Abstract
Objectives
Intraductual papillary mucinous neoplasms (IPMNs) are often multifocal and involve the entire pancreas. Because of the morbidity associated with total pancreatectomy, surgeons will perform segmental pancreatectomy, resecting only the most ‘threatening’ IPMN lesion(s). We sought to determine whether the presence of residual IPMN following segmental pancreatectomy for non-invasive IPMN increases the risk for subsequent development of invasive pancreatic cancer and decreases survival.
Methods
Data on patients undergoing segmental resection of non-invasive IPMN during the period 1991–2010 at a high-volume academic institution were prospectively accrued.
Results
Of 243 patients who underwent segmental resection for IPMN, 191 (79%) demonstrated non-invasive pathology. Of these, 153 (80%) showed the absence and 38 (20%) the presence of residual IPMN at the initial operation. Of the 38 patients with residual IPMN, eight had positive IPMN margins, 23 had radiographic evidence of IPMN, and seven had both. During a mean follow-up of 73 months, 31 (20%) of 153 patients without residual IPMN developed a new radiographic lesion consistent with IPMN and, of these, three (10%) were found to represent invasive cancer. One (3%) of 38 patients with residual IPMN developed invasive cancer. In summary, in 191 initially non-invasive cases of IPMN, four invasive cancers (2%) developed during follow-up. The mean progression-free interval in these four patients was 54 months (range: 20–99 months).
Conclusions
Compared with patients undergoing complete operative IPMN clearance, patients with residual IPMN after segmental pancreatectomy do not demonstrate increased risk for the development of invasive disease or reduced survival. In patients without residual IPMN who later develop new IPMN, the risk for invasive IPMN is increased.
Keywords: pancreatic neoplasia, resection < pancreatic neoplasia, radiologic imaging/intervention < pancreatic neoplasia, outcomes < pancreatic neoplasia, cystic tumours < pancreatic neoplasia
Introduction
Intraductal papillary mucinous neoplasm (IPMN) is a mucin-producing lesion of the pancreatic ductal system with varying degrees of malignant potential.1,2 Its malignant potential is increased in patients with symptoms, including radiographic features (e.g. intramural nodules, main pancreatic duct dilation), and/or atypia on cytopathology. Such high-risk occurrences of IPMN should be resected in fit patients. Historically, complete surgical clearance of IPMN was thought to represent optimal therapy. This principle, however, was based on very limited data. The surgical approach to IPMN in patients with high-risk lesions continues to evolve.
Many cases of IPMN demonstrate multi-centric disease involving multiple, often non-contiguous, regions of the pancreatic gland, which presents a dilemma.3 In such patients in whom surgical resection is indicated, the surgeon may be confronted with the need to choose between performing a segmental pancreatectomy, leaving residual IPMN, or a total pancreatectomy with its attendant considerable morbidity.4–6 A similar scenario may be encountered intraoperatively. In performing a segmental pancreatectomy for IPMN, the surgeon may be confronted with a positive margin based on an intraoperative frozen section. The existing literature supports the extension of the resection to a negative margin in the setting of invasive or high-grade dysplastic disease at the margin.7,8 The issue of whether to extend resection for low-grade non-invasive IPMN at the margin is controversial. Reasonable attempts to clear IPMN at the margin with additional segmental resection are acceptable, particularly if the IPMN involves the main pancreatic duct. The efficacy of extended or even total pancreatic resection in an effort to clear a low-grade IPMN at the margin when there is no gross evidence of main duct dilation remains unclear. In light of this, operative strategy in such patients has evolved from the complete surgical clearance of IPMN to the selective removal of the most threatening lesion(s). This practice of resection of selective lesion(s) may leave residual IPMN in the pancreatic remnant and/or at the margin of resection.
If the decision is made to leave lower-grade IPMN at the margin or residual disease in the remnant pancreas, surveillance must ensue. The most appropriate and effective surveillance strategy is unknown.7–11 Unfortunately, the risk for progression to invasive cancer and the timing of this process in patients with residual IPMN following segmental pancreatectomy are unknown. Whether the natural histories of patients with and without IPMN left in the pancreatic remnant following resection differ is unclear. Currently, surveillance strategies of patients with radiologically or pathologically evident residual IPMN rely heavily on clinical history, interval imaging and cytologic sampling. Changes in the size, character or distribution of existing lesions, the development of new or worsening symptoms, and the development of new cysts or positive cytology in the remnant gland are commonly used as indications for re-resection. These recommendations are based largely on published data regarding the initial diagnosis and resection of IPMN.12 Ultimately, the issue of when to perform additional pancreatic resection is unclear.
Despite the complete clearance of IPMN at the time of segmental pancreatectomy, invasive IPMN has been documented to appear within the pancreatic remnant during follow-up. This potential for the development of de novo invasive disease in a patient in whom the removal of non-invasive IPMN by segmental pancreatectomy was considered to be complete commits both the surgeon and patient to longterm surveillance strategies. Further, the risk for and timeframe of the development of invasive disease in a patient with remnant pancreatic tissue are unknown, which leaves the interval and total duration of surveillance arbitrary.
We sought to determine the risk and timeframe for the development of invasive cancer following segmental pancreatectomy for non-invasive IPMN. In particular, we sought to evaluate the effects of residual IPMN (at the margin or radiologically evident) in comparison with outcomes in patients in whom all visible disease is cleared in the setting of segmental pancreatectomy. We hypothesized that non-invasive IPMN at the margin of resection or radiologically evident IPMN in the remnant pancreas following resection increases the risk for subsequent development of invasive cancer and decreases survival compared with that in patients in whom no evidence of IPMN in the pancreatic remnant is seen.
Materials and methods
Patient selection
Data for this study were catalogued prospectively and reviewed retrospectively in compliance with patient confidentiality protocols as determined by the guidelines set forth by Indiana University School of Medicine's Institutional Review Board. All patients were referred to Indiana University Hospital for treatment between September 1991 and January 2010. All patients were diagnosed with IPMN based on surgical pathology and underwent pancreaticoduodenectomy, distal pancreatectomy, central pancreatectomy or excision/enucleation performed by an experienced pancreatic surgeon at Indiana University Hospital.
Analysis of these data revealed that 243 patients with IPMN underwent a segmental pancreatectomy. Of these, 52 patients were found to have invasive disease on initial surgical pathology and were excluded from analysis. Patients who underwent total (or near total) pancreatectomy as initial management surgery were also excluded. Electronic medical records were queried for demographic, clinical, serologic, endoscopic, pathologic and radiologic data. Additionally, patient survival and follow-up were determined using electronic medical records and the social security death index registry.
Because patients with invasive carcinoma at first operation were excluded, the phrase ‘IPMN recurrence’ was not used. Rather, when documenting an invasive IPMN which developed after initial surgery for a non-invasive IPMN, these lesions were referred to as a progression to invasive cancer or de novo invasive IPMN. Patients developing de novo invasive IPMN had documented invasive carcinoma by biopsy or invasive adenocarcinoma as determined by radiographic findings and clinical course (e.g. mass in the pancreas with hepatic metastases).
Pathologic analysis
All pathology was reviewed by staff pathologists at Indiana University Hospital. Surgical pathology was determined as consistent with IPMN when it showed a mucinous lesion within the pancreas that communicated with the pancreatic ducts and lacked the ovarian stroma consistent with a mucinous cystic neoplasm. Staging of the initial non-invasive IPMN defined it as low-grade or high-grade. Low-grade IPMN encompassed either adenoma (dilated pancreatic duct lined by mucinous epithelium, fulfilling one or none of the criteria for low-grade dysplasia [also called ‘duct ectasia’]) or borderline/moderate (fulfilling one or none of the following criteria: epithelial tufting; nuclear pseudostratification; nuclear atypia, and mitotic figures [also called ‘borderline’]) dysplasia categories. High-grade dysplasia was characterized by cribiform or solid growth usually associated with high-grade nuclear atypia (also called ‘non-invasive intraductal carcinoma’ or ‘carcinoma in situ’).9 On cytopathology, criteria for cytologic atypia included at least one of the following: increased nuclear : cytoplasmic ratio; increased nuclear size; nuclear crowding, and hyperchromasia.
Statistical analysis
Data analysis was carried out using GraphPad Prism 4.00 (GraphPad Software, Inc., La Jolla, CA, USA) and Microsoft Excel 2007 (Microsoft, Inc., Redmond, WA, USA). Overall and progression-free survival were evaluated using the Kaplan–Meier test and statistical differences were evaluated using the log-rank test. All means and proportions were compared using either Student's t-test or Fisher's exact test as appropriate. Statistical significance was accepted at P < 0.05.
Results
During the study period, 243 patients underwent segmental pancreatectomy for pathologically confirmed IPMN. Of these, 191 were found to have non-invasive pathology. The mean follow-up for these patients was 66 months. These patients were separated into two groups consisting of those with and those without evidence of residual IPMN following initial resection. These groups are compared according to type of operation performed, pathology and demographics in Table 1. Of the 191 total patients analysed, 153 (80%) had no residual disease in the pancreatic remnant. These patients had a mean follow-up of 73 months. Conversely, 38 (20%) patients were known at the time of initial resection (based on preoperative imaging and/or intraoperative pathology) to have IPMN in the pancreatic remnant (Fig. 1). Specifically, 23 had preoperative radiographic evidence of residual IPMN, eight had positive IPMN at the margin of resection, and seven had both positive margins and radiologic evidence of IPMN in the pancreatic remnant (Table 2). The mean follow-up in this group was 41 months. The overall 5-year progression-free survival of patients known at the time of initial resection to have IPMN in the pancreatic remnant was 88%, compared with 83% in patients with no residual IPMN burden (P > 0.05) (Fig. 2A). Of the 38 patients with known remnant disease, only one (3%) developed invasive carcinoma (Patient D, Table 3). This patient was initially found to have low-grade IPMN at the margin of resection involving the main pancreatic duct during his initial operation and was diagnosed with invasive disease 36 months later. He was found to be unresectable at re-operation as a result of the vascular involvement of invasive cancer, but remains alive at 62 months after his initial operation (26 months from the diagnosis of invasive cancer). Of 30 patients with positive radiographic evidence of disease, none developed invasive carcinoma during follow-up. No decrease in progression-free survival was noted in patients with radiographic evidence of residual disease within the pancreatic remnant compared with patients without evidence of residual disease (Fig. 2B).
Table 1.
Clinicopathologic features of patients based on pancreatic remnant status
| Patients without remnant disease | Patients with remnant disease | All patients | P-value | |
|---|---|---|---|---|
| Patients, n | 153 | 38 | 191 | – |
| Mean follow-up, months | 73 | 41 | 66 | <0.001 |
| Mean age, years | 67 | 71 | 68 | 0.09 |
| Gender, % male | 47 | 54 | 49 | NS |
| ASA grade, mean (range) | 2.92 (2–3) | 2.97 (2–4) | 2.93 | NS |
| Operation, n (%) | ||||
| Pancreaticoduodenectomy | 94 (61) | 23 (61) | 117 (61) | NS |
| Distal pancreatectomy | 45 (29) | 12 (32) | 57 (30) | NS |
| Central pancreatectomy | 5 (3) | 2 (5) | 7 (4) | NS |
| Enucleation | 9 (6) | 1 (3) | 10 (5) | NS |
| IPMN grade, n (%) | NS | |||
| Low grade | 128 (84) | 34 (89) | 162 (85) | NS |
| High grade | 25 (16) | 4 (11) | 29 (15) | NS |
| Mean size of initial lesion, cm | 2.2 | 2.4 | 2.2 | NS |
ASA, American Society of Anesthesiologists; NS, not significant
Figure 1.
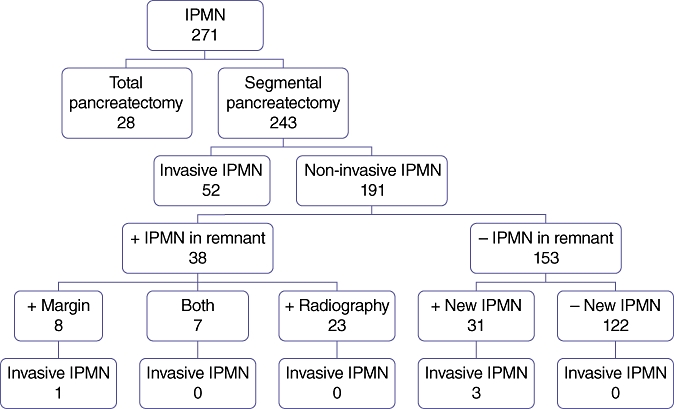
Distribution of all patients included in this study, showing numbers of patients with residual disease and numbers of patients who progressed to invasive cancer. IPMN, intraductal papillary mucinous neoplasm
Table 2.
Characteristics of 38 patients with residual intraductal papillary mucinous neoplasm (IPMN)
| Residual IPMN status | n = 38 | Progression to invasive cancer |
|---|---|---|
| Positive margin onlya, n (%) | 8 (21) | 1b |
| Radiographic lesion only, n (%) | 23 (61) | – |
| Positive margin and radiographic lesiona, n (%) | 7 (18) | – |
All positive margins were low grade
This patient had a low-grade positive margin within the main pancreatic duct
Figure 2.
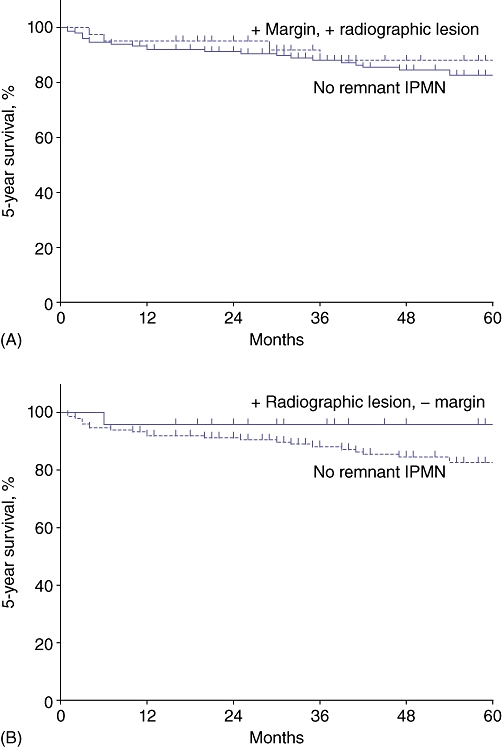
Kaplan–Meier curves demonstrating no significant difference in 5-year progression-free survival in (A) patients with known remnant disease (positive margin and/or radiographically apparent lesion) and patients without evidence of residual disease, and (B) patients with radiographic evidence of residual branch duct disease and patients without evidence of residual disease. IPMN, intraductal papillary mucinous neoplasm
Table 3.
Characteristics of patients who developed invasive cancer during surveillance
| Patient | Pathology at initial operation | Residual disease | Pathology of new lesion from FNA | Progression-free interval, months | Postoperative survival, months | Symptom of invasive disease | Re-resection |
|---|---|---|---|---|---|---|---|
| A | Low grade | None | Non-diagnostic | 61 | 175a | Abdominal pain | R0; curative resection |
| B | High grade (CIS) | None | Adenocab | 20 | 31 | None | Inoperable because of distant metastasis |
| C | High grade (CIS) | None | Benign IPMN | 99 | 132 | Abdominal | Inoperable because of local invasion |
| D | High grade (CIS) | Margin + low grade | Non-diagnostic | 36 | 62a | Recurrent pancreatitis | R1; vascular invasion |
Patient remains alive at current follow-up
FNA was performed in an omental lesion
FNA, fine needle aspiration; CIS, carcinoma in situ; IPMN, intraductal papillary mucinous neoplasm
Of the 153 patients without evidence of disease in the remnant gland, 31 (20%) developed a new lesion in the pancreatic remnant according to surveillance imaging. One of these patients was re-operated twice for two new lesions, resulting in a total of 32 lesions. No difference in 5-year progression-free survival was noted between patients developing a new lesion during surveillance (87%) and those with no evidence of a new lesion (82%) (P > 0.05) (Fig. 3). Three (9%) of these 32 new lesions were eventually found to represent invasive cancer. The characteristics of these lesions are outlined in Table 4. When encountered, invasive disease was diagnosed at a mean of 60 months (range: 20–99 months) after operation compared with 33 months (range: 7–145 months) for new non-invasive lesions. Two patients had unresectable disease and died at 31 months and 132 months, respectively, after initial operation. One patient underwent re-resection (Patient A, Table 3) and was found to have microinvasive disease. This patient developed an additional new lesion 36 months after undergoing a second resection and submitted to a third resection (completion pancreatectomy), which revealed low-grade IPMN; this patient remains alive at 175 months of follow-up. Follow-up was performed elsewhere for 29 of the 153 patients and therefore no comment can be made on any changes in the pancreatic remnant in this subgroup; however, these patients remain alive.
Figure 3.
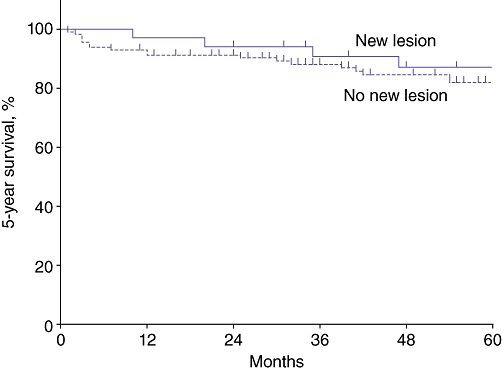
Similar rates of 5-year progression-free survival were observed in patients with no residual disease who developed a new radiographically visible lesion during follow-up and those who remained free of new lesions
Table 4.
Characteristics of patients developing new lesions during surveillance
| Non-invasive(n = 29a) | Invasive (n = 3) | |
|---|---|---|
| Time to new lesion development, months, median (range) | 33 (7–145) | 60 (20–99) |
| FNA/biopsy/cytology, n (%) | 17 (59) | 3 (100) |
| Pathology, n (%) | ||
| Acellular/non-diagnostic | 5 (29) | 1 (33) |
| Cellular/Scant mucin | 5 (29) | 0 |
| Low-grade IPMN/mucin | 6 (35) | 1 (33) |
| High-grade atypia | 1 (6) | 1 (33) |
| Reoperations, n (%) | 8 (28) | 1 (33) |
| No intraductal papillary mucinous neoplasm | 3 | – |
| Low-grade | 3 | – |
| High-grade (CIS) | 2 | – |
| Invasive | – | 1 |
One patient developed an additional new intraductal papillary mucinous neoplasm following re-resection
CIS, carcinoma in situ
A total of 11 patients underwent re-resection. Two of these patients each underwent two pancreatic re-resections, making a total of 13 re-resections. The pathologic results of these re-resections are summarized in Table 5. Five (38%) of these lesions represented low-grade IPMN. Four (31%) of the re-resections demonstrated no IPMN. Two (15%) of the re-resections demonstrated invasive disease, and an additional two (15%) procedures showed high-grade dysplasia. Fine needle aspiration (FNA) was performed in 10 of the 13 cases prior to re-resection. The results are summarized in Table 6. In both patients who were found to have invasive disease, FNA results were false-negative in that they failed to capture high-grade dysplasia or invasive cytology.
Table 5.
Final histologic grade of intraductal papillary mucinous neoplasm (IPMN) in 11 patients undergoing re-resection
| Pathology | Re-resections (n = 13)a | Indications for re-resection | |
|---|---|---|---|
| Symptoms | Radiographic features of concern | ||
| No IPMN, n (%) | 4 (31) | 3 | 1 |
| Low-grade, n (%) | 5 (38) | 3 | 3 |
| High-grade (CIS), n (%) | 2 (15) | 1 | 2 |
| Invasive, n (%) | 2 (15) | 2 | 2 |
Two patients underwent two re-resections each
CIS, carcinoma in situ
Table 6.
A comparison of preoperative cytopathology in patients undergoing attempted re-resection, based on ultimate pathologic findings
| Pathologic diagnosis | Non-invasive (n = 11) | Invasive (n = 2) |
|---|---|---|
| Not performed, n (%) | 3 (27) | – |
| Acellular/non-diagnostic, n (%) | 1 (9) | 2 (100) |
| Cellular/scant mucin, n (%) | 2 (18) | – |
| Low-grade IPMN/mucin, n (%) | 5 (45) | – |
| High-grade dysplasia, n (%) | – | – |
IPMN, intraductal papillary mucinous neoplasm
In summary, four (2%) of all patients subjected to segmental pancreatic resection for non-invasive IPMN progressed to invasive cancer during follow-up. The clinicopathologic characteristics of these patients are outlined briefly in Table 3. The mean interval to the development of the invasive lesions was 54 months (range: 20–99 months). Patient D, who was found to have low-grade dysplasia at the margin, developed recurrent invasive disease at 36 months. This was determined to be unresectable, but the patient remains alive at 62 months after initial surgery. The remaining three patients showed no residual pathologic or radiologic evidence of IPMN in the remnant following the initial pancreatic resection. All four of the patients eventually diagnosed with invasive disease underwent preoperative FNA, but only one (25%) of the FNA results – of an omental lesion – was positive for high-grade dysplasia. It should be noted that three (75%) of these patients were symptomatic, each with significant abdominal pain.
Discussion
The incidence, outcome and natural history of de novo invasive IPMN after segmental pancreatectomy for non-invasive IPMN are not known. Because of the multifocal nature of IPMN, at initial resection pancreatic surgeons are often confronted with low-grade IPMN at the resection margin, or radiographic findings showing a residual IPMN burden in the pancreatic remnant. This may provoke substantial anxiety in the patient and surgeon, and may result in costly and burdensome surveillance strategies. Although the appropriate surveillance protocol in these patients is vigorously debated, typical surveillance involves cross-sectional imaging at least annually.13,14 In addition, patients with lesions that demonstrate changes in radiologic character often undergo repeated invasive cytologic sampling with endoscopic ultrasound (EUS) and FNA. Given the premalignant character of IPMN and its predilection for progression to pancreatic cancer, we hypothesized that residual IPMN following segmental pancreatectomy for non-invasive IPMN would increase the risk for subsequent development of invasive cancer and diminish survival compared with no evidence of IPMN in the remnant gland. Our findings do not support our initial hypothesis. Rather, they suggest that, in the setting of resection for non-invasive IPMN, patients with residual IPMN that is apparent either at the margin or as radiographically identified lesions are not at increased risk for development of de novo invasive adenocarcinoma and have longterm survival similar to that of patients with pathologic and radiographic IPMN clearance at initial resection.
An additional and unexpected result in our study was found in the subgroup of patients without residual disease after their initial pancreatic resection. In this subgroup, 32 new lesions developed, three (9%) of which were eventually pathologically confirmed as representing invasive disease. This figure of 9% represents a large increase in the risk for development of invasive disease in patients who have developed new radiographic lesions suspicious for IPMN. However, 5-year survival from the initial operation in these 32 patients with new lesions was not lower than that in patients without new lesions. This is likely to reflect the small number of deaths caused by de novo invasive lesions (n = 2), the long interval to the development of these invasive lesions and the indolent nature of some subtypes of invasive IPMN.15,16
Currently, the decision to perform re-resection in the setting of IPMN is based primarily on radiologic changes in existing lesions, the development of new lesions, cytologic findings of concern, or symptoms of concern. In this series, 11 patients underwent re-resection (n = 13 re-resections). Of the re-resections performed, four (31%) yielded invasive cancer or high-grade dysplasia and the remaining nine (69%) demonstrated either low-grade IPMN or no IPMN at all. Positive cytology can give excellent confirmatory information; however, negative test results are complicated by the method's inherent low sensitivity.17 In this study, FNA results alone did not represent an effective indicator of which patients harboured invasive pathology. Of the four patients found to have either invasive or high-grade lesions on re-operation, none demonstrated atypia on preoperative FNA of the IPMN. This suggests that current strategies to identify and intervene in the very small proportion of patients who will progress to invasive cancer may be inadequate.
In our study, the results of FNA provided very little insight into the underlying pathology in this setting. Other studies have highlighted limitations in the sensitivity of EUS-FNA in determining malignancy.6 This compounds the problem of determining the efficacy of re-operation in this group of patients. Despite this, EUS remains an important tool in this setting. In addition to its ability to sample IPMN cells/fluid, the real-time imaging afforded by EUS often makes it the optimal method by which to characterize and define any mass or mural nodularity, and differentiate it from dependent cyst mucin and debris. Furthermore, aspiration allows for cyst fluid to be sent for cytopathology, and the analysis of markers including carcinoembryonic antigen (CEA), KRas, mucin and amylase to aid in the diagnosis of recurrent or de novo IPMN, as well as molecular analyses to aid in testing for malignancy. For these reasons, EUS remains an appropriate adjunct in most patients undergoing surveillance, particularly when they develop progressive symptoms and/or a significant change on cross-sectional imaging. Although cytopathology was found to be relatively insensitive in this study, the study sample is small and cytopathology has shown utility in other studies.12,17–19 Although not commonly used in the setting of remnant surveillance, endoscopic retrograde cholangiopancreatography (ERCP), with or without pancreatic ductoscopy, may be employed on occasion to interrogate the main pancreatic duct within the head of the pancreas in the setting of a patient who has previously undergone a distal pancreatectomy. In the setting of pancreaticoduodenectomy, reconstruction of the pancreatic remnant using a pancreaticogastrostomy will theoretically allow for more accessibility via ERCP. This has not been particularly useful in our experience and therefore we employ an EUS rendezvous procedure when ERCP access to the pancreaticojejunostomy is complicated.
Of the nearly 200 patients in this series, roughly one-fifth had known residual disease after pancreatic resection and fewer than half of these had a positive margin. The only patient to develop an invasive lesion in this subgroup was the patient with residual low-grade dysplasia involving the main pancreatic duct at the surgical margin. This provides further support for the conclusion of several other investigators – that a margin positive for low-grade IPMN is not an indication for re-resection – but highlights the exception whereby margin-positive disease that involves the main duct may present additional risk for the future development of cancer.6–8 Our findings confirm the practice of not pursuing significant extensions of pancreatic resection, particularly total pancreatectomy, in order to achieve a completely negative margin when low-grade IPMN is present. Further, our data confirm the practice of extended resection whenever feasible in order to achieve a clear margin if the main pancreatic duct is involved.
Although the role of operative margins in IPMN has been previously examined, very few data exist on the impact of residual radiologic disease in the pancreatic remnant. We noted 30 patients in whom lesions apparent on radiography and suspicious for IPMN remained within the residual pancreas. This group demonstrated 5-year progression-free survival similar to that in patients without residual IPMN and none of them went on to develop invasive cancer. This suggests an extremely indolent clinical course in these patients and would support the practice of not pursuing total pancreatectomy in patients with multi-centric disease that appears to be low risk based on clinical and radiologic features, but, rather, of targeting the highest-risk lesions for segmental resection.
Importantly, the initial IPMN resection pathology in our study may be important in predicting the risk for subsequent de novo invasive cancer development. Three of 29 (10%) patients with high-grade dysplastic IPMNs at initial segmental resection developed invasive cancer on follow-up; thus, high-grade dysplasia at initial resection may confer an increased risk for the subsequent development of de novo invasive IPMN, even in the setting of complete clearance on initial resection.
The overall risk for progression to invasive cancer in this series was very low. Only four patients went on to develop invasive cancer during surveillance of their pancreatic remnant. We demonstrated a 2% overall risk for the development of invasive cancer at 5 years of follow-up. The average interval from initial surgery to the development of new invasive lesions was 54 months (range: 20–99 months). Further, three (75%) of these patients demonstrated significant symptoms consistent with pancreatic disease. Given the low risk for developing de novo invasive IPMN and the substantial disease-free interval until the detection of new invasive lesions, we would cautiously suggest that reliance on clinical follow-up combined with less aggressive radiologic surveillance may be appropriate in selected patients.
Although we feel our data support the conclusions reported here, we must note several important limitations to this analysis. Firstly, this represents a retrospective analysis conducted over a significant period of time. Although the majority of patients were followed using a standardized clinical algorithm, there was some variability in the surveillance approach, as well as in the initial operative approach.
Despite the large number of initial operations for IPMN, relatively few patients underwent re-resection to completion pancreatectomy, which renders statistical analysis in this subgroup challenging as a result of its small size. Mean overall follow-up was 41 months in patients with known residual disease; therefore we did not achieve the 5-year follow-up mark in this subgroup. In addition, because of the indolent nature of IPMN, the surveillance period in this study may render longterm conclusions about the natural history of this disease somewhat limited.
Additionally, the availability and resolution of cross-sectional imaging have improved substantially over time. It is likely that some of the patients whose records date from the earlier period of this study and who were considered to have no evidence of residual disease may have harboured very small cystic lesions which might have been identified with more advanced imaging (e.g. magnetic resonance imaging or magnetic resonance cholangiopancreatography). In other words, lesions described as ‘new’ may actually represent ‘newly discovered’ lesions. These improvements in the quality of imaging will only increase the amount of lesions discovered. This further highlights the importance of determining an efficient surveillance schedule for patients with known residual radiographic disease.
This series provides evidence that complete clearance of IPMN, by removal of all radiographic lesions, although optimal in focal disease, may not be requisite in the setting of low-risk, multi-centric disease. Our study also further confirms the practice of avoiding extended resections in the setting of low-grade IPMN present at the margin that does not involve the main duct. Exceptions to this include multi-centric, high-risk lesions and invasive IPMN.
The oncologic risk to patients with non-invasive IPMN (without a positive high-grade margin) for developing invasive IPMN is < 2% in the first 4–5 years following surgery. This finding holds even in the setting of margins positive for low-grade dysplasia and in patients with radiographic evidence of IPMN within the pancreatic remnant. Furthermore, if imaging detects a new IPMN in the residual pancreas, 5-year progression-free survival is not decreased in these patients compared with those without new lesions.
In conclusion, in the face of non-invasive pathologic or radiographic residual disease following segmental pancreatectomy, the overall risk for progression to invasive cancer is low. Leaving low-grade IPMN at the margins or radiographically low-risk IPMN in the remnant does not substantially increase the risk for the development of invasive cancer compared with complete clearance of pathologically or radiographically detectable disease. In patients with no residual pancreatic remnant disease, the development of new radiographic lesions increases the risk for developing invasive disease. Three of the 31 (9%) patients in this study who developed new radiographic lesions went on to develop new invasive disease within 5 years. Thus, these patients may represent a high-risk subgroup that may need to be followed more closely. In addition, 10% of patients with high-grade dysplastic IPMN at initial segmental resection developed invasive cancer on follow-up; hence high-grade dysplasia at initial resection may confer an increased risk for the subsequent development of de novo invasive IPMN. Although it is important, the accuracy of cytopathology remains low in the setting of residual IPMN in the pancreatic remnant and thus cytopathology alone should not be relied upon to guide the decision of whether or not to operate. This experience highlights the importance of obtaining a thorough clinical history and examination, and suggests that more frequent imaging might be reserved for patients with: (i) newly developed cysts in the pancreatic remnant (particularly if the patient is symptomatic); (ii) high-grade dysplasia on initial resection pathology, or (iii) main duct IPMN-positive margins at initial resection.
Conflicts of interest
None declared.
References
- 1.Sohn TA, Yeo CJ, Cameron JL, Iacobuzio-Donahue CA, Hruban RH, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an increasingly recognized entity. Ann Surg. 2001;234:313–321. doi: 10.1097/00000658-200109000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakashima A, et al. Invasive intraductal papillary mucinous neoplasm of the pancreas: comparison with pancreatic ductal adenocarcinoma. J Surg Oncol. 2009;100:13–18. doi: 10.1002/jso.21290. [DOI] [PubMed] [Google Scholar]
- 3.Crippa S, Partelli S, Massimo F. Extent of surgical resections for intraductal papillary mucinous neoplasms. J Gastrointest Surg. 2010;2:347–351. doi: 10.4240/wjgs.v2.i10.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simons JP, Shah SA, Ng SC, Whalen GF, Tseng JF. National complication rates after pancreatectomy: beyond mere mortality. J Gastrointest Surg. 2009;13:1798–1805. doi: 10.1007/s11605-009-0936-1. [DOI] [PubMed] [Google Scholar]
- 5.Reddy S, Wolfgang CL, Cameron JL, Eckhauser F, Choti MA, Schulick RD, et al. Total pancreatectomy for pancreatic adenocarcinoma. Ann Surg. 2009;250:282–287. doi: 10.1097/SLA.0b013e3181ae9f93. [DOI] [PubMed] [Google Scholar]
- 6.McPhee JT, Hill JS, Whalen GF, Zayaruzny M, Litwin DE, Sullivan ME, et al. Perioperative mortality for pancreatectomy: a national perspective. Ann Surg. 2007;246:246–253. doi: 10.1097/01.sla.0000259993.17350.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raut CP, Cleary KR, Starkel GA, Abbruzzese JL, Wolff RA, Lee JH, et al. Intraductal papillary mucinous neoplasms of the pancreas: effect of invasion and pancreatic margin status on recurrence and survival. Ann Surg Oncol. 2006;13:582–594. doi: 10.1245/ASO.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Fujii T, Kato K, Kodero Y, Kanada M, Nagai S, Yamada S, et al. Prognostic impact of pancreatic margin status in the intraductal papillary mucinous neoplasms of the pancreas. Surgery. 2010;148:285–290. doi: 10.1016/j.surg.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Nara S, Shimada K, Sakamoto Y, Esaki M, Kosuge T, Hiraoka N. Clinical significance of frozen section analysis during resection of intraductal papillary mucinous neoplasm: should a positive pancreatic margin for adenoma or borderline lesion be resected additionally? J Am Coll Surg. 2009;209:614–621. doi: 10.1016/j.jamcollsurg.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 10.White R, D'Angelica M, Katabi N, Tang L, Klimstra D, Fong Y, et al. Fate of the remnant pancreas after resection of non-invasive intraductal papillary mucinous neoplasm. J Am Coll Surg. 2007;204:987–993. doi: 10.1016/j.jamcollsurg.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt CM, Glant J, Winter JM, Kennard J, Dixon J, Zhao Q, et al. Total pancreatectomy (R0 resection) improves survival over subtotal pancreatectomy in isolated neck margin-positive pancreatic adenocarcinoma. Surgery. 2007;142:572–580. doi: 10.1016/j.surg.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt CM, White PB, Waters JA, Yiannoutosos CT, Cummings OW, et al. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg. 2007;246:644–651. doi: 10.1097/SLA.0b013e318155a9e5. [DOI] [PubMed] [Google Scholar]
- 13.Waters JA, Schmidt CM, Pinchot JW, White PB, Cummings OW, Pitt HA, et al. CT vs. MRCP: optimal classification of IPMN type and extent. J Gastrointest Surg. 2008;12:101–109. doi: 10.1007/s11605-007-0367-9. [DOI] [PubMed] [Google Scholar]
- 14.Waters JA, Schmidt CM. Intraductal papillary mucinous neoplasm – when to resect? Adv Surg. 2008;42:87–108. doi: 10.1016/j.yasu.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Yopp AC, Allen PJ. Prognosis of invasive intraductal papillary mucinous neoplasms of the pancreas. J Gastrointest Surg. 2010;2:359–362. doi: 10.4240/wjgs.v2.i10.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chari ST, Yadav D, Smyrk TC, DiMagno EP, Miller LJ, Raimondo M, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500–1507. doi: 10.1053/gast.2002.36552. [DOI] [PubMed] [Google Scholar]
- 17.Wiesenauer CA, Schmidt CM, Cummings OW, Yiannoutsos CT, Howard TJ, Wiebke EA, et al. Preoperative predictors of malignancy in pancreatic intraductal papillary mucinous neoplasms. Arch Surg. 2003;138:610–617. doi: 10.1001/archsurg.138.6.610. [DOI] [PubMed] [Google Scholar]
- 18.Pitman MB, Michaels PJ, Deshpande V, Brugge WR, Bounds BC. Cytological and cyst fluid analysis of small (<or = 3 cm) branch duct intraductal papillary mucinous neoplasms adds value to patient management decisions. Pancreatology. 2008;8:277–284. doi: 10.1159/000134276. [DOI] [PubMed] [Google Scholar]
- 19.Pitman MB, Genevay M, Yaeger K, Chebib I, Turner BG, Mino-Kenudson M, et al. High-grade atypical epithelial cells in pancreatic mucinous cysts are a more accurate predictor of malignancy than ‘positive’ cytology. Cancer Cytopath. 2010;118:434–440. doi: 10.1002/cncy.20118. [DOI] [PubMed] [Google Scholar]


