Abstract
Background
Deep infections are devastating complications of TKA often treated with component explantation, intravenous antibiotics, and antibiotic-impregnated cement spacers. Historically, the spacers have been static, which may limit patients’ ROM and ability to walk. Several recent reports describe dynamic spacers, which may allow for improved ROM and make later reimplantation easier. However, because of several dynamic spacer problems noted at our institution, we wanted to assess their associated failures, reinfection rates, and functionality.
Questions/purposes
We therefore asked whether there were differences between static and dynamic spacers in (1) reinfection rates, (2) complications directly related to the spacer, and (3) final patient functionality as measured by Knee Society objective scores and ROM.
Patients and Methods
We retrospectively identified 111 patients (115 knees) with 34 dynamic spacers (30%) and 81 static spacers (70%). Reinfection rates, complications requiring additional surgery, and final Knee Society scores and ROM were collected for all patients.
Results
Reinfection rates were comparable between groups. In the dynamic spacer cohort, there were four complications; however, these could all be explained by surgical technical errors or patient weightbearing compliance. All patients with failed results eventually underwent successful two-stage exchange arthroplasty. Final Knee Society scores and ROM were also similar between groups.
Conclusions
Reinfection rates, Knee Society scores, and ROM were comparable between the static and dynamic spacer groups. Meticulous surgical technique and proper patient selection should be used to avoid any complications with any spacers.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
TKA has reported survivorships of greater than 90% in reports with mean 10- to 20-year followups [6, 26, 31]. However, despite improvements in preoperative and perioperative methods to reduce infection [5, 9, 10, 33, 38, 47, 50, 54], its incidence after TKA remains around 1% to 2% in large studies [29, 30]. The revision TKA burden in the United States accounted for 46,000 procedures during 2005 [28], with some estimates that up to 25% of revisions are due to infection [3]. Although serial irrigation and débridement or single-stage revision procedures may be used in specific scenarios to treat acute TKA infections, staged revision arthroplasty is generally regarded as the gold standard treatment for deep periprosthetic infections [34, 35]. However, controversy exists concerning many of the specifics of this treatment method. At our institution, patients undergo an initial explantation procedure with the placement of a temporary antibiotic-laden cement spacer, which remains in place for a minimum of 6 weeks while the patient undergoes intravenous antibiotic therapy. Spacers were originally custom-made and nonarticulating, static cement blocks of various shapes [11, 14, 35, 42], which had the advantages of being cost-effective and simple to use. However, reports have documented shortcomings of nonarticulating spacers, including the potential for increased bone loss [24, 41, 49], decreased ROM, and flexion contractures at final revision surgery [14, 42], which may lead to a difficult surgical exposure and reduced patient functionality postoperatively.
Therefore, attempts have been made to design and utilize a more functional dynamic spacer that allows the patient to have some, albeit limited, knee ROM during the interim (preimplantation) period [11, 13–15, 19, 22–24, 42, 49]. Purported advantages of these articulating spacers include allowing the patient to ambulate more easily and maintain some ROM and functionality while possibly not creating as difficult of an exposure during the ultimate revision procedure [11, 14, 15, 19, 42]. These advantages do not come without some cost because these articulating spacers have the potential for decreased implant stability and durability [27, 46] and inability to effectively handle extreme bone loss [24, 41].
Because of the issue of which type of spacer is more appropriate for use, we asked whether there were differences between the two types of spacers in terms of (1) reinfection rates, (2) complications directly related to the spacers, and (3) final patient functionality as measured by Knee Society scores and ROM specifically.
Patients and Methods
We reviewed a database of all patients at our institution who underwent two-stage revision TKA for deep periprosthetic infection between 2000 and 2009 and identified 111 patients (115 knees). Patients were then stratified into two groups based on what type of antibiotic-laden cement spacer was used in the interim between the first- and second-stage procedures: those who received dynamic spacers (34 knees, 30%) and those who received static spacers (81 knees, 70%) (Table 1). The indications for the use of each spacer are described below. Minimum followup was 12 months after the reimplantation procedure (dynamic spacer group: mean, 27 months; range, 12–72 months; static spacer group: mean, 66 months; range, 12–121 months). Appropriate Institutional Review Board approval was obtained for this study.
Table 1.
Comparison of the demographic and clinical data between the dynamic and static spacer groups.
| Variable | Dynamic spacer group | Static spacer group |
|---|---|---|
| Number of knees | 34 | 81 |
| Age (years)* | 62 (59–65) | 61 (58–64) |
| Body mass index (kg/m2)* | 31.25 (28.60–33.90) | 36.70 (35.12–38.28) |
| Spacer duration (days)* | 93 (74–112) | 107 (82–132) |
| Preoperative Knee Society objective score (points)* | 62 (58–66) | 73 (71–75) |
| Preoperative flexion contracture > 5° | ||
| Number | 7 | 4 |
| Flexion contracture (°)† | 11 (5–40) | 10 (5–20) |
| Preoperative ROM (°)* | 99 (93–104) | 83 (89–97) |
| Tibial bone loss (number of knees) | ||
| Type 1 | 17 (50%) | 30 (38%) |
| Type 2a or 2b | 13 (38%) | 38 (46%) |
| Type 3 | 4 (12%) | 13 (16%) |
| Femoral bone loss (number of knees) | ||
| Type 1 | 18 (53%) | 16 (20%) |
| Type 2a or 2b | 16 (47%) | 49 (60%) |
| Type 3 | 0 | 16 (20%) |
| Type 1 tibial and femoral (number of knees) | 16 (47%) | 16 (20%) |
| Type 2 or 3 tibial or femoral (number of knees) | 18 (53%) | 65 (80%) |
* Values are expressed as mean, with 95% confidence interval in parentheses; †values are expressed as mean, with range in parentheses.
To assess whether there were differences in various factors between the dynamic and static spacer groups that might bias the results, we compared the following factors: gender, age, body mass index, duration of spacer use, type of organism (high-virulence versus low-virulence group), preoperative Knee Society objective scores [25], ROM, and bone loss (Table 1). Two factors were different between the two groups: bone loss and preoperative Knee Society objective scores. More patients had severe bone loss in the static spacer group (65 patients, 80%; 95% confidence interval [CI], 70%–88%) than in the dynamic spacer group (18 patients, 53%; 95% CI, 31%–63%). There were no patients who received a dynamic spacer with Type III femoral bone loss and only a small percentage (four, 12%) who had Type III tibial bone loss. In these four patients, all had either Type I or II femoral bone loss, and a dynamic spacer was placed in light of Type III tibial bone loss in an effort to retain functionality. We acknowledge, however, dynamic spacers should typically not be placed in light of any Type III bone loss. Although there was a difference in preoperative Knee Society objective scores, the difference was small and not clinically relevant.
The criteria of Leone and Hanssen [32] were used to diagnose infection in all patients. Only deep incisional and joint space infections were considered periprosthetic infections. All patients underwent two-stage revision using a previously described protocol [55]. Frozen sections were taken to ensure the patient was infection-free before reimplantation [2, 7, 8, 36]. The mean duration between explantation and reimplantation was 93 days (95% CI, 74–112 days) in those with dynamic spacers and 107 days (95% CI, 82–131 days) in those with static spacers. All patients ultimately underwent reimplantation arthroplasty. After reimplantation, patients were discharged with either no additional antibiotics or a 4- to 6-week course of appropriate oral antibiotics in consultation with our infectious disease specialist, as previously described [55], based on original cultures and sensitivities when an organism was identified (Table 2) or empirically when no organism grew. After reimplantation arthroplasty, as described above, patients were seen in the office at 1, 3, and 6 months and yearly thereafter for followup. All patients were ambulatory on discharge with the use of a walking assist device. Outpatient rehabilitation and physical therapy consisted of strengthening and ROM exercises. Patients were encouraged to complete 6 weeks of this at a supervised outpatient physical therapy center.
Table 2.
Cultured organisms and infection categorization.
| Organism | Dynamic spacer group | Static spacer group |
|---|---|---|
| Gram positive | ||
| Methicillin-resistant Staphylococcus aureus | 8 | 26 |
| Coagulase-negative Staphylococcus aureus | 5 | 10 |
| Enterococcus species | 2 | 0 |
| Methicillin-sensitive Staphylococcus aureus | 8 | 3 |
| Streptococcus viridans | 1 | 0 |
| Vancomycin-resistant Enterococcus species | 2 | 0 |
| Corynebacterium species | 1 | 0 |
| Streptococcus, Group B (agalactiae) | 1 | 1 |
| Streptococcus, Group D | 2 | 1 |
| Gram negative | ||
| Pseudomonas aeruginosa | 1 | 0 |
| Eikenella corrodens | 0 | 1 |
| Negative cultures | 12 | 43 |
| Multiple organisms | 6 | 9 |
Between September 2000 and October 2006, all patients received static spacers. After 2006, patients received either a dynamic or static spacer. Patients received a dynamic spacer at the discretion of the operating surgeon. This was performed if there was no draining sinus tract and minimal bone loss [12] and if the patient had a previously well-functioning TKA. Over the course of this study, three different types of dynamic spacers were utilized: Biomet StageOne™ (Biomet Inc, Warsaw, IN), Interspace® Knee (Tecres SpA, Verona, Italy, distributed by Exactech, Inc, Gainesville, FL), and PROSTALAC® (DePuy Orthopaedics, Warsaw, IN). The PROSTALAC® design consists of an all-cement tibial component and a metal-and-cement femoral component, with a cam and post mechanism for added stability and metal skids on the femoral articulating surface. The Interspace® and StageOne® are all-cement designs, with the increased congruency in the Interspace® tibial implant. All spacers were implanted according to manufacturer instructions. This involved component removal, débridement of remaining cement, and irrigation. After this, dynamic spacers were molded to appropriate size. After the mold cured, additional bone cement was affixed to the spacer to provide fixation. We evaluated stability in extension, 90° flexion, and midrange. We attempted to recreate the original joint tension by using a combination of assessing appropriately sized components and differences in cement mantle thicknesses. If after all balancing efforts were attempted the joint was still too loose, a static spacer was used. All patients who received a dynamic spacer were instructed to restrict weightbearing to 50% with use of an ambulatory aid. Patients who received static spacers wore a knee immobilizer for a minimum of 2 weeks; patients with dynamic spacers did not wear knee immobilizers. For all spacers, 2 g vancomycin and 2.4 g tobramycin powder were added per bag of poly(methylmethacrylate) (typically, three bags were used).
Two authors (AJJ, SAS) reviewed the clinical records and radiographs to assess function as measured by Knee Society objective scores and complication rates. We monitored for medical complications (including deep vein thrombosis, urinary tract infection, and pulmonary embolism) and surgical complications (hematoma formation, local skin irritation and breakdown at the operative site, and any signs of superficial infection). Spacer complications included dislocations, subluxations, fractures, and soft tissue compromise that led to reoperations. Any additional operative treatment before scheduled reimplantation was considered a major complication.
Two authors (AJJ, QN) reviewed all radiographs to assess bone loss (Table 1) [12]. Patients were grouped by minimal bone loss (defined as only Type 1 tibial and femoral bone loss) or severe bone loss (all other patients).
Data were recorded in an Excel® spreadsheet (Microsoft Corp, Redmond, WA). Demographics (including bone loss, gender, age, body mass index, duration of spacer use, type of organism, preoperative Knee Society objective scores, and ROMs) were compared using the 95% CI of the mean. Reinfection rates and complication rates were compared using the 95% CI of the proportion. Final postoperative Knee Society objective scores and ROM were again evaluated using the 95% CI of the mean. All values were calculated using SigmaStat® Version 3.0 (Systat Inc, San Jose, CA).
Results
We found no difference in reinfection rates after two-stage revision arthroplasty. Six patients in the dynamic spacer cohort (17%; 95% CI, 8%–34%) and 14 patients in the static spacer cohort (17%; 95% CI, 10%–27%) were reinfected and underwent further débridement.
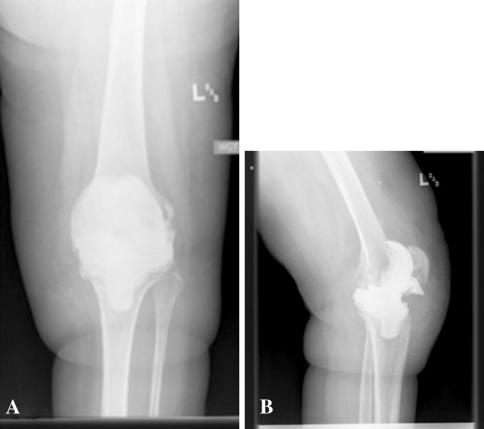
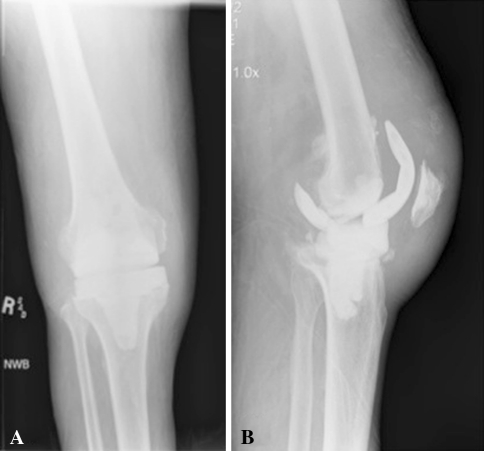
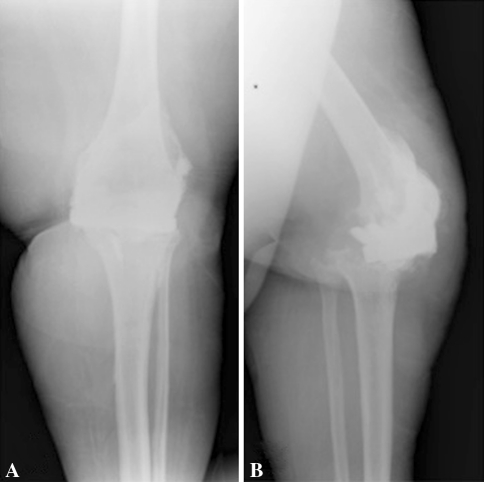
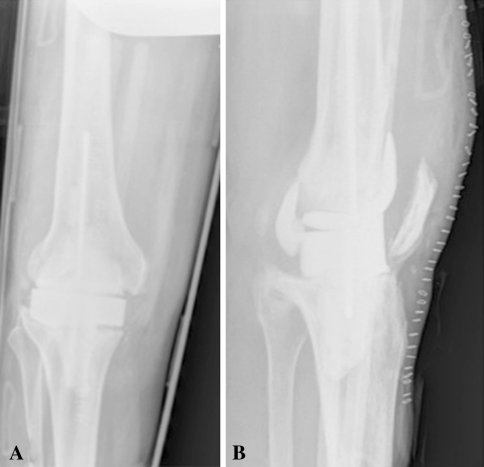
There were more complications directly related to the spacer that led to reoperation in the patients who had dynamic spacers. There were four patients who had mechanical failure of the dynamic spacer (12%; 95% CI, 5%–27%) and no failures of static spacers (0%; 95% CI, 0%–5%). None of the dynamic spacer failures occurred in patients who had Type III tibial bone loss. All failures occurred with a single implant, that being the spacer with the least congruent tibial component. In addition, surgical technical errors in implantation could partially or fully explain the failure, as will be described. The first patient was a 56-year-old woman who had a dislocated femoral component (Fig. 1). The second patient was a 73-year-old man who had a fractured femoral component (Fig. 2). The third patient was a 65-year-old woman who had a subluxated tibial component (Fig. 3). The last patient was a 61-year-old man who had a fractured femoral component (Fig. 4). The two patients who had spacer fractures admitted to full weightbearing. All patients had Knee Society objective scores greater than 80 points at most recent followup.
Fig. 1A–B.
(A) AP and (B) lateral radiographs of the knee of a 56-year-old woman, who presented approximately 2 weeks after spacer placement with extreme pain and inability to move her knee, show complete femoral component dislocation. The reverse tibial slope can be seen, which may have decreased the flexion gap spacing and in turn led to femoral component subluxation. The patient required a femoral spacer component. After final reimplantation, she was infection free at 1-year followup.
Fig. 2A–B.
(A) AP and (B) lateral radiographs of the knee of a 73-year-old man, who presented approximately 4 weeks after spacer placement with pain and swelling of the knee, show a fractured femoral component with insufficient cement anteriorly and posteriorly, which led to the generation of a cantilevered bending moment on weightbearing and ultimately fracture of the posterior condyle that required additional surgery for a new femoral spacer. After final reimplantation arthroplasty, the patient required surgery for hematoma formation, lysis of adhesions, and polyethylene exchange. At 1 year after this surgery, the patient remained free of infection.
Fig. 3A–B.
(A) AP and (B) lateral radiographs of the knee of a 65-year-old woman show complete anterior subluxation of the tibial component secondary to the lack of a cement stem on the tibial component, which led to anterior skin breakdown. The patient ultimately required a gastrocnemius muscle flap for soft tissue coverage and partial resection of the tibia and fibula. One year after flap surgery and final reimplantation, the patient remained infection free.
Fig. 4A–B.
(A) AP and (B) lateral radiographs of the knee of a 61-year-old man, who presented with knee pain approximately 8 weeks after spacer placement, show multiple femoral component fractures. During spacer placement, the patient’s knee dimensions were between those for the femoral component sizes: the larger spacer would have overstuffed the joint and overhung medially and laterally, and the other spacer would not have provided adequate bone coverage. Because a larger femoral component would have required additional cement for fixation and to fill the gap space between the component and femur, which could ultimately result in increased bone loss, the decision was made to use the smaller femoral component size. At the time of spacer fracture, the patient was taken to the operating room. Frozen cultures taken at this time showed no evidence of acute inflammation, and the decision was made to complete the two-stage revision and reimplant a new prosthesis. He remained infection free at 1-year followup.
Postoperative Knee Society objective scores and ROMs improved to 83 points (range, 48–99 points; 95% CI, 79–87 points) and 99° (range, 60°–120°; 95% CI, 92°–104°), respectively, for the dynamic spacer group and 84 points (range, 48–100 points; 95% CI, 81–87 points) and 95° (range, 30°–130°; 95% CI, 90°–101°), respectively, for the static spacer group. The ROM for the patients who had complications with the dynamic spacers ranged from 95° to 120°.
Discussion
Articulating spacers have the potential advantages of allowing the patient to ambulate more easily and maintain some ROM and functionality during the lag period between explantation and reimplantation. Possible disadvantages include decreased implant stability and durability, inability to effectively handle extreme bone loss, and questionable ability to be used to treat patients with much bone loss or with highly virulent infections. We therefore compared static and dynamic spacers to find out whether there were differences between the two spacers in terms of reinfection rates, complications directly related to the spacer, and final patient functionality as measured by Knee Society objective scores and ROM.
There are several limitations to our study. First, it was neither prospective nor randomized. Because of this, there may have been a selection bias by the type of patients chosen to receive a dynamic spacer. To assess this potential bias, we compared various potential confounding factors between the two groups. Gender, age, and body mass index were comparable between the groups. There was a slightly lower Knee Society objective score in the dynamic spacer cohort (62 versus 73 points). Despite this difference, we do not believe this constitutes a clinically important difference in functionality. However, there was a difference in the amount of bone loss between the two groups, with the group that had static spacers having a higher proportion of patients who had severe bone loss when compared to the group that had dynamic spacers (80% versus 53%). This would potentially bias against the static spacer group. Due to historical patient noncompliance with knee immobilization braces at our institution, patients were instructed to 50% weightbear with the use of a walking aid. We currently recommend toe-touch weightbearing, in addition to 2 to 3 weeks of strictly enforced knee immobilization. Additionally, the number of highly virulent infections, duration of spacer placement, and reinfection rate were not different between the two groups. Next, although the followup was short in some patients (12-month minimum followup), we do not believe this affects our results. The spacer complication rate is independent of followup time because, once the patient undergoes reimplantation arthroplasty, there will be no further complications directly related to the spacer type. Further studies should prospectively assess outcomes in similar patients to determine whether there is a greater risk for reoperation in patients who receive dynamic spacers.
Numerous studies have reported on reinfection rates after two-stage revision TKA, with results ranging from 2% to 30% (Table 3) [16–22, 37, 39, 40, 45, 48, 53]. Our results are consistent with these findings. The majority of patients (43%) in our study were infected by highly virulent organisms, which can be difficult to eradicate [4, 39, 43] and may have accounted for the 17% reinfection rate. Previous reports have compared reinfection rates between dynamic or static spacers (Table 4) [11, 14, 15, 23, 42]. Our study differs from most of these reports in that we report no difference in reinfection rates based on spacer type.
Table 3.
Reported reinfection rates for two-stage revision TKA.
| Study | Number of patients | Mean followup (months) | Reinfection rate (%) |
|---|---|---|---|
| von Foerster et al. [53] | 104 | 76 | 27 |
| Goksan and Freeman [18] | 18 | 60 | 6 |
| Gacon et al. [16] | 29 | 42 | 17 |
| Hirakawa et al. [21] | 55 | NR | 18 |
| Haddad et al. [19] | 45 | 48 | 2 |
| Mont et al. [40] | 69 | 63 | 7 |
| Haleem et al. [20] | 96 | 86 | 16 |
| MacAvoy and Ries [37] | 13 | 28 | 30 |
| Hofmann et al. [22] | 50 | 74 | 12 |
| Souillac et al. [48] | 28 | 24 | 14 |
| Mittal et al. [39] | 35 | 51 | 25 |
| Peters et al. [45] | 53 | 49 | 24 |
| Ghanem et al. [17] | 109 | 67 | 21 |
NR = not reported.
Table 4.
Comparison studies of dynamic versus static spacers.
Patient safety should be the primary concern for the treating physician. Despite the functional improvements reported in the literature with dynamic spacers [11, 15, 23, 42], we believe the benefits outweigh the risks [13, 46, 51, 52] for their use only in select patients implanted with precise technique. Several failure mechanisms were observed in this study that led to reoperation. These mechanisms were fracture, dislocation, and subluxation. Prior reports have suggested material properties of poly(methylmethacrylate), such as bending and fatigue strength, are weakened by the addition of antibiotic powders [1, 27, 44]. Two of the four failures observed in our study were due to fracture. Numerous factors could have contributed to these femoral component fractures, including patient noncompliance, poor component fit (secondary to limited sizing options) leading to stress concentrations and inappropriate fatigue cycling of the component, or poor component design. Although the two spacer fractures may have been secondary to patient noncompliance with weightbearing, the most severe failure involved component subluxation leading to skin breakdown that ultimately required flap coverage.
If surgeons are going to use dynamic spacers, we encourage them to use meticulous technique and to follow the manufacturer’s instructions. In addition, we offer some guidelines from our experience in an attempt to minimize these complications. Avoid dynamic spacer use in patients who have severe bone loss and in those who have a reverse tibial slope after resection arthroplasty. Ensure there is sufficient cement to provide adequate spacer support. Always emphasize the importance of decreased weightbearing status on the dynamic spacers. If the patient has a history of poor compliance or dementia, the use of a static spacer might prevent failures related to weightbearing status. A static spacer might be considered for the highly active patient who could put more stress on the spacer. Tibial components should be implanted with some type of cement keel for stability. We stress using caution when the patient is between spacer sizes; while a femoral component that is too large may result in increased cement use for fixation and the potential for increased bone loss at resection arthroplasty, an undersized component may be more likely to fracture.
Periprosthetic infections can be devastating complications after TKA, which are a burden to effectively treat. Patients treated for these infections are often subjected to multiple procedures. Dynamic spacers conceptually provide the patient with an advantage in terms of maintaining joint mobility during the revision process, and we believe the complications of their use noted in this report were mostly attributable to an identifiable surgical-technical error. Although reinfection rates were comparable between the two groups, in an attempt to minimize patient morbidity and reduce the risks associated with additional surgical procedures, we encourage careful patient selection, meticulous surgical technique, and surgeon comfort level and preference when determining which type of spacer to use in the treatment of deep periprosthetic infections after TKA.
Footnotes
MAM is a consultant for Stryker Orthopaedics (Mahwah, NJ), Wright Medical Technology Inc (Arlington, TN), and Salient Surgical Technologies Inc (Dover, NH) and receives royalties from Stryker Orthopaedics. The remaining authors certify that they have no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Baleani M, Persson C, Zolezzi C, Andollina A, Borrelli AM, Tigani D. Biological and biomechanical effects of vancomycin and meropenem in acrylic bone cement. J Arthroplasty. 2008;23:1232–1238. doi: 10.1016/j.arth.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Banit DM, Kaufer H, Hartford JM. Intraoperative frozen section analysis in revision total joint arthroplasty. Clin Orthop Relat Res. 2002;401:230–238. doi: 10.1097/00003086-200208000-00026. [DOI] [PubMed] [Google Scholar]
- 3.Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468:45–51. doi: 10.1007/s11999-009-0945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradbury T, Fehring TK, Taunton M, Hanssen A, Azzam K, Parvizi J, Odum SM. The fate of acute methicillin-resistant Staphylococcus aureus periprosthetic knee infections treated by open debridement and retention of components. J Arthroplasty. 2009;24:101–104. doi: 10.1016/j.arth.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 5.Brown AR, Taylor GJ, Gregg PJ. Air contamination during skin preparation and draping in joint replacement surgery. J Bone Joint Surg Br. 1996;78:92–94. [PubMed] [Google Scholar]
- 6.Buechel FF., Sr Long-term followup after mobile-bearing total knee replacement. Clin Orthop Relat Res. 2002;404:40–50. doi: 10.1097/00003086-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Della Valle CJ, Bogner E, Desai P, Lonner JH, Adler E, Zuckerman JD, Di Cesare PE. Analysis of frozen sections of intraoperative specimens obtained at the time of reoperation after hip or knee resection arthroplasty for the treatment of infection. J Bone Joint Surg Am. 1999;81:684–689. doi: 10.2106/00004623-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Della Valle CJ, Sporer SM, Jacobs JJ, Berger RA, Rosenberg AG, Paprosky WG. Preoperative testing for sepsis before revision total knee arthroplasty. J Arthroplasty. 2007;22:90–93. doi: 10.1016/j.arth.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Der Tavitian J, Ong SM, Taub NA, Taylor GJ. Body-exhaust suit versus occlusive clothing: a randomised, prospective trial using air and wound bacterial counts. J Bone Joint Surg Br. 2003;85:490–494. doi: 10.1302/0301-620X.85B4.13363. [DOI] [PubMed] [Google Scholar]
- 10.Eiselt D. Presurgical skin preparation with a novel 2% chlorhexidine gluconate cloth reduces rates of surgical site infection in orthopaedic surgical patients. Orthop Nurs. 2009;28:141–145. doi: 10.1097/NOR.0b013e3181a469db. [DOI] [PubMed] [Google Scholar]
- 11.Emerson RH, Jr, Muncie M, Tarbox TR, Higgins LL. Comparison of a static with a mobile spacer in total knee infection. Clin Orthop Relat Res. 2002;404:132–138. doi: 10.1097/00003086-200211000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Engh GA, Ammeen DJ. Bone loss with revision total knee arthroplasty: defect classification and alternatives for reconstruction. Instr Course Lect. 1999;48:167–175. [PubMed] [Google Scholar]
- 13.Evans RP. Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Clin Orthop Relat Res. 2004;427:37–46. doi: 10.1097/01.blo.0000143739.07632.7c. [DOI] [PubMed] [Google Scholar]
- 14.Fehring TK, Odum S, Calton TF, Mason JB. Articulating versus static spacers in revision total knee arthroplasty for sepsis. The Ranawat Award. Clin Orthop Relat Res. 2000;380:9–16. doi: 10.1097/00003086-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Freeman MG, Fehring TK, Odum SM, Fehring K, Griffin WL, Mason JB. Functional advantage of articulating versus static spacers in 2-stage revision for total knee arthroplasty infection. J Arthroplasty. 2007;22:1116–1121. doi: 10.1016/j.arth.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Gacon G, Laurencon M, Velde D, Giudicelli DP. [Two stages reimplantation for infection after knee arthroplasty: apropos of a series of 29 cases] [in French] Rev Chir Orthop Reparatrice Appar Mot. 1997;83:313–323. [PubMed] [Google Scholar]
- 17.Ghanem E, Azzam K, Seeley M, Joshi A, Parvizi J. Staged revision for knee arthroplasty infection: what is the role of serologic tests before reimplantation? Clin Orthop Relat Res. 2009;467:1699–1705. doi: 10.1007/s11999-009-0742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goksan SB, Freeman MA. One-stage reimplantation for infected total knee arthroplasty. J Bone Joint Surg Br. 1992;74:78–82. doi: 10.1302/0301-620X.74B1.1732271. [DOI] [PubMed] [Google Scholar]
- 19.Haddad FS, Masri BA, Campbell D, McGraw RW, Beauchamp CP, Duncan CP. The PROSTALAC functional spacer in two-stage revision for infected knee replacements: prosthesis of antibiotic-loaded acrylic cement. J Bone Joint Surg Br. 2000;82:807–812. doi: 10.1302/0301-620X.82B6.10486. [DOI] [PubMed] [Google Scholar]
- 20.Haleem AA, Berry DJ, Hanssen AD. Mid-term to long-term followup of two-stage reimplantation for infected total knee arthroplasty. Clin Orthop Relat Res. 2004;428:35–39. doi: 10.1097/01.blo.0000147713.64235.73. [DOI] [PubMed] [Google Scholar]
- 21.Hirakawa K, Stulberg BN, Wilde AH, Bauer TW, Secic M. Results of 2-stage reimplantation for infected total knee arthroplasty. J Arthroplasty. 1998;13:22–28. doi: 10.1016/S0883-5403(98)90071-7. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125–131. doi: 10.1097/01.blo.0000149241.77924.01. [DOI] [PubMed] [Google Scholar]
- 23.Hsu YC, Cheng HC, Ng TP, Chiu KY. Antibiotic-loaded cement articulating spacer for 2-stage reimplantation in infected total knee arthroplasty: a simple and economic method. J Arthroplasty. 2007;22:1060–1066. doi: 10.1016/j.arth.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 24.Incavo SJ, Russell RD, Mathis KB, Adams H. Initial results of managing severe bone loss in infected total joint arthroplasty using customized articulating spacers. J Arthroplasty. 2009;24:607–613. doi: 10.1016/j.arth.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–14. [PubMed] [Google Scholar]
- 26.Keating EM, Meding JB, Faris PM, Ritter MA. Long-term followup of nonmodular total knee replacements. Clin Orthop Relat Res. 2002;404:34–39. doi: 10.1097/00003086-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Klekamp J, Dawson JM, Haas DW, DeBoer D, Christie M. The use of vancomycin and tobramycin in acrylic bone cement: biomechanical effects and elution kinetics for use in joint arthroplasty. J Arthroplasty. 1999;14:339–346. doi: 10.1016/S0883-5403(99)90061-X. [DOI] [PubMed] [Google Scholar]
- 28.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 29.Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23:984–991. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468:52–56. doi: 10.1007/s11999-009-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lachiewicz PF, Falatyn SP. Clinical and radiographic results of the Total Condylar III and Constrained Condylar total knee arthroplasty. J Arthroplasty. 1996;11:916–922. doi: 10.1016/S0883-5403(96)80132-X. [DOI] [PubMed] [Google Scholar]
- 32.Leone J, Hanssen A. Management of infection at the site of a total knee arthroplasty. J Bone Joint Surg Am. 2005;87:2335–2348. doi: 10.2106/00004623-200510000-00026. [DOI] [PubMed] [Google Scholar]
- 33.Lipsett PA. Do we really need laminar flow ventilation in the operating room to prevent surgical site infections? Ann Surg. 2008;248:701–703. doi: 10.1097/SLA.0b013e31818bb525. [DOI] [PubMed] [Google Scholar]
- 34.Lonner JH, Barrack R, Fitzgerald RH, Jr, Hanssen AD, Windsor ER. Infection in total knee arthroplasty. Part I. Classification, prophylaxis, and diagnosis. Am J Orthop (Belle Mead NJ) 1999;28:530–535. [PubMed] [Google Scholar]
- 35.Lonner JH, Barrack R, Fitzgerald RH, Jr, Hanssen AD, Windsor ER. Infection in total knee arthroplasty. Part II. Treatment. Am J Orthop (Belle Mead NJ) 1999;28:592–597. [PubMed] [Google Scholar]
- 36.Lonner JH, Desai P, Dicesare PE, Steiner G, Zuckerman JD. The reliability of analysis of intraoperative frozen sections for identifying active infection during revision hip or knee arthroplasty. J Bone Joint Surg Am. 1996;78:1553–1558. doi: 10.2106/00004623-199610000-00014. [DOI] [PubMed] [Google Scholar]
- 37.MacAvoy MC, Ries MD. The ball and socket articulating spacer for infected total knee arthroplasty. J Arthroplasty. 2005;20:757–762. doi: 10.1016/j.arth.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 38.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250–280. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 39.Mittal Y, Fehring TK, Hanssen A, Marculescu C, Odum SM, Osmon D. Two-stage reimplantation for periprosthetic knee infection involving resistant organisms. J Bone Joint Surg Am. 2007;89:1227–1231. doi: 10.2106/JBJS.E.01192. [DOI] [PubMed] [Google Scholar]
- 40.Mont MA, Waldman BJ, Hungerford DS. Evaluation of preoperative cultures before second-stage reimplantation of a total knee prosthesis complicated by infection: a comparison-group study. J Bone Joint Surg Am. 2000;82:1552–1557. doi: 10.2106/00004623-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Nickinson RS, Board TN, Gambhir AK, Porter ML, Kay PR. Two stage revision knee arthroplasty for infection with massive bone loss: a technique to achieve spacer stability. Knee. 2011 January 4 [Epub ahead of print]. [DOI] [PubMed]
- 42.Park SJ, Song EK, Seon JK, Yoon TR, Park GH. Comparison of static and mobile antibiotic-impregnated cement spacers for the treatment of infected total knee arthroplasty. Int Orthop. 2010;34:1181–1186. doi: 10.1007/s00264-009-0907-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parvizi J, Pawasarat IM, Azzam KA, Joshi A, Hansen EN, Bozic KJ. Periprosthetic joint infection: the economic impact of methicillin-resistant infections. J Arthroplasty. 2010;25(6 Suppl):103–107. doi: 10.1016/j.arth.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Persson C, Baleani M, Guandalini L, Tigani D, Viceconti M. Mechanical effects of the use of vancomycin and meropenem in acrylic bone cement. Acta Orthop. 2006;77:617–621. doi: 10.1080/17453670610012692. [DOI] [PubMed] [Google Scholar]
- 45.Peters CL, Erickson JA, Gililland JM. Clinical and radiographic results of 184 consecutive revision total knee arthroplasties placed with modular cementless stems. J Arthroplasty. 2009;24:48–53. doi: 10.1016/j.arth.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 46.Pietsch M, Hofmann S, Wenisch C. Treatment of deep infection of total knee arthroplasty using a two-stage procedure. Oper Orthop Traumatol. 2006;18:66–87. doi: 10.1007/s00064-006-1163-5. [DOI] [PubMed] [Google Scholar]
- 47.Pryor F, Messmer PR. The effect of traffic patterns in the OR on surgical site infections. AORN J. 1998;68:649–660. doi: 10.1016/S0001-2092(06)62570-2. [DOI] [PubMed] [Google Scholar]
- 48.Souillac V, Costes S, Aunoble S, Langlois V, Dutronc H, Chauveaux D. [Evaluation of an articulated spacer for two-stage reimplantation for infected total knee arthroplasty: 28 cases] [in French] Rev Chir Orthop Reparatrice Appar Mot. 2006;92:485–489. doi: 10.1016/S0035-1040(06)75835-4. [DOI] [PubMed] [Google Scholar]
- 49.Su YP, Lee OK, Chen WM, Chen TH. A facile technique to make articulating spacers for infected total knee arthroplasty. J Chin Med Assoc. 2009;72:138–145. doi: 10.1016/S1726-4901(09)70039-5. [DOI] [PubMed] [Google Scholar]
- 50.US Center for Disease Control and Prevention. Surgical site infection (SSI) event: guidelines and procedures for monitoring SSI. October 2010. Available at: http://www.cdc.gov/nhsn/PDFs/pscManual/9pscSSIcurrent.pdf. Accessed March 14, 2011.
- 51.Thiel GS, Berend KR, Klein GR, Gordon AC, Lombardi AV, Della Valle CJ. Intraoperative molds to create an articulating spacer for the infected knee arthroplasty. Clin Orthop Relat Res. 2011;469:994–1001. doi: 10.1007/s11999-010-1644-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Villanueva-Martinez M, Rios-Luna A, Pereiro J, Fahandez-Saddi H, Villamor A. Hand-made articulating spacers in two-stage revision for infected total knee arthroplasty: good outcome in 30 patients. Acta Orthop. 2008;79:674–682. doi: 10.1080/17453670810016704. [DOI] [PubMed] [Google Scholar]
- 53.Foerster G, Kluber D, Kabler U. [Mid- to long-term results after treatment of 118 cases of periprosthetic infections after knee joint replacement using one-stage exchange surgery] [in German] Orthopade. 1991;20:244–252. [PubMed] [Google Scholar]
- 54.Zywiel MG, Daley JA, Delanois RE, Naziri Q, Johnson AJ, Mont MA. Advance pre-operative chlorhexidine reduces the incidence of surgical site infections in knee arthroplasty. Int Orthop. 2011;35:1001–1006. doi: 10.1007/s00264-010-1078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zywiel MG, Johnson AJ, Stroh DA, Martin J, Marker DR, Mont MA. Prophylactic oral antibiotics reduce reinfection rates following two-stage revision total knee arthroplasty. Int Orthop. 2011;35:37–42. doi: 10.1007/s00264-010-0992-x. [DOI] [PMC free article] [PubMed] [Google Scholar]