Abstract
Background
One of the radiographic hallmarks in patients with atypical femoral insufficiency fractures after prolonged bisphosphonate treatment is generalized cortical hypertrophy. Whether cortical thickening in the proximal femur is caused by long-term alendronate therapy, however, remains unknown.
Questions/purposes
We asked whether long-term alendronate use of 5 years or more results in progressive thickening of the subtrochanteric femoral cortices.
Patients and Methods
We retrospectively evaluated changes in cortical thickness and cortical thickness ratio (ratio of cortical to femoral shaft diameter) at the subtrochanteric region of the proximal femur in baseline and latest hip dual-energy xray absorptiometry (DXA) scans of 131 patients. The mean followup was 7.3 years. Patients were divided into two groups: control (no history of alendronate, 45 patients) and alendronate (history of alendronate ≥ 5 years, 86 patients). We determined cortical thickness and cortical thickness ratio at 3.5 and 4.0 cm below the tip of the greater trochanter, representing the subtrochanteric region.
Results
After a minimum of 5 years followup, mean cortical thickness decreased approximately 3% in the alendronate and control groups. The cortical thickness at the subtrochanteric femoral region changed less than 1 mm in greater than 90% of the patients with long-term alendronate treatment. We observed no differences in mean changes of cortical thickness and percent changes of cortical thickness between the two groups.
Conclusions
Long-term alendronate treatment did not appear to cause thickened femoral cortices within the detection limits of our method. This finding contrasts with the notion that long-term alendronate treatment leads to generalized cortical thickening.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Alendronate is a bisphosphonate agent that has been widely used for the treatment of osteoporosis [21, 24]. Two randomized controlled studies showed that alendronate increases bone mineral density (BMD) and reduces the risk of osteoporotic fractures [3, 4]. The mechanism of this medication involves the induction of osteoclast apoptosis, thus reducing bone resorption [28]. Several reports have raised a concern of the association between long-term use of alendronate and the occurrence of low-energy subtrochanteric/femoral shaft fractures [8, 9, 16, 17]. Although the pathogenesis of these fractures is not fully understood, it has been suggested the low-energy femoral fracture is related to profound osteoclast inhibition and the resulting suppression of bone turnover and bone remodeling, leading to an atypical femoral insufficiency fracture [11, 12, 16, 23].
As a large number of patients receive long-term bisphosphonate treatment, recognizing and distinguishing this type of fracture is important. The American Society for Bone and Mineral Research Atypical Femoral Fractures Task Force recently described several radiographic features to define an atypical femoral insufficiency fracture, including noncomminuted, transverse, or short oblique fractures with medial spiking of the femoral cortex; a local periosteal reaction; and generalized increases in cortical thickness of the femur [23]. The local periosteal reaction is consistent with chronic healing of stress fractures. The generalized thickening of the femoral cortex is believed to be a result of impaired ability of bone to remodel, leading to an accumulation of microdamage and compromised bone strength [1, 10, 26]. By using high-resolution peripheral quantitative CT (HR-pQCT), Seeman et al. [22] found the cortical thickness at the distal radius and distal tibia were increased after 12 months of alendronate treatment. In addition, a study by Lenart et al. [9] showed that the normalized cortical thickness (defined as a ratio between cortical thickness and femoral shaft diameter) of patients with atypical femoral insufficiency fractures was approximately 80% thicker than that of patients with osteoporotic femoral fractures. The question of a potential predisposition to cortical thickening in the subtrochanteric region of patients with long-term alendronate therapy, however, remains unknown.
To address this question, we (1) asked whether long-term alendronate treatment leads to a change in cortical thickness at the subtrochanteric area of the proximal femur and (2) compared the differences in change of cortical thickness between patients with or without alendronate therapy.
Patients and Methods
After institutional review board approval, we retrospectively reviewed medical records from the Osteoporosis Prevention Center, New York, NY, for eligible patients who were diagnosed with postmenopausal osteoporosis or osteopenia and who received osteoporosis treatment with alendronate (alendronate group). The indications for oral bisphosphonates in postmenopausal osteoporosis/osteopenia included: (1) a vertebral or hip fracture, (2) hip or lumbar spine bone mineral density T-score less than −2.5, (3) low bone mass (T-score between −1 to −2.5) and a US-adapted WHO 10-year probability of a hip fracture of 3% or greater or 10-year probability of any major osteoporosis-related fracture of 20% or greater [13, 25]. The contraindications were: (1) women who are pregnant or planning a pregnancy, and (2) patients with chronic kidney disease Stages 4 or 5, (3) low serum calcium, (4) severe esophageal diseases, or (5) unable to stay upright for an hour [13]. To compare the results of this study group, we also collected data from patients who fit the criteria for osteoporosis treatment yet declined to take medication (control group). Data were collected in a prospective fashion. Only patients who had serial DXA scans performed with an interval of 5 years or more between baseline and latest followup were enrolled in the study. We excluded patients with secondary causes of osteoporosis, such as rheumatoid arthritis or glucocorticoid use; other metabolic bone diseases, such as Paget’s disease of bone or osteomalacia; any significant history of hip osteoarthritis; and a history of treatment with any osteoporosis medications other than alendronate. Of the 131 postmenopausal women eligible for this study, 86 had a history of alendronate therapy of greater than 5 years (alendronate group), whereas 45 fit the treatment criteria but declined medication (control group).
We collected baseline demographic data of the patients from registry records. The mean ages of patients in the alendronate and control groups were 62 and 63 years, respectively. The mean intervals between DXA in the alendronate and control groups were 87 and 88 months, respectively. There were no differences in age, race, interval between DXA tests, and baseline BMD and T score at the hip between the two groups. Body mass index and baseline BMD and T score at the lumbar spine were higher in the control group (Table 1). The minimum followup was 5 years (mean, 7.3 years; range, 5–12 years). After a minimum of 5 years followup, the mean total hip BMD remained stable (an accepted minimal clinically significant difference of BMD change = 3% [14]) in the alendronate group whereas it decreased approximately 3.7% in the control group suggesting a difference (p < 0.001) between the two groups. For lumbar spine BMD, the mean percent change between baseline and latest DXA differed (p < 0.001) between the two groups: it increased approximately 6.1% for the alendronate group but it was stable for the control group (Table 1). No patients were lost to followup. No patients were recalled specifically for this study; all data were obtained from medical records and imaging. All patients provided informed consent and were enrolled in an osteoporosis registry at the time of initial evaluation.
Table 1.
Demographic characteristics
| Variable | Alendronate (n = 86) | Control (n = 45) | p Value |
|---|---|---|---|
| Age at baseline (years)* | 62.4 ± 8.5 | 63.1 ± 8.9 | 0.639 |
| Race (number of patients) | 0.416 | ||
| Caucasian | 44 (97.8%) | 85 (96.5%) | |
| Asian | 0 (0.0%) | 2 (2.3%) | |
| Hispanic | 1 (2.2%) | 0 (0.0%) | |
| African American | 0 (0.0%) | 1 (1.2%) | |
| BMI (kg/m2)* | 22.9 ± 4.0 | 24.7 ± 3.7 | 0.011 |
| Time between DXA (months)* | 87.1 ± 21.9 | 88.1 ± 22.6 | 0.797 |
| Baseline total hip BMD (g/cm2)* | 0.614 ± 0.073 | 0.629 ± 0.048 | 0.156 |
| Baseline total hip T score* | −2.1 ± 0.7 | −2.0 ± 0.4 | 0.153 |
| Baseline LS BMD (g/cm2)*,† | 0.797 ± 0.124 | 0.870 ± 0.097 | 0.001 |
| Baseline LS T score*,‡ | −2.2 ± 1.1 | −1.6 ± 0.9 | < 0.001 |
| Percent change in total hip BMD*,$ | 0.0 ± 5.9 | −3.7 ± 4.4 | < 0.001 |
| Percent change in LS BMD*,†,$ | 6.1 ± 8.5 | −1.4 ± 7.0 | < 0.001 |
* Data are expressed as mean ± SD; † number of patients for alendronate group is 75; ‡ number of patients for alendronate group is 78; BMI = body mass index; DXA = dual-energy xray absorptiometry; BMD = bone mineral density; LS = lumbar spine; $ BMD is considered to be stable if the percent change is less than 3% between baseline and latest followup.
All patients were positioned and scanned by an experienced technician using a standardized protocol. The DXA machine (Hologic Software Version 12.7.4.2, Bedford, MA, USA) was calibrated and tested on site daily, with limits for precision before use of 1.5% for BMD and distance measurements. Measurements were obtained from DXA images. The images from patients’ DXA scans were uploaded using a computerized imaging system to a picture archiving and communication system (PACS; Philips Medical Systems EasyVision DX/CL/RG with core software by Sectra Imtec AB v. 11.1, Linköping, Sweden). Baseline and latest hip DXAs were used to determine the cortical thickness and the cortical thickness ratio at levels of 3.5 and 4.0 cm below the tip of the greater trochanter, representing the subtrochanteric region of the proximal femur. To standardize the measurements in each DXA image, a scale bar of 45 mm was included digitally by the DXA machine at the time the image was created. This scale bar was measured in PACS and used as a calibration tool for our measurements. Each image was uploaded at a resolution of 115 pixels/inch and thus had a minimum measurable distance of 0.22 mm.
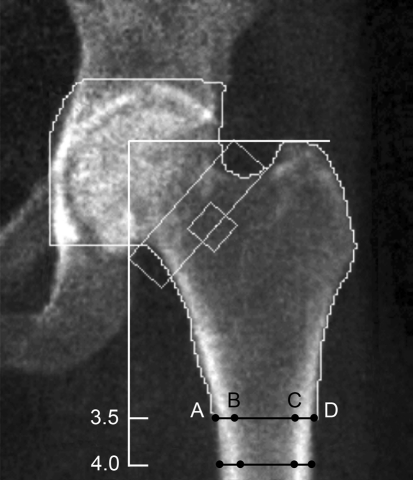
Two of us (JPK, KA), blinded to the groups, measured cortical thickness in the following way. First, a line perpendicular to the femoral shaft axis was drawn at the level of the greater trochanter. Thereafter, two pairs of four points were plotted on the femoral shaft at the levels of 3.5 and 4.0 cm below the tip of the greater trochanter (Fig. 1). Two points were placed on the outer surfaces of the femoral cortices; the other two were placed on the inner or medullary surfaces of the femoral cortices. By using the distance tool in the PACS software, the femoral shaft and medullary diameters were obtained. Femoral shaft diameter was defined as the distance between the outer surfaces of the femoral cortices (Fig. 1, Line AD). Medullary diameter was defined as the distance between the medullary surfaces of the femoral cortices (Fig. 1, Line BC). All measurements were recorded in millimeters. Cortical thickness was calculated by subtracting the medullary diameter from the femoral shaft diameter. The cortical thickness ratio was defined as the percentage of the cortical thickness to its femoral shaft diameter. Endosteal widening was calculated by subtracting baseline medullary canal diameter from end medullary canal diameter. Periosteal apposition was calculated by subtracting baseline femoral shaft diameter from end femoral shaft diameter. All distances were measured four times, twice independently with a 1-week gap between first and second measurements by each observer. The mean value of each measurement then was tabulated. Intraclass correlation coefficients were calculated to assess intrarater and interrater reliabilities of the DXA measurements. The intraclass correlation coefficients (r) for intraobserver and interobserver reliability were more than 0.96 and 0.76, respectively. The mean absolute difference and SD between the first and second measurements of each observer was 0.2 ± 0.2 mm.
Fig. 1.
A DXA scan that was uploaded to a PACS is shown. Measurements were obtained at 3.5 cm and 4.0 cm from the tip of the greater trochanter. Cortical thickness was calculated by subtracting the medullary diameter from the femoral shaft diameter (AD − BC). The cortical thickness ratio was defined as the percentage of the cortical thickness to its femoral shaft diameter [(AD − BC)/AD].
Baseline proximal femoral measurements were compared between alendronate and control groups using the unpaired t-test (Table 2). There were no differences in baseline femoral shaft diameter between the alendronate and control groups (p = 0.707 and p = 0.744 at 3.5 and 4.0 cm below the greater trochanter, respectively); however, the mean medullary diameter was larger in the alendronate group (p = 0.010 and p = 0.016 at 3.5 and 4.0 cm below the greater trochanter, respectively). Therefore, the mean baseline cortical thicknesses and cortical thickness ratios were higher (p < 0.001) in the control group than in the alendronate group at 3.5 and 4.0 cm below the greater trochanter (Table 2). The baseline cortical thickness in both groups was approximately 5 mm at 3.5 and 4.0 cm below the greater trochanter. As a minimum measurable distance of PACS was 0.22 mm, our method of measuring cortical thickness therefore can only detect changes in cortical thickness when it is changed greater than 4.4% [(0.22/5) × 100]. This value is a detection limit or systematic error of our measurement method.
Table 2.
Baseline and end measurements
| Measurement | Baseline | End | ||||
|---|---|---|---|---|---|---|
| Alendronate | Control | p Value | Alendronate | Control | p Value | |
| 3.5 cm below GT | ||||||
| Femoral shaft diameter (mm) | 13.4 ± 1.2 | 13.4 ± 1.2 | 0.707 | 13.2 ± 1.3 | 13.4 ± 1.2 | 0.517 |
| Medullary canal diameter (mm) | 8.2 ± 1.3 | 7.7 ± 1.0 | 0.010 | 8.3 ± 1.3 | 7.8 ± 0.9 | 0.008 |
| Cortical thickness (mm)* | 5.2 ± 0.7 | 5.8 ± 0.9 | < 0.001 | 4.9 ± 0.7 | 5.6 ± 1.1 | < 0.001 |
| Cortical thickness ratio (%)† | 38.8 ± 5.9 | 42.8 ± 5.3 | < 0.001 | 37.2 ± 5.8 | 41.6 ± 6.0 | < 0.001 |
| 4.0 cm below greater trochanter | ||||||
| Femoral shaft diameter (mm) | 12.7 ± 1.1 | 12.7 ± 1.0 | 0.744 | 12.7 ± 1.1 | 12.7 ± 1.0 | 0.957 |
| Medullary canal diameter (mm) | 7.1 ± 1.2 | 6.6 ± 1.0 | 0.016 | 7.2 ± 1.2 | 6.7 ± 1.0 | 0.016 |
| Cortical thickness (mm)* | 5.6 ± 0.8 | 6.2 ± 0.8 | < 0.001 | 5.4 ± 0.8 | 6.0 ± 0.8 | < 0.001 |
| Cortical thickness ratio (%)† | 44.4 ± 6.1 | 48.4 ± 5.4 | < 0.001 | 43.1 ± 6.1 | 47.0 ± 5.8 | < 0.001 |
Data are expressed as mean ± SD; * cortical thickness was calculated as follows: femoral shaft diameter – medullary canal diameter; † cortical thickness ratio was calculated as follows: (cortical thickness/femoral shaft diameter) × 100.
As there are no data on an accepted minimal clinically significant difference in percent changes of cortical thickness after long-term bisphosphonate treatment, we arbitrarily selected 1 mm or a 20% change from a cortical thickness of 5 mm as our minimal clinically significant difference. Our proposed minimal clinically significant difference is much smaller than an 80% difference of the cortical thickness between osteoporotic and atypical femoral insufficiency fractures, which was reported in a previous study [9]. In addition, this 1-mm range covers the entire area of an ill-defined cortical margin. Thus, we can accurately measure changes in cortical thickness greater than this 1-mm or 20%-difference range.
The cortical thickness, cortical thickness ratio, and percent change in cortical thickness were assessed for normality with the Kolmogorov-Smirnov test. Descriptive analyses were performed on the change in cortical thickness and percent change in cortical thickness. These two measurements were presented as mean ± SD and number of patients in each interval. The intervals were stratified based on our detection limit (approximately 5%) and our proposed minimal clinical significance of 1 mm or 20% change in cortical thickness. Subsequently, the change in cortical thickness and percent change in cortical thickness between the alendronate and control groups were compared using the unpaired t-test (two-sided). Analyses were performed using SPSS® Software Version 14.0 (SPSS Inc, Chicago, IL, USA).
Results
The cortical thickness at 3.5 and 4.0 cm below the greater trochanter remained stable (defined as change less than 1 mm from baseline) in greater than 90% of the patients who received alendronate treatment for 5 years or greater (Table 3). The mean percent changes in cortical thickness decreased 3.3% and 2.9% at levels of 3.5 and 4.0 cm below the greater trochanter, respectively. When calculating for the percent change of cortical thickness based on our detection limit of 5%, approximately 40% of the patients had no change in cortical thickness whereas 39.5% and 46.5% of the patients had decreased cortical thickness greater than our detection limit at 3.5 and 4.0 cm below the greater trochanter, respectively. Only 1% to 2% of the patients had increased cortical thickness greater than 1 mm or 20% change compared with baseline (Table 3).
Table 3.
Change in cortical thickness at the subtrochanteric area in patients with prolonged bisphosphonates treatment
| Measurements compared with baseline | 3.5 cm below greater trochanter | 4.0 cm below greater trochanter |
|---|---|---|
| Change in cortical thickness | ||
| Mean ± SD (mm) | −0.2 ± 0.5 | −0.2 ± 0.5 |
| Range (mm) | −1.8 to 0.9 | −1.1 to 1.3 |
| Increase (≥ 1 mm) | 0 (0%) | 2 (2.3%) |
| No change* | 80 (93.0%) | 83 (96.5%) |
| Decrease (≤ −1 mm) | 6 (7.0%) | 1 (1.2%) |
| Percent change of cortical thickness† | ||
| Mean ± SD (%) | −3.3 ± 9.2 | −2.9 ± 8.0 |
| Range (%) | −26.7 to 17.5 | −20.5 to 24.1 |
| Increase in cortical thickness > 20% | 0 (0%) | 1 (1.2%) |
| Increase between 5% to 20% | 15 (17.4%) | 9 (10.5%) |
| No change** | 37 (43.0%) | 36 (41.9%) |
| Decrease between −5% to −20% | 29 (33.7%) | 39 (45.3%) |
| Decrease in cortical thickness > 20% | 5 (5.8%) | 1 (1.2%) |
Values are expressed as number of patients; † percent change of cortical thickness = (change of cortical thickness/baseline cortical thickness) × 100; * no change is designated as the absolute difference between baseline and end cortical thickness less than 1 mm; ** no change is designated as the absolute percent change in cortical thickness less than 5%.
We observed no differences in the mean changes of cortical thickness and cortical thickness ratio between the alendronate and control groups. At the latest followup after a minimum interval of 5 years between DXAs, the mean femoral cortical thickness at the subtrochanteric area was thinner (p < 0.001) in the alendronate group than in the control group at 3.5 and 4.0 cm below the greater trochanter (4.9 versus 5.6 mm and 5.4 versus 6.0 mm, respectively) (Table 2). When comparing the followup parameters with baseline values, the mean cortical thickness at 3.5 cm below the greater trochanter decreased approximately 0.2 mm for the alendronate and control groups (p = 0.838) (Table 4). Similarly, the mean cortical thickness at 4.0 cm below the greater trochanter decreased approximately 0.2 mm for both groups (p = 0.572). When calculating percent changes in cortical thickness over a minimum of 5 years followup, the mean percent change of cortical thickness decreased approximately 3% in both groups (p = 0.931 and p = 0.709 at 3.5 and 4.0 cm below the greater trochanter, respectively) (Table 4). Post hoc analysis showed that our study has 58% and 98% power to detect percent changes of 5% and 20% in cortical thickness, respectively. There were also no differences between the alendronate and control groups regarding the degree of endosteal widening and periosteal apposition over a minimum of 5 years’ followup (Table 4).
Table 4.
Change in measurement parameters after a minimum 5 years’ followup
| Measurements compared with baseline | Alendronate (n = 86) | Control (n = 45) | p Value |
|---|---|---|---|
| 3.5 cm below greater trochanter | |||
| Endosteal widening (mm)* | 0.1 ± 0.6 | 0.1 ± 0.5 | 0.904 |
| Periosteal apposition (mm)† | −0.1 ± 0.7 | 0.0 ± 0.5 | 0.562 |
| Change of cortical thickness (mm) | −0.2 ± 0.5 | −0.2 ± 0.6 | 0.838 |
| Percent change of cortical thickness (%)# | −3.3 ± 9.2 | −2.6 ± 10.3 | 0.931 |
| 4.0 cm below greater trochanter | |||
| Endosteal widening (mm)* | 0.2 ± 0.6 | 0.2 ± 0.5 | 0.972 |
| Periosteal apposition (mm)† | 0.0 ± 0.6 | 0.0 ± 0.8 | 0.602 |
| Change of cortical thickness (mm) | −0.2 ± 0.5 | −0.2 ± 0.6 | 0.572 |
| Percent change of cortical thickness (%)# | −2.9 ± 8.0 | −3.0 ± 8.5 | 0.709 |
Data are expressed as mean ± SD; * endosteal widening was calculated by subtracting baseline medullary canal diameter from end medullary canal diameter; † periosteal apposition was calculated by subtracting baseline femoral shaft diameter from end femoral shaft diameter; # percent change of cortical thickness = (change of cortical thickness/baseline cortical thickness) × 100.
Discussion
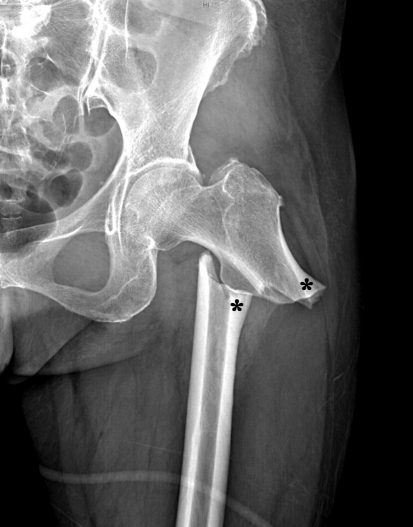
Several reports have suggested an association between long-term treatment with bisphosphonates, especially alendronate, and the development of atypical low-energy subtrochanteric/femoral shaft fractures [9, 15, 16]. It is hypothesized that long-term bisphosphonate therapy results in oversuppression of bone turnover, leading to an accumulation of microdamage and increased susceptibility of bone to fracture from low-energy injury [1, 10, 26]. These atypical femoral insufficiency fractures are rare and usually are associated with a few months of prodromal pain at the fracture site, no history of acute trauma, and characteristic radiographic findings [28], including generalized cortical thickening, a transverse fracture line, and medial cortical beaking (Fig. 2) [23]. In this study, we evaluated the long-term effect of alendronate on cortical thickness of the proximal femur and compared the differences in cortical thickness change between patients with or without alendronate therapy.
Fig. 2.
A radiograph of a patient with an atypical subtrochanteric femoral insufficiency fracture is shown. The asterisks indicate cortical hypertrophy of the femoral cortices at the proximal and distal fragments.
There are some limitations to this study. The first, and perhaps the main, limitation relates to our measurements from two-dimensional DXA scans. Although the minimum measurable distance in PACS was 0.22 mm, the cortical margin of the DXA image was not well defined and may have affected the selection of measurement points. In this study, we proposed a value of 1 mm or approximately a 20% change in cortical thickness as our minimal clinically significant difference. This value is based on the fact that we are interested only in a major change of cortical thickness, and we can accurately measure changes greater than this 1 mm or 20% difference. In addition, this 1 mm range covers the entire area of blurred cortical margin. Advanced imaging technologies such as HR-pQCT would give a more accurate and more detailed view of the proximal femoral geometry; however, these tools have not been used routinely in clinical practices. Second, the scans did not include the femoral shaft region. A systematic review revealed that of 141 cases with atypical femoral insufficiency fractures, 58 (40%) occurred at the femoral shaft and 41 (30%) at the subtrochanteric area, whereas the precise fracture site was not specified in 42 cases [7]. Such subtrochanteric fractures are located at the site measured in our study (Fig. 2). Nevertheless, we acknowledge our results cannot be generalized to the change of cortical thickness at the femoral shaft area. Third, although the DXA images covered the subtrochanteric region in all patients, we did not have data on patients’ heights. It is possible that the measured points we used in each patient may represent different locations of the subtrochanteric region unique for each individual. However, our outcome of interest is the within-patient change of the cortical thickness from baseline to latest DXA. Thus, we believe that measuring potentially different points of the subtrochanteric region would not change the conclusion of the study. Finally, because data on prior alendronate treatment were obtained retrospectively, we do not have accurate details on adherence and compliance to those treatments. Nonetheless, the BMD responses in our patients with long-term alendronate treatment are consistent with a compliant population based on previous reports [6, 19].
We found the majority of patients treated with alendronate at a minimum of 5 years had stable or decreased cortical thickness. Although there is substantial evidence that shows the effect of alendronate on the change in BMD, few studies have shown its influence on cortical thickness. Two recent studies evaluated change in cortical thickness and found that alendronate increased the mean percent change in cortical thickness when compared with placebo [5, 22]. Both studies, however, investigated the treatment effect of alendronate for only 1 to 2 years. In addition, they determined change in cortical thickness at the distal tibia and radius, which are not sites of concern for atypical femoral insufficiency fractures. Beck et al. [2] conducted a post hoc analysis of subjects treated with alendronate (38 patients) and placebo (39 patients) for up to 24 months. Hip structure analysis software was used to examine bone geometry including cortical thickness of the proximal femur from DXA scans. The authors found, after 24 months of alendronate treatment, that the mean percent change in cortical thickness at the femoral shaft increased 1.82%, whereas it decreased approximately 0.31% with placebo. In contrast, we found no differences in percent changes of cortical thickness between the alendronate and control groups. Our study, however, investigated the long-term effect of alendronate at a minimum of 5 years. It is postulated the protective effect of alendronate was overcome by progressive bone loss from the aging process; therefore, the overall cortical thickness at the proximal femur was reduced. In addition, based on the figure included in the study by Beck et al., they measured cortical thickness at the femoral shaft level (lower than levels that we measured). We, therefore, propose that our results were different than theirs because of different location and duration of bisphosphonate treatment.
Our data suggest long-term alendronate therapy does not alter the cortical thickness of the proximal femur compared with untreated controls. The effect size of this study (less than 1% difference) was much lower than our detection limit (5%) and proposed minimally clinically significant change (20%). When performing post hoc analysis based on a 5% to 20% range of percent changes in cortical thickness, our sample size was adequately powered (> 80%) to detect a difference in this range. Thus, we believe that there is no real difference of percent change in cortical thickness at the subtrochanteric femoral region between patients with long-term alendronate therapy and untreated controls. Instead, we suggest patients with atypical femoral insufficiency fractures may have had preexisting thickened femoral cortices before initiation of alendronate treatment. Thickening may not be a result of the suppression of bone attributable to alendronate. Another possibility is that patients with atypical femoral insufficiency fractures may respond to alendronate differently as compared with those without fractures. Among all types of bisphosphonates associated with atypical femoral insufficiency fractures, alendronate remains the most commonly reported in the current literature. A recent systematic review of case reports and case series studies revealed 119 of 141 patients (84.4%) with atypical femoral insufficiency fractures had a history of long-term alendronate therapy [7]. As most bisphosphonates have long half-lives, once administered they accumulate in the bone and continue to be released for months or years after treatment is stopped [18, 20]. Evidence shows there is some residual benefit to antifracture efficacy even after bisphosphonates are discontinued [27]. Therefore, it is recommended osteoporosis treatment with bisphosphonates may be reassessed or stopped for a drug holiday after a course of some years. The duration of a drug holiday depends on the patient’s fracture risk and the pharmacokinetics of the bisphosphonates used [28]. For example, patients with mild risk for fragility fractures might stop the medication after 5 years of alendronate treatment and remain off as long as BMD is stable and no fracture occurs. Conversely, patients with high risk for fragility fractures should be considered for an alternative medication such as raloxifene, denosumab, or teriparatide during the holiday period from bisphosphonates.
Long-term alendronate treatment does not cause thickened femoral cortices at the subtrochanteric region in the detection limits of our method. Percent changes in cortical thickness were similar in patients with or without a history of long-term alendronate use. As alendronate reduces fracture risk, concerns regarding the association between bisphosphonates and femoral insufficiency fractures should not preclude the use of these agents in the treatment of osteoporosis. Nevertheless, we believe these drugs should not be used for a long duration. Biochemical bone markers can be used to help monitor when to stop and resume bisphosphonate treatment. Further investigation will be required to determine the pathogenesis of atypical femoral insufficiency fractures in patients receiving long-term bisphosphonates.
Acknowledgments
We thank Huong Do and Joseph T. Nguyen from the Department of Research, Epidemiology and Biostatistics Core at the Hospital for Special Surgery for help and guidance in data review and statistical analysis. We thank Margaret Eckert, Sylvia Hom, and Catherine Sutton from the Osteoporosis Prevention Center for help in searching the osteoporosis registry and collecting patient records. We also thank Matthew Seah for assistance with the study.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Armamento-Villareal R, Napoli N, Diemer K, Watkins M, Civitelli R, Teitelbaum S, Novack D. Bone turnover in bone biopsies of patients with low-energy cortical fractures receiving bisphosphonates: a case series. Calcif Tissue Int. 2009;85:37–44. doi: 10.1007/s00223-009-9263-5. [DOI] [PubMed] [Google Scholar]
- 2.Beck TJ, Lewiecki EM, Miller PD, Felsenberg D, Liu Y, Ding B, Libanati C. Effects of denosumab on the geometry of the proximal femur in postmenopausal women in comparison with alendronate. J Clin Densitom. 2008;11:351–359. doi: 10.1016/j.jocd.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures: Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541. doi: 10.1016/S0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 4.Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC. Liberman UA; Alendronate Phase III Osteoporosis Treatment Study Group. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189–1199. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- 5.Burghardt AJ, Kazakia GJ, Sode M, Papp AE, Link TM, Majumdar S. A longitudinal HR-pQCT study of alendronate treatment in postmenopausal women with low bone density: relations among density, cortical and trabecular microarchitecture, biomechanics, and bone turnover. J Bone Miner Res. 2010;25:2558–2571. doi: 10.1002/jbmr.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delmas PD. Treatment of postmenopausal osteoporosis. Lancet. 2002;359:2018–2026. doi: 10.1016/S0140-6736(02)08827-X. [DOI] [PubMed] [Google Scholar]
- 7.Giusti A, Hamdy NA, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy: a systematic review of case/case series studies. Bone. 2010;47:169–180. doi: 10.1016/j.bone.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Koh JS, Goh SK, Png MA, Kwek EB, Howe TS. Femoral cortical stress lesions in long-term bisphosphonate therapy: a herald of impending fracture? J Orthop Trauma. 2010;24:75–81. doi: 10.1097/BOT.0b013e3181b6499b. [DOI] [PubMed] [Google Scholar]
- 9.Lenart BA, Neviaser AS, Lyman S, Chang CC, Edobor-Osula F, Steele B, Meulen MC, Lorich DG, Lane JM. Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos Int. 2009;20:1353–1362. doi: 10.1007/s00198-008-0805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res. 2000;15:613–620. doi: 10.1359/jbmr.2000.15.4.613. [DOI] [PubMed] [Google Scholar]
- 11.Mashiba T, Mori S, Burr DB, Komatsubara S, Cao Y, Manabe T, Norimatsu H. The effects of suppressed bone remodeling by bisphosphonates on microdamage accumulation and degree of mineralization in the cortical bone of dog rib. J Bone Miner Metab. 2005;23(Suppl):36–42. doi: 10.1007/BF03026321. [DOI] [PubMed] [Google Scholar]
- 12.Mashiba T, Turner CH, Hirano T, Forwood MR, Johnston CC, Burr DB. Effects of suppressed bone turnover by bisphosphonates on microdamage accumulation and biomechanical properties in clinically relevant skeletal sites in beagles. Bone. 2001;28:524–531. doi: 10.1016/S8756-3282(01)00414-8. [DOI] [PubMed] [Google Scholar]
- 13.National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2008.
- 14.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 15.Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008;22:346–350. doi: 10.1097/BOT.0b013e318172841c. [DOI] [PubMed] [Google Scholar]
- 16.Odvina CV, Levy S, Rao S, Zerwekh JE, Rao DS. Unusual mid-shaft fractures during long-term bisphosphonate therapy. Clin Endocrinol (Oxf) 2010;72:161–168. doi: 10.1111/j.1365-2265.2009.03581.x. [DOI] [PubMed] [Google Scholar]
- 17.Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- 18.Papapoulos SE, Cremers SC. Prolonged bisphosphonate release after treatment in children. N Engl J Med. 2007;356:1075–1076. doi: 10.1056/NEJMc062792. [DOI] [PubMed] [Google Scholar]
- 19.Pols HA, Felsenberg D, Hanley DA, Stepan J, Munoz-Torres M, Wilkin TJ, Qin-sheng G, Galich AM, Vandormael K, Yates AJ, Stych B. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study Group. Osteoporos Int. 1999;9:461–468. doi: 10.1007/pl00004171. [DOI] [PubMed] [Google Scholar]
- 20.Rodan G, Reszka A, Golub E, Rizzoli R. Bone safety of long-term bisphosphonate treatment. Curr Med Res Opin. 2004;20:1291–1300. doi: 10.1185/030079904125004475. [DOI] [PubMed] [Google Scholar]
- 21.Russell RG. Bisphosphonates: the first 40 years. Bone. 2011;49:2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 22.Seeman E, Delmas PD, Hanley DA, Sellmeyer D, Cheung AM, Shane E, Kearns A, Thomas T, Boyd SK, Boutroy S, Bogado C, Majumdar S, Fan M, Libanati C, Zanchetta J. Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res. 2010;25:1886–1894. doi: 10.1002/jbmr.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster D, Einhorn TA, Genant HK, Geusens P, Klaushofer K, Koval K, Lane JM, McKiernan F, McKinney R, Ng A, Nieves J, O’Keefe R, Papapoulos S, Sen HT. van der Meulen MC, Weinstein RS, Whyte M; American Society for Bone and Mineral Research Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25:2267–2294. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 24.Tosteson AN, Burge RT, Marshall DA, Lindsay R. Therapies for treatment of osteoporosis in US women: cost-effectiveness and budget impact considerations. Am J Manag Care. 2008;14:605–615. [PubMed] [Google Scholar]
- 25.Unnanuntana A, Gladnick BP, Donnelly E, Lane JM. The assessment of fracture risk. J Bone Joint Surg Am. 2010;92:743–753. doi: 10.2106/JBJS.I.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Visekruna M, Wilson D, McKiernan FE. Severely suppressed bone turnover and atypical skeletal fragility. J Clin Endocrinol Metab. 2008;93:2948–2952. doi: 10.1210/jc.2007-2803. [DOI] [PubMed] [Google Scholar]
- 27.Watts NB, Chines A, Olszynski WP, McKeever CD, McClung MR, Zhou X, Grauer A. Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos Int. 2008;19:365–372. doi: 10.1007/s00198-007-0460-7. [DOI] [PubMed] [Google Scholar]
- 28.Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95:1555–1565. doi: 10.1210/jc.2009-1947. [DOI] [PubMed] [Google Scholar]