Abstract
Background
The navigation system was introduced to orthopaedic surgery in the 1990s. More recently, CT-based navigation systems have been used more commonly in spine and joint replacement surgery because of their precision.
Questions/purposes
The aim of our study was to evaluate the accuracy and efficacy of navigation-assisted excision of bone and soft tissue tumors.
Methods
From 2006 to 2009, we performed navigation-assisted surgery in 16 patients, 11 males and five females, with a mean age of 39 years (range, 13–70 years). We diagnosed nine benign bone tumors and seven malignant bone and soft tissue tumors. In two patients, the malignant soft tissue tumors infiltrated the adjacent bones. Nine excisional biopsies for benign tumors and seven en bloc excisions for malignant tumors were performed. In all cases, the point registration method was performed using 10 skin markers, which were placed around the tumor. Each excisional difference between the preoperative and postoperative plans was evaluated histologically or by postoperative CT.
Results
The mean accuracy of this system, which was determined using skin markers, was 0.93 mm (range, 0.6–1.2 mm). All biopsy and excision samples were evaluated by pathologic examination and postoperative CT imaging. The mean difference between the planned margin and postoperative CT or excised histologic specimen was 0 mm to 4 mm. The mean followup was 34 months (range, 10–54 months). There were no local recurrences, except for excision of skip metastases in a patient with a chordoma.
Conclusion
We report our experience with navigation-assisted surgery for bone and soft tissue tumors. Navigation-assisted surgery could be indicated for sufficiently reliable, accurate, and minimally invasive resections.
Level of Evidence
Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Computer-assisted surgery has been performed for cranial biopsies and tumor resections for two decades [6, 12, 13, 21, 24, 26, 34] before being introduced to the field of orthopaedics [1, 5, 8, 9, 11, 15–17, 19, 23, 29, 30]. Currently, navigation systems often use CT data to navigate pedicle screw applications [1, 11, 17], cup placements for hip arthroplasties [16], TKAs [8, 19], and cruciate ligament reconstructions [9, 23]. Excising bone tumors is sometimes difficult even for an experienced surgeon because the extent and anatomic location of the tumors are variable and cannot be detected by ordinary radiography or fluoroscopy [15, 30]. In such cases, precise excision might not be possible. Some studies have reported on bone and soft tissue tumor resection using navigation [2–4, 7, 14, 21, 25, 28, 32, 33]. The aim of our study was to evaluate the accuracy and efficacy of navigation-assisted excision of bone tumors with skin marker registration.
Patients and Methods
From January 2006 to December 2009, we studied 16 patients (11 males and five females), between 13 and 76 years of age (mean, 39 years), with tumors located at the pelvis (n = 5), femur (n = 6), tibia (n = 3), humerus (n = 1), and tarsus (n = 1). Nine patients were diagnosed with benign bone tumors, four with low-grade malignancies, and three with high-grade malignancies. Tumor types included osteoid osteoma (n = 6), phosphaturic mesenchymal tumor (n = 2), chondroblastoma (n = 1), parosteal osteosarcoma (n = 2), chordoma (n = 1), myxoid liposarcoma (n = 1), recurrent dedifferentiated chondrosarcoma (n = 1), recurrent extraskeletal osteosarcoma (n = 1), and conventional osteosarcoma (n = 1). The two malignant soft tissue tumors infiltrated the adjacent bone.
The StealthStationTM Treatment Guidance System (Medtronic-Sofamor Danek Co, Ltd, Osaka, Japan) was used for computer-navigated surgical treatment. This navigation system consists of a computer workstation, a reference frame with passive markers, a standard probe, and an electro-optical camera connected to the computer workstation, which serves as a position sensor. This system can be run by six types of application software. No specific application has been developed to support the resection of tumors. We used the “Spine” module developed for pedicle screw application. The system uses CT data sets of the region of interest.
The registration procedure in each patient was performed using 10 skin markers provided by Medtronic Sofamor Danek Co Ltd. Each marker was small, donut-shaped, self-adhesive, and filled with a material that could be seen on CT and MRI. On the day before surgery, a CT scan was obtained with the patient in a posture equivalent to that used during the planned surgery. Data were acquired using a multislice CT scanner (Asterion; Toshiba, Japan) at a maximum of 1-mm intervals. The patient’s data were transferred to the navigation computer and reconstructed as 3-D images on a monitor.
The registration process was similar to the image fusion process in frameless procedures termed “paired point-based registration” and was accomplished with reference to the 10 skin-based markers. The registration procedure was performed in the operating room before the start of surgery, using sterile conditions and with the patient under general anesthesia. The accuracy of the image-to-patient registration was termed the registration error. The registration error was calculated using the navigation system software. It accepted a calculated error less than 2 mm, which was verified with the pointer tool using the verification mode provided by the navigation system. Once the registration was complete, the computer-navigated surgery started. A surgeon verified the actual skin location against what was displayed on the monitor.
An excisional biopsy was performed in each of the nine patients with benign tumors with the same distance to the margin (4 mm in diameter). In five patients with low-grade tumors and one patient with a conventional osteosarcoma that showed a good response to chemotherapy, the margin was 10 mm wide. For the three patients with high-grade tumors, the margin was 20 mm wide. Each excised margin was estimated by histologic analysis and postoperative CT. Each elapsed time of registration and placement of the reference frame were measured.
Results
Body mass index (BMI) values for the patients ranged from 17.3 to 28.5 kg/m2. The mean preoperative registration matching time was 12.8 minutes (range, 8–25 minutes). The total mean preparation time was 24 minutes (range, 16–35 minutes). The registration errors for all patients ranged from 0.6 to 1.5 mm (mean ± SD, 0.93 ± 0.25 mm). The trephine size ranged from the same size as the tumor to 4 mm larger than each of the nine benign tumors. One chondroblastoma and two phosphaturic mesenchymal tumors were excised by trephine and required additional curettage. The skin incision for the nine benign tumors had a mean length of 20.6 mm (range, 15–30 mm). In 16 patients, the mean difference of the margin between the surgical plan and the histologic specimen was 2.0 mm (range, 0–4 mm).
The postoperative followups ranged from 10 to 56 months (mean, 37 months). All nine patients with benign tumors were free of disease. Of the seven patients with malignant tumors, one with an extraskeletal osteosarcoma died of lung metastases 10 months after surgery, whereas in a patient with a chordoma, a second opposite-side skip lesion was observed 12 months after the first surgery. The latter patient underwent a second operation and survived for an additional 39 months with a few different lesions. The other five patients were continuously disease-free after surgery (Table 1).
Table 1.
Clinical data and treatment results
| Case number | Age/Sex | Diagnosis | Location (bone lesion) | BMI |
Tumor size†† (mm) | Surgery | Preparation time (min) |
Registration error (mm) | Difference (mm) | Followup (months) | Local Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | 70/F | Phosphaturic mesenchymal tumor | R-tibia | 19.0 | 10 × 10 × 8 | Excision | 22 | 0.6 | 2 | 51 | No |
| B2 | 20/M | Osteoid osteoma | L-femur | 18.8 | 4 × 4 × 4 | Excision | 22 | 0.7 | 2 | 50 | No |
| B3 | 52/M | Phosphaturic mesenchymal tumor | R-femur | 23.7 | 10 × 10 × 8 | Excision | 21 | 1.1 | 2 | 38 | No |
| B4 | 20/M | Chondroblastoma | L-ilium | 21.3 | 16 × 15 × 14 | Excision | 22 | 0.8 | 0 | 40 | No |
| B5 | 19/M | Osteoid osteoma | L-humerus | 28.1 | 8 × 7 × 6 | Excision | 25 | 1.2 | 3 | 54 | No |
| B6 | 13/F | Osteoid osteoma | L-tibia | 23.4 | 4 × 4 × 4 | Excision | 16 | 0.8 | 2 | 35 | No |
| B7 | 24/M | Osteoid osteoma | R-talus | 19.7 | 6 × 6 × 5 | Excision | 23 | 1.2 | 1 | 24 | No |
| B8 | 10/M | Osteoid osteoma | R-femur | 17.3 | 7 × 6 × 6 | Excision | 35 | 1.5 | 2 | 24 | No |
| B9 | 20/M | Osteoid osteoma | L-femur | 22.6 | 6 × 6 × 6 | Excision | 19 | 0.9 | 1 | 20 | No |
| M1 | 45/M | Chondrosarcoma* | L-ilium, S2, S3 | 25.8 | 25 × 15 × 15 | WE | 29 | 0.8 | 4 | 52 | No |
| M2† | 58/M | Chordoma | S3, 4, L-ilium† | 21.0 | 32 × 22 × 18 | WE | 30 | 1.0 | 3 | 51 | No |
| M3 | 70/M | Parosteal osteosarcoma | R-tibia | 28.5 | 42 × 30 × 15 | WE | 18 | 1.0 | 2 | 48 | No |
| M4 | 62/F | Extraskeletal osteosarcoma* | Sacrum | 22.2 | 32 × 30 × 8 | WE | 24 | 0.6 | 3 | 10 | No |
| M5 | 76/F | Myxoid liposarcoma | R-ilium | 21.3 | 33 × 32 × 18 | WE | 30 | 0.9 | 4 | 42 | No |
| M6 | 30/M | Parosteal osteosarcoma | R-femur | 22.0 | 35 × 26 × 16 | WE | 20 | 0.7 | 2 | 34 | No |
| M7 | 40/F | Osteosarcoma | R-tibia, femur†† | 19.5 | 12 × 10 × 10 | WE | 27 | 1.1 | 0 | 24 | No |
B = benign; M = malignant; * relapsed case; †skip lesion; ††the size of the bone tumor and bone lesion of the soft tissue tumor; WE = wide excision; Preparation time = the time from placement of the reference frame to completion; Registration error, the accuracy of image-to-patient registration; Difference = the difference between the planned margin and the postoperative computed tomography or excised histological actual margin; BMI, body mass index = weight (kg)/(height × height [m]); †a few lesions appeared on the opposite side of the ilium after surgery.
Patient 1
A 70-year-old woman had a 5-year history of general body pain and muscle weakness. Laboratory data indicated a consistently low serum phosphorus and high serum alkaline phosphatase levels. A bone biopsy revealed osteoid accumulation on the bone’s surface indicative of osteomalacia. Venous blood was used for whole-body mapping of intact fibroblast growth factor 23 (FGF-23) [27].
The results showed high levels of FGF-23 in the venous blood drained from the right lower leg. No lesions were detected on the radiographs of the right leg and foot. However, MRI using homogeneously low signal intensity on T1-and T2-weighted sequences, a 10-mm lesion was clearly seen on the proximal tibia (Fig. 1A–B). No accumulation was observed in this tumor using scintigraphy with technetium (99mTc) or thallium (201Tl), or with positron emission tomography (18F-FDG-PET).
Fig. 1A–F.
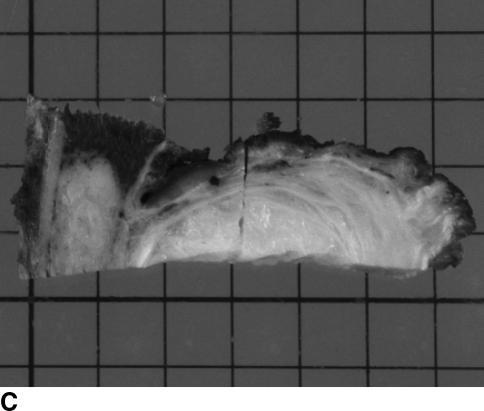
(A) A radiograph of the right tibia of a 70-year-old woman with a phosphaturic mesenchymal tumor showed no remarkable lesions. (B) On coronal T2-weighted MRI, the lesion showed a homogeneously low signal intensity. (C) The data were transferred and recorded on the system computer and reconstructed as 3-D images on a TV monitor. (D) The trephine was guided by the Kirschner wire and a 12-mm-diameter area was excised. (E) A specimen excised en bloc. This was not the real center of the tumor. The 2.0-mm wire was slight, fine, and eccentrically bent. (F) A postoperative CT scan showed that the tumor was filled with bone cement.
This osteomalacia was caused by the benign tumor. Navigation-assisted surgery was performed, and the tumor was successfully excised en bloc using a trephine 12 mm in diameter and additional curettage was required (Fig. 1C–F). The histologic diagnosis was hemangiopericytoma with positive FGF-23 immunostaining and without malignancy [10]. After surgery, the patient’s serum phosphorus level returned to the normal range in a few weeks. She had no evidence of recurrence 51 months after surgery.
Patient 2
A 76-year-old woman was examined using abdominal CT, and an unexpected soft tissue mass was observed on her right hip. MRI revealed a soft tissue tumor with a bony lesion that had homogeneously low signal intensity on T1-weighted and high signal intensity on T2-weighted sequences that was enhanced with gadolinium. A needle biopsy yielded the diagnosis of low-grade myxoid liposarcoma. The extent of the iliac lesion was close to the joint and could not be detected on radiographs or with fluoroscopy. Because of the patient’s age and the low-grade tumor status, we planned minimally invasive surgery with a 10-mm margin [31]. A planned excision line was made for the CT image (Fig. 2A). After registration, several Kirschner wires were placed on the excision lines (Fig. 2B). En bloc excision was performed successfully using chisels outside the wires. Although the actual result of the histologic margin was 6 mm, she was disease-free for 42 months after surgery (Fig. 2C).
Fig. 2A–C.
A 76-year-old woman had myxoid liposarcoma with a bony lesion on the right hip. (A) A planned excision line was made for the CT image. (B) Each Kirschner wire was placed on the excision line. The excision was made using chisels on the outside of each wire. (C) The tumor was excised with margins of at least 6 mm. The patient was disease-free for 42 months after surgery.
Patient 3
A 40-year-old woman had a painful, swollen knee for 2 months. The primary lesion was found in the proximal tibia and a skip lesion was found in the distal femur. The primary and skip lesions showed heterogeneous signal intensity on T1- and T2-weighted sequences, and they were enhanced with the contrast medium. The skip lesion was approximately 14 mm in diameter (maximum). A biopsy was performed only of the primary lesion, which was diagnosed as being a conventional osteosarcoma.
After seven courses of preoperative chemotherapy with caffeine, both lesions showed good response [31]. We planned a 10-mm-wide resection of the primary lesion and computer-assisted surgery (CAS) for the skip lesion (Fig. 3A). The skip lesion was excised using the navigation system with a margin of at least 10 mm (Fig. 3B). Only the excised cortical bone was reimplanted using the intraoperative irradiated auto-bone graft method, whereas the joint was replaced with a custom-made TKA system [18]. There was no evidence of disease recurrence for 24 months after the surgery.
Fig. 3A–B.
A 40-year-old woman had osteosarcoma of the right tibia and (A) a femoral skip lesion that showed bone formation on the CT scan. A planned excision line was made for the CT image. (B) The tumor was excised successfully with margins of at least 10 mm.
Discussion
To complete a tumor resection safely, a surgeon has to examine the extent of the malignant bone and soft tissue. Evaluation of a patient’s MRI and/or CT images is necessary for preoperative planning of tumor resection and reconstruction. The tumor’s 3-D position could not have been recognized accurately by surgeons before introduction of the computer navigation system. Navigation procedures for tumor surgery based on CT data provide the advantages of high precision and reduced radiation exposure for surgeons.
Two basic types of image guidance systems currently are available: a CT-based system and a fluorography-based system. The advantages of the CT-based system include the ability to do preoperative planning and the availability of 3-D reconstructions, whereas the disadvantage of this method is that it requires accurate preoperative registration [27]. In bone tumor surgery, CT-based navigation often is preferred over fluorography-based navigation because of clear recognition of the position and/or tumor margin on the bone, both of which are difficult to obtain with fluorography, and because it allows for preoperative planning of resection surgery on the basis of 3D-CT imaging.
For routine CT-based navigation, paired point-based registration uses fiducial markers that are fixed invasively to the surface of the involved bone. In the current study, instead of the bone-fixed markers, multiple skin-point markers were used to avoid an invasive marking procedure. The drawback of this method was potentially inaccurate registration owing to improper placement of the skin markers and positional shifting of the markers by the skin sliding during surgery.
To overcome these risks, 10 skin markers were placed randomly but carefully to cover the surrounding tumor. This system shows the 3-D virtual real image and has little error. Regarding the registration error using fiducial skin markers, early trials showed displacements from the CT data as large as 2 to 4 mm at the time of surgery, and this was concluded to be an unacceptable accuracy; thus, use of the noninvasive fiducial was inadequate [12].
In neurosurgery, skin shifting is a problem when head pins are used [22]. The use of bone-anchored screw markers has been recommended for better accuracy [14]. In the current study, four Kirschner wires were used preoperatively as fiducial markers and patient image registration was performed as described by Cho et al. [7]. According to Wong et al., registration of paired points and surface matching is needed to touch the bone directly around the lesion and for extensive skin incisions [35].
To improve skin marker accuracy, it is important to match the same positions of the skin markers from the CT data acquisition with those obtained during surgery. In our patients, acquisition of CT data was made in a position equivalent to that of the planned surgery. Consequently, our registration errors might be acceptable (mean, 0.93 mm) in this small study. Another reason for the good registration accuracy might be because our patients were not obese [21].
Registration using skin markers must be performed carefully at sites without respiratory motion and with CT data obtained with the patient in a position identical to that at surgery, eg, hand and forearm surgery. However, a new combined system of CT and navigation has been introduced that can be used for repeated examinations in the same operating room using a new corrective data set. This system does not require a long registration time but does allow for repeated reexamination of the CT scans during the operation [25].
Furthermore, image fusion technology has been used in orthopaedic oncology; however, more practice and new software choices are needed to apply this technology [7, 35]. Osteoid osteomas are commonly treated with CT-guided radiofrequency ablation (RFA), but the main disadvantage in patients with suspected tumors is the lack of histologic verification [20]. RFA for bone and soft tissue tumors is not yet approved for use in Japan.
Wide excision is the most common treatment in malignant bone and soft tissue tumor surgery. Evaluation of the surgical margin is useful for determining the curative success of surgical treatment for musculoskeletal sarcoma and the degree to which later surgeries will be reduced by preoperative therapy [31]. This navigation procedure also might be applicable to resection of malignant bone and soft tissue tumors with bony extension to determine the correct resection margin when the tumor extent cannot be clarified by fluoroscopy or plain radiographs. It also could be used for minimally invasive bone resection. Although a navigation system is useful for tumor surgery, it is expensive and requires additional preparation time. In our study, the preoperative preparation time using this system was 16 to 35 minutes, and the planning time depended on the complexity of the case. More clinical experience is required to elucidate the indication and efficacy of navigation-assisted surgery. Additional research and technological advances of navigation systems will ensure the continual advancement of bone tumor surgery standards.
In appropriately chosen patients, the use of computer navigation for removal of bone and soft tissue tumors with bony extensions using a registration technique involving skin marker fiducials can yield successful and accurate resections.
Acknowledgments
We thank Kunio Takaoka, MD, PhD, formerly of the Osaka City University Graduate School of Medicine, and Isao Yamamoto (Medtronic-Sofamor Danek Co. Ltd) for assistance with operation of the navigation system. We also thank the late Akitoshi Inatani and his father, who donated the navigation system to the Osaka City University Graduate School of Medicine.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with the ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Yodogawa Christian Hospital and Osaka City University in Japan.
References
- 1.Amiot LP, Labelle H, DeGuise JA, Sati M, Brodeur P, Rivard CH. Computer-assisted pedicle screw fixation: a feasibility study. Spine (Phila Pa 1976) 1995;20:1208–1212. doi: 10.1097/00007632-199505150-00019. [DOI] [PubMed] [Google Scholar]
- 2.Araki N, Myoui A, Kuratsu S, Hashimoto N, Inoue T, Kudawara I, Ueda T, Yoshikawa H, Masaki N, Uchida A. Intraoperative extracorporeal autogenous irradiated bone grafts in tumor surgery. Clin Orthop Relat Res. 1999;368:196–206. doi: 10.1097/00003086-199911000-00024. [DOI] [PubMed] [Google Scholar]
- 3.Arand M, Hartwig E, Kinzl L, Gebhard F. Spinal navigation in tumor surgery of the thoracic spine: first clinical results. Clin Orthop Relat Res. 2002;399:211–218. doi: 10.1097/00003086-200206000-00026. [DOI] [PubMed] [Google Scholar]
- 4.Athwal GS, Pichora DR, Ellis RE, Rudan JF. A computer-assisted guidance technique for the localization and excision of osteoid osteoma. Orthopedics. 2004;27:195–197. doi: 10.3928/0147-7447-20040201-11. [DOI] [PubMed] [Google Scholar]
- 5.Berlemann U, Langlotz F, Langlotz U, Nolte LP. [Computer-assisted orthopedic surgery: from pedicle screw insertion to further applications] [in German] Orthopade. 1997;26:463–469. [PubMed] [Google Scholar]
- 6.Bucholz R, Marzouk S, Levy A. In Alexander E 3rd, Maciunas RJ, eds. Advanced Neurosurgical Navigation. New York, NY: Thieme Medical Publishers; 1999:345–355.
- 7.Cho HS, Oh JH, Han I, Kim HS. Joint-preserving limb salvage surgery under navigation guidance. J Surg Oncol. 2009;100:227–232. doi: 10.1002/jso.21267. [DOI] [PubMed] [Google Scholar]
- 8.Delp SL, Stulberg SD, Davies B, Picard F, Leitner F. Computer assisted knee replacement. Clin Orthop Relat Res. 1998;354:49–56. doi: 10.1097/00003086-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Dessenne V, Lavallée S, Julliard R, Orti R, Martelli S, Cinquin P. Computer-assisted knee anterior cruciate ligament reconstruction: first clinical tests. J Image Guid Surg. 1995;1:59–64. doi: 10.1002/(SICI)1522-712X(1995)1:1<59::AID-IGS9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 10.Folpe AL, Fanburg-Smith JC, Billings SD, Bisceglia M, Bertoni F, Cho JY, Econs MJ, Inwards CY, Jan de Beur SM, Mentzel T, Montgomery E, Michal M, Miettinen M, Mills SE, Reith JD, O’Connell JX, Rosenberg AE, Rubin BP, Sweet DE, Vinh TN, Wold LE, Wehrli BM, White KE, Zaino RJ, Weiss SW. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol. 2004;28:1–30. doi: 10.1097/00000478-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Glossop ND, Hu RW, Randle JA. Computer-aided pedicle screw placement using frameless stereotaxis. Spine (Phila Pa 1976) 1996;21:2026–2034. doi: 10.1097/00007632-199609010-00021. [DOI] [PubMed] [Google Scholar]
- 12.Hauser R, Westermann B, Probst R. Noninvasive tracking of patient’s head movements during computer-assisted intranasal microscopic surgery. Laryngoscope. 1997;107:491–499. doi: 10.1097/00005537-199704000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Heermann R, Schwab B, Issing PR, Haupt C, Lenarz T. Navigation with the StealthStationTM in skull base surgery: an otolaryngological perspective. Skull Base. 2001;11:277–285. doi: 10.1055/s-2001-18634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hufner T, Kfuri M, Jr, Galanski M, Bastian L, Loss M, Pohlemann T, Krettek C. New indications for computer-assisted surgery: tumor resection in the pelvis. Clin Orthop Relat Res. 2004;426:219–225. doi: 10.1097/01.blo.0000138958.11939.94. [DOI] [PubMed] [Google Scholar]
- 15.Hufner T, Pohlemann T, Tarte S, Gänsslen A, Geerling J, Bazak N, Citak M, Nolte LP, Krettek C. Computer-assisted fracture reduction of pelvic ring fractures: an in vitro study. Clin Orthop Relat Res. 2002;399:231–239. doi: 10.1097/00003086-200206000-00028. [DOI] [PubMed] [Google Scholar]
- 16.Jaramaz B, DiGioia AM, III, Blackwell M, Nikou C. Computer assisted measurement of cup placement in total hip replacement. Clin Orthop Relat Res. 1998;354:70–81. doi: 10.1097/00003086-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Kamimura M, Ebara S, Itoh H, Tateiwa Y, Kinoshita T, Takaoka K. Accurate pedicle screw insertion under the control of a computer-assisted image guiding system: laboratory test and clinical study. J Orthop Sci. 1999;4:197–206. doi: 10.1007/s007760050094. [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi N, Matsumoto S, Manabe J. New method of evaluating the surgical margin and safety margin for musculoskeletal sarcoma, analysed on the basis of 457 surgical cases. J Cancer Res Clin Oncol. 1995;121:555–563. doi: 10.1007/BF01197769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krackow KA, Phillips MJ, Bayers-Thering M, Serpe L, Mihalko WM. Computer-assisted total knee arthroplasty: navigation in TKA. Orthopedics. 2003;26:1017–1023. doi: 10.3928/0147-7447-20031001-11. [DOI] [PubMed] [Google Scholar]
- 20.Lindner NJ, Ozaki T, Roedl R, Gosheger G, Winkelmann W, Wörtler K. Percutaneous radiofrequency ablation in osteoid osteoma. J Bone Joint Surg Br. 2001;83:391–396. doi: 10.1302/0301-620X.83B3.11679. [DOI] [PubMed] [Google Scholar]
- 21.Maurer CR, Jr, Maciunas RJ, Fitzpatrick JM. Registration of head CT images to physical space using a weighted combination of points and surfaces. IEEE Trans Med Imaging. 1998;17:753–761. doi: 10.1109/42.736031. [DOI] [PubMed] [Google Scholar]
- 22.Mitsui T, Fujii M, Tsuzaka M, Hayashi Y, Asahina Y, Wakabayashi T. Skin shift and its effect on navigation accuracy in image-guided neurosurgery. Radiol Phys Technol. 2010;4:37–42. doi: 10.1007/s12194-010-0103-0. [DOI] [PubMed] [Google Scholar]
- 23.Moody JE, Nikou C, Picard F, Levison T, Jaramaz B, DiGioia AM, 3rd, Reverte CF. Computer-integrated anterior cruciate ligament reconstruction system. J Bone Joint Surg Am. 2002;84(suppl 2):99–101. doi: 10.2106/00004623-200200002-00012. [DOI] [PubMed] [Google Scholar]
- 24.Neumann AM, Jr, Pasquale-Niebles K, Bhuta T, Sillers MJ. Image-guided transnasal endoscopic surgery of the paranasal sinuses and anterior skull base. Am J Rhinol. 1999;13:449–454. doi: 10.2500/105065899781329746. [DOI] [PubMed] [Google Scholar]
- 25.Rajasekaran S, Karthik K, Chandra VR, Rajkumar N, Dheenadhayalan J. Role of intraoperative 3D C-arm-based navigation in percutaneous excision of osteoid osteoma of long bones in children. J Pediatr Orthop B. 2010;19:195–200. doi: 10.1097/BPB.0b013e328333997a. [DOI] [PubMed] [Google Scholar]
- 26.Schlöndorff G, Mösges R, Meyer-Ebrecht D, Krybus W, Adams L. [CAS (computer assisted surgery): a new procedure in head and neck surgery] [in German] HNO. 1989;7:187–190. [PubMed] [Google Scholar]
- 27.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shoda N, Nakajima S, Seichi A, Kan A, Iwasaki M, Kitagawa T, Kawaguchi H, Nakamura K. Computer-assisted anterior spinal surgery for a case of recurrent giant cell tumor. J Orthop Sci. 2002;7:392–396. doi: 10.1007/s007760200065. [DOI] [PubMed] [Google Scholar]
- 29.Stöckle U, König B, Schaser K, Melcher I, Haas NP. [CT and fluoroscopy based navigation in pelvic surgery] [in German] Unfallchirurg. 2003;106:914–920. doi: 10.1007/s00113-003-0677-7. [DOI] [PubMed] [Google Scholar]
- 30.Stöckle U, Krettek C, Pohlemann T, Messmer P. Clinical applications-pelvis. Injury. 2004;35(suppl 1):S-A46–S-A56. doi: 10.1016/j.injury.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Tsuchiya H, Tomita K, Mori Y, Asada N, Morinaga T, Kitano S, Yamamoto N. Caffeine-assisted chemotherapy and minimized tumor excision for nonmetastatic osteosarcoma. Anticancer Res. 1998;18:657–666. [PubMed] [Google Scholar]
- 32.Royen BJ, Baayen JC, Pijpers R, Noske DP, Schakenraad D, Wuisman PI. Osteoid osteoma of the spine: a novel technique using combined computer-assisted and gamma probe-guided high-speed intralesional drill excision. Spine (Phila Pa 1976) 2005;30:369–373. doi: 10.1097/01.brs.0000152531.49095.34. [DOI] [PubMed] [Google Scholar]
- 33.Westendorff C, Hoffmann J, Troitzsch D, Dammann F, Reinert S. Ossifying fibroma of the skull: interactive image-guided minimally invasive localization and resection. J Craniofac Surg. 2004;15:854–858. doi: 10.1097/00001665-200409000-00029. [DOI] [PubMed] [Google Scholar]
- 34.Wirtz CR, Knauth M, Hassfeld S, Tronnier VM, Albert FK, Bonsanto MM, Kunze S. Neuronavigation-first experiences with three different commercially available systems. Zentralbl Neurochir. 1998;59:14–22. [PubMed] [Google Scholar]
- 35.Wong KC, Kumta SM, Chiu KH, Antonio GE, Unwin P, Leung KS. Precision tumour resection and reconstruction using image-guided computer navigation. J Bone Joint Surg Br. 2007;89:943–947. doi: 10.1302/0301-620X.89B7.19067. [DOI] [PubMed] [Google Scholar]