Abstract
Introduction
Chemotherapy-induced hepatic injuries (CIHI) are an increasing problem facing hepatic surgeons. It may be possible to predict the risk of developing CIHI by analysis of genes involved in the metabolism of chemotherapeutics, previously established as associated with other forms of toxicity.
Methods
Quantitative reverse transcriptase-polymerase chain reaction methodology (q-RT-PCR) was employed to quantify mRNA expression of nucleotide excision repair genes ERCC1 and ERCC2, relevant in the neutralization of damage induced by oxaliplatin, and genes encoding enzymes relevant to 5-flurouracil metabolism, [thymidylate synthase (TS), thymidine phosphorylase (TP) and dihydropyrimidine dehydrogenase (DPD)] in 233 hepatic resection samples. mRNA expression was correlated with a histopathological injury scored via previously validated methods in relation to steatosis, steatohepatitis and sinusoidal obstruction syndrome.
Results
Low-level DPD mRNA expression was associated with steatosis [odds ratio (OR) = 3.95, 95% confidence interval (CI) = 1.53–10.19, P < 0.003], especially when stratified by just those patients exposed to chemotherapy (OR = 4.48, 95% CI = 1.31–15.30 P < 0.02). Low expression of ERCC2 was associated with sinusoidal injury (P < 0.001). There were no further associations between injury patterns and target genes investigated.
Conclusions
Predisposition to the development of CIHI may be predictable based upon individual patient expression of genes encoding enzymes related to the metabolism of chemotherapeutics.
Keywords: colorectal metastases < liver, chemotherapy < liver, basic science < liver
Introduction
Chemotherapy-induced hepatic injuries (CIHI) comprising sinusoidal injuries and chemotherapy associated fatty liver diseases [chemotherapy associated simple steatosis (CASS) and chemotherapy associated steatohepatitis (CASH)] are an increasing problem facing hepatic surgeons operating on patients with colorectal liver metastases (CRLM) who have been subjected to chemotherapy.1
Significance of CIHI
Steatosis
Simple steatosis (fatty degeneration of hepatocytes) increases the overall rate of surgical complications up to twofold after a hepatic resection,2 and is an independent predictor of morbidity.3 Infective complications3–5 and increased blood loss2,6,7 are particular problems after resection of steatotic livers.
Steatohepatitis
The presence of steatohepatitis (fatty degeneration of hepatocytes with inflammatory changes) increases the risk of hepatic failure after a major hepatic resection.8–10 Steatohepatitis is also clearly associated with an increased overall post-operative mortality with an almost 10-fold increased 90-day mortality after hepatic resection in patients with this injury (mortality 14.7% vs. 1.6% in a cohort of 406 patients11).
Sinusoidal injury
Morbidity after a hepatic resection is significantly higher in patients with sinusoidal obstruction syndrome (up to 40% vs. 6.3% in a cohort of 90 patients12) although mortality is unchanged.13 Blood loss appears to be the most important mechanism through which sinusoidal injury increases morbidity. Oxaliplatin is particularly associated with the development of sinusoidal injury14 and is also associated with increased transfusion requirements compared with patients receiving 5-flurouracil (5FU) and leucovorin or no chemotherapy.15
Interaction between genetic parameters and toxicity
The clinical significance of CIHI is only now being realized, in parallel with the greater utility of chemotherapy before a hepatic resection. While much work has already been done to examine the toxic side effects of chemotherapy on other organ systems resulting in, for example, hematological, gastrointestinal16 or neuropathic toxicity,17 considerably less is known about the relationship between chemotherapy and hepatic toxicity.
Toxicity in other organ systems has been associated with various genetic polymorphisms and varying expression of certain key enzymes involved in the metabolism of chemotherapy or the manifestations of drug action. Specifically, the nucleotide excision repair (NER) system is known to be key in regulating the toxicity of platinum-based compounds such as oxaliplatin to tumoural tissue, and both ERCC1 and ERCC2 levels have been shown to be genetic susceptibility factors.18,19 Toxicity to normal host hepatic tissue based on varying expression of these enzymes has not been examined.
With respect to 5FU, varying expression of thymidylate synthase (TS) (inhibition of which forms the key mechanism of action of 5FU) is known to be of prime importance in the tumoral response and host toxicity.16 Dihydropyrimidine dehydrogenase (DPD) is the most important enzyme responsible for the metabolism of 5FU,20,21 and patients with reduced activity of this enzyme are at an increased risk of toxicity from 5FU. Similarly, varying expression of tumoural and host thymidine phosphorylase (TP) [also known as endothelial cell growth factor (ECGF)] has been correlated with response and toxicity, although often with conflicting results.22
The genes described above are prime candidates warranting evaluation to explore hepatic toxicity from 5FU and oxaliplatin containing chemotherapies. We hypothesize that low expression of any of these enzymes may predispose to the development of CIHI. Here, we correlate mRNA expression of these enzymes with the development of significant CIHI (namely, steatosis >33%, presence of steatohepatitis and sinusoidal injury involving >1/3 of the hepatic lobule) as assessed histologically using validated scoring systems23 on retrospective tissue samples.
Methods
Patients
Consecutive patients having undergone hepatic resection for any pathology between September 2001 and November 2009 were retrospectively identified from a prospectively collected tissue bank at Peter MacCallum Cancer Centre. Additional patients who had undergone hepatic resection for CRLM at The Alfred Hospital between May 1995 and September 2009 were identified from a database detailing these patients' clinical and operative outcomes. Chemotherapeutic administration data were collected from previously collated databases, retrieved from review of clinical history or extracted from dispensing databases of the pharmacy departments of the relevant hospitals.
Histological assessment
Haematoxylin and Eosin (H&E) stained slides of non-tumorous liver were available for all patients, and Masson trichrome stained slides were generated from formalin-fixed paraffin-embedded (FFPE) tissue blocks containing non-tumorous liver from either the tissue bank specimens or the pathology archive from Peter MacCallum and The Alfred Hospitals, respectively. Both H&E and Masson's slides were independently and blindly scored for the degree of steatosis, presence or absence of steatohepatitis [as defined by non-alcoholic fatty liver disease (NAFLD) activity score >223 with >5% steatosis], and degree of sinusoidal dilation,24 by two independent pathologists blinded to the patient's history. For the purpose of correlation, significant steatosis was considered >33%, and significant sinusoidal injury was considered grade 2 (centrilobular involvement up to 2/3 of the lobule) or grade 3 (complete lobular involvement).18
mRNA expression
RNA was extracted from either fresh frozen or FFPE tissue as available. Briefly, fresh frozen normal hepatic tissue was lysed in Trizol (Invitrogen, Carlsbad, CA, USA) and RNA extracted using the RNeasy minikit (Qiagen, Hilden, Germany) according to the manufacturer's instructions, or from FFPE blocks using the RNeasy MinElute kit (Qiagen) according to the manufacturer's instructions. Reverse transcription (RT) to cDNA was carried out first by mixing 0.5 µg sample RNA (concentration 100 ng/µl) and 0.5 µg random hexamers (500 µg/ml, Promega, Madison, WI, USA) in a 6-µl reaction then incubating at 70° for 5 min. To this, 0.5 µl (200 U/µl) M-MLV RT (Promega) was added with 20 µl M-MLV buffer (Promega), 5 µl of 10 mM dNTP and diethylpyrocarbonate water (DEPC) to a total of 50 µl, and subsequently incubated at 42° for 2 h.
Expression of each of the target genes was assessed by quantitative real-time RT-polymerase chain reaction (PCR). Target gene primers were designed to cross intron/exon boundaries and obtained from Geneworks (Melbourne, Australia), primer sequences are given in supplementary Table S1. Samples were assessed in duplicate in a Roche Lightcycler 480 real-time quantitative PCR machine and compared with the housekeeping gene GAPDH. Reactions consisted of 5 µl SYBR green reagent (Roche, Mannheim, Germany), 1 µl of 100 µM target primer and 0.5 µl of sample cDNA made up to a total volume 10 µl with milliQ water. A gastric cancer cell line, AGS, was used as a positive control (having previously demonstrated expression of all targets). A no template negative control was utilized with each run. After an initial activating step for 15 min at 95°, 50 cycles of 95° (15 s), 60° (30 s) and 72° (30 s) were carried out followed by a final melt stage at 95°. Single products were confirmed using melt-curve analysis for each sample then a CT value was generated using the second derivative maximum method. Target: reference ratios were then calculated as described elsewhere.25 Ratios were normalized to AGS for each sample.
Statistical analysis
mRNA expression data were discarded for patients in whom the CT value indicated that only primer had been amplified when the sample had been run in duplicate on three separate occasions. Associations between presence or absence of significant injuries and mRNA expression of target genes was tested by Fisher's exact test with expression levels considered as categorical variables, groups formed by dividing samples into halves by mRNA expression ratios (representing high or low expressors). Odds ratios (ORs) [with 95% confidence intervals (CIs)] were generated accordingly. The ratio of target gene to housekeeping gene mRNA expression was also assessed as a continuous variable, with differences detected using the t-test. Analyses were done among all patients and among only those treated with chemotherapy followed by surgery. In both analyses results were considered significant when P < 0.05. Patients who had a repeat hepatectomy at a later date than their primary heptatectomy had tissue included from both operations.
Results
The cohort consisted of 221 patients and 233 samples of non-tumoural hepatic tissue (12 patients provided two samples from two separate hepatectomies), 62.4% of which was FFPE, the remainder being fresh frozen tissue. High-grade steatosis (steatosis >33%) was found in 18.0% of the cohort overall. High-grade sinusoidal dilation (grade 2 or 3, involving >1/3 lobule) was seen in 15.4% and the rate of steatohepatitis was 4.3% (Table 1).
Table 1.
Prevalence of hepatic injury among hepatic samples
| Injury | Definition | Per cent (N samples) |
|---|---|---|
| Steatosis | ||
| Grade 0 | 0% | 24.4% (57) |
| 1 | >0–5% | 30.8% (72) |
| 2 | >5–33% | 26.9% (63) |
| 3 | >33–66% | 12.0% (28) |
| 4 | >66% | 6.0% (14) |
| Sinusoidal injury | ||
| Grade 0 | Nil | 70.5% (165) |
| 1 | up to 1/3 lobular involvement | 14.1% (33) |
| 2 | >1/3–2/3 lobular involvement | 6.8% (16) |
| 3 | >2/3 to complete lobular involvement | 8.6% (20) |
| Steatohepatitis | ||
| Presence | steatosis > 5%, NAFLD activity score* > 2 | 4.3% (10) |
NAFLD activity score = sum of scores for: Mallory bodies + lobular inflammation + hepatocyte ballooning + perisinusoidal fibrosis (each factor scored 0 = absent, 1 = focal involvement of some lobules, 2 = focal involvement of most lobules, 3 = focal involvement of most or all, with diffuse involvement of some or most).23
Sixty per cent of patients (N = 133) had been exposed to chemotherapy. 5FU was the most common agent used, and 1/3 of the cohort was also known to have had oxaliplatin administered (concurrently with 5FU). No patient in this cohort was documented as having been administered irinotecan (Table 2). Chemotherapy was ceased before hepatic resection in all cases by at least 4 weeks.
Table 2.
Agents used when chemotherapy administered
| Agent | Given per cent (N) | Not given per cent (N) | Data missing per cent (N) |
|---|---|---|---|
| Chemotherapy in cohort overall | 60% (133) | 40% (88) | |
| 5-FU | 70% (93) | 0.7% (1) | 29.3% (39) |
| Oxaliplatin + 5FU | 33%* (44) | 34% (45) | 33% (44) |
| Irinotecan | 0% | 77.4% (103) | 22.6% (30) |
All those who had oxaliplatin also had 5-FU.
RNA extracted from FFPE blocks was of inferior quality to that extracted from fresh-frozen tissue, and the raw CT value of each target gene was significantly higher for samples that were extracted from FFPE blocks (data not shown). However, the ratio of target: reference expression was not significantly different when compared between FFPE and fresh-frozen samples (supplementary Table S2). Therefore, the ratio data describing the sample as high or low expressing were combined without distinction.
The rates of injury when comparing high vs. low expression of target genes for all patients demonstrated a statistically significantly higher rate of steatosis in patients with low expression of DPD (OR = 3.95, 95%CI 1.53–10.19, P < 0.003), Fig. 1 and Table 3. This was also true for the subgroup of patients who were administered chemotherapy (OR = 4.48, 95%CI 1.31–15.30, P = 0.02). The proportion of samples that were from FFPE blocks was 57.9% for DPD (not statistically significantly different from cohort overall – where 62.4% of samples were from FFPE tissues).
Figure 1.
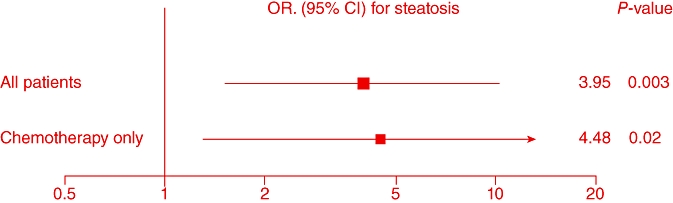
Odds ratios (ORs) of low dihydropyrimidine dehydrogenase (DPD) mRNA expression and risk of high grade steatosis
Table 3.
mRNA expression and odds ratio (OR) for high-grade steatosis [significant associations (P < 0.05) are in bold text]
| N | Steatosis | Expression: | OR (95% CI) | P-value | |||
|---|---|---|---|---|---|---|---|
| Low | High | ||||||
| DPD | All patients | 134 | + | 19 | 7 | 3.95 (1.53–10.19) | 0.003 |
| − | 44 | 64 | |||||
| Chemo only | 75 | + | 14 | 4 | 4.48 (1.31–15.30) | 0.02 | |
| − | 25 | 32 | |||||
| ERCC1 | All patients | 107 | + | 9 | 8 | 1.23 (0.44–3.47) | 0.70 |
| − | 43 | 47 | |||||
| Chemo only | 60 | + | 6 | 5 | 1.47 (0.40–5.48) | 0.56 | |
| − | 22 | 27 | |||||
| ERCC2 | All patients | 60 | + | 7 | 7 | 1.0 (0.33–3.31) | 1.0 |
| − | 23 | 23 | |||||
| Chemo only | 37 | + | 5 | 6 | 0.83 (0.20–3.43) | 0.80 | |
| − | 13 | 13 | |||||
| TS | All patients | 55 | + | 6 | 2 | 4.42 (0.81–24.28) | 0.07 |
| − | 19 | 28 | |||||
| Chemo only | 33 | + | 4 | 1 | 4.0 (0.40–40.43) | 0.21 | |
| − | 14 | 14 | |||||
| TP | All patients | 85 | + | 7 | 8 | 0.93 (0.30–2.83) | 0.89 |
| − | 34 | 36 | |||||
| Chemo only | 53 | + | 4 | 6 | 0.84 (0.21–3.42) | 0.81 | |
| − | 19 | 24 | |||||
When the target: reference ratios were considered as a continuous variable, again lower expression of DPD was associated with steatosis in a statistically significant way, and sinusoidal injury was associated with lower levels of ERCC2, Table 4.
Table 4.
mRNA expression and injury patterns as a continuous variable
| Cohort | Injury | Number of samples | Ratio target: GAPDH (range) | Standard deviation | P-value | |
|---|---|---|---|---|---|---|
| DPD | All samples | Steatosis | 21 | 5.733 (0.124–20.633)* | 6.705 | <0.02 |
| No steatosis | 71 | 2.677 (0.102–21.900) | 4.300 | |||
| Chemo only | Steatosis | 18 | 3.662 (0.124–15.842) | 4.909 | <1.0 | |
| No steatosis | 38 | 3.005 (0.102–21.900) | 4.524 | |||
| ERCC2 | All samples | Sinusoidal injury | 6 | 14.522 (0.178–65.662) | 28.566 | <0.01 |
| No SI | 37 | 4.603 (0.103–67.905) | 11.442 | |||
| Chemo only | Sinusoidal injury | 3 | 34.130 (2.707–65.662) | 44.438 | <0.001 | |
| No SI | 25 | 4.357 (0.103–67.905) | 13.420 | |||
NB: higher ratio target: GAPDH represents lower expression of that target.
There were no further associations between injury patterns, and mRNA expression of ERCC1, ERCC2, DPD or TS and TP.
Discussion
The liver is one of the most metabolically active organs in the human body, with multiple enzyme systems called upon to detoxify, activate, metabolize and/or excrete ingested xenobiotics.24 These enzyme systems are complex, polymorphic, inducible and there is much potential for alternate pathways of metabolism. Some enzyme cascades of relevance in other organs (in which there is more limited ability to detoxify substances using other pathways) may be less relevant in the development of hepatic toxicity. The liver may simply divert the metabolism of any given agent through alternative enzyme systems when the primary system is deficient or absent. Thus, enzymes expected to be of relevance based on correlations from other organ or tissue toxicity may not be found to be important in hepatic toxicity.
Here we have demonstrated a significant correlation between low mRNA expression of DPD and steatosis. This appears to be true for steatosis in general: low expression of DPD is associated with a 3.95-fold increase in the rate of steatosis in our cohort overall (P < 0.003). The effect is more pronounced when analysing just those patients having received chemotherapy, with an OR for development of steatosis of 4.48 (P < 0.02). As far as we know, this is the first time this association has been demonstrated. Low expression of DPD has been correlated previously with other forms of organ toxicity,26,27 and to this list, hepatic toxicity in the form of steatosis may now be added. There was no correlation between DPD expression and sinusoidal injury or steatohepatitis in this cohort. While an association has been shown, causation remains to be proven, as these tissues were collected at the time of hepatic resection after treatment with chemotherapy. Therefore, whether the low expression of DPD predisposes to steatosis, or whether steatosis results in lower expression of DPD, remains unproven.
DPD is the rate-limiting enzyme in the metabolism of 5FU25, and its effect on detoxifying this agent in the liver appears to be important, therefore, in the prevention of liver injury, manifesting as steatosis when this enzyme is only present at low levels. The reasons as to why this injury pattern predominates, and sinusoidal injuries do not appear to be associated remain unclear. Steatosis is a ‘common denominator’ pathological phenotype,28 and is the end result of many injurious agents. Exactly why the liver exhibits a steatotic response when it is unable to detoxify 5FU efficiently is unknown.
In spite of its importance in other organ toxicity, we found no correlation between TS and hepatic toxicity. 5FU is more effective against tumoral tissue and more toxic to normal tissue generally in the presence of low expression of TS29. However, livers examined in this cohort did not exhibit any pathological response when patients with low hepatic expression of TS were exposed to 5FU. This could be because normally, hepatic tissue is a stable tissue type, not actively involved in regeneration and DNA synthesis. Theoretically, 5FU acting through inhibition of TS (thereby inhibiting DNA synthesis) may not affect organs that are not actively synthesizing DNA. Of course, after hepatic resection, the liver does indeed switch on its regenerative capacity. However, in this immediate post-operative setting, patients are not administered 5FU, and any effect from earlier inhibition of DNA synthesis may no longer be in effect.
High-grade sinusoidal injury is associated with low levels of ERCC2 although the evidence for this is weakened in this cohort by small numbers of patients. The association between ERCC2 (key in the repair of damage induced by oxaliplatin), and sinusoidal injury (which is the hallmark injury induced by oxaliplatin) clearly deserves further investigation, but no decisive statements about causality or predisposition can be made at this stage.
The major limitation of this work is its retrospective nature and in particular the fact that tissue samples are collected only after patients have received chemotherapy. This limits our ability to ascribe causation to the findings we have demonstrated, and we are only claiming an association between expression levels and injury patterns as described. A more powerful investigation into the development of CIHI requires the collection of tissue before treatment with chemotherapy, and a comparison with tissue taken after treatment. This work is currently underway in our laboratory.
Another limitation of any study assessing mRNA expression is the potential that the protein level and protein activity and, therefore, effects on the cell may be determined by post-translational events which may be more important than the mRNA expression level. Nonetheless, mRNA expression has been used extensively for these candidate genes. Further work correlating protein level and protein activity with injury development validating the associations we have demonstrated here is warranted.
Conclusion
Low expression of hepatic DPD mRNA is associated with the hepatic steatosis, particularly when associated with the administration of chemotherapy. ERCC2 deserves further investigation as a candidate enzyme associated with sinusoidal injury. It may be possible to predict the development of such injury patterns based on analyses such as these, linking candidate genes with injury phenotypes.
Acknowledgments
Charles Pilgrim receives stipend support from The Royal Australasian College of Surgeons Foundation for Surgery Reg Worcester Research Fellowship and the Surgeon Scientist Scholarship and Melbourne Research Scholarship, University of Melbourne.
Conflicts of interest
None declared.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Table S1 PCR primers.
Table S2 Fresh frozen vs. FFPE target: reference ratio for each target gene.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274–286. doi: 10.1002/bjs.5719. [DOI] [PubMed] [Google Scholar]
- 2.McCormack L, Petrowsky H, Jochum W, Furrer K, Clavien PA. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy: a matched case-control study. Ann Surg. 2007;245:923–930. doi: 10.1097/01.sla.0000251747.80025.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez D, Malik HZ, Bonney GK, Wong V, Toogood GJ, Lodge JP, et al. Steatosis predicts postoperative morbidity following hepatic resection for colorectal metastasis. Br J Surg. 2007;94:1395–1402. doi: 10.1002/bjs.5820. [DOI] [PubMed] [Google Scholar]
- 4.Kooby DA, Fong Y, Suriawinata A, Gonen M, Allen PJ, Klimstra DS, et al. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034–1044. doi: 10.1016/j.gassur.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Garcea G, Maddern GJ. Liver failure after major hepatic resection. J Hepatobiliary Pancreat Surg. 2008 doi: 10.1007/s00534-008-0017-y. Published online only. [DOI] [PubMed] [Google Scholar]
- 6.Behrns KE, Tsiotos GG, DeSouza NF, Krishna MK, Ludwig J, Nagorney DM. Hepatic steatosis as a potential risk factor for major hepatic resection. J Gastrointest Surg. 1998;2:292–298. doi: 10.1016/s1091-255x(98)80025-5. [DOI] [PubMed] [Google Scholar]
- 7.Marcos A, Fisher RA, Ham JM, Shiffman ML, Sanyal AJ, Luketic VA, et al. Liver regeneration and function in donor and recipient after right lobe adult to adult living donor liver transplantation. Transplantation. 2000;69:1375–1379. doi: 10.1097/00007890-200004150-00028. [DOI] [PubMed] [Google Scholar]
- 8.Sahajpal A, Vollmer CM, Jr, Dixon E, Chan EK, Wei A, Cattral MS, et al. Chemotherapy for colorectal cancer prior to liver resection for colorectal cancer hepatic metastases does not adversely affect peri-operative outcomes. J Surg Oncol. 2007;95:22–27. doi: 10.1002/jso.20632. [DOI] [PubMed] [Google Scholar]
- 9.Bilchik AJ, Poston G, Curley SA, Strasberg S, Saltz L, Adam R, et al. Neoadjuvant chemotherapy for metastatic colon cancer: a cautionary note. J Clin Oncol. 2005;23:9073–9078. doi: 10.1200/JCO.2005.03.2334. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez FG, Ritter J, Goodwin JW, Linehan DC, Hawkins WG, Strasberg SM. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200:845–853. doi: 10.1016/j.jamcollsurg.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 12.Nakano H, Oussoultzoglou E, Rosso E, Casnedi S, Chenard-Neu MP, Dufour P, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008;247:118–124. doi: 10.1097/SLA.0b013e31815774de. [DOI] [PubMed] [Google Scholar]
- 13.Karoui M, Penna C, Amin-Hashem M, Mitry E, Benoist S, Franc B, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1–7. doi: 10.1097/01.sla.0000193603.26265.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 15.Aloia T, Sebagh M, Plasse M, Karam V, Levi F, Giacchetti S, et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006;24:4983–4990. doi: 10.1200/JCO.2006.05.8156. [DOI] [PubMed] [Google Scholar]
- 16.Schwab M, Zanger UM, Marx C, Schaeffeler E, Klein K, Dippon J, et al. Role of genetic and nongenetic factors for fluorouracil treatment-related severe toxicity: a prospective clinical trial by the German 5-FU Toxicity Study Group. J Clin Oncol. 2008;26:2131–2138. doi: 10.1200/JCO.2006.10.4182. [DOI] [PubMed] [Google Scholar]
- 17.Lecomte T, Landi B, Beaune P, Laurent-Puig P, Loriot MA. Glutathione S-transferase P1 polymorphism (Ile105Val) predicts cumulative neuropathy in patients receiving oxaliplatin-based chemotherapy. Clin Cancer Res. 2006;12:3050–3056. doi: 10.1158/1078-0432.CCR-05-2076. [DOI] [PubMed] [Google Scholar]
- 18.Shirota Y, Stoehlmacher J, Brabender J, Xiong YP, Uetake H, Danenberg KD, et al. ERCC1 and thymidylate synthase mRNA levels predict survival for colorectal cancer patients receiving combination oxaliplatin and fluorouracil chemotherapy. J Clin Oncol. 2001;19:4298–4304. doi: 10.1200/JCO.2001.19.23.4298. [DOI] [PubMed] [Google Scholar]
- 19.Lunn RM, Helzlsouer KJ, Parshad R, Umbach DM, Harris EL, Sanford KK, et al. XPD polymorphisms: effects on DNA repair proficiency. Carcinogenesis. 2000;21:551–555. doi: 10.1093/carcin/21.4.551. [DOI] [PubMed] [Google Scholar]
- 20.van Kuilenburg AB, Meinsma R, Zonnenberg BA, Zoetekouw L, Baas F, Matsuda K, et al. Dihydropyrimidinase deficiency and severe 5-fluorouracil toxicity. Clin Cancer Res. 2003;9:4363–4367. [PubMed] [Google Scholar]
- 21.van Kuilenburg AB. Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluorouracil. Eur J Cancer. 2004;40:939–950. doi: 10.1016/j.ejca.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Maring JG, Groen HJ, Wachters FM, Uges DR, de Vries EG. Genetic factors influencing pyrimidine-antagonist chemotherapy. Pharmacogenomics J. 2005;5:226–243. doi: 10.1038/sj.tpj.6500320. [DOI] [PubMed] [Google Scholar]
- 23.Mendler MH, Kanel G, Govindarajan S. Proposal for a histological scoring and grading system for non-alcoholic fatty liver disease. Liver Int. 2005;25:294–304. doi: 10.1111/j.1478-3231.2005.01052.x. [DOI] [PubMed] [Google Scholar]
- 24.Hussaini SH, Farrington EA. Idiosyncratic drug-induced liver injury: an overview. Expert Opin Drug Saf. 2007;6:673–684. doi: 10.1517/14740338.6.6.673. [DOI] [PubMed] [Google Scholar]
- 25.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Kuilenburg AB, Haasjes J, Richel DJ, Zoetekouw L, Van Lenthe H, De Abreu RA, et al. Clinical implications of dihydropyrimidine dehydrogenase (DPD) deficiency in patients with severe 5-fluorouracil-associated toxicity: identification of new mutations in the DPD gene. Clin Cancer Res. 2000;6:4705–4712. [PubMed] [Google Scholar]
- 27.Van Kuilenburg AB, Meinsma R, Zoetekouw L, Van Gennip AH. High prevalence of the IVS14 + 1G>A mutation in the dihydropyrimidine dehydrogenase gene of patients with severe 5-fluorouracil-associated toxicity. Pharmacogenetics. 2002;12:555–558. doi: 10.1097/00008571-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Salt WB., 2nd Nonalcoholic fatty liver disease (NAFLD): a comprehensive review. J Insur Med. 2004;36:27–41. [PubMed] [Google Scholar]
- 29.Popat S, Matakidou A, Houlston RS. Thymidylate synthase expression and prognosis in colorectal cancer: a systematic review and meta-analysis. J Clin Oncol. 2004;22:529–536. doi: 10.1200/JCO.2004.05.064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


