History and Physical Examination
A 41-year-old-woman presented with a 10-year history of left lower leg pain distributed from the midportion of the lower leg to the foot and ankle. She had no history of relevant trauma or constitutional symptoms suggesting systemic illness. Shortly after the pain began she was seen at another hospital; no radiographs were taken but she had an MR scan (Fig. 1). The MR scan showed cortical thickening and bone marrow edema, suggesting chronic inflammation. The patient experienced little relief of her leg pain with 4 weeks of rest. The patient tolerated the pain for 8 years, then her leg pain worsened and she underwent an open biopsy of the bone at the midshaft of the left tibia at another hospital. The bone biopsy reportedly revealed chronic inflammation and osteoporosis. She was diagnosed as having low-grade osteomyelitis at the time. After biopsy the pain immediately subsided. However, the pain recurred 1 year after the biopsy and subsequently progressed. She presented to us 1 year later (2 years after the initial biopsy). There was no relevant familial history. A physical examination showed no swelling, redness, or warmth. There was localized tenderness over the anterior midshaft of the left tibia. The ranges of motion of bilateral hips, knees, and ankles were full.
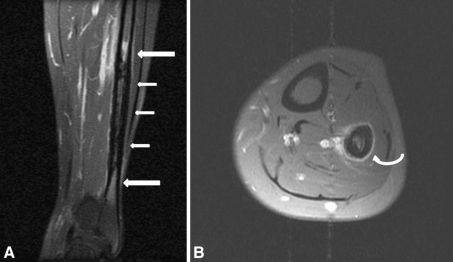
Fig. 1A–B.
MR images of the left lower leg were taken when the patient’s pain began in 1997. (A) Cortical thickening (small arrows) and bone marrow edema (large arrows) of the diaphysis of the left fibula are seen on this coronal MR image. (B) An axial gadolinium-enhanced fat-suppressed image shows periosteal reaction (curved arrow) of the left fibula.
Other than a hemoglobin level of 10.6 g/dL, she had normal laboratory findings. No leukocytosis was evident, and the erythrocyte sedimentation rate (ESR) was 20 mm/hour (normal range, 0–20 mm/hour), C-reactive protein was 0.01 mg/dL (normal range, 0–0.5 mg/dL), and serum alkaline phosphatase (ALP) was 41 IU/L (normal range, 30–115 IU/L).
Plain radiographs, MRI, and technetium-99 bone scan (Tc-99 MDP bone scan) were obtained (Figs. 1–6). Based on the history, physical examination, laboratory studies, and imaging studies, what is the differential diagnosis at this point?
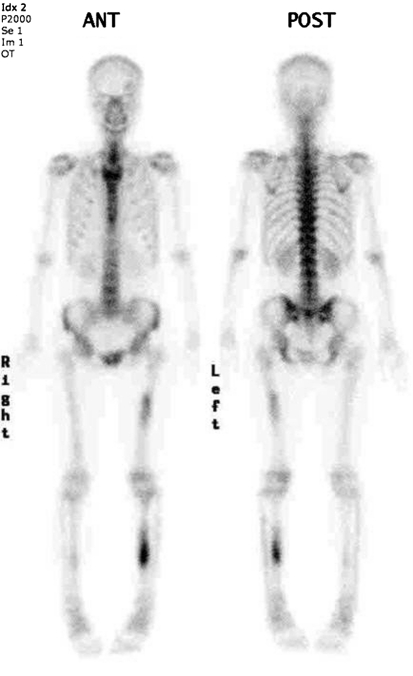
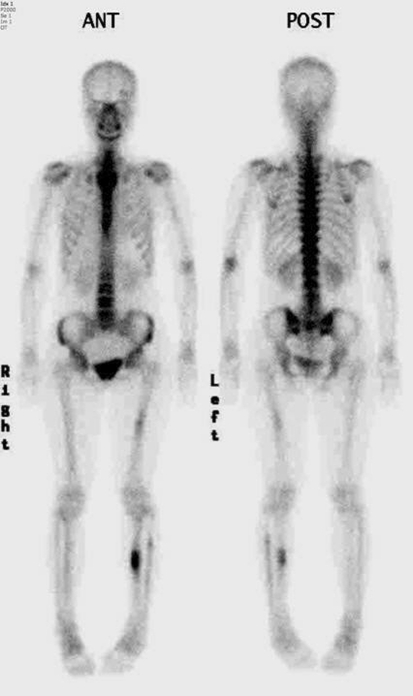
Fig. 6.
A followup bone scan was performed in March 2007, 10 years after onset, and showed stable hot uptake of the left tibia, much increased uptake on the left femur, and a new subtle uptake on the right tibia.
Imaging Interpretation
We reviewed the plain radiographs, MRI, and Tc-99 MDP bone scans obtained at our institution and those obtained previously.
When the patient’s pain began in 1997, MRI of the left lower leg showed an area of cortical thickening and irregular periosteal reaction in the distal fibular shaft with mild surrounding marrow edema (Fig. 1). No plain radiograph was taken at the time.
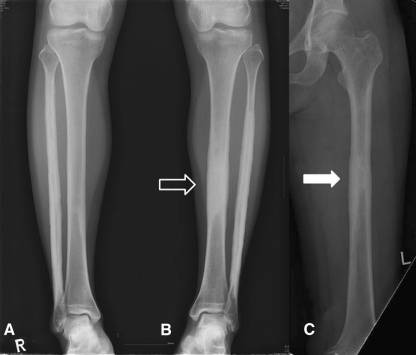
Eight years later, when her symptoms increased, plain radiographs (Fig. 2) revealed cortical thickening, thick wavy benign-appearing periosteal reaction, and increased density of the intramedullary bone in the left fibular diaphysis. Similar, but less prominent findings were seen in the right fibular and left midtibial diaphyses. There was suggestion of early subtle changes in the right midtibial diaphysis. MRI (Fig. 3) showed an area of cortical thickening and intramedullary low signal in the left midtibial shaft correlating with the increased density seen on the plain films with prominent surrounding marrow edema. Similar, but less striking findings were seen in the right tibia and both fibular shafts. The Tc-99 MDP bone scan (Fig. 4) of the left tibia showed increased uptake of the radiotracer in the lesion with areas of lesser uptake in the left fibula and the left midfemoral diaphysis.
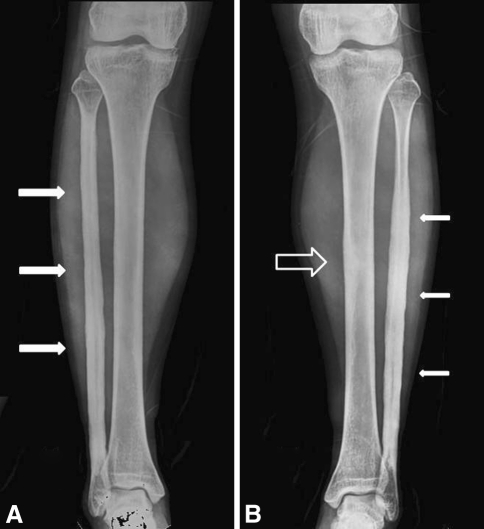
Fig. 2A–B.
Radiographs of both lower legs were obtained when the patient’s left lower leg pain worsened in February 2005, 8 years after onset. (A) Cortical thickening and increased intramedullary bone density were observed at the right fibula (arrows). There was suggestion of early subtle changes in the right midtibial diaphysis. (B) Cortical thickening, periosteal reaction, and increased intramedullary bone density were observed at the left fibula (arrows) and the left midtibial diaphysis (hollow arrow). It was most prominent at the left fibula.
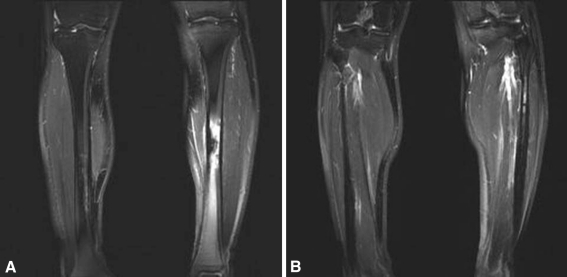
Fig. 3A–B.
Coronal fat-suppressed T2-weighted images were taken in March 2005, 8 years after onset. (A) Increased intramedullary bone density with surrounding bone marrow edema of the midtibiae had developed during the 8 years since the previous MRI, especially on the left side. (B) The left fibular lesion persisted.
Fig. 4.
A Tc-99 MDP bone scan obtained in February 2005, 8 years after onset, shows prominent uptake on the left tibia, and mild uptake on the left fibula and left femur also was observed. Uptake on the right tibia and fibula was not obvious.
Two years later (10 years after initial onset of symptoms), the patient presented to our institution. On the repeat radiographs in 2007 (Fig. 5), all of the previously described lesions showed progression with near obliteration of the intramedullary canal in the left tibia, and obvious, but less striking involvement of the right tibial and left midfemoral diaphyses. A repeat Tc-99 MDP bone scan (Fig. 6) showed stable findings in the left tibia with increased uptake in the left femur and a new subtle area of uptake in the right midtibial diaphysis.
Fig. 5A–C.
Plain radiographs of the (A) right lower leg, (B) left lower leg, and (C) left femur were taken when the patient was first seen by us in 2007, 10 years after onset. It had been 2 years since her first biopsy. Hyperostosis in the left tibia had progressed with near obliteration of the intramedullary canal (hollow arrow) and newly emerged increased radiodensity was observed on the diaphysis of the left femur (arrow).
Imaging studies showed all lesions were on the diaphysis and invaded asynchronously. The most obvious characteristic was of endosteal hyperostosis, but cortical hyperostosis, periosteal reaction, and adjacent bone marrow edema also were observed on MR scans.
Differential Diagnosis
Ribbing disease
Camurati-Engelmann disease
Intramedullary osteosclerosis
Erdheim-Chester disease
Chronic multifocal sclerosing osteomyelitis
Hardcastle syndrome
We performed an incisional biopsy of the midshaft of the left tibia in the region of the dense bone. The bone was so hard the biopsy was difficult to perform even with power drills. No signs of inflammation were evident.
Based on the history, physical examination, laboratory studies, imaging studies, and histologic picture, what is the diagnosis and how should the patient be treated?
Histology Interpretation
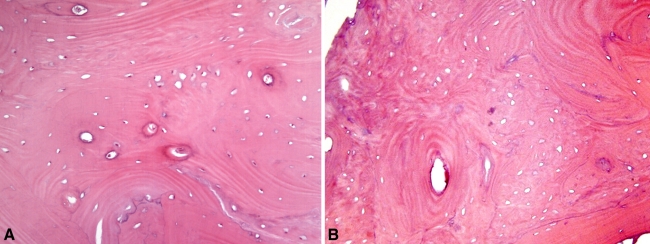
Grossly, the soft tissue specimen was white-gray and contained bony fragments. Permanent histologic sections revealed thickened trabeculae of lamellar bone with various sizes of the Haversian system (Fig. 7A). Some regions had empty lacunae (Fig. 7B).
Fig. 7A–B.
(A) The permanent histologic section showed thickened trabeculae and a variably sized Haversian system at the sclerotic bony lesion. (B) Some portions of the histologic specimen showed many empty lacunae which could suggest dead bone fragments. Those empty lacunae may be the result of bone necrosis from the previous biopsy (Stain, hematoxylin and eosin; original magnification, ×200).
Diagnosis
Ribbing Disease
Discussion and Treatment
Clinical and radiologic features of our patient showed asynchronous bilateral diaphyseal involvement of long bones with surrounding bone marrow edema. Those features combined with the absence of inflammatory signs confirmed laboratory findings, and analysis of the biopsy specimen suggested Ribbing disease.
We considered the primary differential considerations to be Camurati-Engelmann disease, intramedullary osteosclerosis, Erdheim-Chester disease, chronic multifocal sclerosing osteomyelitis, and Hardcastle syndrome. Camurati-Engelmann disease, an autosomal dominant disorder, manifests as pain, progressive leg weakness, elongated extremities, and gait disturbances [10], and is characterized by a symmetric and bilateral distribution with an onset during childhood, whereas Ribbing disease is either unilateral or asynchronous bilateral as in our patient. In addition, Camurati-Engelmann disease is associated with gait and neurologic abnormalities, which are absent with Ribbing disease. Radiographically, Ribbing disease resembles Camurati-Engelmann disease, but Ribbing disease involves only the long bones, whereas Camurati-Engelmann disease can affect the long bones and other bones formed by intramembranous ossification, such as the skull [19].
Intramedullary osteosclerosis is an abnormal intramedullary new bone formation located mainly in the shaft of a long bone, especially the tibia, in adults [1]. It is a rare condition and is more common in females. Furthermore, it shows no familial effect and no relationship with infection, trauma, or systemic illness. Laboratory findings are typically normal [1, 2, 5]. Some studies suggests absence of an hereditary pattern, female predominance, mechanical pain, and absence of periosteal hyperostosis favor intramedullary osteosclerosis [2, 5]. However, several other studies suggest these differential points are not diagnostically useful in many cases [9, 12, 17]. The presence or absence of associated reactive marrow edema can be used to distinguish intramedullary sclerosis from Ribbing disease. Whereas intramedullary sclerosis is characterized chiefly by increased bone density, Ribbing disease usually shows edema and enhancement on T2-weighted and postgadolinium T1-weighted MR images, respectively, in the adjacent marrow and surrounding soft tissues [2, 5, 9, 12, 17].
Erdheim-Chester disease is a rare condition characterized by an abnormal increase in histiocytes, and usually occurs during middle age. It has some histiocytic disorder-favoring signs like exophthalmos, diabetes insipidus, and fever. In addition, biopsy specimens of Erdheim-Chester disease lesions differ from those of Ribbing disease. Erdheim-Chester disease lesions have histiocyte proliferation and immunonegativity for S-100 protein [20]. Radiographically, the metaphysis is involved in most patients with Erdheim-Chester disease, and in some cases a bone mass is present, especially on the ribs or knees [20]. The laboratory findings of chronic multifocal sclerosing osteomyelitis are characterized by elevated ESR and leukocyte count, but laboratory findings for our patient were within normal limits except for a moderately diminished hemoglobin level. Furthermore, histologic findings did not indicate the presence of an infectious condition in our patient.
Hardcastle syndrome is characterized by diaphyseal medullary stenosis of long bones surrounding cortical thickening. However, contrary to Ribbing disease, Hardcastle syndrome manifests as a history of pathologic fracture after minor trauma, radiographs showing multiple permeative cortical radiolucencies and longitudinal metaphyseal striations, and malignant transformation between the second and fifth decades of life [6, 13].
Ribbing disease is a rare condition of unknown etiology reflected by a sclerosing dysplasia of the diaphyses of long bones. It was first described by Ribbing in 1949 [14]. The tibia and femur usually are involved, but it also affects the fibula, radius, and ulna. The disease is asymmetric or asynchronous bilateral, and is suspected to show an autosomal recessive trait [2, 17, 19]. It occurs after puberty and is more common in females [19]. The typical presenting symptom is bone pain [3], which may be associated with swelling [17, 18]. Furthermore, the pain appears to be resistant to medication [2, 18], and to be associated with physical activity [12]. In the absence of specific treatment, it can resolve spontaneously with time or progress [9, 12]. Radiographically, the cortical thickening involves the diaphyses of long bones, with sparing of the epiphysis [12]. The medullary canals may be narrowed. On MR images, the intramedullary and bone marrow edema are characteristic of Ribbing disease [12, 21]. Histologically, the most commonly described findings are increased cortical thickness and Haversian canal narrowing [3, 8, 10, 11, 14], but the histologic findings of Ribbing disease are not pathognomonic [3]. Our patient had atypical regions of bone with empty lacunae, perhaps as a result of necrosis from the previous biopsy. However, as nonspecific destructive and reparative changes have been reported in a patient with asynchronous progressive diaphyseal dysplasia [16], we did not think we could completely exclude the possibility, although probably low, of a new pathogenesis with clinical features of Ribbing disease but with bone necrosis. Laboratory findings are usually within normal values [3, 18].
Given the absence of known etiology but based on the belief that bone marrow edema causes pain, many authors have advocated intramedullary decompression, whether by reaming, curettage, or by making a window at the lesion, for pain relief [3, 4, 7, 9, 10, 15, 21].
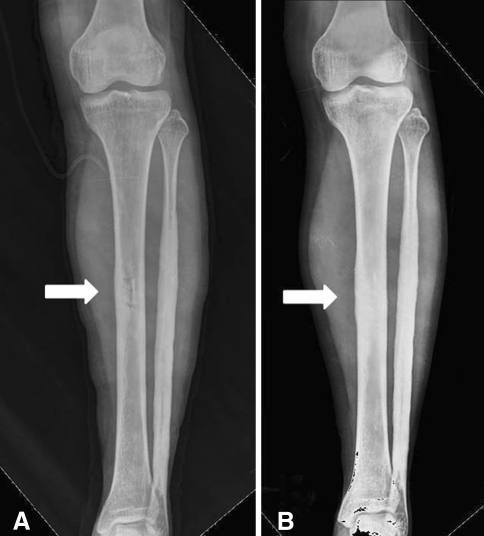
We treated our patient with intramedullary reaming. When the patient returned to our clinic 2 months later, her left lower leg pain had subsided. Comparisons of plain radiographs taken before (Fig. 2) and after (Fig. 8A) the first biopsy showed that the tibial medullary canal had been opened. However, endosteal hyperostosis then slowly progressed and almost completely obliterated the medullary canal as observed on the followup radiograph taken 1 year after the first biopsy (Fig. 8B), and her pain redeveloped at approximately the same time. We presume that decompression resulted in pain relief and that obliteration of the medullary canal caused recurrence of the pain. Approximately 1 year after the initial intramedullary reaming on the left, pain also developed in her right lower leg and she had progressive diaphyseal hyperostosis observed on radiographs. She subsequently underwent intramedullary reaming of the right tibia shaft, and the last followup plain radiograph showed a well-maintained open medullary canal in both tibiae, with relief of symptoms. Medullary decompression appears to be important in pain relief although it does not correct the primary cause of disease.
Fig. 8A–B.
Radiographs were taken immediately after the first biopsy in March 2005. (A) The tibial medullary canal was decompressed by a cortical window (arrow) created during the biopsy (see Fig. 2B for the prebiopsy radiograph). (B) A followup radiograph obtained 1 year after the first biopsy shows endosteal hyperostosis has progressed resulting in obliteration of the medullary canal (arrow).
Acknowledgments
We thank Sung Hwan Hong MD, PhD and Ja-Young Choi MD, PhD for advice on the imaging, and Hyun-Soo Kim MD for advice on the histology.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent licensing arrangements, etc) that might pose a conflict of interest with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution has approved the human protocol for this investigation and all investigations were conducted in conformity with ethical principles of research.
References
- 1.Abdul-Karim FW, Carter JR, Makley JT, Morrison SC, Helper SD, Joyce MJ, Linke TF. Intramedullary osteosclerosis: a report of the clinicopathologic features of five cases. Orthopedics. 1988;11:1667–1675. doi: 10.3928/0147-7447-19881201-08. [DOI] [PubMed] [Google Scholar]
- 2.Balkissoon AR, Hayes CW. Case 14: intramedullary osteosclerosis. Radiology. 1999;212:708–710. doi: 10.1148/radiology.212.3.r99se39708. [DOI] [PubMed] [Google Scholar]
- 3.Beals RK, Pearson JM, Mansoor A. Ribbing disease: a case report, a review of the literature, and a description of novel treatment. J Bone Joint Surg Am. 2002;84:2050–2055. [PubMed] [Google Scholar]
- 4.Bettini G, Boni V. Etiopathogenetic, clinical and radiographic considerations on Ribbing’s disease. (Report of 2 cases of familial nature)][in Italian. Arch Putti Chir Organi Mov. 1962;16:58–72. [PubMed] [Google Scholar]
- 5.Chanchairujira K, Chung CB, Lai YM, Haghighi P, Resnick D. Intramedullary osteosclerosis: imaging features in nine patients. Radiology. 2001;220:225–230. doi: 10.1148/radiology.220.1.r01jl24225. [DOI] [PubMed] [Google Scholar]
- 6.Douya H, Yokoyama R, Beppu Y, Hasegawa T. Malignant fibrous histiocytoma associated with diaphyseal medullary stenosis. Clin Orthop Relat Res. 2002;400:211–216. doi: 10.1097/00003086-200207000-00026. [DOI] [PubMed] [Google Scholar]
- 7.Fallon MD, Whyte MP, Murphy WA. Progressive diaphyseal dysplasia (Engelmann’s disease): report of a sporadic case of the mild form. J Bone Joint Surg Am. 1980;62:465–472. [PubMed] [Google Scholar]
- 8.Furia JP, Schwartz HS. Hereditary multiple diaphyseal sclerosis: a tumor simulator. Orthopedics. 1990;13:1267–1274. doi: 10.3928/0147-7447-19901101-16. [DOI] [PubMed] [Google Scholar]
- 9.Gaeta M, Vinci S, Costa C, Oliviero R, Mazziotti S. MRI in Ribbing disease: a case report. Acta Orthop. 2009;80:622–624. doi: 10.3109/17453670903316876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenspan A. Sclerosing bone dysplasias: a target-site approach. Skeletal Radiol. 1991;20:561–583. doi: 10.1007/BF01106087. [DOI] [PubMed] [Google Scholar]
- 11.Kaftori JK, Kleinhaus U, Naveh Y. Progressive diaphyseal dysplasia (Camurati-Engelmann): radiographic follow-up and CT findings. Radiology. 1987;164:777–782. doi: 10.1148/radiology.164.3.3615880. [DOI] [PubMed] [Google Scholar]
- 12.Mukkada PJ, Franklin T, Rajeswaran R, Joseph S. Ribbing disease. Indian J Radiol Imaging. 2010;20:47–49. doi: 10.4103/0971-3026.59754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norton KI, Wagreich JM, Granowetter L, Martignetti JA. Diaphyseal medullary stenosis (sclerosis) with bone malignancy (malignant fibrous histiocytoma): Hardcastle syndrome. Pediatr Radiol. 1996;26:675–677. doi: 10.1007/BF01356833. [DOI] [PubMed] [Google Scholar]
- 14.Ribbing S. Hereditary, multiple, diaphyseal sclerosis. Acta Radiol. 1949;31:522–536. doi: 10.3109/00016924909138232. [DOI] [PubMed] [Google Scholar]
- 15.Rubin P. On organizing a dynamic classification of bone dysplasias. Arthritis Rheum. 1964;7:693–708. doi: 10.1002/art.1780070610. [DOI] [PubMed] [Google Scholar]
- 16.Sakai T, Matsui Y, Katoh S, Yukata K, Hamada D, Takata Y, Yokoi H, Yasui N. Asynchronous progressive diaphyseal dysplasia. Mod Rheumatol. 2005;15:450–453. doi: 10.1007/s10165-005-0440-8. [DOI] [PubMed] [Google Scholar]
- 17.Seeger LL, Hewel KC, Yao L, Gold RH, Mirra JM, Chandnani VP, Eckardt JJ. Ribbing disease (multiple diaphyseal sclerosis): imaging and differential diagnosis. AJR Am J Roentgenol. 1996;167:689–694. doi: 10.2214/ajr.167.3.8751682. [DOI] [PubMed] [Google Scholar]
- 18.Shier CK, Krasicky GA, Ellis BI, Kottamasu SR. Ribbing’s disease: radiographic-scintigraphic correlation and comparative analysis with Engelmann’s disease. J Nucl Med. 1987;28:244–248. [PubMed] [Google Scholar]
- 19.Vanhoenacker FM, Beuckeleer LH, Hul W, Balemans W, Tan GJ, Hill SC, Schepper AM. Sclerosing bone dysplasias: genetic and radioclinical features. Eur Radiol. 2000;10:1423–1433. doi: 10.1007/s003300000495. [DOI] [PubMed] [Google Scholar]
- 20.Veyssier-Belot C, Cacoub P, Caparros-Lefebvre D, Wechsler J, Brun B, Remy M, Wallaert B, Petit H, Grimaldi A, Wechsler B, Godeau P. Erdheim-Chester disease: clinical and radiologic characteristics of 59 cases. Medicine (Baltimore) 1996;75:157–169. doi: 10.1097/00005792-199605000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Ziran N, Hill S, Wright ME, Kovacs J, Robey PG, Wientroub S, Collins MT. Ribbing disease: radiographic and biochemical characterization, lack of response to pamidronate. Skeletal Radiol. 2002;31:714–719. doi: 10.1007/s00256-002-0552-6. [DOI] [PubMed] [Google Scholar]