Abstract
Aims
To report the clinical and diagnostic findings of a patient with Acanthamoeba keratitis resistant to both polyhexamethylene biguanide (PHMB)-hexamidine and chlorhexidine-hexamidine treatment.
Methods
Slit-lamp biomicroscopy, corneal cell scraping and histopathology were performed on a 39-year-old woman presenting with corneal ulcer in her left eye.
Results
The patient was successfully treated with PHMB-chlorhexidine association therapy. Subsequent perforating keratoplasty remained clear at the last follow-up visit after 7 months and increased visual acuity to 20/20 with correction.
Conclusions
This case emphasizes the proteiform aspects of Acanthamoeba drug resistance, and suggests that PHMB-chlorhexidine association might represent an additional option for cases resistant to standard therapy.
Key Words: Acanthamoeba keratitis, Biguanide, Diamidine
Introduction
Acanthamoeba keratitis is a chronic infection caused by free-living amoebas, which is primarily related to contact lens use. It typically presents as a corneal ring infiltrate associated with severe pain. The infection is commonly misdiagnosed for non-infectious or bacterial, fungal or viral keratitis, as it happened in this case. A provisional diagnosis may be done based upon clinical presentation; however, definitive diagnosis requires histology, culture, and/or identification of Acanthamoeba nucleic acid by polymerase chain reaction [1]. Treatment of Acanthamoeba keratitis is based on the association of a biguanide (polyhexamethylene or chlorhexidine) with a diamidine (hexamidine or propamidine) [2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12]. Acanthamoeba keratitis is estimated to affect 1 per 250,000 people in the United States; although rates vary among studies: from 1.65–2.01 per million in the general population to 1 per 10,000 in contact lens users [2].
Case Report
A 39-year-old woman was admitted to the Cornea Unit of the San Raffaele Hospital for consultation concerning infectious keratitis in her left eye (OS). Visual acuity was counting fingers at 1 m. Ocular history was positive for mild myopia in OO (–1 sph), soft contact lens use, and fresh water exposure while wearing lenses. One month before, she had visited her ophthalmologist due to pain in her OS, and an epithelial defect was noticed. She had previously been treated with antibiotic-steroid association eye drops. After an initial improvement, the symptoms had returned, and when we visited her she complained of severe pain, especially in her OS.
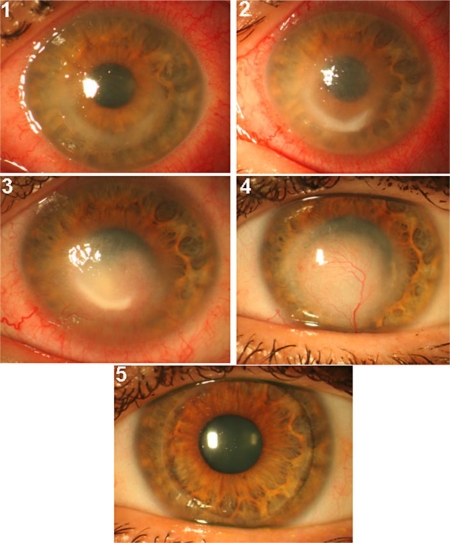
On observation of the OS, we noticed a ring infiltrate that was more evident on the inferior half of the cornea, which stained with fluorescein, and perikeratic hyperemia. The anterior chamber and the pupil were normal, and the crystalline lens was transparent (fig. 1, panel 1). Examination of the right eye was unremarkable. We performed cornea scraping, which stained positive for calcofluor white confirming an Acanthamoeba infection and started therapy with polyhexamethylene biguanide (PHMB) 0.02% and hexamidine 0.1% every 1 h. The treatment was tapered down to PHMB and hexamidine every 2 h after 5 days and subsequently to 4 times per day.
Fig. 1.
Slit-lamp picture of the evolution of Acanthamoeba keratitis. Panel 1: cornea at the first visit. Note the ring infiltrate. Panel 2: corneal infiltrate did not improve following a PHMB-desomedine regimen every 1 h after 3 months. Panel 3: the corneal ulcer showed no signs of improvement at 2 months after therapy switch to hexamidine and chlorhexidine every 1 h. Panel 4: the corneal ulcer finally receded, leaving a vascularized leucoma 4 months following combined double-biguanide PHMB-chlorhexidine treatment. Note that the conjunctival hyperemia has also resolved. Panel 5: perforating corneal graft remained transparent 7 months after transplantation and no sign of recurrence was observed.
After 3 months, the patient did not show further improvement. Visual acuity was stable at counting fingers at approximately 1 m, the cornea infiltrate was denser and corneal edema had appeared in the central cornea (fig. 1, panel 2). Corneal scraping was re-performed yielding a positive result for Acanthamoeba.
The patient was then started on hexamidine and chlorhexidine every 1 h. After 2 months, the ulcer still showed no sign of improvement. Visual acuity did not improve (counting fingers at 1 m), the eye was inflamed, the infiltrate was thicker and stained with fluorescein, and the epithelium was irregular (fig. 1, panel 3). A third corneal scraping was carried out, which was again positive for Acanthamoeba.
Treatment was then switched to PHMB and chlorhexidine every 2 h for 2 months, which was gradually tapered over the following 4 months. At this point, natural visual acuity had improved to 20/100 (20/40 with pinhole), and the patient reported a significant reduction of pain. The eye showed reduced inflammation, the infiltrate had become a leucoma with neovessels and the epithelium was stable (fig. 1, panel 4). Corneal débridement has been shown to increase drug penetration in Acanthamoeba keratitis [13], and this has possibly contributed to the treatment efficacy. However, since we performed a corneal scraping before any treatment changes, we think that the better efficacy of the last treatment (i.e. double-biguanide) is confirmed. There were no ocular signs of active infection and anti-amoebic therapy was suspended. Eight months after therapy was stopped, no signs of recurrent infection could be detected, and the patient referred no pain. We hence proposed penetrating keratoplasty to improve vision. The removed cornea was Acanthamoeba-free at histopathology examination (calcofluor white stain). The last reportable visit, 7 months after penetrating keratoplasty, revealed a clear corneal graft and no recurrence of the infection (fig. 1, panel 5); visual acuity was 20/40 with −4.5 sph and −4 cyl at 110, and reached 20/20 with contact lenses.
Discussion
Treatment of Acanthamoeba keratitis is based on the association of a biguanide (PHMB or chlorhexidine, 0.02% solution) with a diamidine (hexamidine or propamidine, 0.1% solution) [2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12] and should be carried out for weeks or months, or even longer [5, 8]. The development of drug resistance has been described during treatment [6, 14, 15]. Higher drug concentrations up to 0.06% for PHMB and up to 0.2% for chlorhexidine have been proposed in therapeutic regimens, including those in association with oral ketoconazole and topical steroids [16].
Some authors suggest that even biguanide monotherapy may be effective [17]. However, to the best of our best knowledge, there has been no report of Acanthamoeba keratitis improvement with the association of two biguanide agents.
In this case, we started treatment with a biguanide-diamidine association. The infection did not improve even when we changed biguanide (chlorhexidine instead of PHMB). The infection finally responded to the PHMB-chlorhexidine association therapy. This led to the healing of the ulcer and prompted us to subsequently perform penetrating keratoplasty, after which visual acuity reached 20/40 with correction.
We therefore suggest that double-biguanide topical treatment might be an option in cases of Acanthamoeba keratitis that are resistant to standard biguanide-diamidine association therapy.
Disclosure Statement
The authors have no proprietary and/or conflicts of interest to disclose.
Acknowledgment
The authors would like to thank Michael John of the Vita-Salute San Raffaele University for the English language editing of the manuscript.
Footnotes
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/OA-license), applicable to the online version of the article only. Distribution for non-commercial purposes only.
References
- 1.Dart JK, Saw VP, Kilvington S. Acanthamoeba keratitis: Diagnosis and Treatment-Update 2009. Am J Ophthalmol. 2009;148:487–499. doi: 10.1016/j.ajo.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Auran JD, Stan MB, Jakobiec FA. Acanthamoeba keratitis. A review of the literature Cornea. 1987;6:2–26. [PubMed] [Google Scholar]
- 3.Elder MJ, Dart JK. Chemotherapy for Acanthamoeba keratitis. Lancet. 1995;345:791–792. doi: 10.1016/s0140-6736(95)90670-3. [DOI] [PubMed] [Google Scholar]
- 4.D'Aversa G, Stern GA, Driebe WT. Diagnosis and successful medical treatment of Acanthamoeba keratitis. Arch Ophthalmol. 1995;113:1120–1123. doi: 10.1001/archopht.1995.01100090046021. [DOI] [PubMed] [Google Scholar]
- 5.Seal DV, Hay J, Kirkness CM. Chlorhexidine or polyhexamethylene biguanide for Acanthamoeba keratitis. Lancet. 1995;345:136. doi: 10.1016/s0140-6736(95)90106-x. [DOI] [PubMed] [Google Scholar]
- 6.Narasimhan S, Madhavan HN, Lily T. Development and application of an in vitro susceptibility test for Acanthamoeba species isolated from keratitis to polyhexamethylene biguanide and chlorexidin. Cornea. 2002;21:201–205. doi: 10.1097/00003226-200203000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Niederkorn JY. The role of the innate and adaptive immune responses in Acanthamoeba keratitis. Arch Immunol Ther Exp (Warsz) 2002;50:53–59. [PubMed] [Google Scholar]
- 8.Duguid IG, Dart JK, Morlet N, Allan BD, Matheson M, Ficker L, Tuft S. Outcome of Acanthamoeba keratitis treated with polyhexamethyl biguanide and propamidine. Ophthalmology. 1997;104:1587–1592. doi: 10.1016/s0161-6420(97)30092-x. [DOI] [PubMed] [Google Scholar]
- 9.Hay J, Kirkness CM, Seal DV, Wright P. Drug resistance and Acanthamoeba keratitis: the quest for alternative protozoal chemotherapy. Eye. 1994;8:555–563. doi: 10.1038/eye.1994.137. [DOI] [PubMed] [Google Scholar]
- 10.Elder MJ, Kilvington S, Dart JKG. A clinicopathologic study of in vitro sensitivity testing and Acanthamoeba keratitis. Invest Ophthalmol Vis Sci. 1994;35:1059–1064. [PubMed] [Google Scholar]
- 11.Seal DV, Hay J, Kirkness C, Morrell A, Booth A, Tullo A, Ridgway A, Armstrong M. Successful medical therapy of Acanthamoeba keratitis with topical chlorexidine and propamidine. Eye. 1996;10:413–421. doi: 10.1038/eye.1996.92. [DOI] [PubMed] [Google Scholar]
- 12.Tirado-Angel J, Gabriel MM, Wilson LA, Ahearn DG. Effects of polyhexamethylene biguanide and chlorexidine on four species of Acanthamoeba in vitro. Curr Eye Res. 1996;15:225–228. doi: 10.3109/02713689608997418. [DOI] [PubMed] [Google Scholar]
- 13.Ishibashi Y, Matsumoto Y, Kabata T, Watanabe R, Hommura S, Yasuraoka K, Ishii K. Oral itraconazole and topical miconazole with débridement for Acanthamoeba keratitis. Am J Ophthalmol. 1990;109:121–126. doi: 10.1016/s0002-9394(14)75974-4. [DOI] [PubMed] [Google Scholar]
- 14.Wysenbeek YS, Blank-Porat D, Harizman N, Wygnanski-Jaffe T, Keller N, Avni I. The reculture technique: individualizing the treatment of Acanthamoeba keratitis. Cornea. 2000;19:464–467. doi: 10.1097/00003226-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Bang S, Edell E, Eghrari AO, Gottsch JD. Treatment with voriconazole in 3 eyes with resistant Acanthamoeba keratitis. Am J Ophthalmol. 2010;149:66–69. doi: 10.1016/j.ajo.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Mathers W. Use of higher medication concentrations in the treatment of Acanthamoeba keratitis. Arch Ophthalmol. 2006;124:923. doi: 10.1001/archopht.124.6.923. [DOI] [PubMed] [Google Scholar]
- 17.Lim N, Goh D, Bunce C, Xing W, Fraenkel G, Poole TRG, Ficker L. Comparison of polyhexamethylene biguanide and chlorexidine as monotherapy agents in the treatment of Acanthamoeba keratitis. Am J Ophthalmol. 2008;145:130–135. doi: 10.1016/j.ajo.2007.08.040. [DOI] [PubMed] [Google Scholar]