Abstract
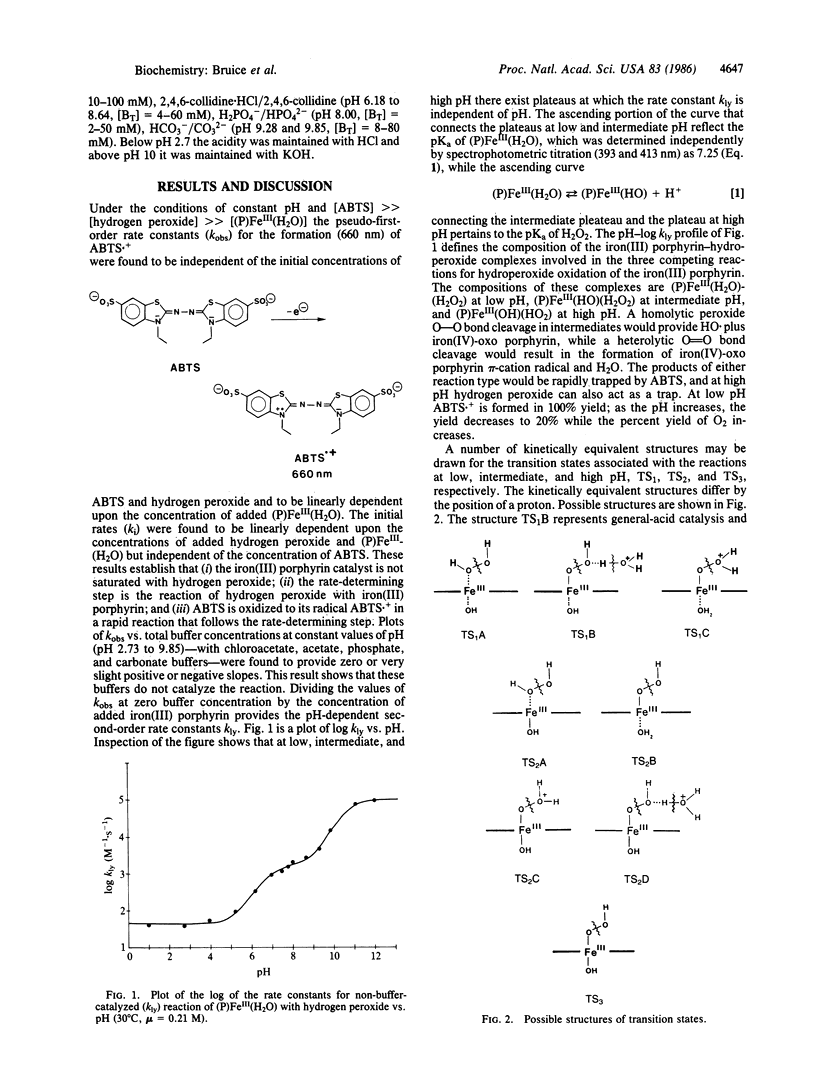
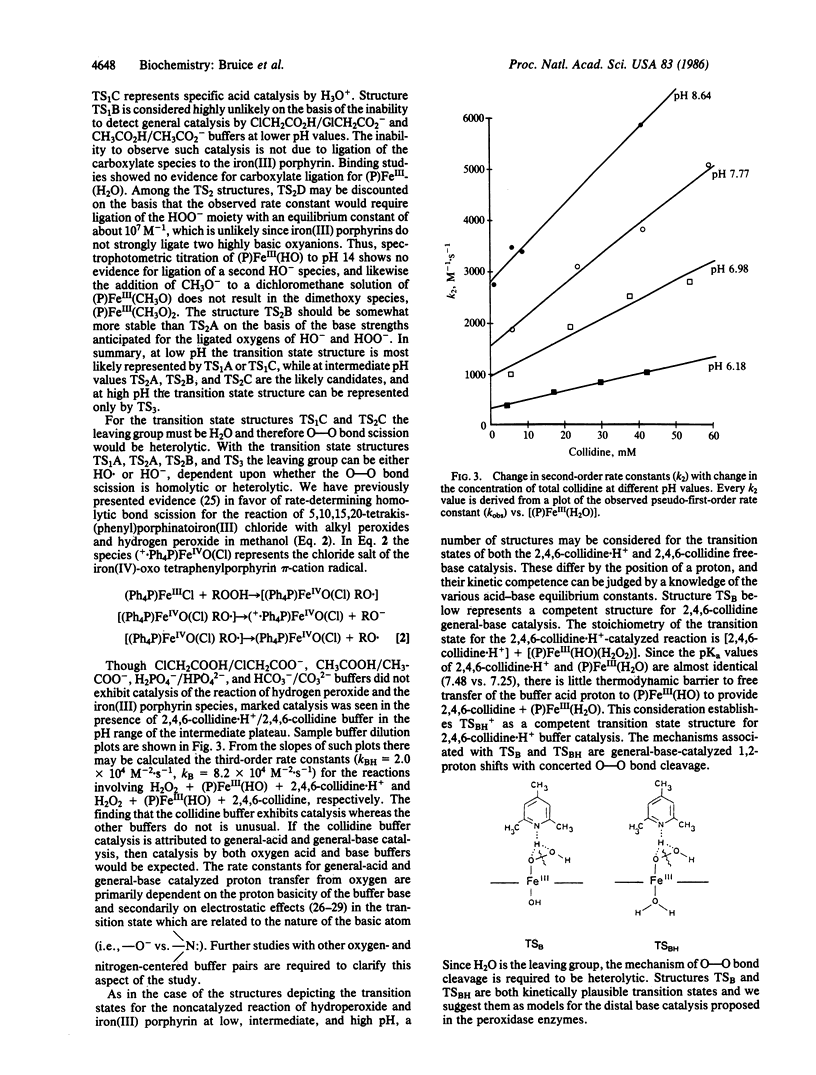
The reaction of hydrogen peroxide with 5, 10,15,20-tetrakis(2,6-dimethyl-3-sulfonatophenyl)porphinato- iron(III) hydrate [(P)FeIII(H2O)] has been investigated in water between pH 1 and pH 12. The water-soluble (P)FeIII(H2O) neither aggregates nor forms a mu-oxo dimer. The pH dependence and rate-limiting second-order rate constants (kly) for oxygen transfer from H2O2 and HO2- to the iron(III) porphyrin were determined by trapping of the resultant higher-valent iron-oxo porphyrin species with 2,2'-azinodi(3-ethylbenzthiazoline)-6-sulfonate (ABTS). Reactions were monitored spectrophometrically by following the appearance of the radical ABTS.+. From a plot of the logarithm of the determined second-order rate constants for reaction of hydrogen peroxide with iron(III) porphyrin vs. pH, the composition of the transition states can be assigned for the three reactions that result in oxygen transfer to yield a higher-valent iron-oxo porphyrin species. The latter not only reacts with ABTS to provide ABTS.+ in a peroxidase-type reaction but also reacts with hydrogen peroxide to provide O2 in a catalase-type reaction. The nitrogen base 2,4,6-collidine serves as a catalyst for oxygen transfer from hydrogen peroxide to the (P)FeIII-(H2O) and (P)FeIII(HO) species. The preferred mechanism involves a 1,2-proton shift concerted with heterolytic cleavage of the peroxide O-O bond. An analogous mechanism is believed to occur in the peroxidase enzymes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake R. C., 2nd, Coon M. J. On the mechanism of action of cytochrome P-450. Evaluation of homolytic and heterolytic mechanisms of oxygen-oxygen bond cleavage during substrate hydroxylation by peroxides. J Biol Chem. 1981 Dec 10;256(23):12127–12133. [PubMed] [Google Scholar]
- Brown S. B., Dean T. C., Jones P. Catalatic activity of iron(3)-centred catalysts. Role of dimerization in the catalytic action of ferrihaems. Biochem J. 1970 May;117(4):741–744. doi: 10.1042/bj1170741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrook R. W., Martin-Wixtrom C., Saeki Y., Renneberg R., Hildebrandt A., Werringloer J. The peroxidatic function of liver microsomal cytochrome P-450: comparison of hydrogen peroxide and NADPH-catalysed N-demethylation reactions. Xenobiotica. 1984 Jan-Feb;14(1-2):87–104. doi: 10.3109/00498258409151400. [DOI] [PubMed] [Google Scholar]
- Hatzikonstantinou H., Brown S. B. Catalase model systems. Decomposition of hydrogen peroxide catalysed by mesoferrihaem, deuteroferrihaem, coproferrihaem and haematoferrihaem. Biochem J. 1978 Sep 15;174(3):893–900. doi: 10.1042/bj1740893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrycay E. G., O'Brien P. J. Cytochrome P-450 as a microsomal peroxidase utilizing a lipid peroxide substrate. Arch Biochem Biophys. 1971 Nov;147(1):14–27. doi: 10.1016/0003-9861(71)90304-3. [DOI] [PubMed] [Google Scholar]
- Hrycay E. G., O'Brien P. J. Microsomal electron transport. I. Reduced nicotinamide adenine dinucleotide phosphate-cytochrome c reductase and cytochrome P-450 as electron carriers in microsomal NADPH-peroxidase activity. Arch Biochem Biophys. 1973 Jul;157(1):7–22. doi: 10.1016/0003-9861(73)90383-4. [DOI] [PubMed] [Google Scholar]
- Hrycay E. G., O'Brien P. J. Microsomal electron transport. II. Reduced nicotinamide adenine dinucleotide--cytochrome b5 reductase and cytochrome P-450 as electron carriers in microsomal NADH-peroxidase activity. Arch Biochem Biophys. 1974 Jan;160(1):230–245. doi: 10.1016/s0003-9861(74)80030-5. [DOI] [PubMed] [Google Scholar]
- Jones P., Mantle D., Davies D. M., Kelly H. C. Hydroperoxidase activities of ferrihemes: heme analogues of peroxidase enzyme intermediates. Biochemistry. 1977 Sep 6;16(18):3974–3978. doi: 10.1021/bi00637a006. [DOI] [PubMed] [Google Scholar]
- Jones P., Prudhoe K., Robson T., Kelly H. C. Kinetics of formation of the peroxidatic intermediate from deuteroferriheme and hydrogen peroxide. Biochemistry. 1974 Oct 8;13(21):4279–4284. doi: 10.1021/bi00718a006. [DOI] [PubMed] [Google Scholar]
- Jones P., Robson T., Brown S. B. The catalase activity of ferrihaems. Biochem J. 1973 Oct;135(2):353–359. doi: 10.1042/bj1350353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadlubar F. F., Morton K. C., Ziegler D. M. Microsomal-catalyzed hydroperoxide-dependent C-oxidation of amines. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1255–1261. doi: 10.1016/0006-291x(73)91122-4. [DOI] [PubMed] [Google Scholar]
- Kelly H. C., Davies D. M., King M. J., Jones P. Pre-steady-state kinetics of intermediate formation in the deuteroferriheme-hydrogen peroxide system. Biochemistry. 1977 Aug 9;16(16):3543–3549. doi: 10.1021/bi00635a007. [DOI] [PubMed] [Google Scholar]
- McCarthy M. B., White R. E. Functional differences between peroxidase compound I and the cytochrome P-450 reactive oxygen intermediate. J Biol Chem. 1983 Aug 10;258(15):9153–9158. [PubMed] [Google Scholar]
- Nordblom G. D., White R. E., Coon M. J. Studies on hydroperoxide-dependent substrate hydroxylation by purified liver microsomal cytochrome P-450. Arch Biochem Biophys. 1976 Aug;175(2):524–533. doi: 10.1016/0003-9861(76)90541-5. [DOI] [PubMed] [Google Scholar]
- Portsmouth D., Beal E. A. The peroxidase activity of deuterohemin. Eur J Biochem. 1971 Apr 30;19(4):479–487. doi: 10.1111/j.1432-1033.1971.tb01338.x. [DOI] [PubMed] [Google Scholar]



