Abstract
Background
Revision of failed two-stage revision TKA for infection is challenging, and amputation often is the only alternative.
Questions/purposes
We asked whether reinfection after two-stage revision for infection could be controlled with an aggressive revision protocol and intraarticular antibiotic infusion.
Methods
We retrospectively reviewed 18 patients (12 women, six men) who underwent revision for failed reimplantation between January 1999 and January 2008. Mean time from revision for infection to rerevision for reinfection was 5 months (range, 1–18 months). All knees were treated with an individualized protocol that included aggressive exposure, extensive débridement, uncemented components, closure with muscle flaps (seven knees) and other plastic surgery procedures (three knees), and direct antibiotic infusion through Hickman catheters for 6 weeks. Ten knees had one-stage revision; five had débridement, cement spacer, and revision surgery 3 to 4 months later; and three had extensive soft tissue reconstruction before revision surgery. The minimum followup was 2.3 years (mean, 6.1 years; range, 2.3–12.0 years).
Results
The mean Knee Society scores improved from 33 preoperatively to 76. Seventeen of the 18 had control of infection and achieved durable fixation and a closed wound. One patient had recurrent infection 13 months after one-stage revision, was revised, and remained asymptomatic 28 months postoperatively after redébridement and vancomycin infusion for 6 weeks. In one patient, soft tissue closure was not obtained and the patient required amputation.
Conclusions
Extensile exposure, débridement, and soft tissue flaps for closure combined with uncemented fixation of revision implants and antibiotic infusion into the knee controlled reinfection after revision TKA.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Revision of total joint arthroplasty for infection is difficult surgery, and recurrent infection, loosening, and pain contribute to the high failure rate [6, 30, 34]. Resistant organisms are especially difficult to treat in TKA infection, resulting in failure rates ranging from 24% to 82% [10, 18, 25, 33]. Revision for infection after previous revision for infection adds another dimension of difficulty to management of these challenging cases. Studies indicate management of reinfection after at least one revision for infection is attended by a high incidence of succeeding infection as well as loosening, instability, and pain [6, 30, 34].
Although it would be difficult to document, it generally is accepted by practitioners in this field that adequate exposure and complete débridement are major factors in the management of multiply failed TKA. If cement has been pressure-injected into the medullary canals of the femur and tibia, direct visualization by means of bivalve osteotomy or endoscopic instruments often is required for exposure and direct access to the inner surfaces of the bone. Adequate exposure of the synovial cavity and its extensions in the form of popliteal cysts and abscesses often requires extensile exposure such as tibial tubercle osteotomy or extensive soft-tissue stripping and extension of the incision proximally between the vastus medialis and rectus femoris muscles [39].
Although cemented fixation of revision TKA can achieve durable fixation [36], fixation of revision implants can be a problem in both hip and knee arthroplasty [5, 28]. However, in revision THA, fixation of implants with a cementless technique has been an important factor in improving mechanical success [5]. Similarly, cementless fixation in revision TKA has achieved stable fixation of both implants in 94% to 97% of cases [37, 40, 41].
Soft tissue damage caused by repeated surgical exposure, draining sinuses, and subcutaneous abscesses causes especially difficult problems in closure and sealing of the joint. Muscle pedicle and subfascial skin flaps often are necessary to achieve watertight closure in these complex cases that involve recurrent failure and infection.
One reason for the high failure rate is the difficulty in delivering antibiotics in high enough concentrations and for the proper duration to control infection in the joint. Intravenous antibiotics achieve adequate joint levels only briefly [13, 17, 22, 23, 26, 35]; and antibiotic-loaded cement spacers are depleted of available antibiotics after 3 to 7 days and thereafter become a potential nidus for growth of infecting organisms [2, 26, 32].
In veterinary practice, direct intraarticular injection of antibiotics has been used for decades, and the reported intraarticular concentration is many orders of magnitude higher than that achieved by intravenous administration of a much higher dose [13]. Direct infusion of antibiotics also has been used in humans to salvage acutely and chronically infected TKA and has had a high rate of infection control (95%) in knees infected with resistant bacteria [42], a category of infection that ordinarily has a high failure rate [20, 22].
For treating reinfection after two-stage revision TKA for infection, the senior author (LAW) developed a surgical protocol that included tibial tubercle osteotomy for exposure when necessary in stiff knees to avoid extensive soft tissue stripping, bivalve osteotomy of the femur and tibia to extirpate extensive cement mantles, cementless fixation, closure with muscle flaps and subfascial skin flaps in cases with deficient capsule and skin, and direct infusion of antibiotics with sealed indwelling catheters. The purpose of this study was to determine the success rate of this aggressive approach to infection in a group of knees that clearly were failures of previous two-stage revision for infection.
We therefore sought to determine (1) the rate of success in controlling the infection; (2) the safety of the method of antibiotic delivery by determining complications of using the catheters and by observing the serum vancomycin concentration; and (3) the rate of success using a cementless fixation technique for the revision arthroplasty.
Patients and Methods
We retrospectively reviewed all 18 patients (18 knees) treated for reinfection after previous two-stage revision of infected TKA between January 1999 and January 2008. Twelve patients were women and six patients were men. Mean time from the original TKA until the initial revision was 7 months (range, 1.5–13 months) and from revision to rerevision was 5 months (range, 1–18 months). All knees were reinfected with the original organism(s). The infecting organisms included methicillin-resistant Staphylococcus aureus (11 patients, 11 knees), methicillin-resistant Staphylococcus epidermidis (two patients, two knees), methicillin-sensitive S aureus (two patients, two knees), and mixed Proteus mirabilis and Escherichia coli (three patients, three knees). All of the Staphylococcus organisms were sensitive to vancomycin in concentrations of 2 to 5 μg/mL, and the three E coli and P mirabilis organisms were sensitive to gentamicin in concentrations of 2 μg/mL. The minimum followup was 2.3 years (mean, 6.1 years; range, 2.3–12.0 years). No patients were lost to followup. No patients were recalled specifically for this study; all data were obtained from medical records.
We treated all knees with the same protocol that included extensive débridement, revision TKA with uncemented components, and direct antibiotic infusion. Surgical treatment included thorough removal of nonabsorbable sutures, complete synovectomy, and débridement of abscesses and popliteal cysts. Vascularized osteoperiosteal flap osteotomy was used to expose diaphyseal cement mantles that extended into the diaphysis and could not be removed from the open end of the bone, leaving the cement bed directly and completely visible for inspection. We meticulously removed cement using three-phase débridement of the bone surfaces starting with rongeurs followed by curettes and finishing with a high-torque reamer to burr away all surfaces exposed to cement. During débridement, hand-pump irrigation with a saline solution of vancomycin (1 g/L), polymyxin (30,000 U/L), and Bacitracin (50,000 U/L) was performed repeatedly. After the débridement was completed, the area was redraped, the surgical team regowned and gloved, and the instruments were washed and soaked in the same type of antibiotic solution used for irrigation. Tibial tubercle osteotomy was used for exposure in 13 knees. Seventeen knees required bivalve osteotomy of the femur, tibia, or both to expose the cement mantle and débride the endosteal surfaces. Five knees had necrosis of the quadriceps tendon, patella, and patellar tendon and had débridement and removal of a portion of the quadriceps tendon, the entire patella, and the patellar tendon. Seven knees, including all the knees that had patella and patellar tendon resection, had muscle flaps for closure of capsular and soft tissue defects. Novel muscle flaps, including lateral transfer of the vastus medialis and medial transfer of the vastus lateralis, were necessary in seven knees. In two knees, a medial gastrocnemius flap was sutured to the transferred vastus medialis muscle to achieve extensor continuity through the knee. Ten knees (56%) had one-stage revision (Fig. 1). Five knees (28%) had débridement, cement spacer, and definitive revision arthroplasty 3 to 4 months later; and three knees (16%) had multiple extensive soft tissue reconstruction including tissue expanders to produce enough skin for closure and external fixators to achieve adequate limb length before their definitive revision arthroplasty. Two of the patients (two knees) required débridement of the edge of a muscle flap and repeat closure within the first week postoperatively. Three patients (three knees) had open drainage of hematoma and reclosure during the first 2 weeks postoperatively. If the bone and soft tissue quality had adequate circulation to sustain healing, and adequate soft tissue was available for closure, then we performed revision TKA using nonporous, fluted, diaphyseal-engaging titanium stems and porous-coated implants applied directly to available bone. No cement was used to fix the implants to bone, and no bone graft was used to fill bone defects. In cases in which bone stock and soft tissue were not deemed adequate for stable fixation of the implants and secure closure of the joint, implants were not inserted, Hickman catheters were inserted for delivery of antibiotics, and closure completed using available skin and muscle flaps, allowing the extremity to shorten if necessary. These patients were managed postoperatively to achieve bone healing of the osteotomies, restore leg length, and gain skin for closure. Three patients (three knees) underwent external fixation for gradual lengthening to regain limb length, and three patients (three knees) had subfascial soft tissue expanders to provide skin for closure. We used a Constavac (Stryker Corp., Kalamazoo, MI, USA) drain for 24 to 48 hours postoperatively, but the blood was not reinfused.
Fig. 1.
Lateral radiograph performed at 6 weeks after revision with uncemented implants and Hickman catheters for antibiotic infusion. The infection resolved, and the patient has progressed to full weightbearing.
To improve the chances of maintaining intraarticular access for 6 weeks, we inserted two Hickman catheters (CR Bard Inc, Salt Lake City, UT, USA) in all patients. These catheters are silicone tubes with a fibrous cuff that allows fibrous tissue ingrowth to seal the entry point and prevent ingress and egress of fluid around the catheter. The catheters were inserted through the lateral thigh, penetrating the vastus lateralis muscle and entering the suprapatellar area of the knee (Fig. 2). The fibrous cuff was placed approximately 5 mm deep to the dermis. We sutured each catheter to the skin surface with silk sutures on two sides, and the injection portals were taped to the surface of the skin. The external portals each were fitted with a Luer lock module and cap to allow injection with a syringe. The junctions were sealed with Betadine ointment. Postoperatively the patients received 1 g vancomycin or 80 mg gentamicin intravenously every 12 hours for at least 48 hours postoperatively. The intravenous antibiotics were discontinued after 48 hours if intraarticular administration was established. Intraarticular infusion of antibiotics began in the evening of the first day after surgery. We administered 100 mg vancomycin or 20 mg gentamicin in 3 mL saline daily as a test dose, and the concentration and volume were increased daily if the wound remained sealed and quiescent. When the wound was stable and dry, the dosage was increased to 500 mg vancomycin or 80 mg gentamycin in 8 mL saline. The dose was given every 12 or 24 hours depending on the patient’s ability to tolerate the antibiotic in the knee. If irritation and redness occurred, we decreased the volume and concentration. The injection was alternated between the two catheters to keep them open. The catheters were not flushed but were capped and clamped to maintain a reliable seal. Vancomycin is unstable in solution and must not be injected in the knee in concentrations greater than 100 mg/mL. To avoid precipitation of the concentration used in the knees, the dosage was limited to 50 mg/mL. Eight milliliters of the solution (500 mg vancomycin or 80 mg gentamicin) was injected once or twice daily for 6 weeks, and the serum peak and trough levels were measured twice weekly. We modified the frequency and dosage to maintain the serum trough levels between 3 and 10 μg/mL for vancomycin and 1 and 2 μg/mL for gentamicin.
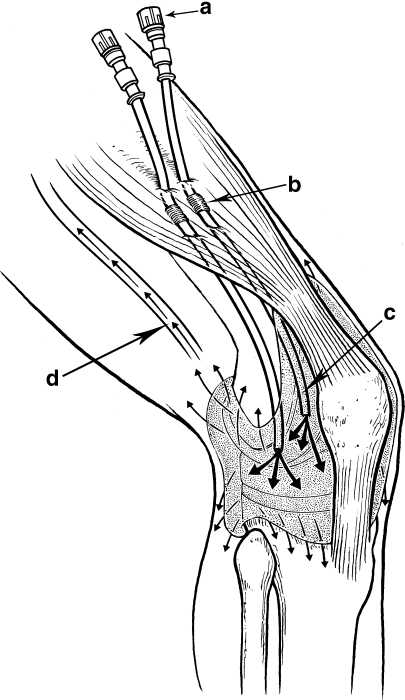
Fig. 2.
Illustration of the infusion system using Hickman catheters. This drawing illustrates the injection portals (a) that are outside the skin, the fibrous cuffs that are approximately 5 mm deep to the dermis (b), the catheters inside the synovial cavity of the knee (c), and outflow of the antibiotic through the synovial membrane and into the regional veins (d). The fibrous cuffs seal the catheters so that contaminants do not enter the knee and joint fluid does not leak out. Reprinted with permission from Whiteside LA, Peppers M, Nayfeh TA, Roy ME. Methicillin-resistant Staphylococcus aureus in TKA treated with revision and direct intraarticular antibiotic infusion. Clin Orthop Relat Res. 2011;469:26–33.
After 6 weeks of treatment, the Hickman catheters were removed surgically. Six of the 18 knees lost one of the catheters because of occlusion during the 6-week infusion interval. Two patients had removal of one of the two catheters for leakage. One knee had traumatic avulsion of both catheters 8 days after surgery and required general anesthesia to insert new catheters. None of the patients had breakage of the catheters, secondary infection, or chronic drainage or fistula formation from the catheters.
All knees were allowed full weightbearing after their final implants were inserted but were supported with a walker for 4 to 6 weeks. Supervised physical therapy was started the first day after surgery and continued through 6 weeks. Gentle active and passive ROM exercises and quadriceps strengthening were begun and progressed as soon as the patient could cooperate.
The patients were seen at 2 weeks for suture removal, at 6 weeks for removal of the Hickman catheters, and again 2 weeks later for suture removal from the tube site. They returned at 3 months for physical evaluation and radiographs and then at yearly intervals for physical evaluation and radiographs. We evaluated all knees for tenderness, erythema, and induration at 3 months postoperatively. Serum C-reactive protein (CRP) concentration and sedimentation rate were evaluated at 3 months. Because the patients were only 3 months postoperative from their revision surgery and had recently had catheter removal, we considered CRP level less than 25% above normal and sedimentation rate less than 50% elevated signs of resolved infection. Other signs of resolved infection included absence of erythema and tenderness and absence of radiographic signs of bone absorption. After 3 months, no additional laboratory tests were obtained by our office and the patients were followed with CRP and sedimentation rates by their infectious disease consultants. Knee scores were determined using the Knee Society Clinical Rating System [8].
To evaluate the quality of the débridement postoperatively, one of us (LAW) evaluated AP, lateral, and skyline patellar radiographs immediately postoperatively and at 1-month and 3-month followup intervals. None had retained cement or debris on their followup radiographs. The same radiographs were scrutinized for evidence of migration or displacement of the bivalve osteotomies and tibial tubercle osteotomy, and fixation was evaluated by appearance of radiolucent lines at every followup visit. We identified and measured radiolucent lines with a ruler accurate to 0.5 mm. The tibial tubercle and fibular head were chosen as landmarks to measure tibial component migration, and the distances from the undersurface of the tibial baseplate to the top of the fibular head and to the tibial tubercle were measured on each radiographic examination. We chose the medial and lateral epicondyles as the femoral bone landmarks for femoral component migration. Femoral component position was measured relative to the femoral bone landmarks by drawing a line that joined the distal surface of the implant and measuring the distance between this line and the medial and lateral epicondyles. We defined radiographic signs of migration as a radiolucent line that increased by more than 1 mm on one side of a diaphyseal stem or greater than 1-mm change in distance relative to one of the bone landmarks on two successive radiographic examinations. We defined a stable arthroplasty as a total knee with no sign of migration of either the femoral or tibial implant over a period of 2 years.
Results
Infection control and clinical signs of success were obtained in 17 of 18 knees. One patient had recurrent infection 13 months after one-stage débridement, revision, and primary closure of the knee. This knee was débrided again, infused with vancomycin for 6 weeks with no implant in place, and reimplanted with cementless implants 6 weeks after catheter removal. The CRP and sedimentation rate were normal at reimplantation with no sign of infection at 28 months followup. One knee failed to obtain soft tissue closure, drained continuously, and finally had above-knee amputation 2 months after beginning treatment. CRP and sedimentation rate were within normal limits at 2-year followup in 16 of the 17 patients. One patient, who has chronic gingivitis, stasis dermatitis, and arteriosclerotic coronary artery disease, had 24% elevation of CRP and a high normal sedimentation rate at 1-year followup. His knee was asymptomatic and benign to examination. Aspiration revealed no white blood cells in the synovial fluid. No patient required chronic suppressive antibiotics. Two patients received oral tetracycline for 3 months after surgery as routine management by their infectious disease consultant.
Serum vancomycin levels within appropriate ranges indicated the safety and efficacy of intraarticular antibiotic delivery through a catheter. Mean serum vancomycin peak level at 1 month postoperatively was 4.1 ± 1.2 μg/mL, and mean trough level was 3.3 ± 1 μg/mL. Mean serum peak gentamicin level was 1.1 ± 1 μg/mL and trough level was 0.2 ± 0.1 μg/mL. Three patients with vancomycin and one with gentamicin infusion required temporary cessation of antibiotic infusion and resumption at a lower dosage because of excessively high serum antibiotic levels or rising blood urea nitrogen and creatinine levels.
No rerevision was performed for loosening or instability with cementless fixation technique. The bivalve osteotomies for cement removal did not fracture or displace, and radiographs revealed no retained cement in any knee. No complications occurred with tibial tubercle osteotomy. All patients except the one requiring amputation achieved full weightbearing by 3 months postoperatively. Knee Society score was 33 ± 11 preoperatively and 76 ± 10 2 years postoperatively. Extensor function was compromised markedly in all knees that required resection of the patella and patellar tendon with extension lag ranging from 5° to 65°. No radiographic signs of implant migration were detected in 16 knees, whereas one knee had migration of the femoral component proximally, a complete radiolucent line around the femoral stem, and 2° varus angulation during the first year after surgery. Two subsequent yearly radiographs revealed no further femoral component migration. One knee had a complete radiolucent line around the tibial component on the AP radiograph that was less than 1 mm and did not widen. All knees had at least one radiographic view showing a less than 1-mm radiolucent line under the tibial porous surface at 2 years postoperatively, and all knees had a less than 1-mm radiolucent line under the nonporous-coated anterior femoral flange. None of these lines widened. Six knees had anterior gaps of 2 to 5 mm since the time of surgery and have remained radiographically stable throughout the followup period.
Discussion
This series demonstrates the magnitude of deficiency and the scope of surgical effort necessary to manage reinfection after two-stage revision for infected TKA and illustrates that it can be treated with a high success rate when a well-planned series of surgical procedures is combined with intraarticular antibiotic infusion. Direct antibiotic infusion was developed to channel very high intraarticular antibiotic concentration into the joint for revision of infected TKA [20, 23, 29]. However, the types of cases in this series involve problems that cannot be solved only with high levels of antibiotics, but also require aggressive exposure and limb salvage techniques and often multiple procedures to prepare the extremity for reimplantation of the arthroplasty components. We therefore determined the success rate of intraoperative and postoperative protocols for reinfection in a group of knees with previous two-stage revision for infection. We evaluated (1) the rate of success in controlling the infection; (2) any complications of antibiotic delivery through a catheter by observing the serum vancomycin concentration; and (3) the rate of success using a cementless fixation technique for the revision arthroplasty.
Our study is limited by several factors. First, we had a small number of cases, although they were all prospectively followed with a set protocol and no patients were lost to followup. Reinfection is relatively uncommon and therefore even in a referral practice, it is difficult to accumulate a large number of cases. Second, we had no comparative group using alternate protocols. The complex and varied nature of the surgical procedures necessarily required individualized treatments. Third, we had a relatively short minimum followup time of 2 years (mean, 6.1 years) but longer than comparative studies [6, 14] (Table 1).
Table 1.
Outcomes of treatment for reinfection after previous revision for infected TKA
| Study | Number of knees | Number of amputations | Number of arthrodeses | Number of pseudarthroses | Number of resection arthroplasties | Number with continued antibiotic cement spacers | Number with suppressive oral antibiotics | Number of functioning TKAs with resolved infections | Mean followup (months) |
|---|---|---|---|---|---|---|---|---|---|
| Hanssen et al. [6] | 24 | 4 | 10 | 3 | 1 | 0 | 5 | 1 (4%) | 47 |
| Maheshwari et al. [14] | 35 | 5 | 2 | 0 | 1 | 3 | 0 | 24 (69%) | 59 |
| Current study | 18 | 1 | 0 | 0 | 0 | 0 | 0 | 17 (94%) | 73 |
Clinically the infection appeared controlled in 17 of 18 knees. Extensile exposure using tibial tubercle osteotomy to open the joint and bivalve osteotomy of the femur and tibia to access retained cement were successful in all cases. Osteotomy, although tedious and time-consuming, was essential to exposure and did not result in complications in this series. Despite all efforts in one knee, soft tissue coverage failed and amputation was required. Although knee function was restored with the planned series of reconstructive procedures, it was compromised in a sizeable proportion of these cases because of loss of the patella and other elements of the extensor apparatus.
Two-stage revision, using intravenous antibiotics and an antibiotic-loaded cement spacer to deliver antibiotics into the joint, is considered the conservative surgical approach to infected TKA [4, 6, 9, 26], but its clinical results are disappointing. Reinfection rates varying from 11% to 24% have been reported in centers that specialize in care of these difficult cases using two-stage débridement and reimplantation [7, 10, 12, 18, 19, 33]. Repeat revision for reinfection adds another dimension of challenge to managing the infected TKA. It has not been reported often, but the few reported series illustrate the difficulty of this condition. One series had a 31% failure rate after repeat two-stage revision for recurrent infection [14]. In another series of 24 reinfected revision TKAs, only one achieved an uninfected knee prosthesis [6] (Table 1). Although one series reported no failures to control the infection in repeated revision for infection [3], in that series of 12, only two were reinfected with the original organism, so 10 cases were not failures of revision for infection. Our success rate in this especially challenging condition suggests direct antibiotic infusion offers a substantial benefit in this situation when combined with an aggressive regimen of débridement, soft tissue coverage, and cementless fixation of implants.
Previous studies of antibiotic infusion directly into the joint have reported remarkable success, including revision for infection with methicillin-resistant S. aureus [42]. Antibiotics injected into synovial joints are absorbed in a manner similar to antibiotics injected intramuscularly and produce similar serum concentration [13, 27], so some peripheral therapeutic antibiotic effect would be expected. Antibiotics injected directly into the joint produce concentrations substantially higher in the adjacent bone than can be achieved with intravenous administration [17, 35]. This suggests local antibiotic concentration in the bone and soft tissue beyond the synovial membrane would more likely be bactericidal with intraarticular than with intravenous administration of antibiotics.
Antibiotic concentrations achieved with antibiotic-loaded cement spacers can be high early but decrease substantially after the first 24 hours [2, 16, 26, 31]. Antibiotic release is minimal after 5 days [11], but the levels remain detectable for as long as 340 days postoperatively [15]. These levels may be too low to be bactericidal in resistant bacteria or gram-negative organisms, even in the first few days, and are likely to be ineffective as time passes [21]. A low concentration of antibiotics in the synovial fluid and on the surface of the cement spacer fosters the development of resistant bacteria [21]. Small colony variants require sustained, high antibiotic concentrations and longer exposure to high antibiotic levels than can be obtained with intravenous antibiotics and antibiotic-loaded cement spacers [21, 24].
Fixation of the implants with one-stage revision and cementless implants was successful in this series. Fixation of implants to bone consistently has been a problem in revision TKA, especially in cases of infection with resistant organisms [10, 18, 25, 33]. Mechanical failure rates of 20% to 40% are reported in centers with high surgical volume and recognized expertise in this field [1, 6, 30, 34]. Cementless fixation with porous devices in revision arthroplasty has had a high rate of success in both hip and knee arthroplasty and has become the predominant mode of fixation in revision THA [5]. Results of the current study using cementless porous-coated devices in difficult cases of infected TKA resemble those of revision THA, and this technique appears to offer an advantage over cemented fixation [5, 38].
Although the reinfected revision TKA protocol may be daunting and require months to complete, we anticipate successful limb salvage and a functioning extremity in the majority of cases. Good soft tissue coverage and revision arthroplasty with cementless implants followed by 6 weeks of antibiotics infused directly into the joint can achieve stable implants and control infection in most cases.
Acknowledgments
We thank William C. Andrea, MS, FAMI, for assistance with the illustration and Diane J. Morton, MS, for assistance with manuscript preparation.
Footnotes
Each author certifies that he or she, or a member of their immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Missouri Bone and Joint Center, Missouri Bone and Joint Research Foundation, St Louis, MO, USA.
References
- 1.Adams SB, Lescun TB. How to treat septic joints with constant intra-articular infusion of gentamicin or amikacin. AAEP Proc. 2000;46:188–192. [Google Scholar]
- 2.Anguita-Alonso P, Rouse MS, Piper KE, Jacofsky DJ, Osmon DR, Patel R. Comparative study of antimicrobial release kinetics from polymethylmethacrylate. Clin Orthop Relat Res. 2006;445:239–244. doi: 10.1097/01.blo.0000201167.90313.40. [DOI] [PubMed] [Google Scholar]
- 3.Backe HA, Jr, Wolff DA, Windsor RD. Total knee replacement infection after 2-stage reimplantation: results of subsequent 2-stage reimplantation. Clin Orthop Relat Res. 1996;331:125–131. doi: 10.1097/00003086-199610000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Haleem AA, Berry DJ, Hanssen AD. Mid-term to long-term followup of two-stage reimplantation for infected total knee arthroplasty. Clin Orthop Relat Res. 2004;428:35–39. doi: 10.1097/01.blo.0000147713.64235.73. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton WG, Cashen DV, Ho H, Hopper RH, Jr, Engh CA. Extensively porous-coated stems for femoral revision: a choice for all seasons. J Arthroplasty. 2007;22(Suppl 1):106–110. doi: 10.1016/j.arth.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Hanssen AD, Trousdale RT, Osmon DR. Patient outcome with reinfection following reimplantation for the infected total knee arthroplasty. Clin Orthop Relat Res. 1995;321:55–67. [PubMed] [Google Scholar]
- 7.Hirakawa K, Stulberg BN, Wilde AH, Bauer TW, Secic M. Results of two-stage reimplantation for infected total knee arthroplasty. J Arthroplasty. 1998;13:22–28. doi: 10.1016/S0883-5403(98)90071-7. [DOI] [PubMed] [Google Scholar]
- 8.Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–14. [PubMed] [Google Scholar]
- 9.Insall JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of infected total knee arthroplasty. J Bone Joint Surg Am. 1983;65:1087–1098. [PubMed] [Google Scholar]
- 10.Kilgus DJ, Howe DJ, Strang A. Results of periprosthetic hip and knee infections caused by resistant bacteria. Clin Orthop Relat Res. 2002;404:116–124. doi: 10.1097/00003086-200211000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Kuechle DK, Landon GC, Musher DM, Noble PC. Elution of vancomycin, daptomycin, and amikacin from acrylic bone cement. Clin Orthop Relat Res. 1991;264:302–308. [PubMed] [Google Scholar]
- 12.Kurd MF, Ghanem E, Steinbrecher J, Parvizi J. Two-stage exchange knee arthroplasty: does resistance of the infecting organism influence the outcome? Clin Orthop Relat Res. 2010;468:2060–2066. doi: 10.1007/s11999-010-1296-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lloyd KC, Stover SM, Pascoe JR, Baggot JD, Kurpershoek C, Hietala S. Plasma and synovial fluid concentrations of gentamicin in horses after intra-articular administration of buffered and unbuffered gentamicin. Am J Vet Res. 1988;49:644–649. [PubMed] [Google Scholar]
- 14.Maheshwari AV, Gioe TJ, Kalore NV, Cheng EY. Reinfection after prior staged reimplantation for septic total knee arthroplasty: is salvage still possible? J Arthroplasty. 2010;25(Suppl):92–97. doi: 10.1016/j.arth.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Masri BA, Duncan CP, Beauchamp CP. Long-term elution of antibiotics from bone-cement: an in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) system. J Arthroplasty. 1998;13:331–338. doi: 10.1016/S0883-5403(98)90179-6. [DOI] [PubMed] [Google Scholar]
- 16.McLaren RL, McLaren AC, Vernon BL. Generic tobramycin elutes from bone cement faster than proprietary tobramycin. Clin Orthop Relat Res. 2008;466:1372–1376. doi: 10.1007/s11999-008-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mills ML, Rush BR, St Jean G, Guaghan EM, Mosier D, Gibson E, Freeman L. Determination of synovial fluid and serum concentrations, and morphologic effects of intraarticular ceftiofur sodium in horses. Vet Surg. 2000;29:398–406. doi: 10.1053/jvet.2000.9141. [DOI] [PubMed] [Google Scholar]
- 18.Mittal Y, Fehring TK, Hanssen A, Marculescu C, Odum S, Osmon D. Two-stage reimplantation for periprosthetic knee infection involving resistant organisms. J Bone Joint Surg Am. 2007;89:1227–1231. doi: 10.2106/JBJS.E.01192. [DOI] [PubMed] [Google Scholar]
- 19.Mortazavi SM, Schwartzenberger J, Austin MS, Purtill JJ, Parvizi J. Revision total knee arthroplasty infection: incidence and predictors. Clin Orthop Relat Res. 2010;468:2052–2059. doi: 10.1007/s11999-010-1308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nayfeh TA, Whiteside LA, Hirsch M. Direct exchange treatment of septic total joint arthroplasty with intra-articular infusion of antibiotics: technique and early results. Orthopedics. 2004;27:987–988. doi: 10.3928/0147-7447-20040901-40. [DOI] [PubMed] [Google Scholar]
- 21.Neut D, Hendriks JG, Horn JR, Mei HC, Busscher HJ. Pseudomonas aeruginosa biofilm formation and slime excretion on antibiotic-loaded bone cement. Acta Orthop. 2005;76:109–114. doi: 10.1080/00016470510030427. [DOI] [PubMed] [Google Scholar]
- 22.Perry CR, Hulsey RE, Mann FA, Miller GA, Pearson RL. Treatment of acutely infected arthroplasties with incision, drainage, and local antibiotics delivered via an implantable pump. Clin Orthop Relat Res. 1992;281:216–223. [PubMed] [Google Scholar]
- 23.Perry CR, Pearson RL. Local antibiotic delivery in the treatment of bone and joint infections. Clin Orthop Relat Res. 1991;263:215–226. [PubMed] [Google Scholar]
- 24.Rusthoven JJ, Davies TA, Lerner SA. Clinical isolation and characterization of aminoglycoside-resistant small colony variants of Enterobacter aerogenes. Am J Med. 1979;67:702–706. doi: 10.1016/0002-9343(79)90269-9. [DOI] [PubMed] [Google Scholar]
- 25.Salgado CD, Dash S, Cantey JR, Marculescu CE. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Relat Res. 2007;461:48–53. doi: 10.1097/BLO.0b013e3181123d4e. [DOI] [PubMed] [Google Scholar]
- 26.Salvati EA, Callaghan JJ, Brause BD, Klein RF, Small RD. Reimplantation in infection: elution of gentamicin from cement and beads. Clin Orthop Relat Res. 1986;207:83–93. [PubMed] [Google Scholar]
- 27.Schurman DJ, Hirshman HP, Kajiyama G, Moser K, Burton DS. Cefazolin concentrations in bone and synovial fluid. J Bone Joint Surg Am. 1978;60:359–362. [PubMed] [Google Scholar]
- 28.Shannon BD, Klassen JF, Rand JA, Berry DJ, Trousdale RT. Revision total knee arthroplasty with cemented components and uncemented intramedullary stems. J Arthroplasty. 2003;18(Suppl 1):27–32. doi: 10.1016/S0883-5403(03)00301-2. [DOI] [PubMed] [Google Scholar]
- 29.Shaw JA. The use of long-term indwelling catheters for local antibiotic administration into infected joints: a concept report. J Orthop Tech. 1995;3:181–184. [Google Scholar]
- 30.Stuart MJ, Larson JE, Morrey BF. Reoperation after condylar revision total knee arthroplasty. Clin Orthop Relat Res. 1993;286:168–173. [PubMed] [Google Scholar]
- 31.Belt H, Neut D, Schenk W, Horn JR, Mei HC, Busscher JH. Gentamicin release from polymethylmethacrylate bone cements and Staphylococcus aureus biofilm formation. Acta Orthop Scand. 2000;71:269–625. doi: 10.1080/000164700317362280. [DOI] [PubMed] [Google Scholar]
- 32.Belt H, Neut D, Uges DR, Schenk W, Horn JR, Mel HC, Busscher HJ. Surface roughness, porosity, wettability of gentamicin-loaded bone cements and their antibiotic release. Biomaterials. 2000;21:1981–1987. doi: 10.1016/S0142-9612(00)00082-X. [DOI] [PubMed] [Google Scholar]
- 33.Volin SJ, Hinrichs SH, Garvin KL. Two-stage reimplantation of total joint infections: a comparison of resistant and non-resistant organisms. Clin Orthop Relat Res. 2004;427:94–100. doi: 10.1097/01.blo.0000143559.34143.3d. [DOI] [PubMed] [Google Scholar]
- 34.Wang CJ, Huang TW, Wang JW, Chen HS. The often poor clinical outcome of infected total knee arthroplasty. J Arthroplasty. 2002;17:608–614. doi: 10.1054/arth.2002.32700. [DOI] [PubMed] [Google Scholar]
- 35.Werner LA, Hardy J, Bertone AL. Bone gentamicin concentration after intra-articular injection or regional intravenous perfusion in the horse. Vet Surg. 2003;32:559–565. doi: 10.1111/j.1532-950X.2003.00559.x. [DOI] [PubMed] [Google Scholar]
- 36.Whaley AL, Trousdale RT, Rand JA, Hanssen AD. Cemented long-stem revision total knee arthroplasty. J Arthroplasty. 2003;18:592–599. doi: 10.1016/S0883-5403(03)00200-6. [DOI] [PubMed] [Google Scholar]
- 37.Whiteside LA. Cementless fixation issues in revision total knee arthroplasty. Instr Course Lect. 1999;48:177–182. [PubMed] [Google Scholar]
- 38.Whiteside LA. Major femoral bone loss in revision total hip arthroplasty treated with tapered, porous-coated stems. Clin Orthop Relat Res. 2004;429:222–226. doi: 10.1097/01.blo.0000150129.65400.78. [DOI] [PubMed] [Google Scholar]
- 39.Whiteside LA. Surgical exposure in total knee arthroplasty. In: Brown TE, Cui Q, Mihalko WM, Saleh KJ, editors. The Knee. Philadelphia: Elsevier; 2008. [Google Scholar]
- 40.Whiteside LA. Two-stage exchange for infected TKA—opposes. Semin Arthroplasty. 2008;19:121–125. doi: 10.1053/j.sart.2007.12.030. [DOI] [Google Scholar]
- 41.Whiteside LA, Bicalho PS. Radiologic and histologic analysis of morselized allograft in revision total knee replacement. Clin Orthop Relat Res. 1998;357:149–156. doi: 10.1097/00003086-199812000-00020. [DOI] [PubMed] [Google Scholar]
- 42.Whiteside LA, Peppers M, Nayfeh TA, Roy ME. Methicillin-resistant Staphylococcus aureus in TKA treated with revision and direct intraarticular antibiotic infusion. Clin Orthop Relat Res. 2011;469:26–33. doi: 10.1007/s11999-010-1313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]