Abstract
Human genetics have revealed that kisspeptin signaling and neurokinin B (NKB) signaling are both required for robust pulsatile gonadotropin-releasing hormone (GnRH) release, and therefore for puberty and maintenance of adult gonadal function. How these two peptides interact to affect GnRH pulse generation remains a mystery. To address the hierarchy of the NKB and kisspeptin signaling pathways that are essential for GnRH release, two experiments were conducted using agonadal, juvenile male monkeys. Pituitary responsiveness to GnRH was first heightened by a pulsatile GnRH infusion to use the in situ pituitary as a bioassay for GnRH release. In the first experiment (n = 3), the kisspeptin receptor (KISS1R) was desensitized by a continuous 99-hour i.v. infusion of kisspeptin-10 (100 μg/h). During the last 4 h of continuous kisspeptin-10 infusion, desensitization of KISS1R was confirmed by failure of an i.v. bolus of kisspeptin-10 to elicit GnRH release. Desensitization of KISS1R was associated with a markedly blunted GnRH response to senktide. The response to senktide was progressively restored during the 72 h following termination of continuous kisspeptin-10. An analogous design was employed in the second experiment (n = 2) to desensitize the NKB receptor (neurokinin 3 receptor, NK3R) by administration of a continuous 48-hour i.v. infusion of senktide (200 μg/h). While a bolus of senktide during the last 3 h of continuous senktide administration failed to elicit GnRH release, thus confirming desensitization of NK3R, the ability of kisspeptin to stimulate GnRH was unimpaired. The foregoing findings support the view that NKB stimulation of GnRH release is upstream from KISS1R.
Key Words: Gonadotropin-releasing hormone, Neurokinin B, Kisspeptin, Monkey, Kisspeptin receptor, KNDy neurons
Introduction
Human genetics have revealed that kisspeptin signaling and neurokinin B (NKB) signaling are both necessary for generating robust pulsatile luteinizing hormone (LH) release and therefore for initiation of puberty and for maintenance of gonadal function in adulthood [1,2,3,4,5]. Interestingly, both neuropeptides are cosynthesized in a population of neurons located in the arcuate (infundibular) nucleus [6,7,8,9,10], and since these cells also express dynorphin [6,8,11,12], they have been termed KNDy neurons [8,13]. Radiofrequency lesions of the arcuate nucleus in the monkey, which would likely destroy the majority of KNDy neurons, abolish gonadotropin secretion [14], while i.v. administration of either kisspeptin or NKB in this species stimulates LH release in a gonadotropin-releasing hormone (GnRH)-dependent manner [9,15,16]. That kisspeptin-induced LH release is mediated via GnRH is further supported by the finding that GnRH release into the median eminence of the monkey is interrupted following local administration of a kisspeptin receptor (KISS1R) antagonist to this region of the hypothalamus [17]. Kisspeptin-induced LH release has also been reported in men and women [18,19].
In studies of non-primate species, a stimulatory action of kisspeptin on LH secretion has also been consistently reported [20,21], and kisspeptin-induced GnRH release into the cerebrospinal fluid has been described in sheep [22]. Moreover, GnRH neurons in rat and mouse express the mRNA encoding KISS1R [22,23,24], and electrophysiological studies of transgenic mice provide compelling evidence that kisspeptin acts to directly excite GnRH perikarya [24,25,26,27] and presumably elicit release of the decapeptide.
Studies of the action of NKB on gonadotropin secretion in non-primate species, on the other hand, are less clear. Genetic disruption of the NKB pathway in mice does not lead to infertility [28], and initial studies of rodents indicated that NKB had an inhibitory or no action on LH release [7,29,30]. However, more recent studies of the rodent [31] and of sheep and goat [12,32] indicate a stimulatory action of this peptide on GnRH/LH release. In addition, an elegant immunohistochemical study has demonstrated the presence of the NKB receptor (neurokinin 3 receptor, NK3R) on GnRH terminals in the median eminence of rat [33,34], although such co-expression has not been observed in sheep hypothalamus [35].
Our current understanding of the neurobiology underlying the interaction of kisspeptin and NKB, and possibly dynorphin [6], to dictate pulsatile GnRH release has been recently reviewed [13,36]. Whether kisspeptin and NKB act independently or hierarchically is unclear, although the latter possibility is supported by findings in rodents and sheep that KNDy neurons express NK3R [7,35,37], and are contacted by axonal boutons immunopositive for NKB [11,37].
In the agonadal juvenile monkey, in which endogenous GnRH release is minimal [38], intermittent i.v. injections of kisspeptin-10 elicit a sustained train of GnRH discharges [16], while similar treatment with the NK3R agonist senktide [39] fails to do so and is associated with a progressive blunting of the GnRH discharges [9]. The apparent desensitization of the NK3R signaling pathway to repetitive senktide stimulation, however, did not compromise KISS1R signaling, as reflected by the robust GnRH discharge observed in response to kisspeptin-10 [9]. Similarly, in the same experimental model, pretreatment with the NK3R antagonist SB222200 [40] abolished senktide-induced GnRH release but did not interfere with the stimulatory action of kisspeptin-10 [9]. These findings are consistent with the view that NK3R signaling in the GnRH releasing pathway is either independent of or upstream from KISS1R.
In the present study, we tested the foregoing possibilities by examining in the male monkey whether GnRH release induced by acute activation of either NK3R or KISS1R signaling was preserved following desensitization of the alternate pathway, achieved with prolonged continuous infusion of a respective agonist. Agonadal juvenile animals were selected because, in the case of the KISS1R pathway, a robust desensitization protocol was already established in our laboratory [41]. In order to use the in situ pituitary of the juvenile monkey as a bioassay for endogenous GnRH release, the sensitivity of the juvenile pituitary was first heightened by intermittent GnRH stimulation as previously described [42,43].
Animals and Methods
Animals
Four juvenile male rhesus monkeys (Macaca mulatta, 16–18 months of age, 2.4–3.1 kg body weight) obtained from the California National Primate Research Center (Davis, Calif., USA) were used. The age of the animals at the end of the study was 24–26 months; the pubertal reactivation of pulsatile GnRH in this primate occurs at around 30–36 months of age [42,44]. Animals were maintained under controlled photoperiod (lights on between 7.00–19.00 h) and at approximately 21°C in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Bilateral castration and implantation of i.v. catheters were performed under sterile conditions with postoperative antibiotic and analgesic therapy exactly as described previously [9,41]. For access to the venous circulation, 2 indwelling i.v. catheters were implanted using an internal jugular and femoral vein, also as previously described [9]. Following catheterization, the monkeys were fitted with a jacket and tether and housed in remote sampling cages to which they had been acclimatized. The routine maintenance of animals housed in these specialized cages, which permit continuous access to the venous circulation with minimal restraint and without sedation or interruption of the light dark cycle, has been described previously [42]. One of the i.v. lines was dedicated to infusion of peptides or vehicle and the other to blood sampling. Catheterization was performed 2 or more weeks after castration and 4–5 weeks before the initiation of continuous infusions. The experimental procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Peptides
Human kisspeptin-10 was synthesized at the Peptide/Protein Core Facility of the Massachusetts General Hospital Endocrine/Reproductive Endocrinology Unit (Boston, Mass., USA). Stock solutions (1 μg/μl) in 5% dimethylsulfoxide (DMSO) in sterile saline were prepared and stored at −20°C. For continuous i.v. administration of kisspeptin-10 (50 μg/ml at 2 ml/h in 0.25% DMSO in sterile saline, i.e. 100 μg or 75 nmol kisspeptin-10/h) or vehicle (0.25% DMSO in sterile saline, 2 ml/h), the respective infusates were prepared and administered exactly as previously described [41]. The dose of kisspeptin-10 used in the present study was previously established to desensitize the KISS1R pathway when administered for 4 days [41].
Senktide, a selective NK3R peptide agonist [39], was also synthesized at the Peptide/Protein Core Facility of the Massachusetts General Hospital Endocrine/Reproductive Endocrinology Unit or obtained from Phoenix Pharmaceuticals, Inc. (Burlingame, Calif., USA). We chose to use this NK3R agonist instead of the native ligand, because concentrated DMSO was required to keep NKB in solution. Stock solutions (1 μg/μl) in sterile saline were prepared and stored at 4°C. For continuous infusion, the stock solution of senktide was diluted to 100 μg/ml with sterile saline the day before the experiment was begun, and administered at a rate of 200 μg/h (237 nmol/h) as described previously for the continuous infusion of kisspeptin-10 [41].
For bolus i.v. administration of kisspeptin-10, a 10 μg dose (10 μg in 1 ml sterile saline; 7.5 nmol) was used as described previously [41]. This dose of kisspeptin is fivefold higher than that which elicits in this experimental model a discharge of LH comparable to that observed spontaneously in adult castrates [16]. A similar strategy was employed to arrive at a bolus i.v. dose of senktide, namely 250 μg (297 nmol), a dose fivefold greater than that which has a similar LH-releasing action approximately equivalent to that of 2 μg kisspeptin-10 [9]. Senktide for bolus i.v. administration was used at a concentration of 250 μg/ml in sterile saline.
GnRH was obtained from Sigma-Aldrich Co. (St. Louis, Mo., USA). A stock solution of this peptide was prepared at 1 mg/ml in sterile saline and stored at −20°C.
Remote i.v. Sampling
Blood samples (1–2 ml) were collected under sterile conditions via the sampling catheter. During periods of sequential sampling, packed blood cells were resuspended with sterile saline and returned to the respective animal. Plasma was stored at −20°C.
In situ GnRH Bioassay
To use pituitary LH secretion as a bioassay for endogenous GnRH release, the responsiveness of the gonadotropes to GnRH stimulation was first enhanced by a pulsatile i.v. infusion of GnRH (0.6 μg over 2 min every hour) as described previously [42,43]. GnRH priming was initiated on the day of catheterization. Robust, adult-like LH response to exogenous GnRH stimulation is usually established by ∼3–4 weeks of pulsatile GnRH treatment [42,43].
LH Assay
Plasma LH levels were measured using a homologous (macaque) RIA as described previously [45]. The sensitivity of the LH assay ranged between 0.08 and 0.32 ng/ml, and the intra- and interassay coefficients of variation for LH at 40% binding were less than 5 and 9%, respectively. LH concentrations below detection were assigned a value equivalent to the sensitivity of the assay.
Experimental Design
The decision to use continuous administration of ligand to desensitize receptor signaling, rather than employing respective receptor antagonists, was based on the ready availability of large quantities of receptor agonists for both KISS1R and NK3R compared with limited availability of potent KISS1R antagonists.
The experimental model employed was essentially identical to that described by us previously [41], and therefore only the main features of experimental design will be described here. Two experiments were performed. They were separated by a period of 5 months because an independent study aimed at establishing the relative potency of two forms of kisspeptin-54 was conducted in the intervening period.
Effect of Continuous Administration of Kisspeptin-10 on NK3R Signaling to Elicit GnRH Release
In this experiment, 2 of the 4 monkeys received the continuous kisspeptin-10 infusion initially followed by continuous vehicle infusion; this sequence was reversed in the remaining 2 animals. Pituitaries were reprimed with pulsatile GnRH treatment for a week between the continuous infusions.
On day 1 of the experiment, GnRH priming was terminated. One hour following the last priming pulse of GnRH, 250 μg senktide was administered as a bolus i.v. injection and, 2 h later, continuous i.v. infusion of kisspeptin-10 (100 μg/h for 99 h) or vehicle (2 ml 0.25% DMSO in saline/h) was initiated. During the last 4 h of the continuous infusions on day 4, the animals received in sequence and at intervals of 1–2 h a bolus i.v. injection of senktide (250 μg), kisspeptin-10 (10 μg) and GnRH (0.6 μg), respectively. After the last of these peptide challenges, the continuous infusion was terminated. One and 3 days later (days 5 and 7), the animals were again challenged with senktide and kisspeptin-10 separated by a 2-hour interval using the same doses administered on day 4 of continuous infusion.
Circulating concentrations of LH were monitored as follows: day 1, samples were collected at frequent intervals to describe the LH response to the last GnRH priming pulse, to the bolus injection of senktide, and to initiation of the continuous kisspeptin-10 (or vehicle) infusion; on days 2, 3 and 4 during continuous infusions, a single sample was collected at approximately 11:00 and 23:00 h; on day 4, samples were collected at frequent intervals to describe the LH response to the bolus injections of senktide, kisspeptin-10 and GnRH during the last 4 h of the continuous infusions. Additional samples were collected to describe the LH response to the senktide and kisspeptin-10 challenges administered 24 and 72 h after termination of the continuous infusions (day 5 and 7, respectively).
Effect of Continuous Administration of Senktide on NK3R and KISS1R Signaling to Elicit GnRH Release
The second experiment (n = 3) was conducted in a manner similar to that described for the first study. One hour following the last GnRH priming pulse, 10 μg kisspeptin-10 was administered as an i.v. injection followed 1 h later by an i.v. injection of 250 μg senktide. One hour later, continuous i.v. infusion of senktide (200 μg/h for 48 h) or vehicle (2 ml sterile saline/h) was initiated. In contrast to the first experiment, where the rate of duration of a kisspeptin infusion required to desensitize KISS1R signaling had been established in an earlier study, analogous parameters for desensitizing N3KR were unknown. Therefore, the dose and duration of senktide administration were intuitively determined by the amount of peptide available to us. During the last 3 h of the continuous infusions, the animals received in sequence and at hourly intervals i.v. injections of 10 μg kisspeptin-10, 250 μg senktide and 0.6 μg GnRH, respectively. After the last of these peptide challenges, the continuous infusions were terminated. One day later (day 3), the animals were again challenged with the bolus injections of kisspeptin-10 followed 1 h later by senktide. Circulating concentrations of LH were monitored in blood samples collected at frequent intervals in essentially the same manner as described for the first experiment.
Statistical Analyses
The significance of differences between mean LH values was determined by multifactor ANOVA with repeated measures followed by Newman-Keuls post hoc test using the GB STAT statistical program (version 6.5.6 Pro; Dynamic Microsystems Inc., Silver Spring, Md., USA). Statistical significance was accepted at p ≤ 0.05. All data are expressed as mean ± SEM.
Results
Effect of Continuous Administration of Kisspeptin-10 on NK3R Signaling to Elicit GnRH Release
Unexpectedly, in 1 of the 4 monkeys, the senktide challenge before initiation of the continuous infusions failed to induce LH release, as did those administered to this animal later in the experiment. Therefore, results from this non-responding monkey were excluded from numerical analyses and graphical presentation.
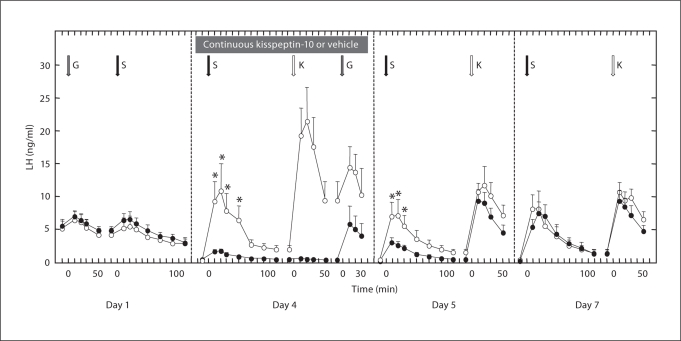
Prior to initiation of either continuous kisspeptin-10 or vehicle infusion to the 3 ‘responders’, senktide elicited an LH discharge similar in magnitude to that induced by GnRH priming (fig. 1). Before initiation of the continuous infusion of kisspeptin-10 on day 1, mean concentrations of the gonadotropin immediately before and 20 min after the senktide bolus were 4.8 ± 0.6 and 6.5 ± 0.9 ng/ml, respectively (fig. 1). Similar profiles of LH release were also noted in response to the senktide bolus prior to initiation of the continuous vehicle infusion and in response to the last priming pulse of GnRH (fig. 1). As previously described [41], initiation of the continuous infusion of kisspeptin-10 (2 h after the senktide challenge) resulted in a robust LH response (data not shown) that was not sustained; by 36 h of continuous kisspeptin-10 exposure, LH concentrations were indistinguishable from those of vehicle. That KISS1R signaling had been desensitized by continuous exposure to kisspeptin-10 was confirmed by the absolute failure of the bolus injection of kisspeptin-10 during the final 4 h of continuous infusion of this peptide to elicit LH release (fig. 1). The LH response to the senktide challenge during the final 4 h of continuous vehicle administration on day 4 was dramatic, with the concentration of this gonadotropin increasing significantly from a basal value of 0.3 ± 0.1 ng/ml to a peak of 9.3 ± 3.0 ng/ml 10 min after the administration of the agonist (fig. 1). In striking contrast, the LH response to senktide during the final hours of continuous exposure to kisspeptin-10 was markedly blunted (fig. 1). The peak LH concentration in response to senktide during continuous infusion of kisspeption-10 (1.8 ± 0.2 ng/ml) or vehicle (9.3 ± 3.0 ng/ml) was significantly different. It is to be noted, that pituitary responsiveness to GnRH was retained during the continuous kisspeptin infusion, as it was during continuous vehicle administration (fig. 1).
Fig. 1.
Left-hand panel shows the LH response (mean plasma concentration ± SEM) to the last i.v. priming pulse of GnRH (G; gray arrow, 0.6 μg) and to a bolus injection of senktide (S; black arrow, 250 μg), administered 1 h later, on day 1 of the first experiment immediately before initiation of the continuous i.v. infusion of either kisspeptin-10 (100 μg/h, black data points) or vehicle (white data points) in agonadal juvenile male rhesus monkeys (n = 3). In the remaining panels, the effect of single sequential bolus injections of senktide, kisspeptin-10 (K; white arrow, 10 μg) or GnRH on LH during the last 4 h (shaded horizontal box) of the 99-hour continuous infusion of kisspeptin-10 or vehicle (day 4) is compared with the LH response to the same bolus injections of senktide and kisspeptin-10 administered 24 h (day 5) and 72 h (day 7) after termination of the respective continuous infusion. Note that the last data point describing the LH response to a given peptide challenge and that for the preinjection value of the subsequent challenge are the same. The greater LH discharge in response to the same senktide challenge on day 4 compared with that on day 1 is most likely due to the continued synthesis of LH after GnRH priming was terminated leading to a larger releasable pool of LH in the pituitary 4 days later. ∗ p ≤ 0.05, mean LH significantly different between continuous kisspeptin and vehicle infusion at time indicated.
The LH response to the senktide challenge had partially recovered (basal and peak values of 0.3 ± 0.05 and 3.1 ± 0.7 ng/ml, respectively) 24 h after terminating the continuous kisspeptin-10 infusion, and was fully restored 3 days later (fig. 1). As previously described for this experimental model [41], full recovery of KISS1R signaling was observed by 24 h of terminating the continuous kisspeptin-10 infusion, as reflected by the similar LH response to bolus injection of kisspeptin-10 at this time in both vehicle- and peptide-treated animals (fig. 1).
Effect of Continuous Administration of Senktide on NK3R and KISS1R Signaling to Elicit GnRH Release
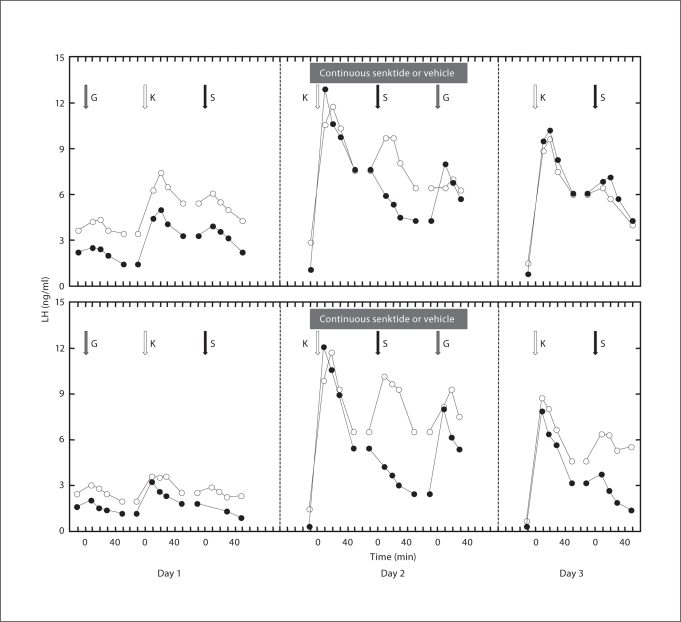
Circulating concentrations of LH during initiation and throughout 48 h, continuous senktide infusion (n = 3) were indistinguishable from those during continuous vehicle treatment. Administration of the kisspeptin challenge during the last 4 h of the continuous senktide infusion elicited a robust LH discharge that was identical to that observed in response to kisspeptin during the continuous administration of vehicle. On the other hand, all 3 monkeys failed to respond to the bolus senktide challenge during the final hours of the continuous senktide infusion. However, desensitization of NK3R was only established in 2 of these animals because in 1 monkey a response to the bolus injection of senktide before initiation and during the continuous infusion of either vehicle or the NK3R agonist was not observed. The individual time courses of LH secretion in response to the last priming infusion of GnRH and to the kisspeptin and senktide challenges before, during the last 4 h, and after termination of the continuous infusion of senktide or vehicle are shown separately for these 2 animals in figure 2. It may also be seen from this figure that an LH response to the senktide challenge had reemerged within 24 h of terminating the continuous infusion of the NK3R agonist.
Fig. 2.
Effect of single bolus injections of GnRH (G; gray arrow, 0.6 μg), kisspeptin-10 (K; white arrow, 10 μg) or senktide (S; black arrow, 250 μg) on plasma LH concentrations prior to initiation (left-hand panels, day 1), during the last 4 h (shaded horizontal box; center panels, day 2) and after termination (right-hand panels, day 3) of the 48-hour continuous i.v. infusion of 200 μg/h of senktide (black data points) or vehicle (white data points) in 2 individual monkeys. Missing data points in bottom left-hand panel during the senktide challenge prior to initiation of the continuous infusion with this agonist are the result of sample loss during processing for storage. The senktide infusions were completed 5 days before initiation of the vehicle infusions. Note that the last data point describing the LH response to a given peptide challenge and that for the preinjection value of the subsequent challenge are the same. The greater LH discharge in response to the same kisspeptin challenge on day 4 compared with that on day 1 is most likely due to the continued synthesis of LH after GnRH priming was terminated leading to a larger releasable pool of LH in the pituitary 4 days later.
Discussion
The finding that, in agonadal juvenile male monkeys, the LH response to bolus senktide administration was markedly blunted when KISS1R signaling was desensitized indicates that a major site of action of NK3R to elicit GnRH release must lie upstream to that of KISS1R. It should be recognized, however, that the LH response to administration of NK3R agonists during other states of primate development or in the presence of gonadal steroid may differ from that observed in the present study. Similarly, only the i.v. route was employed here, and therefore KISS1R-independent actions of NKB agonists at sites within the brain that are not protected by the blood brain barrier cannot be excluded. Nevertheless, the observation that senktide-induced LH release observed in WT female mice in diestrus was not recapitulated in Kiss1–/–, and Kiss1r–/– females [46] suggests that the present findings may define the general hierarchy of NKB and kisspeptin signaling involved in the induction of GnRH release.
While the site of action of NKB to induce GnRH release in the monkey remains to be established, the arcuate nucleus is considered the most likely. This is because studies in non-primate species have demonstrated that KNDy neurons within this mediobasal hypothalamic nucleus express NK3R [7,35,37], and are contacted by axonal boutons immunopositive for NKB [11]. The notion of an upstream site of NKB action on KNDy neurons in the arcuate nucleus to induce GnRH release represents a key component of contemporary models for GnRH pulse generation that have emerged recently from studies in rodents and ruminants [12,13,46]. An essential feature of these models is an integrated network of KNDy neurons formed by reciprocal connections throughout the arcuate nucleus that signal within the KNDy network via NK3R and/or the kappa opioid receptor. It is hypothesized that GnRH pulse generation originates within the network of KNDy neurons as a result of coordinated and alternating stimulatory (NK3R) and inhibitory (kappa opioid receptor) signaling, and that the output of the pulse generator is relayed from the KNDy neurons to the GnRH network by kisspeptin.
Consistent with the foregoing view is the finding that desensitization of NK3R signaling did not influence the ability of kisspeptin to elicit GnRH release. Although the significance of this observation must be tempered by the fact that desensitization of the NK3R pathway was confirmed in only 2 animals, the finding that KISS1R signaling was preserved in the face of disrupted NK3R signaling is consistent with our earlier finding that intermittent hourly infusions of senktide for 4 h, which led to a progressive decrement in the response to the NK3R agonist, did not compromise kisspeptin-induced LH release [9]. It is also consistent with the failure of the NK3R antagonist SB222200 to block kisspeptin-induced LH release in the monkey [9]. Together, these findings indicate that KISS1R signaling to elicit GnRH release in the monkey is independent of NK3R, and are therefore in line with the conclusion developed above that the site of NK3R signaling to elicit GnRH release in the monkey is upstream from that of KISS1R.
The most likely explanation for the small residual response to senktide in the face of KISS1R desensitization is that it was mediated directly on GnRH neurons, because these neurons, in rodent, express the message encoding NK3R [47], and the receptor itself has been identified by immunohistochemistry on GnRH axons in the median eminence [33]. It should be noted, however, that coexpression of GnRH and NK3R has not been observed in sheep hypothalamus [35], and has not been examined in the monkey. Interestingly, recovery of sensitivity in the NK3R pathway following termination of the continuous kisspeptin infusion was delayed relative to that of the KISS1R pathway. This suggests that, in addition to desensitization of KISS1R, an additional, occult, mechanism may have contributed to the abrogation of NK3R signaling during continuous kisspeptin administration. Whether KISS1R-independent release of GnRH/LH in response to NK3R signaling is physiologically relevant remains to be determined.
The reason for the high incidence of monkeys that failed to respond to the bolus injection of senktide under control conditions in the present study is unknown. All animals were Indian rhesus, born in the US, as were those previously studied [9], and responsiveness to senktide could not be related to either age or basal LH levels. Although sources of senktide in this and the earlier study [9] were different, pilot experiments indicated the potency of the two preparations was similar. Certainly, in the monkey, senktide administered by the i.v. route is a much less reliable GnRH secretagogue than kisspeptin. In this regard, studies of the effects of senktide on LH release in the rodent have yielded discordant results [7,29,30,31].
The response to continuous kisspeptin administration in the present study was identical to that previously reported using the same experimental model [41]. There was a dramatic and prolonged stimulation of LH release during the initial 12 h of continuous kisspeptin administration, but by the end of the 2nd day of the infusion, blood levels of this gonadotropin were indistinguishable from those in control. That desensitization of KISS1R had been achieved was confirmed by failure of an acute i.v. injection of kisspeptin-10 to elicit an LH discharge during the last 4 h of the continuous infusion, although, as expected, pituitary responsiveness to GnRH was intact.
Lastly, the failure of continuous administration of senktide at 200 μg/h to initially stimulate LH release in the present study is perhaps surprising, because the dose of senktide employed was sufficient to induce desensitization of NK3R after 44 h of exposure. Any interpretation of this result, however, should be postponed until additional rates of infusion of this agonist have been studied.
In summary, using continuous infusion of respective agonists to desensitize KISS1R or NK3R signaling in the male monkey, evidence is provided to support the view that NK3R is upstream of KISS1R in the signaling cascade within the hypothalamus that leads to pulsatile GnRH release: a mode of secretion that is essential for sustained gonadotropin secretion [48]. Such a hierarchy in these signaling pathways provides an explanation for the findings that both KISS1R signaling and NK3R signaling are obligatory for normogonadotropism in humans [1,2,3,4,5], and implies that kisspeptin agonists may have a therapeutic value in the treatment of hypogonadotropism due to disruption of NK3R signaling.
Acknowledgements
We thank the Primate and Assay Core staff (Ms. Rachel Rosland, Mr. Mike Cicco and Ms. Carolyn Phalin) of the Specialized Cooperative Centers Program in Reproduction and Infertility Research at the University of Pittsburgh Medical School for their expert technical assistance. The work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development through cooperative agreement U54 HD 08610 (T.M.P.) and U54 HD 028138 (S.B.S.) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, and National Institutes of Health Grant R01 HD 013254 to T.M.P.
References
- 1.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick A, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley W, Jr, Apaicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 2.De Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS-1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NL, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guran T, Tolhurst G, Bereket A, Rocha N, Porter K, Turan S, Gribble FM, Kotan LD, Akcay T, Atay Z, Canan H, Serin A, O'Rahilly S, Reimann F, Semple RK, Topaloglu AK. Hypogonadotropic hypogonadism due to a novel missense mutation in the first extracellular loop of the neurokinin B receptor. J Clin Endocrinol Metab. 2009;94:3633–3639. doi: 10.1210/jc.2009-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young J, Bouligand J, Francou B, Raffin-Sanson ML, Gaillez S, Jeanpierre M, Grynberg M, Kamenicky P, Chanson P, Brailly-Tabard S, Guiochon-Mantel A. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab. 2010;95:2287–2295. doi: 10.1210/jc.2009-2600. [DOI] [PubMed] [Google Scholar]
- 6.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CVR, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- 7.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151:301–311. doi: 10.1210/en.2009-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151:4494–4503. doi: 10.1210/en.2010-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Kallo I. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci. 2010;31:1984–1998. doi: 10.1111/j.1460-9568.2010.07239.x. [DOI] [PubMed] [Google Scholar]
- 11.Foradori CD, Amstalden M, Goodman RL, Lehman MN. Colocalization of dynorphin A and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol. 2006;18:534–541. doi: 10.1111/j.1365-2826.2006.01445.x. [DOI] [PubMed] [Google Scholar]
- 12.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda KI, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillations of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30:3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehman MN, Merkley CM, Coolen LM, Goodman RL. Anatomy of the kisspeptin neural network in mammals. Brain Res. 2010;1364:90–102. doi: 10.1016/j.brainres.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plant TM, Krey LC, Moossy J, McCormach JT, Hess DL, Knobil E. The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female rhesus monkey. Endocrinology. 1978;102:52–62. doi: 10.1210/endo-102-1-52. [DOI] [PubMed] [Google Scholar]
- 15.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plant TM, Ramaswamy S, DiPietro MJ. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147:1007–1013. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- 17.Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29:3920–3929. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90:6609–6615. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- 19.Dhillo WS, Chaudhri OB, Thompson EL, Murphy KG, Patterson M, Ramachandran R, Nijher GK, Amber V, Kokkinos A, Donaldson M, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab. 2007;92:3958–3966. doi: 10.1210/jc.2007-1116. [DOI] [PubMed] [Google Scholar]
- 20.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navarro VM, Tena-Sempere M. Kisspeptins and the neuroendocrine control of reproduction. Front Biosci (Schol Ed) 2011;3:267–275. doi: 10.2741/s150. [DOI] [PubMed] [Google Scholar]
- 22.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 24.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149:1979–1986. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Lee K, Herbison AE. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology. 2008;149:4605–4614. doi: 10.1210/en.2008-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C, Roepke TA, Kelly MJ, Ronnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci. 2008;28:4423–4434. doi: 10.1523/JNEUROSCI.5352-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kung TT, Crawley Y, Jones H, Luo B, Gilchrest H, Greenfeder S, Anthes JC, Lira S, Wiekowski M, Cook DN, Hey JA, Egan RW, Chapman RW. Tachykinin NK3-receptor deficiency does not inhibit pulmonary eosinophilia in allergic mice. Pharmacol Res. 2004;50:611–615. doi: 10.1016/j.phrs.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Sandoval-Guzmán T, Rance NE. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res. 2004;1026:307–312. doi: 10.1016/j.brainres.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Corander MP, Challis BG, Thompson EL, Jovanovic Z, Loraine Tung YC, Rimmington D, Huhtaniemi IT, Murphy KG, Topaloglu AK, Yeo GS, O'Rahilly S, Dhillo WS, Semple RK, Coll AP. A study of the effects of neurokinin B upon gonadotropin release in male rodents. J Neuroendocrinol. 2010;22:181–187. doi: 10.1111/j.1365-2826.2009.01951.x. [DOI] [PubMed] [Google Scholar]
- 31.Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2011;300:E202–E210. doi: 10.1152/ajpendo.00517.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman ML, McManus CJ, Nestor CC, Jacobs BH, Goodman RL. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology. 2010;151:3836–3846. doi: 10.1210/en.2010-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489:372–386. doi: 10.1002/cne.20626. [DOI] [PubMed] [Google Scholar]
- 34.Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 2010;1364:116–128. doi: 10.1016/j.brainres.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2010;22:1–12. doi: 10.1111/j.1365-2826.2009.01930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maeda KI, Ohkura S, Uenoyama Y, Wakabayashi Y, Oka Y, Tsukamura H, Okamura H. Neurobiological mechanisms underlying GnRH pulse generation by the hypothalamus. Brian Res. 2010;1364:103–115. doi: 10.1016/j.brainres.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 37.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498:712–726. doi: 10.1002/cne.21086. [DOI] [PubMed] [Google Scholar]
- 38.Plant TM, Witchel SF. Puberty in non-human primates and humans. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. vol 2. New York: Elsevier; 2006. pp. 2177–2230. [Google Scholar]
- 39.Almeida TA, Rojo J, Nieto PM, Pinto FM, Hernandez M, Martín JD, Candenas ML. Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem. 2004;11:2045–2081. doi: 10.2174/0929867043364748. [DOI] [PubMed] [Google Scholar]
- 40.Sarau HM, Griswold DE, Bush B, Potts W, Sandhu P, Lundberg D, Foley JJ, Schmidt DB, Webb EF, Martin LD, Legos JJ, Whitmore RG, Barone FC, Medhurst AD, Luttmann MA, Giardina GAM, Hay DWP. Nonpeptide tachykinin receptor antagonists. II. Pharmacological and pharmacokinetic profile of SB-222200, a central nervous system penetrant, potent and selective NK-3 receptor antagonist. J Pharmacol Exp Ther. 2000;295:373–381. [PubMed] [Google Scholar]
- 41.Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF, Jr, Plant TM. Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology. 2006;147:2122–2126. doi: 10.1210/en.2005-1550. [DOI] [PubMed] [Google Scholar]
- 42.Suter KJ, Pohl CR, Plant TM. The pattern and tempo of the pubertal reaugmentation of open-loop pulsatile gonadotropin-releasing hormone release assessed indirectly in the male rhesus monkey (Macaca mulatta) Endocrinology. 1998;139:2774–2783. doi: 10.1210/endo.139.6.6055. [DOI] [PubMed] [Google Scholar]
- 43.Gay VL, Plant TM. N-methyl-D, L-aspartate elicits hypothalamic gonadotropin-releasing hormone release in prepubertal male rhesus monkeys (Macaca mulatta) Endocrinology. 1987;120:2289–2296. doi: 10.1210/endo-120-6-2289. [DOI] [PubMed] [Google Scholar]
- 44.Plant TM. A study of the role of the postnatal testes in determining the ontogeny of gonadotropin secretion in the male rhesus monkey (Macaca mulatta) Endocrinology. 1985;116:1341–1350. doi: 10.1210/endo-116-4-1341. [DOI] [PubMed] [Google Scholar]
- 45.El Majdoubi M, Ramaswamy S, Sahu A, Plant TM. Effects of orchidectomy on levels of the mRNAs encoding gonadotropin-releasing hormone and other hypothalamic peptides in the adult male rhesus monkey (Macaca mulatta) J Neuroendocrinol. 2000;12:167–176. doi: 10.1046/j.1365-2826.2000.00433.x. [DOI] [PubMed] [Google Scholar]
- 46.Caligioni CS, Yang J, Seminara SB: The stimulatory effect of neurokinin B on GnRH/LH release in female mice is dependent on kisspeptin (abstract 633.3); in Annu Meet Soc Neurosci, San Diego, 2010.
- 47.Todman MG, Han S-K, Herbison AE. Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience. 2005;132:703–712. doi: 10.1016/j.neuroscience.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 48.Belchez PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science. 1978;202:631–633. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]