Abstract
Objective
The optimal strategy for treating hepatocellular carcinoma (HCC), a disease with increasing incidence, in patients with Child–Pugh class A cirrhosis has long been debated. This study evaluated the cost-effectiveness of hepatic resection (HR) or locoregional therapy (LRT) followed by salvage orthotopic liver transplantation (SOLT) vs. that of primary orthotopic liver transplantation (POLT) for HCC within the Milan Criteria.
Methods
A Markov-based decision analytic model simulated outcomes, expressed in costs and quality-adjusted life years (QALYs), for the three treatment strategies. Baseline parameters were determined from a literature review. Sensitivity analyses tested model strength and parameter variability.
Results
Both HR and LRT followed by SOLT were associated with earlier recurrence, decreased survival, increased costs and decreased quality of life (QoL), whereas POLT resulted in decreased recurrence, increased survival, decreased costs and increased QoL. Specifically, HR/SOLT yielded 3.1 QALYs (at US$96 000/QALY) and LRT/SOLT yielded 3.9 QALYs (at US$74 000/QALY), whereas POLT yielded 5.5 QALYs (at US$52 000/QALY). Sensitivity analyses supported these findings at clinically meaningful probabilities.
Conclusions
Under the Model for End-stage Liver Disease (MELD) system, in patients with HCC within the Milan Criteria, POLT increases survival and QoL at decreased costs compared with HR or LRT followed by SOLT. Therefore, POLT is the most cost-effective strategy for the treatment of HCC.
Keywords: hepatocellular carcinoma < liver, outcomes < transplant, resection < liver, radiological intervention – CT/US guided < liver
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer worldwide, with an estimated incidence of 500 000–750 000 cases annually.1 Although HCC is historically a tumour of the Asian and African continents, its incidence has been increasing in the Western world. The annual age-adjusted incidence of HCC in the USA more than tripled between 1975 and 2005 from 1.6 to 4.9 per 100 000 population.2 Survival rates in the USA have also increased over that period, probably as a result of earlier detection and identification of smaller and more treatable tumours. Survival at 1 year in patients treated (via all modalities) for localized HCC may reach 80–90%.2
However, even as survival rates have increased in the USA over the past decade, the best strategy for treating HCC in patients with Child–Pugh class A cirrhosis is still under debate. Despite high recurrence rates, surgical resection has been the standard treatment in cirrhotic patients without extrahepatic disease and with sufficient liver function. Early experiences with liver transplantation (LT) as a curative therapy for HCC resulted in poor results and high recurrence rates; however, tumour characteristics could be used to select the most suitable candidates.3,4 In 1996, Mazzaferro and colleagues described their protocol for identifying which patients with HCC were candidates for transplantation.5 This protocol became what is now commonly known as the Milan Criteria, which indicate that a subject is a transplant candidate if he or she has a single tumour measuring ≤5 cm or three or fewer tumours, each ≤3 cm. Prior to the implementation of the Model for End-stage Liver Disease (MELD) allocation system, wait times were prohibitively long and many patients with HCC died as a result of tumour progression.6 Under the MELD system, candidates with tumours within the Milan Criteria are preferentially transplanted and the number of persons removed from the wait list as a result of tumour progression has declined.7,8 More recently, the use of locoregional therapies (LRTs), particularly radiofrequency ablation (RFA), has increased as both primary therapy for HCC as well as for local tumour treatment in anticipation of transplantation.9–11
Surgeons now have several primary and salvage treatment options available for patients with HCC and compensated cirrhosis. The aim of this study was to evaluate the cost-effectiveness of hepatic resection (HR) or LRT with RFA followed by salvage orthotopic liver transplantation (SOLT) vs. that of primary orthotopic liver transplantation (POLT) for HCC within the Milan Criteria.
Materials and methods
Markov decision model
TreeAge Pro 2009 (TreeAge Software, Inc., Williamstown, MA, USA) was used to construct a Markov-based decision analytic model to simulate outcomes for patients undergoing one of three treatment strategies: (i) HR followed by SOLT; (ii) LRT followed by SOLT, and (iii) POLT. The Markov-based decision analytic technique begins by assigning a group (or groups) of hypothetical patients to one or more health states and simulates their outcomes over prespecified time intervals or cycles. These hypothetical patients can move into and out of various health states after each cycle until all of the patients have reached an ‘absorbing state’ (most commonly death). The absorbing state is, by definition, a state that, once entered, cannot be left. Modelled probabilities of transitioning between health states or staying within the same health state for a given cycle are populated on the basis of a thorough literature review. The accrued time in each health state, and the associated values and costs associated with that health state, are accumulated as patients move through each cycle. A value, most commonly in units of quality-adjusted life years (QALYs), is assigned to the presence of each patient within a health state. These values are accrued over the number of cycles needed for model completion. A cost per QALY for each patient can then be calculated, which allows for direct comparison of the value of each treatment strategy.12,13 This cost-effectiveness analysis was performed and reported according to the guidelines set forth by the Panel on Cost-Effectiveness in Health and Medicine.14,15
Health states
Figure 1 represents the health states considered in the Markov decision model. Patients can undergo one of HR, RFA or POLT. Between interventions, patients enter one of several health states. The selected therapy can be followed by death (the absorbing state), tumour-free survival, survival with recurrence, or need for salvage transplantation (for locoregional or resection choices). The time horizon was 10 years.
Figure 1.
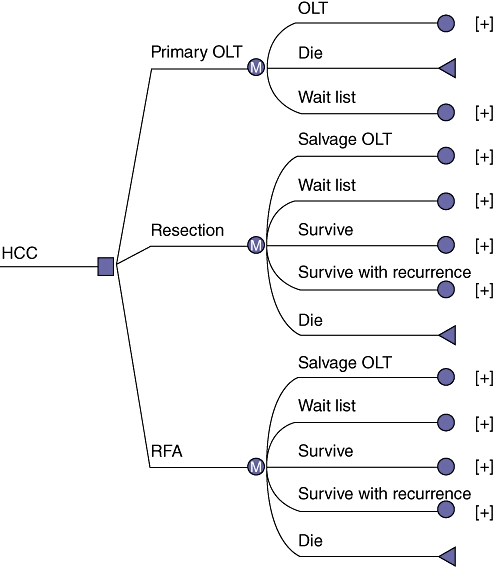
The Markov decision analytic model. Patients with hepatocellular carcinoma (HCC) could undergo one of three therapies including primary orthotopic liver transplantation (OLT), hepatic resection or locoregional therapy with radiofrequency ablation (RFA) followed by salvage OLT. A 10-year time horizon was used in this model with Markov transition states including survival, death, survival with recurrence, and wait list or salvage transplantation (for locoregional and hepatic resection strategies only)
Model assumptions
The model has several assumptions.
Hepatocellular carcinomas considered for these therapies fall within the Milan Criteria, which dictate that a single nodule must measure ≤5 cm or no more than three nodules measuring ≤3 cm must be present. The minimum size is considered to be 2 cm, which allows patients to be eligible for the priority exception points that permit listing as a candidate for LT with the United Network of Organ Sharing (UNOS). Significantly different MELD scores can then result in significantly heterogeneous wait times. To include both of these populations (with tumours measuring ≥2 cm and <2 cm, respectively) in the model would have made it difficult to interpret the results. The model does not consider more detailed tumour features such as vascular invasion, tumour differentiation or anatomic location, which may change the treatment approach. All tumours are assumed to be amenable to all three therapies.
All patients are considered to have Child–Pugh class A cirrhosis.
Locoregional therapy is modeled for RFA only because several randomized controlled trials (RTCs) have demonstrated the superiority of this modality over other locoregional methods.16–18 Additionally, all LRT is assumed to be delivered percutaneously.
All surgical resections of HCC were carried out via a laparotomy.
Tumour recurrences after POLT were considered to occur outside the Milan Criteria or at extrahepatic locations. Therefore, patients who had recurrence after POLT were not reconsidered for salvage transplantation within the model.
Probability data
Table 1 demonstrates the probabilities and rates used in the baseline analysis, as well as the ranges used for all sensitivity analyses. These values are based on a critical review of the available literature on HCC. MEDLINE/PubMed was systematically searched for all articles dating from 1995 to February 2011 that compared HR, LRT and POLT, especially reviews and meta-analyses. Specific search terms included: ‘hepatocellular carcinoma’, ‘hepatic resection’, ‘locoregional therapy’, ‘radiofrequency ablation’ and ‘liver transplantation’. Given the enormity of the literature in these areas, representative studies were selected and data from RCTs and prospective studies were emphasized. Special care was taken to ensure the range of all variables was extracted from the literature. Through this literature survey, a baseline value and range for all variables of interest were obtained.
Table 1.
Transition probability estimates from the literature with ranges used for sensitivity analyses
| Transition probabilities | Baseline value | Range | References |
|---|---|---|---|
| Radiofrequency ablation | |||
| Post-procedure mortality | 1% | 1–3% | |
| Decompensation | 5% | 3–10% | 30,33–41 |
| 5-year survival | 35% | 15–50% | |
| Annual HCC recurrence | 50% | 25–70% | |
| Hepatic resection | |||
| Postoperative mortality | 3% | 1–7% | |
| Decompensation | 5% | 3–10% | 42–49 |
| 5-year survival | 55% | 40–70% | |
| Annual HCC recurrence | 20% | 15–35% | |
| Primary liver transplant | |||
| Waitlist dropout/death | 20% | 10–40% | 24,42–48 |
| Postoperative mortality | 5% | 3–8% | |
| 5-year survival | 70% | 60–80% | |
| Salvage liver transplant | |||
| Waitlist dropout/death | 10% | 10–40% | 24,39,42–48 |
| Postoperative mortality | 8% | 3–10% | |
| 5-year survival | 70% | 60–80% | |
| Cost, US$ | |||
| Compensated cirrhosis/year | 5 000 | 3 000–10 000 | |
| RFA | 5 000 | 2 500–10 000 | |
| Hepatic resection | 35 000 | 25 000–60 000 | 30,35,50,51 |
| Post-RFA/resection care/year | 3 500 | 1 000–5 000 | |
| Liver transplant | 150 000 | 100 000–200 000 | |
| Post-OLT care/year | 12 000 | 10 000–25 000 | |
| Utilities, QALY | |||
| Compensated cirrhosis | 0.7 | 0.5–0.8 | |
| Post-RFA | 0.7 | 0.5–0.8 | |
| Post-RFA recurrence | 0.5 | 0.4–0.8 | 30,40,50–53 |
| Post-resection | 0.7 | 0.5–0.8 | |
| Post-resection recurrence | 0.5 | 0.4–0.8 | |
| Post-liver transplant | 0.8 | 0.6–0.9 | |
HCC, hepatocellular carcinoma; RFA, radiofrequency ablation; OLT, orthotopic liver transplantation; QALY, quality-adjusted life year
Cost data
Table 1 presents all cost estimates and ranges. Cost data were abstracted from published studies of institutional costs and studies in which the Medicare database or similar was used for cost analysis. All costs were approached from a societal basis according to established recommendations.19 This vantage point allows for comparisons with other studies with similar perspectives and the interpretation of results in the public interest (as opposed to that of a particular patient group), and incorporates both positive and negative cost changes to the system as the result of an intervention. To adjust for inflation, all monetary values were adjusted to 2008 US dollars using the Consumer Price Index for medical care (US Bureau of Labor Statistics; http://www.bls.gov). Future costs and health benefits were discounted to account for the cost of spending money now vs. in the future.20 The discount rate was held constant at 3%.
Utilities
The effectiveness of interventions was measured in terms of QALYs. This measure of health value incorporates both quality of life (QoL) and time into a composite statistic that allows for comparison between health interventions. Quality of life is determined by the measurement of health utilities, which usually range from 0 (utility of death) to 1 (utility of perfect health). Utilities represent the actual or tested health preferences of groups of patients, either presently ill or possibly ill in the future21 (Table 1).
Sensitivity analysis
Given the inherent uncertainty of any absolute value within the model, one- and two-way sensitivity analyses were performed to test the model conclusions based on variations in individual model parameters. The ranges utilized for these analyses are described in Table 1.
To integrate additional levels of uncertainty, multi-way probabilistic sensitivity analyses using Monte Carlo methods, which change all probabilities and costs within the model simultaneously,22 provided additional tests of model sensitivity to changes in model parameters.
Results
Base case analysis
Given the relative frequency of hepatitis C virus (HCV) cirrhosis as an indication for LT in patients with HCC, the base case patient in this model was a 56-year-old man with HCV and HCC. The results of the reference case analysis in the Markov model are depicted in Table 2 and Fig. 2. Using a 10-year time horizon, HR with SOLT resulted in costs of US$296 000 to achieve 3.1 QALYs, or approximately US$96 000/QALY. Locoregional therapy with SOLT resulted in costs of US$290 000 to achieve 3.9 QALYs, or approximately US$74 000/QALY. Primary orthotopic LT resulted in costs of US$286 000 to achieve 5.5 QALYs, or approximately US$52 000/QALY. Therefore, the POLT treatment strategy was superior to and dominated both HR followed by SOLT and LRT followed by SOLT.
Table 2.
Results of the base case Markov decision analysis model
| Strategy | Cost, US$ | Incremental cost, US$ | Effectiveness, QALY | Incremental effectiveness, QALY | Cost-effectiveness, US$/QALY |
|---|---|---|---|---|---|
| POLT | 286 000 | – | 5.5 | – | 51.8 |
| RFA | 290 000 | 4 000 | 3.9 | −1.6 | 74.0 |
| Resection | 296 000 | 9 000 | 3.1 | −2.5 | 96.1 |
Both RFA and hepatic resection are dominated by POLT
QALY, quality-adjusted life year; POLT, primary orthotopic liver transplantation; RFA, radiofrequency ablation
Figure 2.
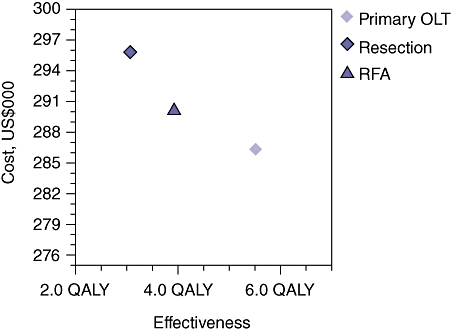
Results of the base case analysis in the Markov model comparing the cost-effectiveness of hepatic resection or locoregional therapy with radiofrequency ablation (RFA) followed by salvage orthotopic liver transplantation (OLT) vs. primary OLT in patients with Child–Pugh class A cirrhosis and hepatocellular carcinoma within the Milan Criteria. Primary OLT is the most cost-effective strategy at US$52 000 per quality-adjusted life year (QALY) (US$286 000 achieves 5.5 QALYs)
Sensitivity analyses
Because the baseline probabilities and costs used in this model vary across centres and regions performing these procedures, one- and two-way sensitivity analyses to test the validity of the conclusions over a range of probabilities and costs were performed. Figure 3 demonstrates the results of a one-way sensitivity analysis in which the probability of transplantation from the POLT wait list is varied. The yearly rate of POLT for HCC on the wait list was varied between 20% and 90%. The threshold value at which POLT is no longer the dominant (superior) strategy is 48%. Therefore, 52% of patients per year on the POLT wait list with HCC would be required to expire or experience tumour progression beyond the Milan Criteria for LRT with SOLT to become the preferable strategy. Figure 4 demonstrates the results of a one-way sensitivity analysis in which annual survival after POLT is varied. The threshold value at which POLT is no longer the dominant strategy is 83%. Therefore, if the mortality rate after POLT exceeds 17% per year, LRT with SOLT becomes the preferable strategy. Figure 5 demonstrates the results of a two-way sensitivity analysis in which the probability of POLT per year on the wait list is simultaneously varied with the probability of tumour-free survival after LRT. Other model parameters remained constant as performed in the base case analysis. Over the range of clinically meaningful values on both the x- and y-axes, only POLT and RFA present cost-effective strategies within this model. As rates of transplantation from the POLT wait list increase, the POLT strategy is clearly the most cost-effective. Only at low probability of transplantation from the wait list for POLT does the RFA strategy become dominant. Primary LT is clearly the dominant strategy at rates within expected values of POLT from the wait list per year (>30%) and rates of tumour-free survival after LRT (<75%). Figure 6 demonstrates the results of a two-way sensitivity analysis in which the yearly probabilities of survival after POLT, and SOLT after HR and LRT, were simultaneously varied. Other model probabilities remained constant as performed in the base case analysis. Again, over the range of clinically meaningful values on both the x- and y-axes, only POLT and RFA present cost-effective strategies within this model. As the annual probability of survival after POLT decreases, RFA becomes the dominant strategy. Put another way, POLT is the dominant strategy unless rates of yearly death after POLT exceed 17%.
Figure 3.
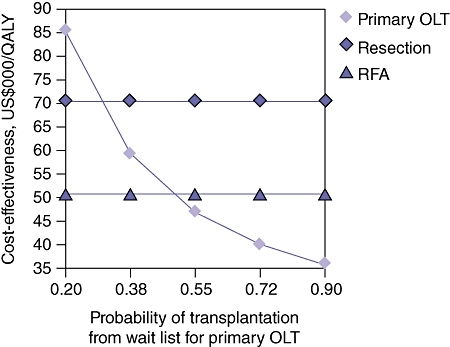
One-way sensitivity analysis varying the annual probability of transplantation from the wait list for primary orthotopic liver transplantation (OLT). The threshold value in which primary OLT is no longer the dominant (most cost-effective) strategy is 48%. At annual probabilities of transplantation from the wait list for primary OLT of <48%, radiofrequency ablation (RFA) becomes the most cost-effective intervention. QALY, quality-adjusted life year
Figure 4.
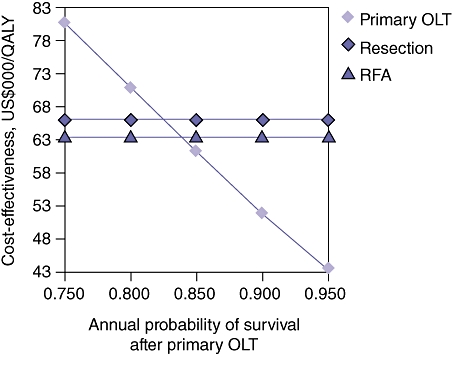
One-way sensitivity analysis varying annual survival after primary orthotopic liver transplantation (OLT). The threshold value at which primary OLT is no longer the dominant (most cost-effective) strategy is 83%. If annual survival after OLT falls to <83%, radiofrequency ablation (RFA) becomes the most cost-effective strategy. QALY, quality-adjusted life year
Figure 5.
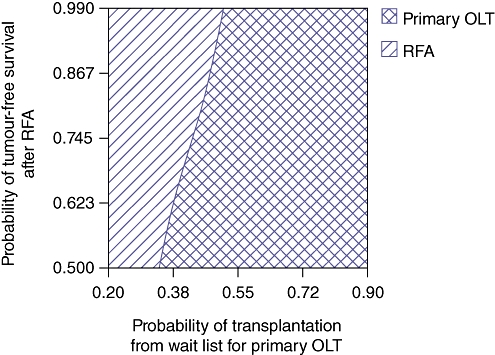
Two-way sensitivity analysis simultaneously varying annual probability of transplantation from the wait list for primary orthotopic liver transplantation (OLT) and probability of tumour-free survival after radiofrequency ablation (RFA). Primary OLT is clearly the dominant strategy at rates of transplantation from the wait list per year of >30% and rates of tumour-free survival after RFA of <75%. Hepatic resection is not cost-effective within these clinically meaningful ranges
Figure 6.
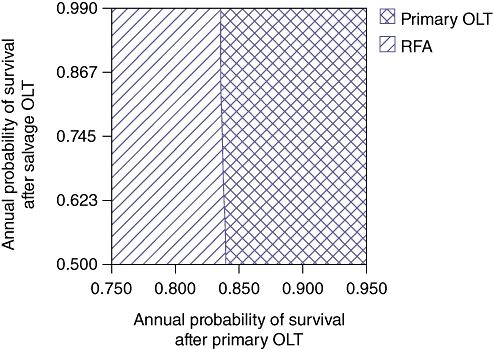
Two-way sensitivity analysis simultaneously varying annual probability of survival after primary orthotopic liver transplantation (OLT) and after salvage OLT. Primary OLT is the dominant treatment strategy at rates of survival >83%. Below this rate, salvage OLT after radiofrequency ablation (RFA) becomes the dominant (most cost-effective) strategy. Hepatic resection is not cost-effective within these clinically meaningful ranges
One-way and two-way sensitivity analyses were performed for a variety of ranges for costs and utilities; POLT emerged as the dominant strategy at all clinically relevant values. The only contexts in which SOLT become the dominant strategy were those in which the cost of POLT exceeded US$350 000 or if the QALYs achieved by POLT dropped to 0.5. Additionally, multi-way probabilistic sensitivity analyses using Monte Carlo methods proved POLT to be the dominant strategy at all clinically relevant values.
Discussion
This study uses a Markov decision analysis model to compare HR or LRT with RFA followed by SOLT with POLT for the treatment of HCC within the Milan Criteria in patients with Child–Pugh class A cirrhosis. Although several studies have compared LT and HR, this is one of the first to simultaneously examine these three treatment strategies in this patient population using a Markov decision analysis model and delineating the effectiveness of each in terms of cost per QALY. In this model, POLT was clearly the most cost-effective strategy, with gains of 5.5 QALYs at a cost of US$52 000/QALY, which is clearly within range of the US$50 000/QALY level that is deemed to represent a cost-effective intervention.23 Additionally, one-, two- and multi-way sensitivity analyses further demonstrated the dominance of this strategy in patients with small HCC and compensated cirrhosis.
Most importantly, the model was robust to a wide range of probabilities in the rate of wait list attrition caused by tumour progression or death. In the USA, the percentage of patients transplanted for HCC ranges from 5% to 20% and the mean wait time for LT for HCC within the Milan Criteria is 90 days.24 One of the primary arguments against POLT for HCC concerns the scarcity of organs and the assumption that offering resection or LRT followed by salvage transplant allows donor organs to be more effectively managed and reduces the likelihood of death on the wait list. In order to account for this assumption, the wait list dropout rate in the POLT strategy was doubled in the model in comparison with that in the SOLT strategy (20% vs. 10%). Despite biasing the model in favour of salvage transplantation in this manner, POLT still provided better overall QoL at reduced costs over the patient's lifetime. Additionally, by increasing the rate of wait list dropout in the POLT strategy, the model was able to account for increases in the percentage of HCC patients represented on the wait list and increases in wait times.
Several studies have examined the impact of LT vs. HR in this patient population using Markov modelling. Sarasin and colleagues used a Markov model to evaluate immediate resection or transplantation in patients with compensated cirrhosis and HCC within the Milan Criteria.25 They demonstrated a survival benefit of 1.0–4.7 years depending on the wait time, with a marginal cost-effectiveness ratio of US$44 454 : 183 840 per additional life year. Locoregional therapies were not considered in their analysis.25 El-Serag and colleagues used a Markov decision analysis model to evaluate 3-year survival in patients with small hepatic nodules (<3 cm) who underwent diagnosis via computed tomography (CT) imaging or image-guided biopsy followed by treatment with LT, HR or trans-hepatic chemoembolization.26 In patients diagnosed with HCC by alpha-feto protein (AFP) elevation and imaging, these authors demonstrated LT to have a 3-year survival benefit over HR. As with previous studies, they noted the shortage of organ supply and wait times as disadvantages to this strategy.26 The present study's results build on these findings26 by including RFA as the LRT and by describing the QALYs for each strategy. By expressing cost as cost per QALY, the cost-effectiveness of these interventions can be utilized by physicians and policymakers to compare the effectiveness of these interventions with that of others reported in the literature.
Although the optimal treatment strategy for HCC in patients with Child–Pugh class A cirrhosis remains under debate, the primary treatment challenge is to diagnose patients before the disease progresses beyond the criteria for surgical or transplantation consideration or to extrahepatic dissemination. Several studies have noted resectability at the time of diagnosis at ranges of 10–20%.9 Sarasin and colleagues found that a targeted biannual surveillance programme in cirrhotic patients with preserved liver function increased life expectancy from 3 months to up to 9 months, depending on the patient's age, the rate of cancer development and the patient's survival rates after surgical intervention.27 They did not recommend screening for all cirrhotic patients, but noted that increases in life expectancy that resulted from the surveillance of patients with compensated cirrhosis were similar to those found in other large-scale cancer screening programmes. In a 2007 analysis, Thompson Coon and colleagues estimated that a biannual surveillance programme using AFP and ultrasound could result in a 10-fold increase in the diagnosis of small (<2 cm) HCC tumours.28 This increased rate of detection would result in HCC tumours being amenable to surgical resection at more than three times the present rate. Unfortunately, surveillance is not uniformly implemented. In a recent analysis conducted by the National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) registry and Medicare, Davila and colleagues noted that surveillance in cirrhotic patients aged >65 years occurs in <20% of patients.29 As optimal treatment strategies are developed, cost-effective and easily applied surveillance methods should be employed to increase the number of patients eligible for curative therapy at the time of diagnosis. Additionally, increased diagnosis of tumours within the Milan Criteria will require additional grafts, probably by increasing rates of both living donation and extended criteria donation.
Within the present model, it is assumed that patients were amenable to all three treatment options at the time of presentation. However, many patients presenting with HCC within the Milan Criteria, in addition to being ineligible for resection, are ineligible for LRT, specifically RFA, because of the anatomic location of the tumour(s). Tumours located high in the dome of the liver, superficially at Glisson's capsule or near to blood vessels or biliary radicals are not amenable to this treatment modality. Laparoscopic, laparoscopic hand-assisted and open RFA may circumvent some of these anatomic considerations, but these techniques were not considered within this model. Increasing the invasiveness of the LRT will not only affect direct and indirect cost estimates attributable to the procedure, but will also impact on patients' perceptions of the procedure, thereby possibly resulting in decreased QoL and an overall increase in cost per QALY.
Bridging strategies with combinations of resection and LRT will need to be tailored. Additionally, the use of chemotherapeutics such as sorafenib (Nexavar®; Bayer HealthCare Pharmaceuticals, Inc., Montville, NJ, USA) may further impact the equation.30 Actual treatment therapy and selection for LT may need to be approached in tiers in which bridging therapy is considered first and in which patients with HCC that is amenable to locoregional or systemic therapies may need to be fully treated with those therapies in order to provide maximal wait time prior to transplantation. Once this therapy is complete or, more notably, if tumours are minimally responsive to bridging therapy, patients would then be considered for surgical intervention. Surgical intervention would potentially include resection, but more ideally transplantation, depending on the underlying liver function, anatomic considerations of the HCC and the availability of donor organs. Increased understanding of tumour biology and gross morphology may indicate those patients who would benefit from one treatment modality over another given their responsiveness to chemotherapy, local treatment therapies and transplantation.31,32
The model demonstrates the dramatic benefit of primary LT for HCC within the Milan Criteria (and >2 cm in size) under the UNOS allocation scheme. When HCC lesions measure <2 cm, other strategies may predominate, given significant differences in MELD scores and therefore wait times for transplantation. Naugler and Sonnenberg recently demonstrated the benefit of immediate LRT (particularly RFA) over monitoring with intent to transplant in the treatment of patients with cirrhosis and HCC lesions of <2 cm.30 They found both a survival benefit and cost savings with immediate treatment with RFA within their model. As surveillance becomes more commonplace, the appropriate strategies for the treatment of very small HCC will be refined.
The necessity of applying assumptions to any decision analytic model creates the possibility that the model does not accurately reflect the nuances and realities of every clinical situation. The model was created on the assumption that each patient would be equally amenable to each of the treatment strategies examined in the model. Clearly, as noted above, not all patients present with surgically curable disease by either partial HR or transplantation. The model assumed that the post-transplantation costs and utilities of patients undergoing transplantation were identical. However, it is clear that complications arising from transplantation may both increase costs and decrease patient-reported QoL values after transplantation, and thus may change model outcomes. Additionally, tumours present in locations in which percutaneous interventions are not appropriate, but in which other mechanisms of applying LRT, by laparoscopic or open techniques, may permit the patient to realize the benefit of oncologic LRT. The model does not account for alternative LRTs utilized by many transplant centres, particularly trans-arterial chemoembolization. Decision analytic models rely heavily on current and historic cost and utility data and are most useful in identifying points of decision in any treatment process. These decisions are associated with discrete costs. The evolution of payment strategies, such as bundled payments, may change the model costs and final model interpretation. Finally, all decision analytic models are only as good as the literature that informs the base case and sensitivity analyses.
Cirrhosis resulting in HCC is a global disease that is becoming more prevalent. Treatment strategies must not only be clinically effective, but must also be cost-effective within the system of medical practice in which cirrhosis and HCC are encountered. The present analysis demonstrates the benefit of LT for HCC patients with Child–Pugh class A cirrhosis within the Milan Criteria. However, as surveillance is applied more consistently, the number of tumours amenable to transplantation will increase, which may further burden the limited supply of deceased donor livers and will certainly require further exploration of extended donation protocols and the expansion of living donation.28 As transplantation continues to emerge as the superior strategy in cost-effectiveness models such as that in the present study, we need to be alert to the possibility of adverse consequences in other transplant candidate populations. This will require ongoing economic and utility analyses to optimize diagnostic and treatment strategies within systems of care with finite health care resources.
Acknowledgments
This study was supported in part by an Institutional National Research Service Award (T32 HS 013833) from the Agency for Healthcare Research and Quality, US Department of Health and Human Services, and by an educational grant from the Novartis Corporation, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Conflicts of interest
None declared.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011 doi: 10.3322/caac.20107. Feb 2011; doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection vs. transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145–151. doi: 10.1097/00000658-199308000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ringe B, Pichlmayr R, Wittekind C, Tusch G. Surgical treatment of hepatocellular carcinoma: experience with liver resection and transplantation in 198 patients. World J Surg. 1991;15:270–285. doi: 10.1007/BF01659064. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 6.Yao FY, Bass NM, Nikolai B, Davern TJ, Kerlan R, Wu V, et al. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transpl. 2002;8:873–883. doi: 10.1053/jlts.2002.34923. [DOI] [PubMed] [Google Scholar]
- 7.Sharma P, Balan V, Hernandez JL, Harper AM, Edwards EB, Rodriguez-Luna H, et al. Liver transplantation for hepatocellular carcinoma: the MELD impact. Liver Transpl. 2004;10:36–41. doi: 10.1002/lt.20012. [DOI] [PubMed] [Google Scholar]
- 8.Ioannou GN, Perkins JD, Carithers RL., Jr Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134:1342–1351. doi: 10.1053/j.gastro.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg. 2009;249:20–25. doi: 10.1097/SLA.0b013e31818eec29. [DOI] [PubMed] [Google Scholar]
- 10.DuBay DA, Sandroussi C, Kachura JR, Ho CS, Beecroft JR, Vollmer CM, et al. Radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. HPB (Oxford) 2011;13:24–32. doi: 10.1111/j.1477-2574.2010.00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarz RE, Smith DD. Trends in local therapy for hepatocellular carcinoma and survival outcomes in the US population. Am J Surg. 2008;195:829–836. doi: 10.1016/j.amjsurg.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Birkmeyer JD, Liu JY. Decision analysis models: opening the black box. Surgery. 2003;133:1–4. doi: 10.1067/msy.2003.21. [DOI] [PubMed] [Google Scholar]
- 13.Inadomi JM. Decision analysis and economic modelling: a primer. Eur J Gastroenterol Hepatol. 2004;16:535–542. doi: 10.1097/00042737-200406000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 15.Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1339–1341. doi: 10.1001/jama.276.16.1339. [DOI] [PubMed] [Google Scholar]
- 16.Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radiofrequency thermal ablation vs. percutaneous ethanol injection. Radiology. 2003;228:235–240. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 17.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomized controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151–1156. doi: 10.1136/gut.2004.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1172–1177. [PubMed] [Google Scholar]
- 20.Weinstein MC, Stason WB. Foundations of cost-effectiveness analysis for health and medical practices. N Engl J Med. 1977;296:716–721. doi: 10.1056/NEJM197703312961304. [DOI] [PubMed] [Google Scholar]
- 21.Torrance GW. Preferences for health outcomes and cost-utility analysis. Am J Manag Care. 1997;3(Suppl.):8–20. [PubMed] [Google Scholar]
- 22.Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making. 1985;5:157–177. doi: 10.1177/0272989X8500500205. [DOI] [PubMed] [Google Scholar]
- 23.Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992;146:473–481. [PMC free article] [PubMed] [Google Scholar]
- 24.Freeman RB, Edwards EB, Harper AM. Waiting list removal rates among patients with chronic and malignant liver diseases. Am J Transplant. 2006;6:1416–1421. doi: 10.1111/j.1600-6143.2006.01321.x. [DOI] [PubMed] [Google Scholar]
- 25.Sarasin FP, Giostra E, Mentha G, Hadengue A. Partial hepatectomy or orthotopic liver transplantation for the treatment of resectable hepatocellular carcinoma? A cost-effectiveness perspective. Hepatology. 1998;28:436–442. doi: 10.1002/hep.510280222. [DOI] [PubMed] [Google Scholar]
- 26.El-Serag HB, Mallat DB, Rabeneck L. Management of the single liver nodule in a cirrhotic patient: a decision analysis model. J Clin Gastroenterol. 2005;39:152–159. [PubMed] [Google Scholar]
- 27.Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in Western patients with Child–Pugh class A cirrhosis. Am J Med. 1996;101:422–434. doi: 10.1016/S0002-9343(96)00197-0. [DOI] [PubMed] [Google Scholar]
- 28.Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, et al. Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis. Health Technol Assess. 2007;11:1–206. doi: 10.3310/hta11340. [DOI] [PubMed] [Google Scholar]
- 29.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52:132–141. doi: 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naugler WE, Sonnenberg A. Survival and cost-effectiveness analysis of competing strategies in the management of small hepatocellular carcinoma. Liver Transpl. 2010;16:1186–1194. doi: 10.1002/lt.22129. [DOI] [PubMed] [Google Scholar]
- 31.Suriawinata A, Thung SN. Molecular signature of early hepatocellular carcinoma. Oncology. 2010;78(Suppl. 1):36–39. doi: 10.1159/000315228. [DOI] [PubMed] [Google Scholar]
- 32.Tsuchiya M, Parker JS, Kono H, Matsuda M, Fujii H, Rusyn I. Gene expression in non-tumoral liver tissue and recurrence-free survival in hepatitis C virus-positive hepatocellular carcinoma. Mol Cancer. 2010;9:74. doi: 10.1186/1476-4598-9-74. doi: 10.1186/1476-4598-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho YK, Kim JK, Kim WT, Chung JW. Hepatic resection vs. radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010;51:1284–1290. doi: 10.1002/hep.23466. [DOI] [PubMed] [Google Scholar]
- 34.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection vs. transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 35.Llovet JM, Mas X, Aponte JJ, Fuster J, Navasa M, Christensen E, et al. Cost-effectiveness of adjuvant therapy for hepatocellular carcinoma during the waiting list for liver transplantation. Gut. 2002;50:123–128. doi: 10.1136/gut.50.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206–1222. doi: 10.1046/j.1365-2168.2002.02168.x. [DOI] [PubMed] [Google Scholar]
- 37.Curley SA, Marra P, Beaty K, Ellis LM, Vauthey JN, Abdalla EK, et al. Early and late complications after radiofrequency ablation of malignant liver tumours in 608 patients. Ann Surg. 2004;239:450–458. doi: 10.1097/01.sla.0000118373.31781.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 39.Bharat A, Brown DB, Crippin JS, Gould JE, Lowell JA, Shenoy S, et al. Pre-liver transplantation locoregional adjuvant therapy for hepatocellular carcinoma as a strategy to improve longterm survival. J Am Coll Surg. 2006;203:411–420. doi: 10.1016/j.jamcollsurg.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Molinari M, Helton S. Hepatic resection vs. radiofrequency ablation for hepatocellular carcinoma in cirrhotic individuals not candidates for liver transplantation: a Markov model decision analysis. Am J Surg. 2009;198:396–406. doi: 10.1016/j.amjsurg.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 41.Porrett PM, Peterman H, Rosen M, Sonnad S, Soulen M, Markmann JF, et al. Lack of benefit of pre-transplant locoregional hepatic therapy for hepatocellular cancer in the current MELD era. Liver Transpl. 2006;12:665–673. doi: 10.1002/lt.20636. [DOI] [PubMed] [Google Scholar]
- 42.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 43.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Longterm survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373–382. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adam R, Azoulay D, Castaing D, Eshkenazy R, Pascal G, Hashizume K, et al. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg. 2003;238:508–518. doi: 10.1097/01.sla.0000090449.87109.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cucchetti A, Vitale A, Gaudio MD, Ravaioli M, Ercolani G, Cescon M, et al. Harm and benefits of primary liver resection and salvage transplantation for hepatocellular carcinoma. Am J Transplant. 2010;10:619–627. doi: 10.1111/j.1600-6143.2009.02984.x. [DOI] [PubMed] [Google Scholar]
- 46.Cheng SJ, Freeman RB, Jr, Wong JB. Predicting the probability of progression-free survival in patients with small hepatocellular carcinoma. Liver Transpl. 2002;8:323–328. doi: 10.1053/jlts.2002.31749. [DOI] [PubMed] [Google Scholar]
- 47.Belghiti J, Carr BI, Greig PD, Lencioni R, Poon RT. Treatment before liver transplantation for HCC. Ann Surg Oncol. 2008;15:993–1000. doi: 10.1245/s10434-007-9787-8. [DOI] [PubMed] [Google Scholar]
- 48.Lee KK, Kim DG, Moon IS, Lee MD, Park JH. Liver transplantation vs. liver resection for the treatment of hepatocellular carcinoma. J Surg Oncol. 2010;101:47–53. doi: 10.1002/jso.21415. [DOI] [PubMed] [Google Scholar]
- 49.Shimozawa N, Hanazaki K. Longterm prognosis after hepatic resection for small hepatocellular carcinoma. J Am Coll Surg. 2004;198:356–365. doi: 10.1016/j.jamcollsurg.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 50.Patel D, Terrault NA, Yao FY, Bass NM, Ladabaum U. Cost-effectiveness of hepatocellular carcinoma surveillance in patients with hepatitis C virus-related cirrhosis. Clin Gastroenterol Hepatol. 2005;3:75–84. doi: 10.1016/s1542-3565(04)00443-4. [DOI] [PubMed] [Google Scholar]
- 51.Andersson KL, Salomon JA, Goldie SJ, Chung RT. Cost-effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2008;6:1418–1424. doi: 10.1016/j.cgh.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stein K, Rosenberg W, Wong J. Cost-effectiveness of combination therapy for hepatitis C: a decision analytic model. Gut. 2002;50:253–258. doi: 10.1136/gut.50.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wright M, Grieve R, Roberts J, Main J, Thomas HC. Health benefits of antiviral therapy for mild chronic hepatitis C: randomized controlled trial and economic evaluation. Health Technol Assess. 2006;10:1–113. doi: 10.3310/hta10210. [DOI] [PubMed] [Google Scholar]


