Abstract
The middle ear response to otitis media includes transformation and hyperplasia of the mucosal epithelium and subepithelial connective tissue. Significant neovascularization is also noted, which occurs both to support the hypertrophied mucosa and to mediate the increased trafficking of leukocytes. We investigated the role of two known potent angiogenic growth factor families, the fibroblast growth factors (FGFs) and vascular endothelial growth factors (VEGFs), in middle ear mucosal angiogenesis. DNA microarrays were used to evaluate the expression of FGFs and VEGFs, as well as their receptors and unique signaling proteins, in the middle ears of mice undergoing a complete course of acute bacterial otitis media. In addition, a member of each family was introduced to the middle ear submucosal compartment of the normal middle ears of guinea pigs, by a continuous-release osmotic minipump system over 1 week. During the course of bacterial otitis media, a significant regulation of a number of genes important for angiogenesis was identified. Histologic evaluation of middle ear mucosa following micropump infusion of both FGF1 and VEGF-A showed significant angiogenesis at the site of infusion in comparison to control saline infusion. These results support a role for FGFs and VEGFs in the neovascularization of the middle ear mucosa during otitis media, and offer a potential avenue for therapeutic intervention.
Key Words: Angiogenesis, Otitis media, Fibroblast growth factor, Vascular endothelial growth factor
Introduction
Otitis media (OM) is a common cause of hearing loss in the pediatric population [Bluestone and Klein, 1996; Kenna, 1998]. Repeated episodes of OM during childhood may lead to delayed development of critical language and cognitive skills as well as a significant drain on medical resources [Roberts et al., 2004; Shekelle et al., 2002]. A better understanding of the pathophysiology of the middle ear (ME) inflammatory response during OM could lead to therapies for this disease.
The inflammatory response of the ME in OM results in transformation and hyperplasia of the mucosal epithelium and subepithelial connective tissue [Ryan et al., 1986]. In the resting state, the ME mucosa (MEM) consists of a simple squamous epithelium overlying a thin lamina propria, which adheres to the underlying periostium [Junqueira and Carneiro, 2003]. This monolayer of cuboidal, nonsecretory, unciliated epithelial cells normally ranges from 15 to 20 μm in thickness. During acute infection, however, the MEM proliferates into a pseudostratified, ciliated, secretory columnar epithelial complex that can measure more than 1000 μm in thickness [Lim and Birck, 1971]. The MEM also undergoes significant neovascularization, presumably necessary to nourish the expanded mucosal tissue [Lim and Birck, 1971; Ryan and Baird, 1993]. This increase in mucosal vasculature provides a substrate for leukocytic infiltration and may be involved in both tissue edema and the generation of ME effusions [Palacios et al., 2002; Van Blitterswijk et al., 1986]. MEM hyperplasia and ME effusions are associated with many of the negative sequelae of OM.
A variety of biological mechanisms has been shown to regulate ME responses during OM. One class of molecules that are likely to play an important role is growth factors, which have been shown to be critical regulators of cellular differentiation and proliferation in many systems [Sporn and Roberts, 1991]. During the hyperplastic response of the MEM, the ME has been shown to produce a number of different families of peptide growth factors [Palacios et al., 2002]. In this paper, we report upon the role of two such growth factor families in promoting the proliferation of the vasculature that occurs in the subepithelial compartment of the MEM.
Two of the best-studied families of angiogenic growth factors are the fibroblast growth factor (FGF) family and the vascular endothelial growth factor (VEGF) family. By interacting with their cognate receptors, these factors stimulate the proliferation, differentiation and migration of endothelial cells during the growth of new blood vessels. Several factors of the 23-member FGF family have been strongly linked to angiogenesis through a variety of processes from extracellular matrix deposition to the proliferation, differentiation and migration of endothelial cells [Presta et al., 2005], including FGF1, FGF2, FGF4, FGF7, FGF8 and FGF9. However, it should be noted that FGFs can affect many other processes, including, of course, the proliferation of fibroblasts and the growth of stroma. Several additional FGFs have been implicated in angiogenesis, but the evidence is less compelling.
The FGFs bind to and activate the 4 members of the high-affinity FGF receptor (FGFR) family, FGFR1–4, all of which are expressed on vascular progenitor and endothelial cells. This interaction can be facilitated by a cofactor, FGFR binding protein, which has also been associated with angiogenesis. In addition, it has been shown that binding of FGFs to a number of low-affinity receptors can also stimulate angiogenesis. These include the αωβ3 integrin, heparin sulfate proteglycans and gangliosides, although these multifunctional receptors are linked to many functions besides angiogenesis.
The VEGFs are much more exclusively related to the growth of blood and lymphatic vessels. The members of the VEGF family include the many splicoforms of VEGF-A, placental growth factor (PGF), VEGF-B, VEGF-C and VEGF-D. The VEGFs bind to and activate a family of high-affinity VEGF receptors (VEGFR1–3). Two isoforms of PGF, both derived from the PLG gene, can also activate VEGFR1. Activation of VEGFR1 and VEGFR2 is associated with the proliferation, migration and differentiation of endothelial cells. VEGFR3 is expressed mainly in the lymphatic endothelium, and is thus involved primarily in lymphogenesis. The neuropilins NRP1 and NRP2 are coreceptors for VEGFR1 and VEGFR2, which increase the binding of VEGFs to these VEGFRs.
The presence of both FGFs and VEGFs and their receptors during experimental OM has been well documented [Chae et al., 2003; Folkman and Klagsbrun, 1987; Jung et al., 1999; Koutnouyan et al., 1994; Mondain and Ryan, 1995; Ryan and Baird, 1991; Sporn and Roberts, 1991], suggesting that they may be involved in the regulation of angiogenesis. Recombinant VEGF has also been shown to contribute to the development of OM with effusion by increasing vascular permeability of the MEM [Kim et al., 2005]. The purpose of this study was to explore whether the expression of genes encoding FGF or VEGF signaling molecules are differentially regulated during OM. In addition, we determined whether the exogenous application of FGF1, which activates FGFR1–4, or of VEGF-A, which activates VEGFR1 and VGFR2, into the ME would induce vascular proliferation. By identifying the underlying signaling mechanisms responsible for angiogenesis during OM, we may be able to develop improved therapies for this disease process.
Materials and Methods
All experiments were performed according to National Institutes of Health guidelines on the care and use of laboratory animals and were approved by the institutional committee for animal experimentation. All animals were obtained from virus antibody-free colonies.
Expression of selected genes involved in angiogenesis was evaluated in mice by DNA microarray. This method was chosen over qPCR or protein assays since we were able to assess the responses of essentially all mouse genes simultaneously. Age-matched C57Bl/6:CB F1 hybrid mice were purchased from Jackson Laboratories (Bar Harbor, Me., USA). F1 hybrid mice were used to reduce the potential effects of recessive mutations that are common in inbred strains. Twenty mice per time point were inoculated bilaterally with the Haemophilus influenzae strain 3655 (nontypeable, biotype II, originally isolated from an OM patient; NTHi), in 5 ml at a concentration of 105–106/ml as described previously [Ebmeyer et al., 2005; Melhus and Ryan, 2003]. Uninoculated animals (time 0) served as controls. All of the mucosal tissue that could be recovered from the ME was harvested from 20 mice at each of the following intervals: 0 (no treatment), 3 and 6 h, then 1, 2, 3, 5 and 7 days after NTHi inoculation, and pooled. The pooled tissue was homogenized in TRIzol™ (Invitrogen, Carlsbad, Calif., USA) and total RNA was extracted. Total RNA quality was assessed using the RNA 6000 Labchip Kit on the Agilent 2100 Bioanalyzer for the integrity of 18S and 28S ribosomal RNA. The mRNA was reverse transcribed using a T7-oligodT primer, then transcribed in vitro using T7 RNA polymerase to generate biotinylated cRNA probes that were hybridized to 2 Affymetrix MU430 2.0 microarrays. This procedure was duplicated for each time point to obtain a second, independent replication. Thus each postinoculation time point represents 2 separate samples consisting of 20 mice each, and 4 Affymetrix arrays, for a total of 320 mice and 16 arrays in the study. Raw intensity data was median normalized and statistical differences in gene transcript expression levels were evaluated using a variance-modeled posterior inference approach (VAMPIRE) [Hsiao et al., 2005]. Specific genes were assessed at individual time points, after Bonferonni correction for multiple tests, using Genespring GX 7.3 (Agilent Technologies, Santa Clara, Calif., USA).
To evaluate the effects of growth factors on the MEM, guinea pigs were used since the MEs of mice are too small to accommodate an osmotic minipump in the subepithelial compartment. Fifty-six Hartley guinea pigs, weighing 200–350 g at the onset of the study, underwent surgery for placement of an Alzet mini-osmotic pump (Model 2001; ALZA Pharmaceuticals, Palo Alto, Calif., USA). The animals were anesthetized using a cocktail of ketamine and xylazine in a 4:1 proportion. An incision was made behind the animal's left ear, and the pump reservoirs were placed against the animal's back muscles in a posterior subcutaneous pocket. The tip of the pump catheter was inserted under the MEM, through a hole in the bulla. The hole was microsurgically drilled with a diamond burr and care was taken so as not to disturb the mucosal layer. A groove was also cut into the exterior wall of the bulla so that the catheter could enter the ME parallel to the MEM. The mucosa was gently freed from the underlying bone with a blunt probe. The catheter was inserted through the hole in the bulla to a depth of approximately 2 mm to rest in the submucosal space between the bone and the mucosa. After insertion, the catheter was stabilized to the styloid process with cyanoacrylate glue.
The catheters were constructed from a 0.025-inch i.d. silastic tube that terminated in a 0.0055-inch i.d. polyimide insertion catheter (No. 54-II; Microlumen, Tampa, Fla., USA). The pumps had a continuous delivery rate of 1 μl/h for 7 days. Each pump was loaded with either FGF1 in 1 of 3 concentrations: 20, 200 or 1000 ng/ml, or VEGF in 50, 500, or 1000 ng/ml (8 subjects per condition). The growth factors were suspended in a solution of phosphate-buffered saline with 1% bovine serum albumin (PBS/BSA). Eight control animals received pumps loaded with PBS/BSA only.
At the conclusion of the surgery, the wound was closed with staples and the animals were returned to the colony. Daily checks were performed on the animals to make sure that they were recovering well and did not develop any infection at the operative site. The animals were anesthetized and sacrificed at the previously determined study time points.
The left bulla of each animal was harvested, taking care to preserve the tip of the catheter in its original position in the bulla. The bullae were opened on the dorsomedial surface. The bone around the tympanic membrane, along with the membrane itself, was removed so that the entry point of the catheter was clearly visible from the inside of the bulla. If the catheter had perforated the mucosa of the bulla or if there was evidence of ME infection, the bulla was discarded. The bullae were then placed in 4% paraformaldehyde.
After a minimum of 24 h in paraformaldehyde, the bullae were rinsed and reacted with diaminobenzidine, which stained all blood vessels dark. The local effect of the growth factors on vessel proliferation at the mucosal area overlying the tip of the catheter was then measured by standard stereometric analytical methods. Using a measurement grid over one ocular of a Leica microscope, at a power of 3.2, an area of 2.3 × 2.3 mm in size and centered on the catheter end was examined. The number of times within the standard area of mucosa that a vessel crossed an intersection point within the grid was counted. We then analyzed an equivalent area, well behind the entrance point of the catheter (the central area) that was the least likely to be affected by the pump infusate. The 2 areas were compared statistically, using the frequency of a vessel crossing a grid junction as a measure of vessel density within the areas being examined. Data were compared using ANOVA against growth factor and dosage with a p value criterion of <0.05.
Results
Regulation of Angiogenic Factors and Receptors during OM
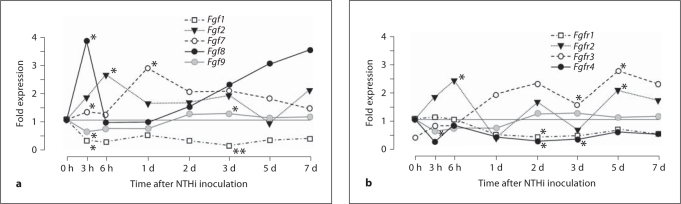
The expression during the course of acute OM, of genes encoding FGFs known to be associated with angiogenesis in other systems, is illustrated in figure 1a and detailed in the online supplementary table (for online suppl. material, see www.karger.com/doi/10.1159/000333805). Fgf1 and Fgf9 were significantly downregulated, while Fgf2, Fgf7 and Fgf8 were significantly upregulated, primarily during the first 24 h after NTHi inoculation. Fgf4 was not differentially regulated, nor was Fgfbp. Of the FGFR genes (fig. 1b, online suppl. table), neither Fgfr1 nor Fgfr2 was consistently regulated, but Fgfr3 was upregulated beginning at 24 h after NTHi inoculation, while Fgfr4 was downregulated during the same period.
Fig. 1.
a Expression of mRNA-encoding members of the FGF family that have been clearly associated with angiogenesis in other systems, assessed by gene array in the mouse ME during NTHi-induced OM. Significant upregulation of FGF mRNAs was observed, especially 3–24 h after ME inoculation. In this and in subsequent figures, of greatest interest are genes that are more strongly regulated (upregulated more than 2-fold, or downregulated to less than 0.5 of preinoculation levels). b Expression of genes encoding FGFRs during OM. Only the Fgfr2 gene was strongly upregulated early in OM. ∗ p < 0.05; ∗∗ p < 0.01. d = Day.
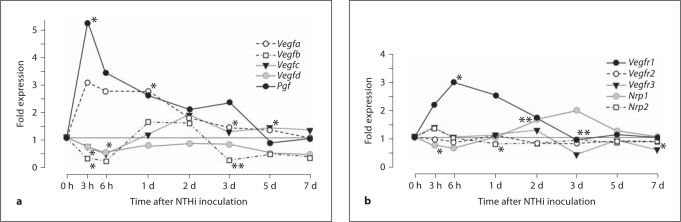
Of the VEGF genes (fig. 2a, online suppl. table), only Vegfa was upregulated on the first day after NTHi inoculation, while Vegfb was downregulated at 3 h, and Vegfd at 6 h. Vegfc was not consistently regulated. The Pgf gene was briskly upregulated 3 h after NTHi inoculation. The VEGFR gene Vegfr1 was upregulated in the first 24 h, while Vegfr2 and Vegfr3 were not regulated (fig. 2b, online suppl. table). Of the two VEGF coreceptors, neuropilins 1 and 2, Nrp1 was downregulated early and upregulated late in OM, while Nrp2 was upregulated early in OM.
Fig. 2.
a ME expression of genes encoding VEGFs during OM. Vegfa and Pgf were significantly upregulated early in OM. b Expression of VEGFr genes. Only Vegfr1 was significantly upregualted early in OM. ∗ p < 0.05; ∗∗ p < 0.01. d = Day.
Influence of Angiogenic Factors on the MEM
The implanted minipumps were well tolerated. Approximately 15% of subjects were lost due to ME infection or to instability of the ME catheter after implantation. Control minipumps delivering 1% BSA in PBS into the subepithelial space of the ME produced no inflammation and minimal stimulation of vessel growth in the mucosa over the catheter tip. Even the area of the opening in the bulla through which the catheter passed showed little evidence of inflammation or neovascularization.
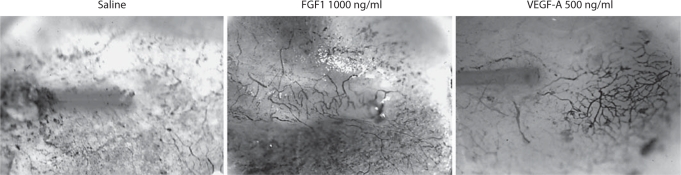
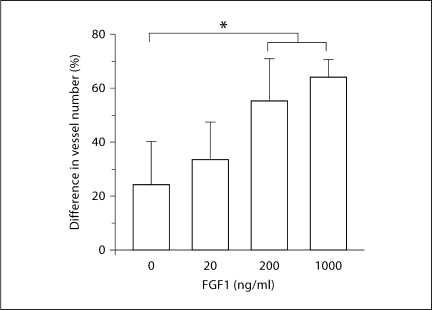
The animals that received FGF1 demonstrated a clear difference in appearance between the higher concentration FGF1 doses and controls. The animals that received FGF1 at concentrations of 200 or 1000 ng/ml exhibited visually obvious, localized proliferation of vessels at the area of the MEM over the tip of the catheter (fig. 3). This visual observation was supported by our stereometric analysis. The difference in vessel density between the area of the MEM over the catheter tip and the control area of the MEM was significantly greater for the FGF1 200 ng/ml (p < 0.05) and 1000 ng/ml (p < 0.05) groups as compared to the saline and 20 ng/ml groups (fig. 4).
Fig. 3.
Representative examples of guinea pig MEs after 1 week of continuous delivery of growth factors to the submucosal compartment of the MEM, via osmotic minipump, at 1 μl/h. The vessels are stained by reaction of erythrocytes with diaminobenzidine. While delivery of saline produced no increase in mucosal vascularization, local angiogenesis is observed after delivery of 1000 ng/ml of FGF1 or 500 ng/ml of VEGF-A. In addition, while the microcatheter is clearly visible underneath the mucosa after saline or VEGF-A delivery, FGF1 induced thickening of the mucosa that obscures the delivery tube.
Fig. 4.
Quantitative evaluation of ME mucosal blood vessels in the region of the microcatheter, after 1 week of continuous FGF1 delivery to the mucosal stroma at varying dosages. At both 200 and 1000 ng/ml, FGF1 significantly increased the number of mucosal blood vessels observed in comparison to saline infusion. ∗ p < 0.05.
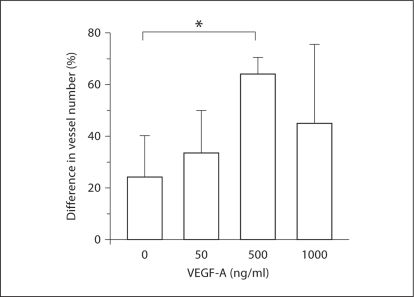
In the VEGF groups, the differences were less clear-cut. There was obvious localized vessel proliferation at the catheter tip in the bullae of the animals that received pumps loaded with VEGF at concentrations of 500 and 1000 ng/ml (fig. 3). The ANOVA showed statistically significant differences in vessel density only between the control condition (BSA) and the 500-ng/ml group (p < 0.05) (fig. 5). The 1000-ng/ml group had less vessel density at the catheter site than the 500-ng/ml group, and although there was a trend toward increased vessel density versus control, this was not statistically significant. We also noticed the presence of small amounts of clear, apparently acellular ME effusion in the MEs that received 1000 ng/ml VEGF into the subepithelial space. This was not observed in any other group.
Fig. 5.
Quantitative evaluation of ME mucosal blood vessels in the region of the microcatheter, after 1 week of continuous VEGF-A delivery to the mucosal stroma at varying dosages. At 500 ng/ml, VEGF-A significantly increased the number of mucosal blood vessels observed in comparison to saline infusion. ∗ p < 0.05.
Discussion
We observed the upregulation of genes encoding members of the FGF and VEGF families and their receptors during OM. In general, this upregulation was observed in the initial hours and days following NTHi inoculation of the ME. This is consistent with the time course of angiogenesis that occurs during experimental acute OM, in which the growth of new vessels occurs primarily during the first few days [e.g. Ryan et al., 1986]. Our gene array data suggest that, of the growth factors examined, FGFs 2, 7 and 8 as well as VEGF-A and PGF are upregulated to a degree most likely to be functionally important (<2-fold) in the ME during OM, and may therefore be the most likely to contribute to angiogenesis. These factors may act through FGFR2, FGFR3 and VEGFR1, which were also upregulated >2-fold during OM.
While we did not further evaluate gene expression, we have previously shown that mRNA and protein for other genes generally follow the pattern of expression observed in the arrays [e.g. Leichtle et al., 2009, 2010].
It should be noted that increased levels of FGF1 and FGF2, as well as their receptors, have previously been demonstrated in experimental acute and chronic OM in the guinea pig [Koutnouyan et al., 1994; Palacios et al., 2002; Ryan and Baird, 1993]. The observation of increased FGF1 expression in these previous studies conflicts with our gene array data from the mouse, which may represent a species difference in the FGF family members that mediate responses in the ME during OM. Since members of the FGF family bind to the same set of receptors, flexibility in function across family members is potentially possible. FGF1 mRNA was observed in cells near blood vessels in the guinea pig MEM. Since the vascular actions of FGF1 are well known, it would be reasonable to speculate that one of its effects in the ME during OM is the enhancement of vascular permeability and angiogenesis.
Although FGFs are known to be strong promoters of angiogenesis, it is reasonable to assume that they are not the only growth factors involved in the vascular aspect of the MEM inflammatory response. FGFs lack a hydrophobic leader signal peptide that is necessary for extracellular transport. Angiogenesis, as Leung et al. [1989] maintain in their paper on VEGF, is best enhanced by the secretion of diffusible factors.
VEGFs are robust promoters of angiogenesis and vascular permeability first isolated from media conditioned by bovine pituitary follicular cells. Consistent with our gene array data, Jung et al. [1999] demonstrated an increased expression of the secretory forms of VEGF-A in the endotoxin-challenged rat MEM. They also showed increased levels of VEGF-A mRNA in the rat MEM as well as in human ME effusion samples taken from patients with chronic OM and OM with effusion. In their rat study, upregulation of VEGF-A mRNA began as quickly as 1 h after endotoxin challenge and peaked within 24 h. From these results it seems that VEGF is critical to the ME inflammatory response. It is mobilized very early, before there are visible signs of inflammation, and it is present as long as the inflammatory reaction persists in chronic OM and OM with effusion.
The demonstration that genes encoding FGFs, VEGFs and their receptors are regulated during OM provides evidence regarding their potential role in OM. However, it does not by itself demonstrate functional involvement. For this reason, we applied these factors directly to the ME. By utilizing the mouse for expression studies, we were able to employ gene arrays that simultaneously measure the regulation of virtually all FGF, VEGF and receptor family members. However, the ME of the mouse is too small to support the delivery of growth factors into the subepithelial compartment of the MEM. While ME luminal delivery would be feasible in this species, our group has found that luminal delivery of FGF2 to the normal ME lumen has little effect upon the mucosa [Ryan et al., 1997], presumably because these factors are unable to access their receptors, which are located in the subepithelial space [Sporn and Roberts, 1991]. Therefore, the larger guinea pig was chosen for subepithelial MEM delivery. This study demonstrates the feasibility of delivering compounds to the subepithelial space of the MEM in a dose-controlled and spatially limited manner using osmotic minipumps. However, it should be noted that species differences, as discussed above and between both animal species and humans, require that our data be interpreted with caution.
We observed that the application of exogenous FGF1 or VEGF-A to the subepithelial compartment of the MEM resulted in the formation of new blood vessels that were spatially related to the site of growth factor application. These results suggest that receptors for VEGF-A and FGF1 are present in the subepithelial layer of the unstimulated MEM. This is consistent with our gene array data, which indicate that transcripts are present in the uninfected ME and that induced changes in expression are rather modest. Our minipump application data indicate that, when complexed with their receptors, both FGFs and VEGFs have the potential to stimulate angiogenesis, creating the neovascular network that nourishes the hypertrophied MEM in the setting of OM, and which forms the basis for the trafficking of leukocytes into and out of the ME.
Interestingly, we also found that the application of VEGF-A to the subepithelial compartment caused a transudation of fluid into the ME lumen. Fluid was not seen in the ME lumen of animals in any other group, indicating that the fluid did not originate from the osmotic minipumps themselves. VEGF-A has been reported to be 50,000 times more potent than histamine at inducing vascular permeability [Connolly et al., 1989]. It thus seems likely that the observed fluid reflects serum transudation from mucosal vessels and movement of serum from the mucosa into the ME lumen. This supports the observation that VEGF plays a role in the generation of ME effusion during OM, as reported by Kim et al. [2005].
Conclusion
The results of this study support a role for FGF1 and VEGF as contributing agents in the neovascularization of the MEM during OM. In vivo, MEM neovascularization is most likely controlled by a complex interaction of a number of different growth factors and their specific receptors. The observed results of this study support this concept and may be pertinent to the treatment of OM. Inhibition of receptors of potential angiogenic growth factors, such as FGFs and VEGFs, may inhibit MEM neovascularization. In the case of VEGFs, inhibitors could also reduce the generation of effusion. This may in turn reduce inflammatory mediators delivered to the ME, ultimately reducing the severity and morbidity of OM.
Supplementary Material
SUPPLEMENTARY TABLE: FGF-VEGF Gene Expression During OM
Acknowledgements
The authors would like to thank Sharon Okamoto (Department of Infectious Diseases, VA Medical Center, La Jolla, Calif., USA) for excellent technical assistance. This work was supported by NIH/NIDCD grants DC000129 and DC000028 (A.F.R.), DC006279 (Stephen I. Wasserman) and the VA Research Service (A.F.R.).
References
- Bluestone CD, Klein JO. Otitis media, atelectasis and Eustachian tube dysfunction. In: Bluestone CD, Stool SE, Kenna MA, editors. Pediatric Otolaryngology. ed 3. Philadelphia: Saunders; 1996. pp. 388–582. [Google Scholar]
- Chae SW, Kim SJ, Kim JL, Jung HH. Expression of vascular endothelial growth factor receptors in experimental otitis media in the rat. Acta Otolaryngol. 2003;123:559–563. doi: 10.1080/00016480310001501. [DOI] [PubMed] [Google Scholar]
- Connolly DT, Heuvelman DM, Nelson R, et al. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebmeyer J, Furukawa M, Pak K, et al. Role of mast cells in otitis media. J Allergy Clin Immunol. 2005;116:1129–1135. doi: 10.1016/j.jaci.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Hsiao A, Ideker T, Olefsky JM, Subramaniam S. VAMPIRE microarray suite: a web-based platform for the interpretation of gene expression data. Nucleic Acids Res. 2005;33:W627–W632. doi: 10.1093/nar/gki443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HH, Kim MW, Lee JH, et al. Expression of vascular endothelial growth factor in otitis media. Acta Otolaryngol. 1999;119:801–808. doi: 10.1080/00016489950180450. [DOI] [PubMed] [Google Scholar]
- Junqueira LC, Carneiro J. Basic Histology. New York: McGraw-Hill; 2003. [Google Scholar]
- Kenna MA. Otitis media with effusion. In: Bailey BJ, Calhoun KH, editors. Head and Neck Surgery – Otolaryngology. ed 2. Philadelphia: Lippincott-Raven; 1998. pp. 1297–1310. [Google Scholar]
- Kim TH, Chae SW, Kim HJ, Jung HH. Effect of recombinant vascular endothelial growth factor on experimental otitis media with effusion. Acta Otolaryngol. 2005;125:256–259. doi: 10.1080/00016480410024677. [DOI] [PubMed] [Google Scholar]
- Koutnouyan HA, Baird A, Ryan AF. Acidic and basic FGF mRNA expression in the middle ear mucosa during experimental acute and chronic otitis media. Laryngoscope. 1994;104:350–358. doi: 10.1288/00005537-199403000-00018. [DOI] [PubMed] [Google Scholar]
- Leichtle A, Hernandez M, Ebmeyer J, Yamasaki K, Lai Y, Radek K, Choung YH, Euteneuer S, Pak K, Gallo R, Wasserman SI, Ryan AF. CC chemokine ligand 3 overcomes the bacteriocidal and phagocytic defect of macrophages and hastens recovery from experimental otitis media in TNF–/– mice. J Immunol. 2010;184:3087–3097. doi: 10.4049/jimmunol.0901167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichtle A, Hernandez M, Pak K, Yamasaki K, Cheng C-F, Webster NJ, Ryan AF, Wasserman SI. TLR4-mediated induction of TLR2 signaling is critical in the pathogenesis and resolution of otitis media. Innate Immun. 2009;14:205–215. doi: 10.1177/1753425909103170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Wun-Jing K, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Lim D, Birck H. Ultrastructural pathology of the middle ear mucosa in serous otitis media. Ann Otol Rhinol Laryngol. 1971;80:838–853. doi: 10.1177/000348947108000611. [DOI] [PubMed] [Google Scholar]
- Melhus A, Ryan AF. A mouse model for acute otitis media. APMIS. 2003;111:989–994. doi: 10.1034/j.1600-0463.2003.1111012.x. [DOI] [PubMed] [Google Scholar]
- Mondain M, Ryan AF. Epidermal growth factor and basic fibroblast growth factor are induced in guinea pig tympanic membrane following traumatic perforation. Acta Otolaryngol. 1995;115:50–54. doi: 10.3109/00016489509133346. [DOI] [PubMed] [Google Scholar]
- Palacios SD, Pak K, Rivkin AZ, Bennett T, Ryan AF. Growth factors and their receptors in the middle ear mucosa during otitis media. Laryngoscope. 2002;112:420–423. doi: 10.1097/00005537-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Presta M, Dell'Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Roberts J, Hunter L, Gravel J, et al. Otitis media, hearing loss, and language learning: controversies and current research. J Dev Behav Pediatr. 2004;25:110–122. doi: 10.1097/00004703-200404000-00007. [DOI] [PubMed] [Google Scholar]
- Ryan AF, Baird A. Growth factors during the proliferation of the middle ear mucosa. Acta Otolaryngol. 1993;113:68–74. doi: 10.3109/00016489309135769. [DOI] [PubMed] [Google Scholar]
- Ryan AF, Luo L, Baird A. Implantation of cells transfected with the FGF-1 gene induces ME mucosal proliferation. In: Lim D, et al., editors. Recent Advances in Otitis Media with Effusion. Amsterdam: Kugler; 1997. pp. 248–250. [Google Scholar]
- Ryan AF, Wasserman SI, Catanzaro A, Harris JP. Secondary immune response in the middle ear: immunological, morphological, and physiological observations. Ann Otol Rhinol Laryngol. 1986;95:242–249. doi: 10.1177/000348948609500307. [DOI] [PubMed] [Google Scholar]
- Shekelle P, Takata G, Chan LS, et al. Diagnosis, natural history, and late effects of otitis media with effusion. Evid Rep Technol Assess. 2002;55:1–5. doi: 10.1037/e439822005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M, Roberts A. Peptide Growth Factors and Their Receptors. New York: Springer; 1991. [Google Scholar]
- Van Blitterswijk C, Ponec M, Van Muijen G, Wijsman M, Koerten H, Grote J. Culture and characterization of rat middle-ear epithelium. Acta Otolaryngol. 1986;101:453–466. doi: 10.3109/00016488609108632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY TABLE: FGF-VEGF Gene Expression During OM