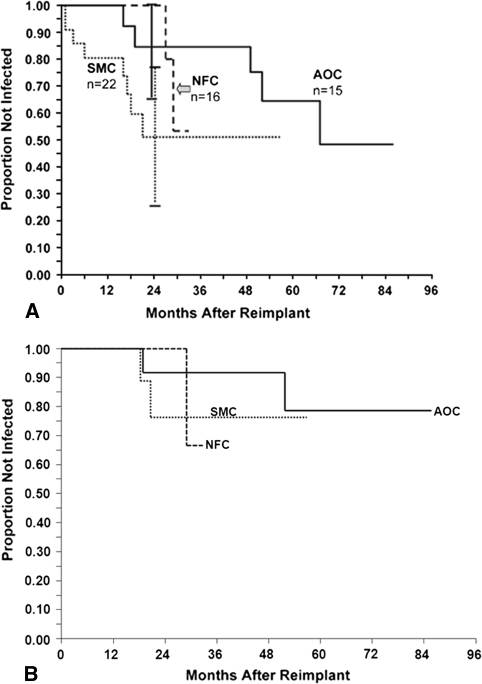
Using the conventional definition of infection control, the overall survival was 88.6% at 24 months. Survival rates with either further surgery to control infection or the need for long-term antibiotic suppression as the end point were similar (p = 0.83) with the three techniques (Fig. 3). The overall reinfection rate was 9.4% (five of 53) at mean 39 months’ followup.
Fig. 3A–B.
Kaplan-Meyer survival analysis of the three spacer techniques (A) without long-term antibiotic suppression and (B) with long-term antibiotic suppression showed no difference (p = 0.17 and p = 0.83, respectively) in infection-free survival.
If long-term antibiotic suppression after reimplantation is not classified as infection control, the overall infection-free survival dropped to 73.8% (95% confidence interval [CI] = 59.3%–88.2%) at 24 months. However, even with this stricter definition, there was no overall difference (p = 0.17) in the infection-free survival of the three spacer techniques (Fig. 3). There was also no difference in the pairwise comparison of infection-free survival in the three spacer techniques (p values for AOC-SMC, AOC-NFC, and NFC-SMC = 0.14, 0.39, and 0.39, respectively).
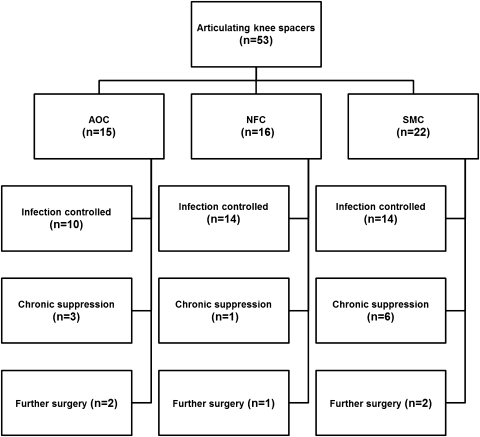
In the AOC spacer group, 13 of 15 (86.7%) had the infection controlled at a mean followup of 73 months (range, 37–105 months) (Fig. 4). One patient had a spacer dislocation causing wound problems and persistent infection and is awaiting an above-knee amputation. One patient had a spacer exchange and subsequently an arthrodesis which failed, necessitating an above-knee amputation. In the NFC spacer group, 15 of 16 (93.8%) had the infection controlled at a mean followup of 19 months (range, 12–32 months). One patient had a spacer exchange and eventually an arthrodesis. In the SMC spacer group, 20 of 22 (90.9%) had the infection controlled at a mean followup of 32 months (range, 14–56 months). One patient had a positive culture on knee aspiration at last followup and was awaiting further treatment.
Fig. 4.
The outcome of the three cohorts in terms of infection control is shown. Ten of 15 (66.7%) AOC spacers were infection-free at a mean followup of 73 months (range, 37–105 months); 14 of 16 (87.5%) NFC spacers were infection-free at a mean followup of 19 months (range, 12–32 months); and 14 of 22 (65%) SMC spacers were infection-free at a mean followup of 32 months (range, 14–56 months).
Mean flexion achieved immediately before reimplantation was similar (p = 0.93) in the three groups: 76.7°, 78.3°, and 79.0° for the SMC, NFC, and AOC spacers, respectively. Mean flexion achieved at the final followup in patients in whom the infection was controlled was also similar (p = 0.92): 95.7°, 98.3°, and 93.8° for SMC, NFC, and AOC spacers, respectively.
Direct costs for all implants, molds, cement, and antibiotics were $3945 for the SMC technique, $3589 for the NFC technique, and $932 for the AOC technique.
The only factors associated with treatment failure were antibiotic-resistant organisms (p = 0.036, hazard ratio [HR] = 3.42) and chronic obstructive pulmonary disease (p = 0.005, HR = 9.09).
Discussion
Articulating antibiotic spacers in two-stage exchange arthroplasty for infected TKAs allow physiologic motion between stages, reduce bone loss [5, 11], and may make reimplantation easier compared to a static spacer. Fehring et al. [11] reported no improvement in ROM with molded cement articulating spacers compared to static spacers while Emerson et al. [10] found improved ROM and function with AOC articulating spacers compared to static spacers. Clearly, different articulating spacer techniques may have different outcomes and no consensus exists on the optimum articulating spacer technique. An ideal articulating spacer technique should eradicate infection, allow enough motion to enhance patient comfort and minimize exposure issues at secondary reimplantation, and minimize dislocation, fracture, wound issues, and bone loss. Our study compared three different articulating spacer techniques in terms of infection control, ROM, and cost.
Our study has a number of limitations. First, our limited numbers in this preliminary study increase the potential for a Type II error. Our selection of spacer type according to the surgeon’s customary preference instead of randomization may have introduced selection bias, although each surgeon used his preferred method for all first-time infected TKAs during this time frame. Third, we did not have uniform criteria for long-term antibiotic suppression after reimplantation, though this option was generally entertained in patients where cultures remained positive at the time of reimplantation, there were multiple patient comorbidities, there was a resistant organism, or some combination of these difficult circumstances. The three groups were not matched for sex, but there is no evidence in the literature of a sex-related difference in infection control. Finally, we made no comparison of relative bone loss for the techniques, one of the espoused advantages of articulating spacer techniques over static spacers [5, 11].
We found comparable infection control with AOC, NFC, and SMC spacer techniques. Hofmann et al. [16] first described the use of an articulating spacer with a recycled AOC and new tibial polyethylene insert. No reinfections were noted in 26 patients at a mean followup of 30 months. More recently, other authors have been able to demonstrate comparable results with this method [8, 15, 17, 20, 26]. We were forced to modify this technique by using a NFC technique instead of the recycled AOC by a 2006 national Veterans Administration directive prohibiting reuse of implantable components. Given the failure to find a difference in infection control between AOC and NFC techniques and the lower cost of the former, we would still recommend use of the AOC technique where permitted. Cement-on-cement articulating spacers can be prepared intraoperatively using silicone molds or can be hand-made or prefabricated [6, 9, 11, 12, 20, 27]. They have the advantage of greater surface area for antibiotic elution but are available in limited sizes and have the risk of a cement component fracture and cement debris. Intraoperative manufacture of cement spacers also has the potential to increase operating room time and indirect expense as a result [20]. Although we could not demonstrate a difference in tourniquet time among the techniques, the tourniquet was often deflated for a period of the procedure with the SMC technique. The added cost of the silicone molds and the potential indirect cost of longer operating room time for the SMC technique as noted by others [20] do not seem justifiable without a demonstrated improvement in reinfection rates.
The overall reinfection rate in our study (9.4% at mean 39 months) is comparable to those in the literature (Table 3). It is unclear whether studies that examine the success of infection control after surgical procedures should simply look at reinfection rates or apply survival methodology; we have chosen to perform both analyses. Similarly, whether chronic suppression can be accurately classified as infection control remains debatable, so we have supplied data points both with and without suppression as an end point. Suppression may be more commonly chosen as an option as antibiotic resistance increases. According to the latest National Nosocomial Infection Surveillance System report [25], there has been a substantial rise in incidence of antibiotic resistance among organisms isolated from intensive care units and antibiotic-resistant bacteria are becoming increasingly common in PPIs. Volin et al. [31] reported an 11.1% reinfection rate in the methicillin-resistant Staphylococcus aureus group compared to 5.4% in the methicillin-sensitive S. aureus group for two-stage exchange arthroplasty in TKA infections, and Salgado et al. [29] found presence of methicillin-resistant S aureus was an independent risk factor for treatment failure (HR = 9.2). Our study supports these findings involving resistant organisms.
Table 3.
Selected prior studies of articulating spacers
| Study | Year | Spacer type | Number of knees | Mean followup (months) | Reinfection/persistent infection rate (%) | Average flexion (°) |
|---|---|---|---|---|---|---|
| Hofmann et al. [16] | 1995 | AOC | 26 | 30 | 0 | 106 |
| Emerson et al. [10] | 2002 | AOC | 22 | 44 | 9 | 108 |
| Hofmann et al. [15] | 2005 | AOC | 50* | 74 | 12 | 104 |
| Cuckler [8] | 2005 | AOC | 44 | 64 | 2.3 | |
| Pietsch et al. [26] | 2006 | AOC | 33 | 28 | 9 | NA |
| Jämsen et al. [20] | 2006 | AOC | 24 | 25 | 9 | 104 |
| Huang et al. [17] | 2006 | AOC | 21 | 52 | 4.7 | 97.6 |
| Fehring et al. [11] | 2000 | Custom-molded cement spacer | 15 | 24 | 6.6 | NA |
| Durbhakula et al. [9] | 2004 | SMC | 24 | 33 | 8.3 | 104 |
| Pitto et al. [27] | 2005 | Preformed cement spacer | 21 | 24 | 0 | 94 |
| Jämsen et al. [20] | 2006 | SMC | 10 | 48 | 20 | 92 |
| Freeman et al. [12] | 2007 | SMC | 48 | 62 | 5.3 | NA |
* This study includes the 26 knees from the authors’ prior study [16]; AOC = autoclaved original component; NFC = new femoral component; SMC = silicon mold component; NA = not available.
Our observations with the limited numbers available do not support any of the articulating spacer techniques as superior for ROM. This is consistent with a study by Jämsen et al. [20] comparing AOC spacer technique with a hand-molded cement spacer technique. The authors found no difference in the ROM at last followup examination.
Not surprisingly, the AOC spacer technique was least expensive in terms of direct costs for all implants, molds, cement, and intraoperative antibiotic usage. Directives that have limited the use of this technique ignore the fact that the implant is reused for a short period of time in a protected weightbearing environment and its structural integrity is not integral for the intended purpose of antibiotic delivery. We did not assess indirect costs related to operative time or the need for repeat hospitalizations or surgery but expect, if the different techniques achieve similar infection control rates, indirect costs would likewise show little difference with larger patient volumes.
Articulating spacers have the ability to control infection in two-stage reimplantations and maintain ROM and can be performed in a low-cost fashion with an AOC technique. Larger multiinstitutional studies performed in a prospective fashion with different techniques utilized at different institutions could better corroborate these preliminary findings. Analysis of such populations, in combination perhaps with accounting of indirect costs, will best establish a single articulating spacer technique that is most efficacious and cost-effective.
Acknowledgments
The authors thank Paul Lender (University of Minnesota) and Penny Tatman, MPH (HealthEast Care System, St Paul, MN) for their help with the statistical analysis.
Footnotes
This material is partially based on work supported (or supported in part) by the Office of Research and Development, Department of Veterans Affairs. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
In support of their research for or preparation of this work, one or more of the authors (TJG) received funding from DePuy Orthopaedics, Inc (Warsaw, IN).
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the University of Minnesota Medical School and the Minneapolis Veterans Affair Medical Center.
References
- 1.Azzam K, McHale K, Austin M, Purtill JJ, Parvizi J. Outcome of a second two-stage reimplantation for periprosthetic knee infection. Clin Orthop Relat Res. 2009;467:1706–1714. doi: 10.1007/s11999-009-0739-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bengston S, Knutson K, Lidgren L. Treatment of infected knee arthroplasty. Clin Orthop Relat Res. 1989;245:173–178. [PubMed] [Google Scholar]
- 3.Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86:688–691. doi: 10.1302/0301-620X.86B5.14887. [DOI] [PubMed] [Google Scholar]
- 4.Booth RE, Lotke PA. The results of spacer block technique in revision of infected total knee arthroplasty. Clin Orthop Relat Res. 1989;248:57–60. [PubMed] [Google Scholar]
- 5.Calton TF, Fehring TK, Griffin WL. Bone loss associated with the use of spacer blocks in infected total knee arthroplasty. Clin Orthop Relat Res. 1997;345:148–154. doi: 10.1097/00003086-199712000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Chevrel G, Schott AM, Fontanges E, Charrin JE, Lina-Granade G, Duboeuf F, Garnero P, Arlot M, Raynal C, Meunier PJ. Effects of oral alendronate on BMD in adult patients with osteogenesis imperfecta: a 3-year randomized placebo-controlled trial. J Bone Miner Res. 2006;21:300–306. doi: 10.1359/JBMR.051015. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JC, Hozack WJ, Cuckler JM, Booth RE. Two-stage reimplantation of septic total knee arthroplasty: report of three cases using an antibiotic-PMMA spacer block. J. Arthroplasty. 1988;3:369–377. doi: 10.1016/S0883-5403(88)80040-8. [DOI] [PubMed] [Google Scholar]
- 8.Cuckler J. The infected total knee: management options. J Arthroplasty. 2005;20:33–36. doi: 10.1016/j.arth.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Durbhakula S, Czajka J, Fuchs M, Uhl R. Antibiotic-loaded articulating cement spacer in the 2-stage exchange of infected total knee arthroplasty. J Arthroplasty. 2004;19:768–774. doi: 10.1016/j.arth.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 10.Emerson R, Muncie M, Tarbox T, Higgins L. Comparison of a static with a mobile spacer in total knee infection. Clin Orthop Relat Res. 2002;404:132–138. doi: 10.1097/00003086-200211000-00023. [DOI] [PubMed] [Google Scholar]
- 11.Fehring TK, Odum S, Calton TF, Mason JB. Articulating versus static spacers in revision total knee arthroplasty for sepsis. The Ranawat Award. Clin Orthop Relat Res. 2000;380:9–16. doi: 10.1097/00003086-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Freeman M, Fehring T, Odum S, Fehring K, Griffin W, Mason JB. Functional advantage of articulating versus static spacers in 2-stage revision for total knee arthroplasty infection. J Arthroplasty. 2007;22:1116–1121. doi: 10.1016/j.arth.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Hall W, Ramachandran R, Narayan S, Jani A, Vijayakumar S. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer. 2004;4:94–95. doi: 10.1186/1471-2407-4-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hebert CK, Williams RE, Levy RS, Barrack RL. Cost of treating an infected total knee replacement. Clin Orthop Relat Res. 1996;331:140–145. doi: 10.1097/00003086-199610000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann A, Goldberg T, Tanner A, Kurtin S. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125–131. doi: 10.1097/01.blo.0000149241.77924.01. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann AA, Kane KR, Tkach TK, Plaster RL, Camargo MP. Treatment of infected total knee arthroplasty using an articulating spacer. Clin Orthop Relat Res. 1995;321:45–54. [PubMed] [Google Scholar]
- 17.Huang HT, Su JY, Chen SK. The results of articulating spacer technique for infected total knee arthroplasty. J Arthroplasty. 2006;21:1163–1168. doi: 10.1016/j.arth.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 18.Insall JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of infected total knee arthroplasty. J Bone Joint Surg Am. 1983;65:1087–1098. [PubMed] [Google Scholar]
- 19.Jacobs C, Christensen C, Berend M. Static and mobile antibiotic-impregnated cement spacers for the management of prosthetic joint infection. J Am Acad Orthop Surg. 2009;17:356–368. doi: 10.5435/00124635-200906000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Jämsen E, Sheng P, Halonen P, Lehto MU, Moilanen T, Pajamäki J, Puolakka T, Konttinen YT. Spacer prostheses in two-stage revision of infected knee arthroplasty. Int Orthop. 2006;30:257–261. doi: 10.1007/s00264-006-0102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jämsen E, Stogiannidis I, Malmivaara A, Pajamäki J, Puolakka T, Konttinen Y. Outcome of prosthesis exchange for infected knee arthroplasty: the effect of treatment approach. Acta Orthop. 2009;80:67–77. doi: 10.1080/17453670902805064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leone J, Hanssen A. Management of infection at the site of a total knee arthroplasty. Instr Course Lect. 2006;55:449–461. [PubMed] [Google Scholar]
- 23.Lombardi A, Karnes J, Berend K. A motion maintaining antibiotic delivery system. J Arthroplasty. 2007;22:50–55. doi: 10.1016/j.arth.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 24.McPherson EJ, Lewonowski K, Dorr LD. Techniques in arthroplasty: use of an articulated PMMA spacer in the infected total knee arthroplasty. J Arthroplasty. 1995;10:87–89. doi: 10.1016/S0883-5403(05)80105-6. [DOI] [PubMed] [Google Scholar]
- 25.National Nosocomial Infections Surveillance System National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–485. doi: 10.1016/j.ajic.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Pietsch M, Hofmann S, Wenisch C. Treatment of deep infection of total knee arthroplasty using a two-stage procedure. Oper Orthop Traumatol. 2006;18:66–87. doi: 10.1007/s00064-006-1163-5. [DOI] [PubMed] [Google Scholar]
- 27.Pitto RP, Castelli CC, Ferrari R, Munro J. Pre-formed articulating knee spacer in two-stage revision for the infected total knee arthroplasty. Int Orthop. 2005;29:305–308. doi: 10.1007/s00264-005-0670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pitto RP, Spika IA. Antibiotic-loaded bone cement spacers in two-stage management of infected total knee arthroplasty. Int Orthop. 2004;28:129–133. doi: 10.1007/s00264-004-0545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salgado C, Dash S, Cantey JR, Marculescu C. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Relat Res. 2007;461:48–53. doi: 10.1097/BLO.0b013e3181123d4e. [DOI] [PubMed] [Google Scholar]
- 30.Sculco TP. The economic impact of infected total joint arthroplasty. Instr Course Lect. 1993;42:349–351. [PubMed] [Google Scholar]
- 31.Volin S, Hinrichs S, Garvin K. Two-stage reimplantation of total joint infections: a comparison of resistant and non-resistant organisms. Clin Orthop Relat Res. 2004;427:94–100. doi: 10.1097/01.blo.0000143559.34143.3d. [DOI] [PubMed] [Google Scholar]