Abstract
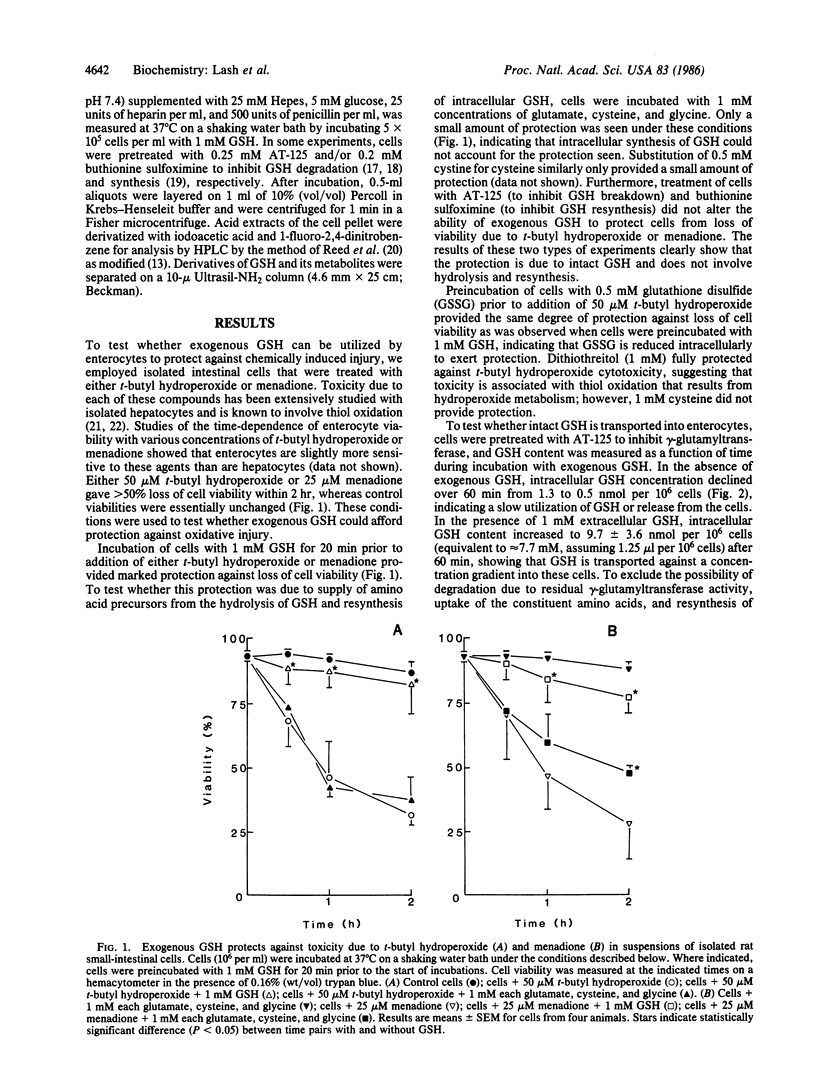
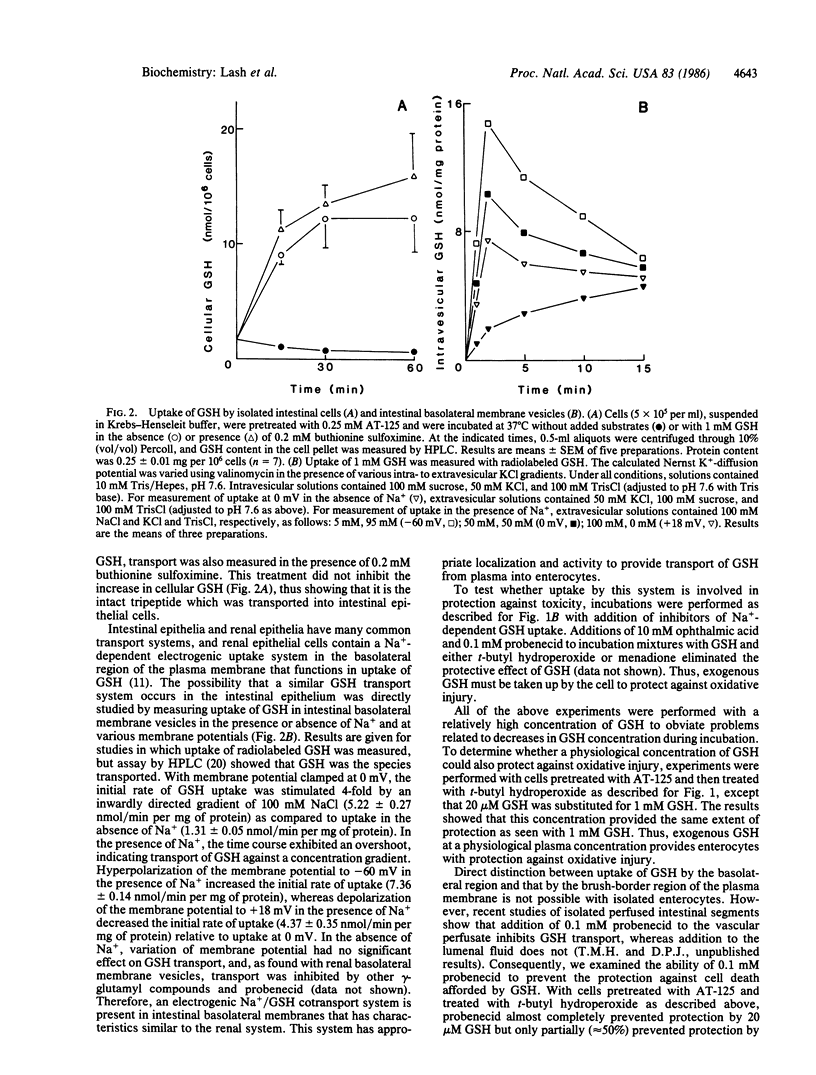
Exogenous GSH provided rat small-intestinal epithelial cells with significant protection against injury induced by t-butyl hydroperoxide or menadione. This protection was found to be dependent upon uptake of intact GSH. Uptake of GSH occurred by a Na+-dependent electrogenic system found in the basolateral membrane. Thus, rat small-intestinal epithelial cells can utilize plasma GSH to support intracellular detoxication systems that function in protection against chemically induced injury.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Babson J. R., Abell N. S., Reed D. J. Protective role of the glutathione redox cycle against adriamycin-mediated toxicity in isolated hepatocytes. Biochem Pharmacol. 1981 Aug 15;30(16):2299–2304. doi: 10.1016/0006-2952(81)90102-7. [DOI] [PubMed] [Google Scholar]
- Bartoli G. M., Sies H. Reduced and oxidized glutathione efflux from liver. FEBS Lett. 1978 Feb 1;86(1):89–91. doi: 10.1016/0014-5793(78)80105-7. [DOI] [PubMed] [Google Scholar]
- Bellomo G., Jewell S. A., Thor H., Orrenius S. Regulation of intracellular calcium compartmentation: studies with isolated hepatocytes and t-butyl hydroperoxide. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6842–6846. doi: 10.1073/pnas.79.22.6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Di Monte D., Ross D., Bellomo G., Eklöw L., Orrenius S. Alterations in intracellular thiol homeostasis during the metabolism of menadione by isolated rat hepatocytes. Arch Biochem Biophys. 1984 Dec;235(2):334–342. doi: 10.1016/0003-9861(84)90206-6. [DOI] [PubMed] [Google Scholar]
- Grafström R., Moldéus P., Andersson B., Orrenius S. Xenobiotic metabolism by isolated rat small intestinal cells. Med Biol. 1979 Oct;57(5):287–293. [PubMed] [Google Scholar]
- Granger D. N., Rutili G., McCord J. M. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981 Jul;81(1):22–29. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Inoue M., Kinne R., Tran T., Arias I. M. Glutathione transport across hepatocyte plasma membranes. Analysis using isolated rat-liver sinusoidal-membrane vesicles. Eur J Biochem. 1984 Feb 1;138(3):491–495. doi: 10.1111/j.1432-1033.1984.tb07943.x. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kinne R., Tran T., Arias I. M. The mechanism of biliary secretion of reduced glutathione. Analysis of transport process in isolated rat-liver canalicular membrane vesicles. Eur J Biochem. 1983 Aug 15;134(3):467–471. doi: 10.1111/j.1432-1033.1983.tb07590.x. [DOI] [PubMed] [Google Scholar]
- Jones D. P., Eklöw L., Thor H., Orrenius S. Metabolism of hydrogen peroxide in isolated hepatocytes: relative contributions of catalase and glutathione peroxidase in decomposition of endogenously generated H2O2. Arch Biochem Biophys. 1981 Sep;210(2):505–516. doi: 10.1016/0003-9861(81)90215-0. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N. Physiological significance of glutathione S-transferases. Am J Physiol. 1980 Dec;239(6):G439–G444. doi: 10.1152/ajpgi.1980.239.6.G439. [DOI] [PubMed] [Google Scholar]
- Lash L. H., Jones D. P. Characteristics of cysteine uptake in intestinal basolateral membrane vesicles. Am J Physiol. 1984 Oct;247(4 Pt 1):G394–G401. doi: 10.1152/ajpgi.1984.247.4.G394. [DOI] [PubMed] [Google Scholar]
- Lash L. H., Jones D. P. Characterization of the membrane-associated thiol oxidase activity of rat small-intestinal epithelium. Arch Biochem Biophys. 1983 Aug;225(1):344–352. doi: 10.1016/0003-9861(83)90039-5. [DOI] [PubMed] [Google Scholar]
- Lash L. H., Jones D. P. Distribution of oxidized and reduced forms of glutathione and cysteine in rat plasma. Arch Biochem Biophys. 1985 Aug 1;240(2):583–592. doi: 10.1016/0003-9861(85)90065-7. [DOI] [PubMed] [Google Scholar]
- Lash L. H., Jones D. P. Renal glutathione transport. Characteristics of the sodium-dependent system in the basal-lateral membrane. J Biol Chem. 1984 Dec 10;259(23):14508–14514. [PubMed] [Google Scholar]
- Lash L. H., Jones D. P. Transport of glutathione by renal basal-lateral membrane vesicles. Biochem Biophys Res Commun. 1983 Apr 15;112(1):55–60. doi: 10.1016/0006-291x(83)91796-5. [DOI] [PubMed] [Google Scholar]
- Linder M., De Burlet G., Sudaka P. Transport of glutathione by intestinal brush border membrane vesicles. Biochem Biophys Res Commun. 1984 Sep 28;123(3):929–936. doi: 10.1016/s0006-291x(84)80223-5. [DOI] [PubMed] [Google Scholar]
- Orrenius S., Ormstad K., Thor H., Jewell S. A. Turnover and functions of glutathione studied with isolated hepatic and renal cells. Fed Proc. 1983 Dec;42(15):3177–3188. [PubMed] [Google Scholar]
- Reed D. J., Babson J. R., Beatty P. W., Brodie A. E., Ellis W. W., Potter D. W. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980 Jul 15;106(1):55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- Reed D. J., Ellis W. W., Meck R. A. The inhibition of gamma-glutamyl transpeptidase and glutathione metabolism of isolated rat kidney cells by L-(alpha S, 5S)-alpha-amino-3-chloro-4, 5-dihydro-5-isoxazoleacetic acid (AT-125; NSC-163501). Biochem Biophys Res Commun. 1980 Jun 30;94(4):1273–1277. doi: 10.1016/0006-291x(80)90557-4. [DOI] [PubMed] [Google Scholar]
- Scalera V., Storelli C., Storelli-Joss C., Haase W., Murer H. A simple and fast method for the isolation of basolateral plasma membranes from rat small-intestinal epithelial cells. Biochem J. 1980 Jan 15;186(1):177–181. doi: 10.1042/bj1860177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schasteen C. S., Curthoys N. P., Reed D. J. The binding mechanism of glutathione and the anti-tumor drug L-(alpha S, 5S)-alpha-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid (AT-125;NSC-163501) to gamma-glutamyltransferase. Biochem Biophys Res Commun. 1983 Apr 29;112(2):564–570. doi: 10.1016/0006-291x(83)91501-2. [DOI] [PubMed] [Google Scholar]
- Sies H., Graf P. Hepatic thiol and glutathione efflux under the influence of vasopressin, phenylephrine and adrenaline. Biochem J. 1985 Mar 1;226(2):545–549. doi: 10.1042/bj2260545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor H., Smith M. T., Hartzell P., Bellomo G., Jewell S. A., Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem. 1982 Oct 25;257(20):12419–12425. [PubMed] [Google Scholar]
- Wefers H., Sies H. Hepatic low-level chemiluminescence during redox cycling of menadione and the menadione-glutathione conjugate: relation to glutathione and NAD(P)H:quinone reductase (DT-diaphorase) activity. Arch Biochem Biophys. 1983 Jul 15;224(2):568–578. doi: 10.1016/0003-9861(83)90244-8. [DOI] [PubMed] [Google Scholar]



