Abstract
Background
C-reactive protein (CRP) serum assays are a standard element of the diagnostic workup for periprosthetic joint infection (PJI). However, because CRP is a marker for systemic inflammation, this test is not specific to PJI.
Questions/purposes
Our purpose was to assess whether synovial fluid and serum assays alone could differentiate between infected and uninfected revision knee arthroplasties and to determine which of these methods had the greatest diagnostic accuracy.
Methods
We collected synovial fluid specimens from 66 patients undergoing revision total knee arthroplasty. Patients were judged uninfected or infected by standardized criteria. Synovial CRP levels were measured using an individual CRP assay (15 samples; 10 infected, five uninfected) and a multiplex immunoassay platform (59 samples; 25 infected, 34 uninfected). Results from preoperative standard serum CRP assays conducted were also collected (55 samples; 25 infected, 30 uninfected). Sensitivity, specificity, and receiver operating characteristic curve analyses were performed for each assay with a diagnosis of infection based on previously established criteria.
Results
Synovial CRP concentrations differed between infected and uninfected joints in the multiplex and serum analyses. The area under the curve was 0.84 for the individual assay, 0.91 for the multiplex assay, and 0.88 for the serum CRP assay. Sensitivity and specificity were 70.0% and 100.0% for the individual enzyme-linked immunosorbent assay, 84.0% and 97.1% for the multiplex assay, and 76.0% and 93.3% for the serum CRP assay.
Conclusions
An assay measuring CRP in synovial fluid may be more accurate in diagnosing PJI than the standard serum CRP assay. We believe such an assay holds promise as a new diagnostic marker for PJI.
Level of Evidence
Level II, diagnostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Numerous tests are available for the diagnosis of periprosthetic joint infection (PJI), but, although evidence-based recommendations for standardizing this diagnosis have been made [10, 43], there remains no established protocol for its definitive diagnosis [4, 22, 44, 52]. To this end, numerous researchers have focused on improving existing methods for diagnosing infection (ie, preoperative and intraoperative cultures [2, 3, 16, 27, 46] and Gram stain [2, 12, 20, 49], serum markers of inflammation [19, 23, 27], synovial white blood cell [WBC] count and polymorphonuclear [PMN] percentages [5, 21, 29, 34, 48], intraoperative frozen section [11, 14, 15, 17, 42] and preoperative tissue biopsy [32, 36], and nuclear medicine techniques [8, 25, 30, 31, 35]) as well as developing novel methods for diagnosing infection (polymerase chain reaction techniques [1, 6, 18, 24, 26], sonication of implants [50], and analysis of circulating cytokines [7, 9, 13, 47]). The more we learn about the histopathologic characteristics of infected versus uninfected joints, the better able we will be to identify clinically relevant characteristics of infections [28, 37, 39–41]. Drawbacks to many of these new diagnostic methods are that many of them are expensive, require specialized equipment, or are relatively time-consuming and therefore are not universally available [4, 44, 52].
The standard serum C-reactive protein (CRP) assay is a simple test that is currently a mainstay in diagnosis of infection [4, 19, 44]. CRP is produced by the liver as an acute-phase reactant, and elevated levels are seen in the case of systemic infection. However, serum CRP concentrations are nonspecific for the diagnosis of localized infection because elevated CRP levels can be present in a number of noninfectious inflammatory processes [19]. Additionally, false-negatives may result when a subclinical infection (ie, a biofilm) does not result in systemic elevation of CRP despite the presence of an active infection [23]. The question arises as to whether the CRP level in the synovial fluid would be more a specific and/or sensitive predictor than the serum level. Because the physiological role of CRP is to activate the complement system for disposal of dying cells, one would expect its levels to be higher at the source of an inflammatory process (ie, the joint) than systemically. Zamani et al. have examined CRP levels in synovial fluid as a method for differentiating inflammatory and noninflammatory primary arthritis [51], but we are not aware of previous studies that have analyzed CRP levels in synovial fluid for the purpose of diagnosing PJI.
The concept of improving the accuracy of a biomarker assay commonly performed on serum samples by performing the same assay on a localized fluid such as synovial fluid has been previously investigated. Martinot et al. [33] compared serum and synovial fluid assays for procalcitonin and found that although serum levels of procalcitonin were useful differentiating septic arthritis from rheumatoid and crystal-induced arthritis, synovial fluid levels of procalcitonin were too low to be measured. They concluded a synovial fluid assay for procalcitonin did not appear to be a feasible method for differentiating between different types of arthritis [22]. We suspected CRP might hold greater promise for identification in synovial fluid than does procalcitonin and undertook this study to evaluate this potential.
We therefore determined whether (1) CRP levels in either synovial fluid or in serum would differentiate between infected and uninfected revision knee arthroplasties; and (2) measuring CRP in synovial fluid instead of in serum would improve the accuracy of the diagnosis of PJI.
Patients and Methods
Before beginning this prospective study, we obtained Institutional Review Board approval for the collection of all patient samples. We collected a total of 66 synovial fluid specimens from 64 patients undergoing revision TKA for septic or aseptic reasons between February 2009 and May 2010. Samples were consecutive with the exception of cases in which a synovial fluid aspiration was dry or was not performed per study protocol. All samples were collected in the operating room before arthrotomy and ranged in volume from 0.5 mL to greater than 2 mL. Samples were transferred to 2-mL sterile cryotubes, immediately placed on ice, and flash-frozen within 30 minutes of collection. Patients underwent revision arthroplasty for loosening, infection, fracture, polyethylene wear, instability, or pain. For all patients, we also reviewed medical charts to collect information on patient demographics, preoperative and intraoperative laboratory findings, clinical presentation, and surgical details.
Samples were divided for the purpose of sensitivity and specificity analyses into two groups: infected and uninfected. This diagnosis was based on criteria currently in place at our institution for differentiating between infected and uninfected arthroplasties. Patients were classified as infected if (1) they presented with a sinus tract or open wound in communication with the joint; (2) purulence was encountered in the joint intraoperatively; (3) preoperative or intraoperative fluid or tissue cultures were positive; or (4) a combination of serologic and aspiration analyses was positive. In cases in which no purulence was mentioned in the operative note, it was assumed that none was present. Positive cultures were defined as (1) at least one culture with “light” growth or greater; (2) at least one culture with “very light” growth and at least one culture of the same organism with the same level of growth or less; or (3) at least three “broth only” or “single isolate” cultures of the same organism. We used antibiotic sensitivity analyses to confirm that the same organism was isolated in multiple cultures. Finally, serologic and aspirate analyses were defined positive if three of the following were true and the patient had not undergone a surgical intervention within 6 weeks: (1) erythrocyte sedimentation rate 30 mm/hr or greater; (2) CRP 10 mg/L or greater; (3) synovial WBC 1760 cells/μL or greater; or (4) synovial PMNs 73% or greater. For patients who had undergone surgery within 6 weeks of aspiration, three of four results were still required to be positive, but thresholds for aspirate analyses were set at synovial WBC 10,700 cells/μL or greater and synovial PMN 89% or greater as previously described [5]. Patients who could not be classified as infected or uninfected as a result of missing data values (five samples from five patients) were eliminated from all subsequent analyses.
Synovial fluid specimens were analyzed according to three separate protocols: (1) an individual assay for CRP (as a preliminary study); (2) a multiplex assay for CRP; and (3) the standard hospital serum assay for CRP. For the first assay, we used GenWay Biotech’s C-Reactive Protein Concentration (San Diego, CA) enzyme-linked immunosorbent assay (ELISA) kit. Samples were thawed on ice and sterilely centrifuged to remove cells. We performed these assays on 15 of the samples in the study (five uninfected and 10 infected). Patients underwent revision for infection, loosening, or instability. The supernatant was diluted either 1:100 or 1:1000 and assayed for the presence of CRP according to the manufacturers’ instructions. Absorbance of each well was measured using a spectrophotometer, and the concentration of CRP in each sample was calculated by comparing absorbance with the absorbance measured for controls with known concentration. In cases in which the absorbance measured was lower than that in the negative control well, we recorded samples as “below detection” and used a 0-mg/mL concentration for purposes of our statistical analysis. In cases in which the absorbance measured was higher than that measured in the 0.1-mg/L control well (the highest control concentration available), we recorded samples as “high” concentrations and, if possible, rediluted the sample to obtain a more accurate reading.
Our secondary analysis was performed using Rules-Based Medicine’s Human Inflammation MAP (Austin, TX). This multiplex ELISA analysis allows for the simultaneous testing of samples to determine concentrations of several proteins; for the present study, we focused on the outcomes of the CRP measurements. Samples were shipped on dry ice to RBM for analysis and digital results returned. This assay was performed on fluid taken from 59 of the 66 samples in the study (34 uninfected and 25 infected). Patients in this group underwent revision arthroplasty for infection, loosening, fracture, polyethylene wear, instability, or pain.
Finally, we collected results of all preoperative serum CRP assays. Preoperative serum CRP assays using Beckman Coulter’s Synchron Lx system (Brea, CA) are routinely done in our institution before revision arthroplasty with standard values ranging from below detection at less than 5 mg/L to above 100 mg/L; we obtained preoperative serum CRP assay findings for 55 of the samples in the study (30 uninfected and 25 infected). There were six patients (four uninfected and two infected) for whom this value was missing from the medical chart.
We used receiver operating characteristic (ROC) curve analyses to determine whether the CRP concentration in synovial fluid could effectively be used to diagnose PJI. This method assesses the diagnostic strength of a test and calculates the best cutoff point for differentiating between a positive and a negative test result. Sensitivity is plotted against 1-specificity and the area under the curve (AUC) is calculated from the plot. An AUC of 0.5 indicates that a test has no diagnostic strength; as the AUC increases (to a maximum of 1), the diagnostic strength improves. A test with an AUC greater than 0.9 is considered an excellent diagnostic. Because our two methods for identifying CRP concentrations were substantially different from each other, we chose to perform two separate analyses rather than to pool our separate data sets. Youden’s J statistic was used in each case to determine optimum cutoff values for diagnosis of infection. According to this rule, a test’s best diagnostic threshold occurs at the value for which the sum of the test’s sensitivity and specificity is maximized. We calculated sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of each assay we evaluated.
Results
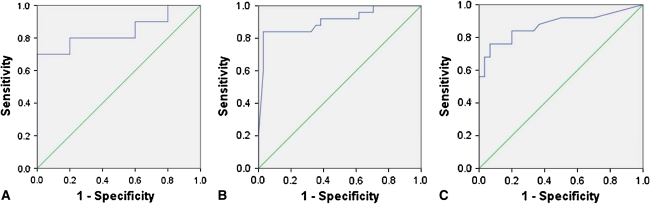
Our first purpose was to assess the ability of each of these three assays to differentiate between infected and uninfected arthroplasties. For the GenWay Biotech ELISA assay, CRP concentrations were similar (p = 0.13) in the two groups: 0.002 mg/L in the uninfected group versus 0.322 mg/L in the infected group. However, in both the RBM multiplex assay and the serum CRP assay, CRP values in the infected group were higher than in the uninfected group (p = 0.002 and p < 0.0001, respectively): for RBM, 1.19 mg/L in the uninfected group versus 22.49 mg/L in the infected group; and for the serum assay, 9.6 mg/L in the uninfected group versus 91.7 mg/L in the infected group (Table 1). Our ROC analysis further confirmed the diagnostic capabilities of each of the three assays. Each assay had an ROC above 0.80, indicating a high-strength diagnostic test. For the GenWay assay, ROC curve analysis demonstrated an AUC = 0.84 (95% confidence interval [CI], 0.64–1.04). For this assay, we identified an optimum diagnostic threshold of 0.057 mg/L, which gave a sensitivity of 70.0% and a specificity of 100.0% (Fig 1A). For the RBM assay, the AUC was 0.91 (95% CI, 0.82–0.99) with a threshold of 3.65 mg/L, giving a sensitivity of 84.0% and a specificity of 97.1% (Fig 1B). Finally, for the serum CRP assay, the AUC was 0.88 (95% CI, 0.77–0.98) with a threshold of 16.5 mg/L, giving a sensitivity of 76.0% and a specificity of 93.3% (Fig 1C). Moreover, we calculated the test accuracy of each of the three assays and found all to be accurate with the RBM assay as the most accurate (accuracy = 0.80 for the GenWay assay, 0.92 for the RBM assay, and 0.86 for the serum assay). All three tests, then, were capable of differentiating between infected and uninfected arthroplasties with a high degree of accuracy (Table 1).
Table 1.
Summary of three assay results
| Fluid | Assay (number; infected, uninfected) | AUC (95% CI) | Threshold (mg/L) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|
| Synovial fluid | Individual ELISA (N = 15; 10, 5) | 0.84 (0.64–1.04) | 0.06 | 70.0 | 100.0 | 100.0 | 62.5 | 80.0 |
| Multiplex ELISA (N = 59; 25, 34) | 0.91 (0.82–0.99) | 3.7 | 84.0 | 97.1 | 95.5 | 89.2 | 91.5 | |
| Serum | Clinical laboratory assay (N = 55; 25; 30) | 0.88 (0.77–0.98) | 16.5 | 76.0 | 93.3 | 90.5 | 82.4 | 85.5 |
AUC = area under the curve; CI = confidence interval; PPV = positive predictive value; NPV = negative predictive value; ELISA = enzyme-linked immunosorbent assay.
Fig. 1A–C.
Receiver operating characteristic (ROC) curves are shown for (A) synovial fluid CRP assay by individual ELISA; (B) synovial fluid CRP assay by multiplex ELISA; and (C) serum CRP assay according to hospital laboratory protocols. The multiplex ELISA had the greatest area under the curve (AUC) followed by the serum CRP assay and the individual ELISA. CRP = C-reactive protein; ELISA = enzyme-linked immunosorbent assay.
Our second purpose was to assess whether measuring CRP in synovial fluid instead of in blood serum improved the diagnostic capabilities of the test. Although we found that all three assays were predicted infection (even with a small sample number, as seen with the individual ELISA assay), we found suggestive (although not definitive) support for the superiority of the RBM analysis over the clinical hospital laboratory analysis. Although both tests predicted infection, the RBM analysis demonstrated a higher AUC, a smaller CI range, and a higher diagnostic accuracy than did the clinical hospital laboratory analysis.
Discussion
Serum CRP analysis has long been an important biomarker for the diagnosis of PJI. However, because CRP is a marker for systemic inflammation, this test is not specific to PJI. We speculated synovial fluid CRP would enhance the sensitivity and specificity. We therefore determined whether (1) CRP levels in either synovial fluid or in serum would differentiate between infected and uninfected revision knee arthroplasties; and (2) measuring CRP in synovial fluid instead of in serum would improve the accuracy of the diagnosis of PJI.
We acknowledge limitations of our study. First, because there is no consensus on the diagnosis of PJI, we lacked an established method for differentiating infected and uninfected cases. We used the diagnostic criteria that have been established at our institution for diagnosing PJI. Given the wide range of previously described algorithms [4, 10, 22, 38, 43, 45, 52] that have been used as benchmarks for novel diagnostic methods, then, it is important to interpret our findings in light of the specific components included in our PJI diagnostic algorithm. Second, for logistic reasons in this preliminary study, we did not use the same clinical laboratory assay and analysis protocol to measure CRP in synovial fluid. As a result, our positive findings in this group may reflect the strength of the assay itself rather than the source of the fluid tested. To confirm this point, future work needs to be done to compare CRP measurements in the hospital laboratory on blood serum and on synovial fluid using the same laboratory parameters for testing. Third was the problem of missing values, which is relevant in two contexts: (1) because we did not alter clinical protocols for this study, not all patients had preoperative serum CRP assays conducted; and (2) dry or inappropriate taps prevented us from including in our study a proportion of the knee arthroplasty revisions performed within the study period. Although the fact that both groups of missing values included both infected and uninfected patients suggests this absence did not contribute substantial bias toward our findings, this limitation should not be ruled out. Fourth, although we report significant findings, our sample size is relatively small and our findings should be confirmed in a larger study and at multiple institutions.
Our first purpose was to determine whether our synovial fluid and serum CRP assay findings were correlated with a diagnosis of PJI. We found this was the case for both the multiplex CRP assay and the hospital clinical laboratory findings. Moreover, although the difference between CRP measurements in infected and uninfected cases for the individual ELISA assay were not significant, we believe this was likely the result of small numbers in this preliminary cohort. The only investigation into the diagnostic role of CRP in joints that we are aware of comes from Zamani et al., who used a high-sensitivity synovial fluid CRP assay to demonstrate differences between noninflammatory and inflammatory arthritis in primary knees. Specifically, they found the synovial fluid CRP assay effectively differentiated osteoarthritis and each of rheumatoid arthritis, crystal-induced arthritis, and septic arthritis. Because the majority of our cohort underwent primary knee arthroplasty for degenerative joint disease and not for one of these etiologies, we can make no statement about differences in synovial fluid versus serum CRP analyses in patients with inflammatory primary arthritis. Future studies should aim at describing standard synovial fluid CRP concentrations for these varying etiologies. Given our current findings, however, it appears that, at least in the revision setting, both synovial fluid and serum CRP assays can be used to differentiate infected and uninfected revision knee arthroplasty cases. We were interested to note, moreover, that Zamani et al. used a high-sensitivity CRP assay for their study. Our preliminary investigations questioned whether we would have to alter manufacturer protocols in carrying out our assays in synovial fluid instead of in serum; we found standard assays were more than sufficient to detect CRP in synovial fluid.
Our second purpose was to determine whether the assays we conducted in synovial fluid performed better in differentiating infected and uninfected cases than did those performed on serum in the hospital clinical laboratory. We found the multiplex analysis appeared to perform better than the serum assay. The fact that the individual assay performed less well than did the serum assay is likely the result of the relatively small number of samples tested using the individual assay. Additional research is needed to better describe the role that a synovial fluid CRP assay might play in differentiating between septic and aseptic failure of arthroplasty components. Our findings should be corroborated in a larger sample size and should be expanded to include patients with failed hip arthroplasties. Some work also needs to be done to streamline and standardize protocols for carrying out assays. In the two assays we performed, we observed very different absolute concentrations for CRP. These numbers, moreover, were substantially lower than standard CRP concentrations as measured in serum. This variability indicates the next step toward incorporating synovial fluid CRP measurements in a clinical diagnostic algorithm for PJI will be to standardize clinical laboratory protocols and establish appropriate thresholds for assay results from infected and uninfected patients. Such standardization would allow for further evaluation of the accuracy and feasibility of a synovial fluid CRP assay in the diagnosis of PJI.
Measuring CRP in joint fluid rather than in blood serum appears to result in a test with higher diagnostic accuracy. Moreover, because CRP is already a routine clinical measurement taken before revision arthroplasty [17], widespread adaptation of this test would be fairly straightforward. Although future studies are needed to confirm our findings in a larger cohort, we believe an assay for synovial fluid CRP levels may serve as a simple method for diagnosing PJI in the future, particularly because many hospitals already have protocols in place to perform similar tests.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article. Separately from the content of this article, J.P. is a consultant for Stryker Orthopaedics and has intellectual properties on SmarTech.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Achermann Y, Vogt M, Leunig M, Wüst J, Trampuz A. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. J Clin Microbiol. 2010;48:1208–1214. doi: 10.1128/JCM.00006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkins BL, Athanasou N, Deeks JJ, Crook DW, Simpson H, Peto TE, McLardy-Smith P, Berendt AR. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group. J Clin Microbiol. 1998;36:2932–2939. doi: 10.1128/jcm.36.10.2932-2939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrack RL, Aggarwal A, Burnett RSJ, Clohisy JC, Ghanem E, Sharkey P, Parvizi J. The fate of the unexpected positive intraoperative cultures after revision total knee arthroplasty. J Arthroplasty. 2007;22(Suppl 2):94–99. doi: 10.1016/j.arth.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 4.Bauer TW, Parvizi J, Kobayashi N, Krebs V. Diagnosis of periprosthetic infection. J Bone Joint Surg Am. 2006;88:869–882. doi: 10.2106/JBJS.E.01149. [DOI] [PubMed] [Google Scholar]
- 5.Bedair H, Ting N, Jacovides C, Saxena A, Moric M, Parvizi J, Della Valle CJ. The Mark Coventry Award: diagnosis of early postoperative TKA infection using synovial fluid analysis. Clin Orthop Relat Res. 2011;469:34–40. doi: 10.1007/s11999-010-1433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergin PF, Doppelt JD, Hamilton WG, Mirick GE, Jones AE, Sritulanondha S, Helm JM, Tuan RS. Detection of periprosthetic infections with use of ribosomal RNA-based polymerase chain reaction. J Bone Joint Surg Am. 2010;92:654–663. doi: 10.2106/JBJS.I.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bottner F, Wegner A, Winkelmann W, Becker K, Erren M, Götze C. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg Br. 2007;89:94–99. doi: 10.1302/0301-620X.89B1.17485. [DOI] [PubMed] [Google Scholar]
- 8.Chacko TK, Zhuang H, Stevenson K, Moussavian B, Alavi A. The importance of the location of fluorodeoxyglucose uptake in periprosthetic infection in painful hip prostheses. Nucl Med Commun. 2002;23:851–855. doi: 10.1097/00006231-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Chana FR, Guisáosla MZ, Sánchez JH, Villanueva MM, Calvo JH, Vaquero JM. Tumor necrosis factor as a biomarker of infection in total knee arthroplasty [in Spanish] Acta Ortop Mex. 2010;24:298–305. [PubMed] [Google Scholar]
- 10.Della Valle C, Parvizi J, Bauer TW, Dicesare PE, Evans RP, Segreti J, Spangehl M, Watters WC, 3rd, Keith M, Turkelson CM, Wies JL, Sluka P, Hitchcock K. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18:760–770. doi: 10.5435/00124635-201012000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Della Valle CJ, Bogner E, Desai P, Lonner JH, Adler E, Zuckerman JD, Di Cesare PE. Analysis of frozen sections of intraoperative specimens obtained at the time of reoperation after hip or knee resection arthroplasty for the treatment of infection. J Bone Joint Surg Am. 1999;81:684–689. doi: 10.2106/00004623-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Della Valle CJ, Scher DM, Kim YH, Oxley CM, Desai P, Zuckerman JD, Di Cesare PE. The role of intraoperative Gram stain in revision total joint arthroplasty. J Arthroplasty. 1999;14:500–504. doi: 10.1016/S0883-5403(99)90108-0. [DOI] [PubMed] [Google Scholar]
- 13.Di Cesare PE, Chang E, Preston CF, Liu C-J. Serum interleukin-6 as a marker of periprosthetic infection following total hip and knee arthroplasty. J Bone Joint Surg Am. 2005;87:1921–1927. doi: 10.2106/JBJS.D.01803. [DOI] [PubMed] [Google Scholar]
- 14.Fehring TK, McAlister JA., Jr Frozen histologic section as a guide to sepsis in revision joint arthroplasty. Clin Orthop Relat Res. 1994;304:229–237. [PubMed] [Google Scholar]
- 15.Feldman DS, Lonner JH, Desai P, Zuckerman JD. The role of intraoperative frozen sections in revision total joint arthroplasty. J Bone Joint Surg Am. 1995;77:1807–1813. doi: 10.2106/00004623-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Font-Vizcarra L, García S, Martínez-Pastor JC, Sierra JM, Soriano A. Blood culture flasks for culturing synovial fluid in prosthetic joint infections. Clin Orthop Relat Res. 2010;468:2238–2243. doi: 10.1007/s11999-010-1254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francés Borrego A, Martínez FM, Cebrian Parra JL, Grañeda DS, Crespo RG, López-Durán Stern L. Diagnosis of infection in hip and knee revision surgery: intraoperative frozen section analysis. Int Orthop. 2007;31:33–37. doi: 10.1007/s00264-005-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallo J, Kolár M, Koukalová D, Sauer P, Lovecková Y, Dendis M, Kesselová M, Petrzelová J, Yapletalová J. Bacterial pathogens of periprosthetic infections and diagnostic possibilities [in Czech] Klin Mikrobiol Infekc Lek. 2006;12:117–123. [PubMed] [Google Scholar]
- 19.Ghanem E, Antoci V, Jr, Pulido L, Joshi A, Hozack W, Parvizi J. The use of receiver operating characteristics analysis in determining erythrocyte sedimentation rate and C-reactive protein levels in diagnosing periprosthetic infection prior to revision total hip arthroplasty. Int. J. Infect. Dis. 2009;13:e444–e449. doi: 10.1016/j.ijid.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Ghanem E, Ketonis C, Restrepo C, Joshi A, Barrack R, Parvizi J. Periprosthetic infection: where do we stand with regard to Gram stain? Acta Orthop. 2009;80:37–40. doi: 10.1080/17453670902804943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghanem E, Parvizi J, Burnett RSJ, Sharkey PF, Keshavarzi N, Aggarwal A, Barrack RL. Cell count and differential of aspirated fluid in the diagnosis of infection at the site of total knee arthroplasty. J Bone Joint Surg Am. 2008;90:1637–1643. doi: 10.2106/JBJS.G.00470. [DOI] [PubMed] [Google Scholar]
- 22.Gollwitzer H, Diehl P, Gerdesmeyer L, Mittelmeier W. Diagnostic strategies in cases of suspected periprosthetic infection of the knee. A review of the literature and current recommendations [in German]. Orthopade. 2006;35:904, 906–908, 910–916. [DOI] [PubMed]
- 23.Johnson AJ, Zywiel MG, Stroh A, Marker DR, Mont MA. Serological markers can lead to false negative diagnoses of periprosthetic infections following total knee arthroplasty. Int Orthop. 2010. Available at: www.ncbi.nlm.nih.gov/pubmed/21181540. Accessed May 3, 2011. [DOI] [PMC free article] [PubMed]
- 24.Kalogianni DP, Goura S, Aletras AJ, Christopoulos TK, Chanos MG, Christofidou M, Skoutelis A, Ioannou PC, Panagiotopoulos E. Dry reagent dipstick test combined with 23S rRNA PCR for molecular diagnosis of bacterial infection in arthroplasty. Anal. Biochem. 2007;361:169–175. doi: 10.1016/j.ab.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Kisielinski K, Cremerius U, Büll U, Hermanns B, Wirtz DC, Niethard FU. First experiences with fluorodeoxyglucose-positron-emission tomography (FDG-PET) in the evaluation of painful total knee and hip joint replacements [in German] Z Orthop Ihre Grenzgeb. 2003;141:153–159. doi: 10.1055/s-2003-38649. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi N, Procop GW, Krebs V, Kobayashi H, Bauer TW. Molecular identification of bacteria from aseptically loose implants. Clin Orthop Relat Res. 2008;466:1716–1725. doi: 10.1007/s11999-008-0263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koudela K, Jr, Geigerová L, Hes O, Koudela K., Sr Comprehensive diagnosis of infection in revision total replacements of large joints [in Slovak] Acta Chir Orthop Traumatol Cech. 2010;77:425–431. [PubMed] [Google Scholar]
- 28.Krohmer G, Koleganova N, Hadjicostas PT, Fink B, Berger I. Degenerative changes of the interface membrane as a possible reason for prosthesis loosening. Histol Histopathol. 2008;23:925–933. doi: 10.14670/HH-23.925. [DOI] [PubMed] [Google Scholar]
- 29.Lee SC, Jung KA, Yoon JY, Nam CH, Hwang SH, Park IS. Analysis of synovial fluid in culture-negative samples of suspicious periprosthetic infections. Orthopedics. 2010;33:725. doi: 10.3928/01477447-20100225-17. [DOI] [PubMed] [Google Scholar]
- 30.Love C, Marwin SE, Palestro CJ. Nuclear medicine and the infected joint replacement. Semin Nucl Med. 2009;39:66–78. doi: 10.1053/j.semnuclmed.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Love C, Marwin SE, Tomas MB, Krauss ES, Tronco GG, Bhargava KK, Nichols KJ, Palestro CJ. Diagnosing infection in the failed joint replacement: a comparison of coincidence detection 18F-FDG and 111In-labeled leukocyte/99mTc-sulfur colloid marrow imaging. J Nucl Med. 2004;45:1864–1871. [PubMed] [Google Scholar]
- 32.Malhotra R, Morgan DA f. Role of core biopsy in diagnosing infection before revision hip arthroplasty. J Arthroplasty. 2004;19:78–87. doi: 10.1016/S0883-5403(03)00453-4. [DOI] [PubMed] [Google Scholar]
- 33.Martinot M, Sordet C, Soubrier M, Puéchal X, Saraux A, Lioté F, Guggenbuhl P, Lègre V, Jaulhac B, Maillefert J-F, Zeisel M, Coumaros G, Sibilia J. Diagnostic value of serum and synovial procalcitonin in acute arthritis: a prospective study of 42 patients. Clin Exp Rheumatol. 2005;23:303–310. [PubMed] [Google Scholar]
- 34.Mason JB, Fehring TK, Odum SM, Griffin WL, Nussman DS. The value of white blood cell counts before revision total knee arthroplasty. J Arthroplasty. 2003;18:1038–1043. doi: 10.1016/S0883-5403(03)00448-0. [DOI] [PubMed] [Google Scholar]
- 35.McKillop JH, McKay I, Cuthbert GF, Fogelman I, Gray HW, Sturrock RD. Scintigraphic evaluation of the painful prosthetic joint: a comparison of gallium-67 citrate and indium-111 labelled leucocyte imaging. Clin Radiol. 1984;35:239–241. doi: 10.1016/S0009-9260(84)80148-8. [DOI] [PubMed] [Google Scholar]
- 36.Meermans G, Haddad FS. Is there a role for tissue biopsy in the diagnosis of periprosthetic infection? Clin Orthop Relat Res. 2010;468:1410–1417. doi: 10.1007/s11999-010-1245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morawietz L, Classen R-A, Schröder JH, Dynybil C, Perka C, Skwara A, Neidel J, Gehrke T, Frommelt L, Hansen T, Otto M, Barden B, Aigner T, Stiehl P, Schubert T, Meyer-Scholten C, König A, Ströbel P, Rader CP, Kirschner S, Lintner F, Rüther W, Bos I, Hendrich C, Kriegsmann J, Krenn V. Proposal for a histopathological consensus classification of the periprosthetic interface membrane. J Clin Pathol. 2006;59:591–597. doi: 10.1136/jcp.2005.027458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morawietz L, Tiddens O, Mueller M, Tohtz S, Gansukh T, Schroeder JH, Perka C, Krenn V. Twenty-three neutrophil granulocytes in 10 high-power fields is the best histopathological threshold to differentiate between aseptic and septic endoprosthesis loosening. Histopathology. 2009;54:847–853. doi: 10.1111/j.1365-2559.2009.03313.x. [DOI] [PubMed] [Google Scholar]
- 39.Nalepka JL, Lee MJ, Kraay MJ, Marcus RE, Goldberg VM, Chen X, Greenfield EM. Lipopolysaccharide found in aseptic loosening of patients with inflammatory arthritis. Clin Orthop Relat Res. 2006;451:229–235. doi: 10.1097/01.blo.0000224050.94248.38. [DOI] [PubMed] [Google Scholar]
- 40.Nordström D, Santavirta S, Antti-Poika I, Konttinen YT. Local immune inflammatory response to infected total hip and knee replacements. Arch Orthop Trauma Surg. 1994;113:159–163. doi: 10.1007/BF00441625. [DOI] [PubMed] [Google Scholar]
- 41.Otto M, Kriegsmann J, Gehrke T, Bertz S. Wear particles: key to aseptic prosthetic loosening? [in German] Pathologe. 2006;27:447–460. doi: 10.1007/s00292-006-0868-4. [DOI] [PubMed] [Google Scholar]
- 42.Pace TB, Jeray KJ, Latham JT., Jr Synovial tissue examination by frozen section as an indicator of infection in hip and knee arthroplasty in community hospitals. J Arthroplasty. 1997;12:64–69. doi: 10.1016/S0883-5403(97)90049-8. [DOI] [PubMed] [Google Scholar]
- 43.Parvizi J, Della Valle CJ. AAOS Clinical Practice Guideline: diagnosis and treatment of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18:771–772. doi: 10.5435/00124635-201012000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Parvizi J, Ghanem E, Menashe S, Barrack RL, Bauer TW. Periprosthetic infection: what are the diagnostic challenges? J Bone Joint Surg Am. 2006;88(Suppl 4):138–147. doi: 10.2106/JBJS.F.00609. [DOI] [PubMed] [Google Scholar]
- 45.Parvizi J, Ghanem E, Sharkey P, Aggarwal A, Burnett RSJ, Barrack RL. Diagnosis of infected total knee: findings of a multicenter database. Clin Orthop Relat Res. 2008;466:2628–2633. doi: 10.1007/s11999-008-0471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47:1403–1409. doi: 10.1086/592973. [DOI] [PubMed] [Google Scholar]
- 47.Shah K, Mohammed A, Patil S, McFadyen A, Meek RMD. Circulating cytokines after hip and knee arthroplasty: a preliminary study. Clin Orthop Relat Res. 2009;467:946–951. doi: 10.1007/s11999-008-0562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spangehl MJ, Masri BA, O’Connell JX, Duncan CP. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am. 1999;81:672–683. doi: 10.2106/00004623-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Tamaki Y, Takakubo Y, Goto K, Hirayama T, Sasaki K, Konttinen YT, Goodman SB, Takagi M. Increased expression of toll-like receptors in aseptic loose periprosthetic tissues and septic synovial membranes around total hip implants. J. Rheumatol. 2009;36:598–608. doi: 10.3899/jrheum.080390. [DOI] [PubMed] [Google Scholar]
- 50.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357:654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 51.Zamani B, Jamali R, Ehteram H. Synovial fluid adenosine deaminase and high-sensitivity C-reactive protein activity in differentiating monoarthritis. Rheumatol Int. 2010. Available at: www.ncbi.nlm.nih.gov/pubmed/20721560. Accessed May 3, 2011. [DOI] [PubMed]
- 52.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]